Abstract
Background
Functional magnetic resonance imaging (fMRI) holds promise as a noninvasive means of identifying neural responses that can be used to predict treatment response before beginning a drug trial. Imaging paradigms employing facial expressions as presented stimuli have been shown to activate the amygdala and anterior cingulate cortex (ACC). Here, we sought to determine whether pretreatment amygdala and rostral ACC (rACC) reactivity to facial expressions could predict treatment outcomes in patients with generalized anxiety disorder (GAD).
Methods
Fifteen subjects (12 female subjects) with GAD participated in an open-label venlafaxine treatment trial. Functional magnetic resonance imaging responses to facial expressions of emotion collected before subjects began treatment were compared with changes in anxiety following 8 weeks of venlafaxine administration. In addition, the magnitude of fMRI responses of subjects with GAD were compared with that of 15 control subjects (12 female subjects) who did not have GAD and did not receive venlafaxine treatment.
Results
The magnitude of treatment response was predicted by greater pretreatment reactivity to fearful faces in rACC and lesser reactivity in the amygdala. These individual differences in pretreatment rACC and amygdala reactivity within the GAD group were observed despite the fact that 1) the overall magnitude of pretreatment rACC and amygdala reactivity did not differ between subjects with GAD and control subjects and 2) there was no main effect of treatment on rACC-amygdala reactivity in the GAD group.
Conclusions
These findings show that this pattern of rACC-amygdala responsivity could prove useful as a predictor of venlafaxine treatment response in patients with GAD.
Keywords: Amygdala, anxiety, facial expressions, fear, neuroimaging, rostral anterior cingulate cortex
The human amygdala is a critical part of a neural circuitry that functions to detect environmental stimuli that predict biologically relevant outcomes (1,2). For example, in humans, the amygdala is particularly sensitive to presented facial expressions of emotion, presumably because these nonverbal cues have predicted important outcomes in the past (3). The amygdala is reciprocally connected with rostal and ventral regions of the anterior cingulate cortex (ACC) (4,5). Furthermore, it has been demonstrated that the ACC is part of a neural circuit that can provide regulatory control over the amygdala (5-12). This control can occur spontaneously (6), can be directed (7-10), or can occur in reaction to a change in environmental contingencies (e.g., extinction) (11,12). Of specific relevance to the present experiment are data showing that greater rostral ACC (rACC) reactivity in response to facial expressions of emotion is correlated with lesser amygdala reactivity (6,7). Thus, facial expressions represent simple stimuli for assessing amygdalaprefrontal reactivity and provide a basis to assess amygdalaprefrontal function in psychopathology (13-23).
The use of facial expressions as stimuli has proven useful for the study of the anxiety disorders. For example, exaggerated amygdala responsivity to fearful facial expressions (13,14,16) as well as hyporesponsivity within the rACC (13,14) has been observed in individuals with posttraumatic stress disorder (PTSD). We sought to extend this work by determining whether amygdala and rACC reactivity to facial expressions could predict treatment outcomes in patients with generalized anxiety disorder (GAD). Such data will be particularly important since functional imaging data involving these structures in adult GAD subjects is lacking.
Generalized anxiety disorder is a chronic disorder that is characterized by excessive, pervasive, and uncontrollable anxiety that is present for a period of at least 6 months (DSM-IV) (24). Venlafaxine is a serotonin and norepinephrine reuptake inhibitor (SNRI) that has been shown to be effective in treating subjects with GAD. For example, Pollack et al. (25) showed in a meta-analysis of five venlafaxine treatment studies of GAD that, on average, 56% of GAD subjects treated with drug for 8 weeks showed considerable improvement (i.e., >50% drop in symptoms). This efficacy rate was significantly greater than the 39% who showed improvement in the placebo-treated groups. Thus, if roughly more than half of the GAD subjects in the present study showed improvement after 8 weeks of venlafaxine treatment, this would provide the requisite variability across subjects to identify pretreatment functional magnetic resonance imaging (fMRI) predictors of beneficial treatment response.
