Abstract
Reactive oxygen species (ROS) increase ligation of Fas (CD95), a receptor important for regulation of programmed cell death. Glutathionylation of reactive cysteines represents an oxidative modification that can be reversed by glutaredoxins (Grxs). The goal of this study was to determine whether Fas is redox regulated under physiological conditions. In this study, we demonstrate that stimulation with Fas ligand (FasL) induces S-glutathionylation of Fas at cysteine 294 independently of nicotinamide adenine dinucleotide phosphate reduced oxidase–induced ROS. Instead, Fas is S-glutathionylated after caspase-dependent degradation of Grx1, increasing subsequent caspase activation and apoptosis. Conversely, overexpression of Grx1 attenuates S-glutathionylation of Fas and partially protects against FasL-induced apoptosis. Redox-mediated Fas modification promotes its aggregation and recruitment into lipid rafts and enhances binding of FasL. As a result, death-inducing signaling complex formation is also increased, and subsequent activation of caspase-8 and -3 is augmented. These results define a novel redox-based mechanism to propagate Fas-dependent apoptosis.
Introduction
Fas (CD95; Apo-1) is a member of the tumor necrosis factor receptor superfamily of death receptors that shares a conserved 80 amino acid death domain (DD) in their cytoplasmic tail critical in apoptosis signaling (Peter et al., 2007). Upon ligation of Fas, the sequential association of Fas-associated DD (FADD), pro forms of caspase-8 and -10, and cellular FADD-like IL-1β–converting enzyme inhibitory protein occurs, leading to the formation of the death-inducing signaling complex (DISC) with resulting oligomerization, processing, and activation of caspase-8 and execution of apoptosis via direct or indirect programs (Wajant, 2002). Fas is constitutively expressed in tissues, and although its role in apoptosis is well established, additional regulatory roles of Fas that include immune cell activation and proliferation have recently been suggested (Tibbetts et al., 2003).
The production of reactive oxygen species (ROS) has traditionally been associated with cellular and tissue injury as a result of the high reactivity of some oxidant species. Compelling data now exist to demonstrate that oxidants are used under physiological settings as signaling molecules that control processes such as cell division, migration, and mediator production (Lambeth, 2004; Janssen-Heininger et al., 2008). Amino acids that are targets for reversible oxidations are cysteines with a low pKa sulfhydryl group, and numerous classes of proteins contain conserved reactive cysteine groups. These cysteines can be reversibly oxidized to sulfenic acids, S-nitrosylated cysteines, or disulfides, or can be irreversibly oxidized to sulfinic or sulfonic acids (Hess et al., 2005; Janssen-Heininger et al., 2008; for review see Forman et al., 2004). S-glutathionylation reflects the formation of a disulfide between the cysteine of glutathione and the cysteine moiety of a protein (also known as protein-mixed disulfide or PSSG [protein S-glutathionylation]) and has emerged as an important mechanism to regulate reversible cysteine oxidations as it occurs in the cellular environment where glutathione concentrations are in the millimolar range (Fernandes and Holmgren, 2004). Under physiological conditions, the thiol transferases glutaredoxin 1 (Grx1) and 2 in mammalian cells specifically catalyze reduction of PSSG, restoring the protein cysteine to the sulfhydryl state (Fernandes and Holmgren, 2004).
