Abstract
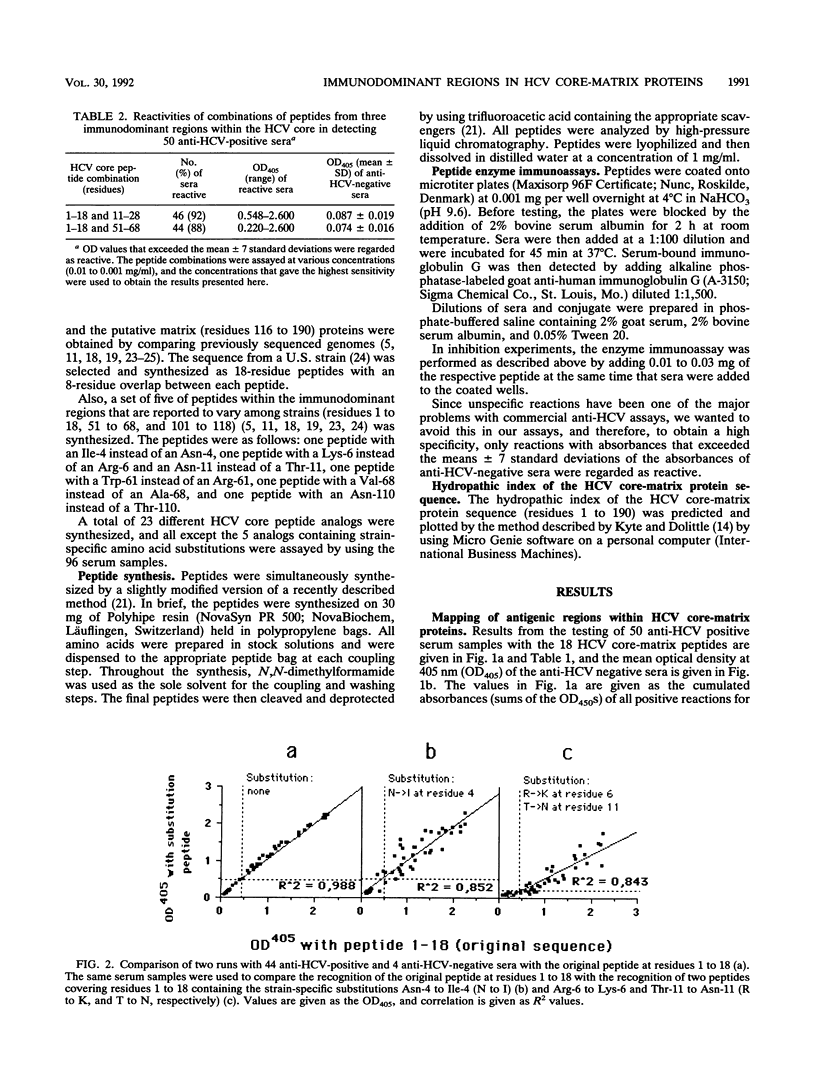
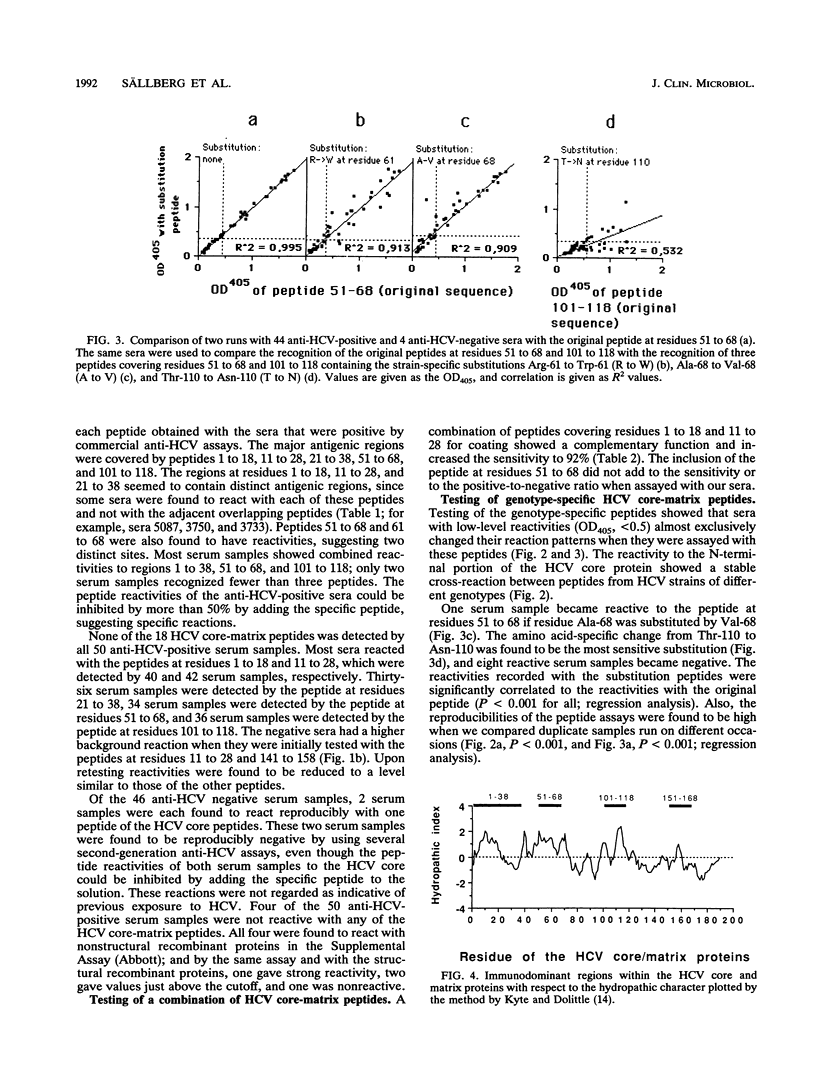
The complete amino acid sequences of hepatitis C virus (HCV) core (residues 1 to 115) and putative matrix (residues 116 to 190) proteins were synthesized as 18-residue-long peptides with an 8-amino-acid overlap. The peptides were assayed with 50 human serum samples with antibodies to HCV (anti-HCV) and 46 serum samples without anti-HCV, as determined by several commercial assays. Immunodominant regions were defined within residues 1 to 18, 11 to 28, 21 to 38, 51 to 68, and 101 to 118. The peptides that covered these regions were recognized by 40 of 50 (80%), 42 of 50 (84%), 36 of 50 (72%), 34 of 48 (68%), and 36 of 48 (72%) of the anti-HCV positive serum samples, respectively. Two anti-HCV negative serum samples were each repeatedly reactive with one peptide, but both were found to be negative by confirmatory anti-HCV assays. Four serum samples that were confirmed to be positive for anti-HCV in commercial assays did not recognize any of the peptides that cover the HCV core-matrix regions. Ninety-two percent of anti-HCV-positive serum samples reacted with a combination of peptides covering residues 1 to 18 and 11 to 28. Testing of peptides that contain the reported genotypic variations of the HCV core within the regions at residues 1 to 18, 51 to 68, and 101 to 118 showed that a change from Thr-110 to Asn-110 decreased the reactivities of eight serum samples. In conclusion, we found that human antibodies to the HCV core-matrix protein(s) are mainly directed to linear determinants and can easily be reproduced by using short synthetic peptides. We also found that such antibodies develop in more than 90% of HCV-infected people.
Full text
PDF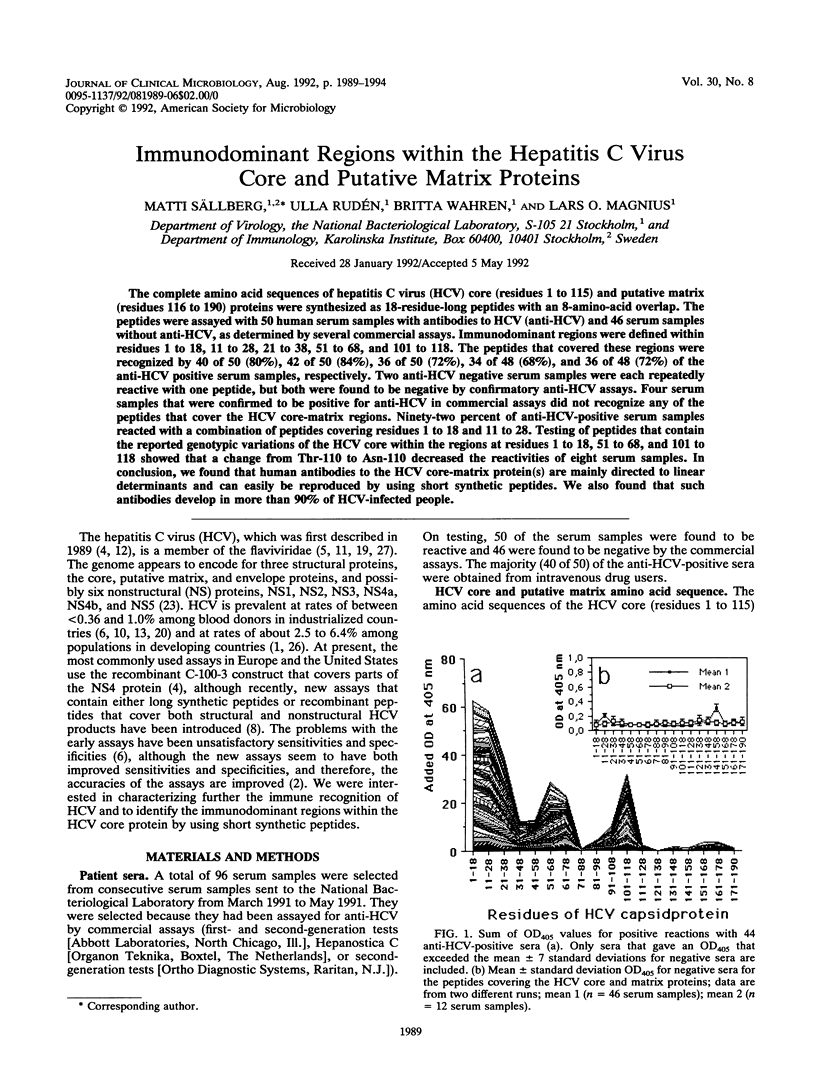


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaudhary R. K., MacLean C. Evaluation of first- and second-generation RIBA kits for detection of antibody to hepatitis C virus. J Clin Microbiol. 