To this end, the current report presents voxelwise regression analyses from an exploratory open-label study aimed at determining whether pretreatment activity in the amygdala and/or rACC would be related to changes in reported anxiety after an 8-week drug treatment trial with venlafaxine. More generally, the present study sought to offer an example of the promise of functional neuroimaging for informing pharmaco-therapy.
Methods and Materials
Participants
Thirty subjects (24 female subjects) shown to be right-handed (26) were studied. Fifteen of these subjects (12 female subjects) answered a local newspaper advertisement seeking individuals with GAD for a venlafaxine treatment study (mean age 27 ± 7 [SD] years; range 19-52). The 15 control subjects who were also recruited via newspaper advertisement (mean age 33 ± 11 [SD] years; range 20-51) did not differ significantly in age [t(28) = 1.79, p > .05] or in years of education [t(28) = .58, p > .05]. All control subjects were screened and found to be free of history of head injury and current DSM-IV Axis I disorders as determined by the Structured Clinical Interview for DSM (SCID) (24). All control subjects scored below 9 on the Hamilton Anxiety Rating Scale (HAM-A) (27) and below 7 on the Raskin Depression Scale (28).
All subjects with GAD met diagnostic criteria for GAD as determined by the SCID and verified by physician interview. In addition to the SCID interview as confirmation of the diagnosis of GAD, subjects also exceeded an accepted scale cutoff on the HAM-A (i.e., ≥18) (27) with scores of 2 or more on item 1 (anxious mood) and item 2 (tension). Further, we specifically recruited subjects with GAD who were low in depressive symptoms. To this end, all subjects with GAD scored below 7 on the Raskin Depression Scale (Table 1). Also, all GAD subjects did not meet diagnostic criteria according to the SCID for depression or any other Axis I disorder. All subjects were medication free for at least 4 weeks if they had been taking fluoxetine and for 2 weeks for all other psychoactive substances.
Table 1.
Pretreatment and Posttreatment Information for GAD and Control Subjects
| Gender | HAM-A |
PSWQ |
Raskin |
|||
|---|---|---|---|---|---|---|
| (Pre) | (Post) | (Pre) | (Post) | (Pre) | (Post) | |
| Subjects with GAD | ||||||
| F | 18 | 5 | 68 | 43 | 5 | 4 |
| M | 19 | 2 | 43 | 37 | 6 | 3 |
| M | 19 | 7 | 64 | 46 | 4 | 5 |
| F | 19 | 6 | 71 | 67 | 5 | 4 |
| F | 21 | 11 | 68 | 42 | 5 | 4 |
| F | 19 | 5 | 66 | 62 | 5 | 4 |
| F | 19 | 9 | 78 | 56 | 4 | 4 |
| F | 21 | 12 | 79 | 78 | 5 | 5 |
| M | 19 | 4 | 69 | 42 | 5 | 4 |
| F | 19 | 9 | 72 | 69 | 4 | 5 |
| F | 19 | 5 | 52 | 52 | 5 | 3 |
| F | 18 | 13 | 78 | 80 | 4 | 3 |
| F | 19 | 11 | 73 | 66 | 6 | 6 |
| F | 18 | 5 | 40 | 38 | 5 | |
| F | 19 | 2 | 74 | 6 | 3 | |
| Control Subjects | ||||||
| F | 1 | 2 | 28 | 28 | 3 | 3 |
| M | 1 | 1 | 36 | 36 | 3 | 3 |
| M | 2 | 0 | 25 | 29 | 3 | 3 |
| F | 0 | 0 | 30 | 36 | 3 | 3 |
| F | 0 | 2 | 33 | 30 | 3 | 3 |
| F | 0 | 0 | 35 | 36 | 3 | 3 |
| F | 1 | 3 | 25 | 28 | 3 | 3 |
| F | 1 | 1 | 31 | 26 | 3 | 3 |
| M | 1 | 5 | 32 | 40 | 3 | 4 |
| F | 1 | 3 | 33 | 33 | 3 | 3 |
| F | 1 | 2 | 30 | 32 | 3 | 3 |
| F | 3 | 3 | 31 | 26 | 3 | 3 |
| F | 2 | 1 | 34 | 33 | 3 | 3 |
| F | 3 | 4 | 35 | 30 | 3 | 4 |
| F | 2 | 0 | 32 | 37 | 3 | 3 |
Control subjects received no treatment but are labeled to be consistent with subjects with GAD. PSWQ was not collected from one GAD subject posttreatment-this subject's data were not included in the PSWQ analysis. Raskin was not collected for one GAD subject posttreatment-this subject's data were not included in correlational analyses corrected for depression.