Various studies exist to support a role of redox regulation of the Fas death pathway. Caspases contain a reactive cysteine critical for enzymatic activity, and a role for nitric oxide in preventing caspase activation has been established based upon findings demonstrating that caspase-3 and -9 are S-nitrosylated under basal conditions to prevent activation (Mannick et al., 1999, 2001; Benhar et al., 2008). In response to a proapoptotic stimulus, such as Fas ligand (FasL), thioredoxin-2 (Trx2)–mediated denitrosylation of caspase-3 occurs, which is a process required for caspase-3 activation and subsequent execution of the apoptotic pathway (Mannick et al., 1999, 2001; Benhar et al., 2008). Fas-mediated apoptosome formation was also shown to involve ROS derived from mitochondrial permeability transition (Sato et al., 2004). Furthermore, Fas-dependent cell death in response to highly reactive oxidants has been reported in association with clustering of Fas (Huang et al., 2003; Shrivastava et al., 2004), whereas conversely antioxidant compounds attenuate Fas-dependent cell death (Huang et al., 2003). Based on those collective observations, we sought to establish the physiological relevance of redox-based regulation of Fas. In this study, we describe a novel mechanism whereby Fas-dependent cell death is regulated. This pathway is initiated via caspase-dependent degradation of Grx1, subsequent increases in S-glutathionylation of cysteine 294 of Fas (which promotes binding of FasL and enhances recruitment into lipid rafts), formation of SDS-resistant high molecular weight (MW) Fas complexes, and DISC, and subsequently further augments activation of caspases, thereby amplifying cell death.
Results
Increases in PSSG by FasL occur independently of generation of ROS but instead are associated with degradation of Grx1
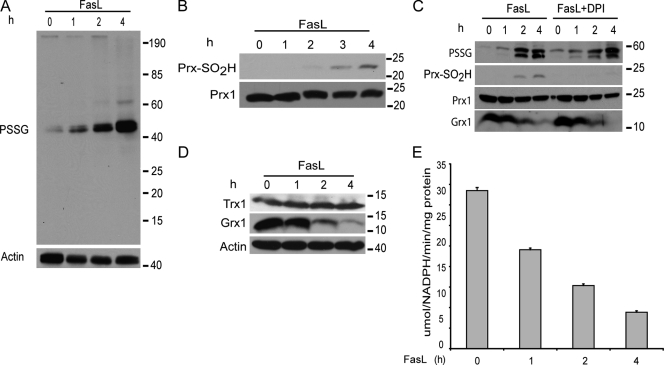
S-glutathionylation represents a redox-based modification of cysteines, which is a regulatory switch that affects cell signaling. Therefore, we addressed whether levels of PSSG were increased after ligation of Fas in lung epithelial cells using nonreducing SDS-PAGE and immunoblot analysis with an antibody directed against glutathione. Results shown in Fig. 1 A demonstrate a marked increase in PSSG that occurred as early as 1 h after Fas ligation.1 Increases in PSSG occurred with significant specificity based on the appearance of two bands of an apparent MW between 40 and 50 kD (Fig. 1, A and C) that comigrated with Fas (not depicted).
Figure 1.
Increases in PSSG and down-regulation of Grx1 in cells stimulated with FasL. (A) Evaluation of PSSG. C10 lung epithelial cells were stimulated with 200 ng/ml FasL + 500 ng/ml cross-linking antibody M2. Cells were harvested at the indicated time points, lysates were resolved on nonreducing SDS-PAGE, and immunoblot analysis was performed using antiglutathione antibody. The bottom panel shows immunoblotting for β-actin as a loading control. (B) Assessment of overoxidation of Prx after ligation of Fas. C10 cells were exposed as described in A, and cell lysates were subjected to SDS-PAGE. The oxidized form of Prx was detected by immunoblot analysis using a specific antibody directed against overoxidized Prx (Prx-SO2H). The bottom panel shows total Prx1 content. (C) Lack of requirement of NADPH oxidase activity in FasL-induced increases in PSSG. C10 cells were treated with FasL in the presence or absence of the inhibitor of 10 µM NADPH oxidase DPI. Lysates were prepared at the indicated times for assessment of PSSG as described in A. Total levels of Prx1, Prx-SO2H, or Grx1 were assessed by immunoblotting. (D) Evaluation of total Grx1 and Trx1 content by immunoblotting in cells exposed to FasL + M2 as described in A. The bottom panel shows β-actin content as a loading control. (E) Evaluation of enzymatic activity of Grx1 in C10 cells exposed to 200 ng/ml FasL + 500 ng/ml M2 for 1–4 h. Results represent triplicate values from two independent experiments. The time-dependent decrease in Grx1 activity was significant at the level of P < 0.05 by Student's t test. Error bars represent SEM.