1991 Oct;29(10):2329–2330. doi: 10.1128/jcm.29.10.2329-2330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba J., Ohba H., Matsuura Y., Watanabe Y., Katayama T., Kikuchi S., Saito I., Miyamura T. Serodiagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. J., Lesniewski R. R., Stewart J. L., Boardway K. M., Gutierrez R. A., Pendy L., Johnson R. G., Alcalde X., Rote K. V., Devare S. G. Detection of antibodies to hepatitis C virus in U.S. blood donors. J Clin Microbiol. 1991 Mar;29(3):551–556. doi: 10.1128/jcm.29.3.551-556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns R. B., Tedder R. S. Human and monoclonal antibodies to hepatitis B core antigen recognise a single immunodominant epitope. J Med Virol. 1986 Jun;19(2):193–203. doi: 10.1002/jmv.1890190213. [DOI] [PubMed] [Google Scholar]
- Hosein B., Fang C. T., Popovsky M. A., Ye J., Zhang M., Wang C. Y. Improved serodiagnosis of hepatitis C virus infection with synthetic peptide antigen from capsid protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3647–3651. doi: 10.1073/pnas.88.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba S., Fukuda M., Okochi K., Irita Y., Tokunaga K., Kiyokawa H., Maeda Y. HCV transmission after receiving anti-c100-negative blood units. Lancet. 1991 Jun 1;337(8753):1354–1354. doi: 10.1016/0140-6736(91)93036-9. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Guay L., Goldfarb J., Olness K., Ndugwa C., Mmiro F., Kataaha P., Allain J. P. Hepatitis C virus antibody in HIV-1 infected Ugandan mothers. Lancet. 1991 Mar 2;337(8740):551–551. doi: 10.1016/0140-6736(91)91334-q. [DOI] [PubMed] [Google Scholar]
- Janot C., Couroucé A. M., Maniez M. Antibodies to hepatitis C virus in French blood donors. Lancet. 1989 Sep 30;2(8666):796–797. doi: 10.1016/s0140-6736(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kühnl P., Seidl S., Stangel W., Beyer J., Sibrowski W., Flik J. Antibody to hepatitis C virus in German blood donors. Lancet. 1989 Aug 5;2(8658):324–324. doi: 10.1016/s0140-6736(89)90500-x. [DOI] [PubMed] [Google Scholar]
- Mimms L., Vallari D., Ducharme L., Holland P., Kuramoto I. K., Zeldis J. Specificity of anti-HCV ELISA assessed by reactivity to three immunodominant HCV regions. Lancet. 1990 Dec 22;336(8730):1590–1591. doi: 10.1016/0140-6736(90)93377-2. [DOI] [PubMed] [Google Scholar]