F, female; GAD, generalized anxiety disorder; HAM-A, Hamilton Anxiety Rating Scale; M, male; PSWQ, Penn State Worry Questionnaire; Post, post-treatment; Pre, pretreatment; Raskin, Raskin Depression Scale.
All subjects provided informed written consent and were monetarily compensated for their participation. This investigation was conducted in accordance with the guidelines of the Human Subjects Committee of the University of Wisconsin-Madison.
Procedure
All GAD subjects were assessed at seven visits over an 8-week period after starting venlafaxine treatment. Compliance with venlafaxine treatment was monitored at these visits through the use of personal diaries kept by subjects throughout the 8-week treatment phase documenting their compliance with treatment. Subjects were titrated from 37.5 mg by mouth every morning to 225 mg by mouth every morning per clinical indication. Most subjects (75%) were at 150 mg by week 4 and remained there for the duration of the study. Hamilton Anxiety Rating Scale and Penn State Worry Questionnaire (PSWQ) (29) scores were administered at each visit to assess treatment efficacy. Control subjects were assessed at similar intervals over 8 weeks but received no treatment. Participants and experimenters were not blind to the participants' group status.
The present report presents scan data before beginning treatment (pretreatment for GAD subjects) and that following 8 weeks of treatment (posttreatment for the GAD subjects), consistent with most previous studies that have also utilized an 8-week period to assess the efficacy of venlafaxine (25). Subjects were also scanned 2 weeks following venlafaxine treatment, but these data are not considered here since most GAD subjects did not achieve their optimal drug level until week 4. Thus, data from both GAD and control subjects' fMRI scans at pretreatment and 8 weeks posttreatment are analyzed here for assessment of between-group effects, whereas pretreatment fMRI data from GAD subjects are used to assess the prediction of treatment outcomes.
One week prior to the pretreatment scan session, all subjects attended a 30-minute fMRI simulation session in a mock scanner to reduce any initial apprehension or anxiety. A week after the mock scan, all subjects participated in a pretreatment fMRI session. Subjects with GAD then began an 8-week treatment trial of venlafaxine extended release (XR) the day following their first scan.
An Avotec goggle system (Avotec, Inc., Stuart, Florida) was used to present visual stimuli. Padding was arranged around the subject's head, which together with use of a bite bar served to minimize head movement. During fMRI scanning, participants passively viewed alternating 18-second blocks of fearful (F), neutral (N), and happy (H) facial expressions during two scan runs, where F and H block order was counterbalanced within and across subjects. Each scan run started and finished with an 18-second fixation (+) baseline block; thus, a typical scan would be as follows: +, N, H, N, F, N, H, N, F, +.
Each block consisted of six repetitions of six identities (three female identities) from a standardized stimulus set (30) (identities used were PE, SW, WF, PF, C, GS). All stimuli were standardized for contrast and luminance. Each expression was displayed for 200 milliseconds, with an intertrial interval (ITI) of 300 milliseconds consisting of a fixation cross on a black background (i.e., two faces per second).
These data were collected as part of a larger project assessing venlafaxine's effects on neural responsivity during multiple fMRI activation paradigms. To this end, during scanning, all subjects participated in the current facial expression study, as well as a subsequent study involving presentation of affective pictures (i.e., International Affective Picture System [IAPS]) (31). The face study was always presented first. The project also involved two further scanning sessions where the same tasks were presented 2 and 8 weeks posttreatment. The test-retest reliability for amygdala reactivity during the face task for the control subjects has been published previously (32).