The current paradigm of redox-based signaling after stimulation of growth factor receptors is the production of ROS by the activation of NADPH oxidases, which causes inhibition of tyrosine phosphatases (Rhee et al., 2000). Overoxidation of the antioxidant molecule peroxiredoxin (Prx) is believed to be critical in promoting signaling by H2O2 (Wood et al., 2003). Results shown in Fig. 1 B demonstrate that oxidized Prx (Prx-SO2H) appeared between 2 and 3 h after stimulation with FasL, which are time points protracted relative to the accumulation of PSSG, which occurred as early as 1 h (Fig. 1 A). Incubation of cells with diphenyliodonium (DPI), an inhibitor of NADPH oxidases, failed to attenuate the increases in cellular PSSG content, whereas overoxidation of Prx was completely inhibited by DPI (Fig. 1 C). These findings suggest that FasL-induced increases in PSSG and Prx-SO2H occur through independent mechanisms and demonstrate that increases in PSSG occur in an NADPH oxidase–independent manner.
Mammalian cells contain Grxs, which under physiological conditions act to reverse S-glutathionylated cysteines, restoring the protein cysteine to the sulfhydryl group. Levels of cytosolic Grx1 were markedly reduced by 2 and 4 h after ligation of Fas (Fig. 1 D) with corresponding decreases in enzymatic activity (Fig. 1 E). Consistent with the lack of effect of PSSG, DPI also failed to restore Grx1 levels in cells stimulated with FasL (Fig. 1 C). In contrast to decreases in Grx1, expression of the related disulfide reductase Trx1 remained unchanged in response to ligation of Fas (Fig. 1 D), demonstrating that the redox changes caused by ligation of Fas showed some specificity toward Grx1.
Activation of caspases is required for degradation of Grx1 and S-glutathionylation of Fas
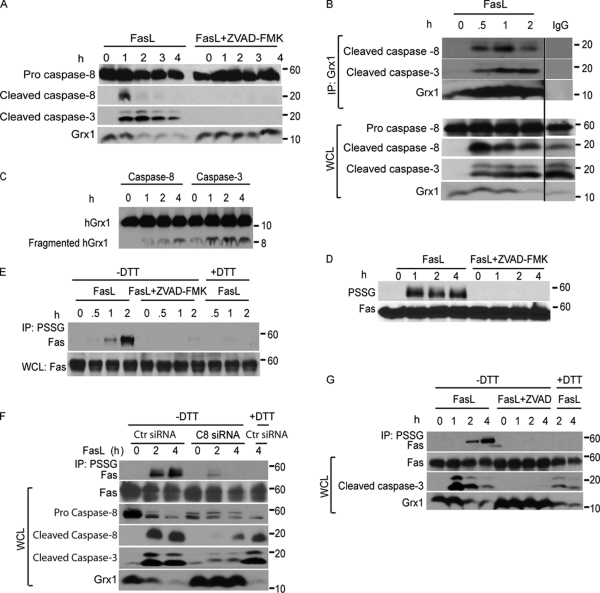
Engagement of Fas causes a rapid activation of caspase-8 and -3 (Hengartner, 2000). Sequence analysis of murine Grx1 suggested that amino acids 43–46 (EFVD) and 56–59 (AIQD) may be putative cleavage sites of caspase-8 and -3, both of which have predicted affinity toward glutamic and aspartic acid residues (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1; Earnshaw et al., 1999). This raised the possibility that upon ligation of Fas, Grx1 was degraded in a caspase-dependent fashion. Indeed, pretreatment of cells with a generic caspase inhibitor, ZVAD-FMK, effectively blocked FasL-induced cleavage of caspase-8 and -3 and completely prevented FasL-induced degradation of Grx1 (Fig. 2 A). Immunoprecipitation (IP) of Grx1 followed by immunoblot analysis of cleaved caspase-8 and -3 demonstrated an association between active caspases and Grx1 in cells after ligation of Fas, whereas in control cells, these associations were not observed (Fig. 2 B). Incubation of recombinant Grx1 with active caspase-8 or -3 in vitro led to the formation of a fragment of ∼8 kD, which was more apparent in response to caspase-3 as compared with caspase-8 (Fig. 2 C). Consistent with the protection against Grx1 degradation (Fig. 2 A), pretreatment of cells with ZVAD-FMK prevented the formation of detectable levels of PSSG after FasL stimulation (Fig. 2 D). Based on our observations that proteins that were S-glutathionylated upon stimulation of cells with FasL comigrated with Fas, we speculated that Fas