- Nasoff M. S., Zebedee S. L., Inchauspé G., Prince A. M. Identification of an immunodominant epitope within the capsid protein of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5462–5466. doi: 10.1073/pnas.88.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Sirchia G., Bellobuono A., Giovanetti A., Marconi M. Antibodies to hepatitis C virus in Italian blood donors. Lancet. 1989 Sep 30;2(8666):797–797. doi: 10.1016/s0140-6736(89)90852-0. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Magnius L. O., Harthus H. P., Noah M., Wahren B. Characterisation of a linear binding site for a monoclonal antibody to hepatitis B core antigen. J Med Virol. 1991 Apr;33(4):248–252. doi: 10.1002/jmv.1890330407. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Magnius L. O., Norrby E., Wahren B. Rapid "tea-bag" peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol Lett. 1991 Sep;30(1):59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. Nucleotide sequence of core and envelope genes of the hepatitis C virus genome derived directly from human healthy carriers. Nucleic Acids Res. 1990 Aug 11;18(15):4626–4626. doi: 10.1093/nar/18.15.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. The putative nucleocapsid and envelope protein genes of hepatitis C virus determined by comparison of the nucleotide sequences of two isolates derived from an experimentally infected chimpanzee and healthy human carriers. J Gen Virol. 1990 Dec;71(Pt 12):3027–3033. doi: 10.1099/0022-1317-71-12-3027. [DOI] [PubMed] [Google Scholar]
- Tibbs C. J., Palmer S. J., Coker R., Clark S. K., Parsons G. M., Hojvat S., Peterson D., Banatvala J. E. Prevalence of hepatitis C in tropical communities: the importance of confirmatory assays. J Med Virol. 1991 Jul;34(3):143–147. doi: 10.1002/jmv.1890340302. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Brauer M. J., Rosenblatt J., Richman K. H., Tung J., Crawford K., Bonino F., Saracco G., Choo Q. L., Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991 Feb;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]