Image Acquisition
A 3-Tesla SIGNA magnetic resonance imaging (MRI) scanner (General Electric Medical Systems, Waukesha, Wisconsin) with a quadrature head coil and high-speed gradients was used to acquire both whole-brain, axial, high-resolution anatomical scans (three-dimensional [3-D] spoiled gradient recalled [SPGR]; 240 mm field of view [FOV], 256 × 192 in-plane resolution; 124 slices, 1.1 mm slice thickness) and functional gradient-echo echo planar imaging (EPI) scans. Eighteen partial brain (amygdala centered) coronal oblique functional slices were obtained (3 mm slice thickness; .5 mm interslice gap; 64 × 64 in-plane resolution; 180 mm FOV; 108 3-D volumes per scan run; repetition time [TR]/echo time [TE]/flip angle # 2000 msec/30 msec/60°). The slices are centered on the amygdala (perpendicular to the anterior commissure-posterior commissure [AC-PC] line and then tilted ~30° in the rostral direction to provide coverage of the amygdala and medial prefrontal cortex including the anterior cingulate cortex). This slice acquisition has been used extensively in our laboratory and previous studies by the authors because it displaces through-plane dephasing, phase cancellation, and phase dispersion in the medial temporal lobe. This results in data in this region relatively free of susceptibility artifacts and dropout, with higher signal-to-noise ratio and between-session reliability (32). A similar acquisition scheme has recently been proposed as optimal for amygdala imaging (33).
Image Analysis
All data processing was performed using AFNI software (http://afni.nimh.nih.gov) (34). Individual subject data were motion corrected, low-pass filtered (cutoff = .15 Hz), and then analyzed using a general linear model (GLM) with separate regressors for each expression type, formed by convolving a stimulus boxcar function with an ideal hemodynamic response function (HRF). The GLM yielded a set of contrast maps for each individual, which were smoothed with a 4-mm full-width at half maximum Gaussian smoothing kernel, converted to percent signal change and normalized into Talairach space. Individual contrast maps were then entered into two types of voxelwise group analyses. Consistent with the aims of the study, we constrained analyses to voxels within two a priori regions of interest: rACC and amygdala. Voxelwise regression analyses were used to assess the association between brain activation contrasts and ratings of treatment response (HAM-A, PSWQ), as well as other potential confounds such as rated depression (Raskin Depression Scale). A mixed-effects GLM with subject as a random factor nested within the fixed factor group (control versus GAD) and scan session as a fixed repeated factor was used to assess group and session differences in brain activation to the different expressions. For all voxelwise analyses, the statistical threshold was set at p = .05 corrected, determined separately for the amygdala (cluster threshold = 72 mm3) and rACC (cluster threshold = 144 mm3) a priori areas of interest by Monte Carlo simulations using AlphaSim (within AFNI) based on their anatomical volumes, respectively.