itself could be a target for S-glutathionylation. Lysates from FasL-treated cells were immunoprecipitated using an antiglutathione antibody followed by detection of Fas by immunoblotting. After FasL stimulation, Fas-SSG (S-glutathionylated Fas) was detectable as early as 1 h after Fas ligation with further increases apparent after 2 h (Fig. 2 E). To confirm the specificity of the immunoreactivity, the S-glutathionylated proteins were reduced with 50 mM DTT. As expected, samples treated with DTT (Fig. 2 E, +DTT) before IP showed no immunoreactivity for Fas, demonstrating the PSSG-specific IP of Fas in response to stimulation with FasL (Fig. 2 E). Lastly, pretreatment of cells with ZVAD-FMK before FasL resulted in no detectable Fas-SSG (Fig. 2 E), which is consistent with the absence of PSSG in cells exposed to ZVAD-FMK within this time frame (Fig. 2 D). To corroborate the requirement of caspases in the degradation of Grx1 and accumulation of Fas-SSG, we treated lung epithelial cells with control or caspase-8–specific siRNA. Results shown in Fig. 2 F demonstrate that FasL-induced degradation of Grx1 and accumulation of Fas-SSG were largely absent in cells with markedly lowered caspase-8 content. Caspase-dependent degradation of Grx1 and accumulation of Fas-SSG were also readily apparent in NIH 3T3 cells (Fig. 2 G), demonstrating that these redox changes occur in cell types other than lung epithelial cells. Lastly, incubation of cells with staurosporine did not cause S-glutathionylation of Fas nor marked degradation of Grx1 despite causing robust cleavage of caspase-3 (Fig. S1 B), suggesting that activation of caspases by other agonists is insufficient to cause the formation of Fas-SSG. In aggregate, these findings demonstrate that after stimulation of cells with FasL, caspase-dependent degradation of Grx1 occurs in association with increases in Fas-SSG. Our data also suggest that caspase activation is necessary but may not be sufficient for degradation of Grx1.
Figure 2.
FasL induces caspase-dependent cleavage of Grx1 and increases PSSG as well as S-glutathionylation of Fas. (A) Immunoblot analysis of cleaved caspase-8 (p18) and -3 fragments (p17 and p19) in C10 cells treated with FasL + M2 as described in Fig. 1 in the presence or absence of 10 µM ZVAD-FMK. The bottom panel shows total cellular content of Grx1. Note that expression of the pro form of caspase-8 remains unchanged during the course of the experiment. (B) Evaluation of the interaction between Grx1 and caspase-8 or -3 in cells. C10 cells were exposed to FasL + M2 as described in Fig. 1 A, and Grx1 was immunoprecipitated (IP) at the indicated times for the evaluation of association with active caspase-8 or -3 fragments via Western blotting. The bottom panel represents a Grx1 immunoblot. Lanes on the right represent lysates from cells treated with FasL + M2 for 2 h but were subjected to IgG IP as a reagent control. All samples were run on the same gel, and the lanes were cut and reassembled for consistency. The bottom panels represent total content of proteins in whole cell lysates (WCL) that were used as the input for IP. Note that expression of the pro form of caspase-8 remains unchanged during the course of the experiment. Black line indicates that intervening lanes have been spliced out. (C) In vitro assessment of cleavage of Grx1 by caspase-8 or -3. 