Results
fMRI Predictor of Treatment Response in Subjects with GAD: Reported Anxiety
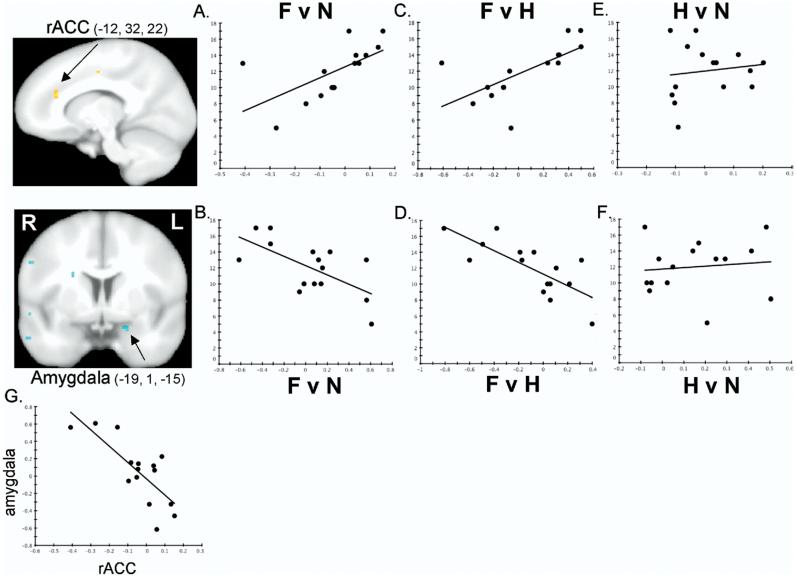
Table 1 presents pretreatment and posttreatment HAM-A scores for all subjects. Individual differences in decrease in HAM-A scores were then correlated with pretreatment amygdala and rACC responsivity of subjects with GAD to fearful faces. Figure 1A depicts a significant positive correlation between drop in HAM-A scores (y axis) and pretreatment rACC (x =-12, y = 32, z = 22) responsivity (x axis) to fearful faces when compared with neutral faces (r = .61, p = .016). Figure 1B shows a concomitant negative correlation between drop in HAM-A scores and pretreatment amygdala (x = -19, y = 1, z =-15) responsivity to fearful faces when compared with neutral faces (r =-.64, p = .01).
Figure 1.
fMRI statistical maps showing that pretreatment responsivity to fearful versus neutral faces is positively correlated in the left rACC (red) and negatively correlated in the left amygdala (blue) with subjects' drop in HAM-A scores after 8 weeks of treatment with venlafaxine. R=right, L=left. Images thresholded at p = .05, corrected.FvN=fear versus neutral contrast in the rACC(A) and amygdala(B). Y axis=drop in HAM-A scores after 8 weeks of treatment (i.e., pretreatment minus posttreatment score); X axis=fMRI percent signal change. Higher rACC reactivity and lower amygdala reactivity predict a greater drop in reported anxiety. Results are similar for the fear versus happy (FvH) contrast(C, D) but not the happy versus neutral (HvN) contrast (E, F) (data presented from the FvN contrast locus), showing that these effects are specific to fearful faces. Axes same as in (A) and (B). (G) rACC and amygdala responses to fearful versus neutral faces are negatively correlated showing that the subjects with higher rACC signal tend to be the subjects with lower amygdala signal. Both axes are fMRI percent signal change. fMRI, functional magnetic resonance imaging; HAM-A, Hamilton Anxiety Rating Scale; rACC, rostral anterior cingulate cortex.
Similar effects were observed when fearful faces were contrasted with happy faces in the rACC (Figure 1C; r = .67, p = .006; x =-7, y = 35, z = 25) and the amygdala (Figure 1D; r = -.75, p = .001; x =-19, y = 1, z =-15). These effects showed strong spatial correspondence with the effects observed for the fearful versus neutral faces contrast, being observed in an immediately adjacent region of the left rACC and the identical locus within the left amygdala.
Figures 1E and 1F further support that these effects are specific to fearful faces since they are not observed in happy versus neutral faces when directly contrasted in rACC (Figure 1E; r = .14, p = .63) or amygdala (Figure 1F; r = .11, p = .69) and these happy versus neutral correlations are significantly different than the correlations reported here for fear (p's ≤ .05).
Figure 1G shows that the subjects showing greater rACC reactivity to fearful faces tend to be the subjects who show lesser amygdala reactivity (fear vs. neutral, r =-.77, p = .001, depicted in Figure 1G; fear vs. happy, r = -.72, p = .002, not shown). Taken together, subjects showing higher rACC and lower amygdala reactivity to fearful faces before starting venlafaxine treatment show the greatest drop in reported anxiety after 8 weeks of treatment.
Finally, these effects were unrelated to any change in depression scores with treatment, as the partial correlations between pretreatment fMRI response to fearful faces and change in HAM-A remained significant after partialing out changes in depression scores (amygdala, fear vs. neutral, r = -.61, p = .027, fear vs. happy, r =-.72, p = .005; rACC, fear vs. neutral, r = .60, p = .03, fear vs. happy, r = .63, p = .02).
Other Brain Regions
The anterior cingulate and amygdala were the clear focus of the present report, as evidenced by the partial brain volume we collected focusing on these areas (see Methods and Materials). For completeness, we assessed whether other brain regions within our collected volume showed a similar predictive pattern of activation. No other brain region showed correlated activation that exceeded a whole-volume correction threshold. To obviate bias, we report that one subcortical region exceeded the threshold based on amygdala volume (hippocampus, x = -27, y = -10, z = -15, 120 mm3 cluster) and one cortical region exceeded the threshold set for the rACC volume (middle temporal gyrus, x = -55, y = -12, z = -12, 184 mm3 cluster).
Reported Worry
Table 1 also presents PSWQ scores for these subjects. Voxel-wise regression analyses showed that amygdala and rACC responsivity were unrelated to changes in reported worry in GAD subjects after venlafaxine treatment (all p's > .05, corrected). Further, the effects reported here for drop in HAM-A scores remain significant after partialing out changes in PSWQ scores (amygdala, fear vs. neutral, r = -.58, p = .029, fear vs. happy, r = -.72, p = .004; rACC, fear vs. neutral, r = .55, p = .04, fear vs. happy, r = .65, p = .012).
GAD Versus Control Subjects
Control subjects did not show any of the above reported relationships between fMRI responsivity and HAM-A or PSWQ scores consistent with their restricted range of scores on these measures (Table 1). Further, though presentation of fearful versus neutral faces produced bilateral activation of the amygdala across all subjects (right, x = 17, y = -5, z = -10, p = .00027, uncorrected; left, x = -19, y = -3, z = -10, p = .0016, uncorrected), there was no group difference between control subjects and subjects with GAD in amygdala or rACC reactivity to fearful versus neutral faces, fearful versus happy faces, or happy versus neutral faces when directly contrasted (all F's < 1). In addition, there was no effect of time (pre vs. post) and no time × group interaction (all F's < 1). Thus, the present report focuses on the finding that individual differences in pretreatment rACC-amygdala fMRI responsivity to fearful faces in subjects with GAD predicted treatment outcomes, though the magnitude of their fMRI responses as a group did not differ from control subjects.
Discussion
Pretreatment fMRI responsivity during presentation of biologically relevant stimuli associated with threat (i.e., fearful faces) predicted treatment response to venlafaxine in subjects with GAD. Specifically, greater rACC and lesser amygdala responsivity to fearful faces predicted greater decreases in anxiety after 8 weeks of treatment. This effect was specific to fearful faces, as no such effect was observed to either happy or neutral faces when directly contrasted (Figure 1E and F).
Given the correlational nature of the present findings, the directional nature of rACC-amygdala interaction during pretreatment facial expression processing and its relationship with treatment outcomes cannot be known. One possibility is that activation of the rACC in response to fearful faces exerts a regulatory influence over the amygdala (6-13) and lower amygdala activation to fearful faces predicts a more beneficial outcome in terms of reported anxiety with venlafaxine. This hypothesis is consistent with anatomical data in nonhuman primates showing that the multiple regions of the ACC that send direct projections to the amygdala include a more dorsal portion of the rACC (4) similar to that identified here in humans, as well as a recent report suggesting that the outputs from the ACC to the amygdala greatly outnumber the amygdala's reciprocal inputs (35). That said, based upon the present data, the opposite alternative explanation is equally plausible; namely, that subjects who respond with a higher amygdala response to fearful faces, in turn, show a lesser rACC response, which predicts a less beneficial outcome in terms of reported anxiety with venlafaxine. Available anatomical data in nonhuman primates support this interpretation as well: the amygdala is known to project to a more dorsal region of the rACC in nonhuman primates (5,35) similar to that identified here in human subjects. Finally, despite the existence of direct reciprocal connections between these two regions, the present effects do not necessitate a functional interaction, as activity at each region could be independently related to change in anxiety.