200 ng recombinant hGrx1 was incubated with 200 U active caspase-8 or -3. At the indicated times, samples were prepared for immunoblot analysis of hGrx1. Fragmented hGrx1 product is ∼8 kD in size. Incubation of heat-inactivated caspase-8 and -3 with hGrx1 for 4 h largely prevented the formation of cleaved fragment (0 h). (D) Increases in overall PSSG are a response to ligation of Fas and are caspase dependent. Cells were incubated as described in A. ZVAD-FMK or vehicle was added to cells 2 h before ligation of Fas as well as 2 h after ligation. Lysates were resolved by nonreducing SDS-PAGE. Antiglutathione antibody was used to detect PSSG on immunoblots. The bottom panel shows total Fas content. (E) Caspase-dependent S-glutathionylation of Fas. C10 cells were incubated with FasL + M2 for 0.5, 1, or 2 h in the presence or absence of ZVAD-FMK. Cell lysates were subjected to nonreducing IP (−DTT) using antiglutathione antibody to IP S-glutathionylated proteins (IP: PSSG) before detection of Fas via Western blotting. As a reagent control to reduce S-glutathionylated proteins before IP, samples were incubated with 50 mM DTT (+DTT). The bottom panel represents Fas content in cell lysates. (F) S-glutathionylation of Fas requires the presence of caspase-8. C10 cells were transfected with control (Ctr) siRNA or caspase-8 (C8)–specific siRNA and 48 h later were incubated with FasL + M2 for 2 or 4 h. The top lane shows assessment of S-glutathionylation of Fas via IP of S-glutathionylated proteins using antiglutathione antibody (IP: PSSG) under nonreducing conditions (−DTT) before detection of Fas via Western blotting. As a reagent control to reduce S-glutathionylated proteins before IP, samples were incubated with 50 mM DTT (+DTT). The bottom panels show total content of Fas, procaspase-8, cleaved caspase-8, cleaved caspase-3, and Grx1 in whole cell lysates. (G) Assessment of caspase-dependent degradation of Grx1 and S-glutathionylation of Fas in NIH 3T3 cells after ligation of Fas. Cells were treated with 500 ng/ml FasL + 1 µg/ml M2 for 1, 2, or 4 h in the presence or absence of ZVAD-FMK. S-glutathionylated proteins were immunoprecipitated as described in E before detection of Fas via Western blotting. The bottom panel represents Fas content, cleaved caspase-3, and Grx1 content in whole cell lysates.
Increased S-glutathionylation of Fas results in enhanced apoptosis in cells lacking Grx1
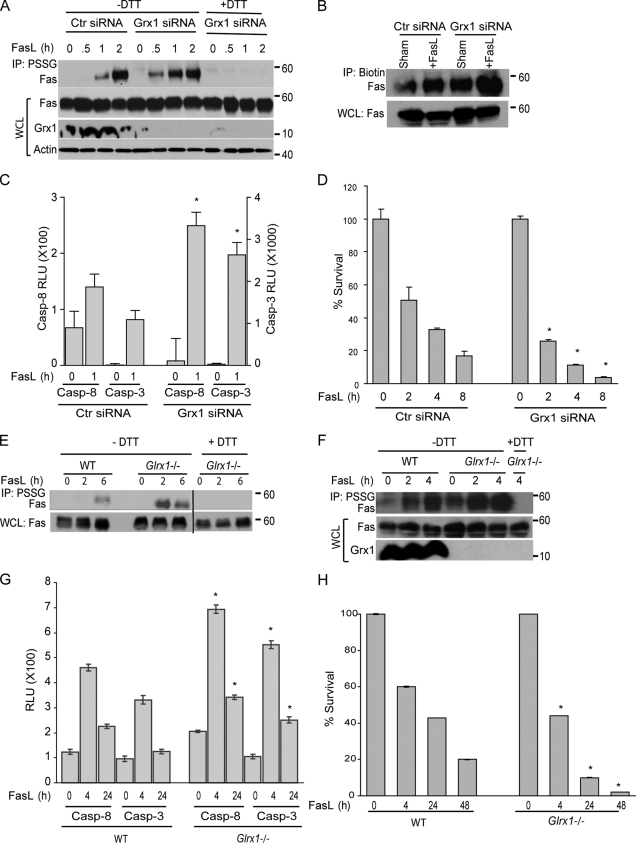
We next examined the impact of modulation of Grx1 on Fas-SSG and the subsequent sensitivity of cells to undergo apoptosis. Transfection of lung epithelial cells with a Grx1-specific siRNA caused a marked decrease in the cellular content of Grx1 (Fig. 3 A). Two independent approaches were used to assess Fas-SSG that encompassed IP with an antibody against glutathione (Fig. 3 A) or preloading of cells with a biotinylated version of glutathione followed by incubation with an antibiotin antibody to IP S-glutathionylated proteins (Fig. 3 B). Results demonstrate that FasL-induced Fas-SSG was enhanced in cells with a lowered expression of Grx1 as compared with controls (Fig. 3, A and B). We were also able to detect Fas-SSG under nonstimulated conditions and increases after knockdown of Grx1 only after labeling of cells with biotinylated glutathione (Fig. 3 B), presumably because of the enhanced sensitivity of detection of PSSG using the latter approach as compared with the antiglutathione antibody. In addition to increasing Fas-SSG, FasL-stimulated activation of caspase-8 and -3 was also enhanced in cells subjected to Grx1 siRNA knockdown compared with controls (Fig. 3 C). Cells with lowered Grx1 content were less viable compared with controls, and Grx1 siRNA–treated cells were more sensitive to FasL-induced death than controls (Fig. 3 D). Similarly, in comparison to C57BL6 (wild type [WT]) controls, primary cultures of lung fibroblasts (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1) or proliferating CD4+ T lymphocytes derived from Glrx1−/− mice (Ho et al., 2007) demonstrated a higher extent of Fas-SSG upon receptor ligation (Fig. 3, E and F). Although we were unable to detect S-glutathionylation of Fas in the absence of FasL in lung fibroblasts (Fig. 3 E), possibly as a result of the aforementioned detection limits of our assay, baseline Fas-SSG was detected in Glrx1−/− CD4+ T lymphocytes before stimulation with FasL (Fig. 3 F). Enhanced FasL-induced activation of caspase-8 and -3 was readily apparent in Glrx1−/− fibroblasts, which were also less viable before, or in response to Fas ligation compared with WT counterparts (Fig. 3, G and H).
Figure 3.
Increased S-glutathionylation of Fas, caspase-8 activity, and cell death in cells lacking Grx1. (A) Assessment of S-glutathionylation of Fas after knockdown of Grx1. C10 cells were transfected with Grx1 siRNA or control (Ctr) siRNA and treated with FasL + M2 for the indicated times. S-glutathionylated proteins were immunoprecipitated using antiglutathione antibody (IP: PSSG). Samples treated with 50 mM DTT to reduce S-glutathionylated proteins (+DTT) were used as reagent controls. The content of Fas, Grx1, and actin in whole cell lysates (WCL) used as the input for IP are shown in the bottom panels. (B) Assessment of S-glutathionylation of Fas after loading of cells with biotinylated glutathione. siRNA-transfected cells were labeled with 5 mM biotinylated glutathione ethyl ester for 1 h before treatment with FasL. After 2 h of FasL + M2 treatment, glutathionylated proteins in lysates were immunoprecipitated using antibiotin antibody followed by immunoblot detection of Fas. The bottom panel shows total Fas expression in whole cell lysates as a loading control. (C) Evaluation of caspase-8 and -3 enzymatic activities in cells after knockdown of Grx1. C10 cells were transfected with control or Grx1 siRNA before stimulation with FasL + M2 for 1 h, and lysates were prepared for evaluation of caspase activity. Results are expressed as mean + SEM relative luminescence units (RLU)/20,000 cells. The graph represents triplicate values obtained from two independent experiments. *, P < 0.05 compared with respective control siRNA groups (ANOVA). (D) Impact of Grx1 knockdown on cell survival. Cells were transfected with control or Grx1 siRNA and exposed as described in A. Cell survival was assessed using the MTT assay. Results are expressed at a percent of survival compared with respective untreated control groups and are presented as mean values + SEM of triplicate values obtained from two independent experiments. Note that survival in the Grx1 siRNA–treated cells was 11% lower