The present study in subjects reporting high levels of anxiety complements previous studies in subjects with depression showing that neural responsivity in the rACC (36,37) and amygdala (38) can predict drug treatment outcomes (18,39). Here we show that inverse rACC-amygdala activity can predict changes in reported anxiety as measured by the HAM-A in adult subjects with GAD who were specifically recruited because of their low levels of depressive symptoms and lack of comorbid diagnosis. Given the high comorbidity between GAD and depression (40) and the fact that amygdala reactivity can be related to the severity of reported depression (41,42), future studies could assess subjects with varying degrees of anxious and depressive symptoms to determine any overlapping and nonoverlapping neural substrates that predict changes in anxious versus depressive symptoms with treatment.
Interestingly, the present findings were observed in a group of subjects with GAD whose overall magnitude of amygdala reactivity did not differ from the control group. This finding adds to existing experimental data assessing amygdala responsivity to fearful face stimuli across different anxiety disorders. To date, exaggerated amygdala responsivity to emotional facial expressions has been documented in PTSD (13,14,16) and social phobia (20,23) but not in simple phobia (43) or GAD (present study). More complex fMRI response patterns have been observed in other studies, perhaps owing to differences in the acuteness of the disorder (PTSD) (44), stimulus presentation parameters (i.e., masked vs. unmasked, PTSD) (16,44), and medication status (panic disorder) (45). The relatively small number of subjects assessed in this initial study calls for caution in the interpretation of the present between-group null effect, since such an effect could be due to insufficient statistical power, though we note that the comparison studies referenced above (some showing positive between-group effects) comprised subject samples of similar size. Alternatively, the lack of group differences reported here could be an artifact of elevated baseline amygdala blood flow in GAD consistent with findings that baseline blood flow correlates negatively with the magnitude of evoked blood oxygenation level-dependent (BOLD) responses (46). This possibility could be explicitly tested using positron-emission tomography (PET) imaging or perfusion imaging in future fMRI studies.
The present results are qualified by the following limitations. For this initial study, we employed an open-label design with no comparison group of subjects with GAD receiving a placebo treatment. Thus, we cannot know if the subjects who improved would have shown this effect irrespective of venlafaxine treatment. That said, we point out that while prior studies predict that 39% of subjects would be expected to improve on a placebo basis (25), 73% of subjects within the present study showed a significant drop in anxiety symptoms with venlafaxine treatment (defined as >50% decrease according to the criteria of Pollack et al. [25]). The present study design is further limited by our use of self-report as a measure of drug compliance. A more rigorous means of documenting compliance in future studies would entail blood/urine samples. Finally, our use of a passive viewing task does not allow us to verify that subjects were attentive to the presented stimuli.
Despite the lack of a between-group main effect in the present study, individual differences in rACC and amygdala activation predicted beneficial treatment outcomes for the subjects with GAD. These pretreatment individual differences in rACC-amygdala reactivity were observed even though all subjects with GAD showed a homogeneous level of pretreatment anxiety (HAM-A scores for all subjects ranged from 18 to 21). Thus, the observed individual differences in pretreatment rACC-amygdala reactivity to fearful faces do not identify the presence of GAD or its accompanying anxiety symptoms per se. Nor can these homogeneous pretreatment levels of anxiety explain the degree of improvement with treatment in some but not other subjects. Taken together, these data suggest that the presentation of fearful faces to assess ACC-amygdala responsivity can identify some subjects reporting high anxiety but low depression symptoms who will benefit from venlafaxine treatment. Perhaps the fMRI response pattern identified here in subjects showing a greater treatment effect (i.e., higher rACC-lower amygdala) indicates an integrity of rACC-amygdala responsivity and/or interaction that can benefit from venlafaxine treatment. Clearly, replication of the present effect, along with further research into the possible mechanisms for such an outcome, is needed. More generally, the present study suggests that there is promise for the use of fMRI as a tool to predict drug treatment outcomes for patients with clinically significant anxiety.
Acknowledgments
Supported by Wyeth Pharmaceuticals to NHK and National Institute of Mental Health (NIMH) (069315 to RJD, NHK, and PJW; 01866 to PJW; and 63984 to JBN).
We thank Thomas Ihde-Scholl, Lesley Tarelton, and Robin Chene for clinical assistance; Michael Anderle and Ron Fischer for technical support; and Jonathan Oler and anonymous reviewers for comments.
NHK is a consultant to Wyeth-Ayerst Pharmaceuticals who supported the current research. In the past 3 years, Dr. Kalin has also consulted to Amgen, Astra Zeneca, Bristol-Myers-Squibb, Corcept, CeNeRx Biopharma, Cypress Biosciences, Cyberonics, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Lilly, Neurocrine Bio, Neuronetics, Pfizer Pharmaceuticals, and Sanofi-Syntholabs. He owns stock in Neurocrine, Corcept, and CeNeRx Biopharma. He is the major owner of Promoter Neuro-sciences. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online.
References
- 1.LeDoux JE. The Emotional Brain. Simon-Schuster; New York: 1996. [Google Scholar]
- 2.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 3.Whalen PJ. Fear, vigilance and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 4.Pandya DN, Van Hoeseon GW, Mesulam M-M. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 5.Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dys-function. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- 6.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 8.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J Cogn Neurosci. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Shin LM, Wright CI, Cannistraro P, Wedig M, McMullin K, Martis B. A functional magnetic resonance imaging study of amygdala and me-dial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 14.Williams LM, Kemp AH, Felmingham K, Barton M, Oliveri G, Peduto A. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 15.Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, et al. FMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- 16.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 17.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD. FMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 18.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 20.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 21.Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. Facial emotion recognition in schizophrenia: Intensity effects and error pattern. Am J Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- 22.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 23.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- 25.Pollack MH, Meoni P, Otto MW, Hackett D. Predictors of outcome following venlafaxine extended-release treatment of DSM-IV generalized anxiety disorder: A pooled analysis of short-and long-term studies. J Clin Psychopharmacol. 2003;23:250–259. doi: 10.1097/01.jcp.0000084025.22282.84. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Raskin A, Schulterbrandt J, Reatig N, McKeon JJ. Replication of factors of psychopathology in interview, ward behavior and self-report ratings of hospitalized depressives. J Nerv Ment Dis. 1969;148:87–98. doi: 10.1097/00005053-196901000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State worry questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 30.Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- 31.Lang PJ, Öhman A, Vaitl D. The International Affective Picture System [photographic slides] The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1998. [Google Scholar]
- 32.Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, et al. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Chen NK, Dickey CC, Yoo SS, Guttmann CRG, Panych LP. Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: Application to imaging of the amygdala. Neuroimage. 2003;19:817–825. doi: 10.1016/s1053-8119(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 34.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 37.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: A potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 38.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 39.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 40.Stein MB, Heimberg RG. Well-being and life satisfaction in generalized anxiety disorder: Comparison to major depressive disorder in a community sample. J Affect Disord. 2004;79:161–166. doi: 10.1016/S0165-0327(02)00457-3. [DOI] [PubMed] [Google Scholar]
- 41.Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 42.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;15:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- 44.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 45.Pillay SS, Gruber SA, Rogowska J, Simpson N, Yurgelun-Todd DA. FMRI of fearful facial affect recognition in panic disorder: The cingulate gyrus-amygdala connection. J Affect Disord. 2006;94:173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Vazquez AL, Cohen ER, Gulani V, Hernandez-Garcia L, Zheng Y, Lee GR, et al. Vascular dynamics and BOLD fMRI: CBF level effects and analysis considerations. Neuroimage. 2006;32:1642–1655. doi: 10.1016/j.neuroimage.2006.04.195. [DOI] [PubMed] [Google Scholar]