than the control siRNA–treated cells in the absence of stimulation with FasL. *, P < 0.05 compared with respective control siRNA groups (ANOVA). (E) Assessment of S-glutathionylation of Fas in lung fibroblasts lacking Glrx1. WT or Glrx1−/− cells were exposed to FasL + M2 for the indicated times, and S-glutathionylation of Fas was determined as described in A. All samples were run on the same gel, and the lanes were cut and reassembled for consistency. Note that S-glutathionylation of Fas in these primary cells is relatively protracted compared with results obtained in the C10 cell line. Black line indicates that intervening lanes have been spliced out. (F) Assessment of S-glutathionylation of Fas in CD4+ T lymphocytes lacking Glrx1. WT or Glrx1−/− cells were exposed to FasL + M2 for the indicated times, and S-glutathionylation of Fas was determined as in A. The bottom panels show content of Fas and Grx1 in whole cell lysates. (G) Caspase-8 and -3 activities in primary lung fibroblasts isolated from WT or Glrx1−/− mice in response to exposure to FasL + M2 for the indicated times. Results are expressed as mean ± SEM relative luminescence units (RLU)/20,000 cells. The graph represents triplicate values obtained from two independent experiments. *, P < 0.05 compared with respective WT groups (ANOVA). Note that activation of caspases in these primary cells is relatively protracted compared with results obtained in the C10 cell line. (H) Comparative assessment of FasL-induced cell death in WT or Glrx1−/− primary lung fibroblasts. Cells were exposed as indicated, and survival was assessed using the MTT assay. Results are expressed as a percent of survival compared with respective untreated control groups and are presented as mean values + SEM of triplicate values obtained from two independent experiments. Note that survival in the Glrx1−/− cells was 13% lower than their WT counterparts in the absence of stimulation with FasL. *, P < 0.05 compared with the WT FasL-treated group (ANOVA). Note that FasL-induced cell death in the primary cells is relatively protracted compared with results obtained in the C10 cell line.
Overexpression of Grx1 prevents increases in S-glutathionylation of Fas and attenuates caspase activation and apoptosis in response to receptor ligation
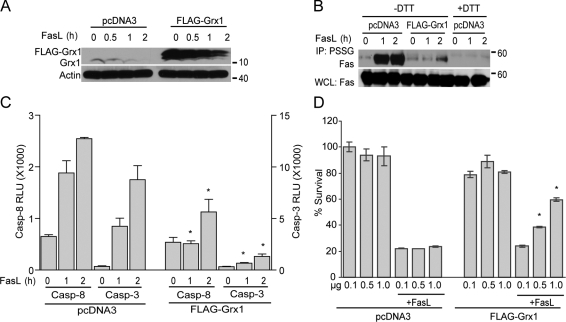
Because Grx1 deficiency caused an increase in FasL-induced caspase activation, Fas-SSG, and enhanced cell death, we speculated that overexpression of Grx1 would reduce levels of Fas-SSG and afford protection from FasL-induced apoptosis. Cells transfected with Flag-Grx1 were stimulated with FasL and examined for the S-glutathionylation status of Fas (Fas-SSG) by IP with antiglutathione antibody. Compared with pcDNA3 vector–transfected cells, overexpression of Grx1 (Fig. 4 A) markedly reduced Fas-SSG (Fig. 4 B) and markedly lowered the activities of caspase-8 and -3 (Fig. 4 C) induced in response to FasL. Overexpression of Grx1 also markedly enhanced survival (Fig. 4 D) in response to ligation of Fas as compared with controls.
Figure 4.
Overexpression of Grx1 decreases S-glutathionylation of Fas, caspase-8 and -3 activities, and cell death. (A) Assessment of Grx1 expression. C10 cells were transfected with pcDNA3 or Flag-Grx1 plasmids (1 µg/4 × 105 cells). Cells were stimulated with FasL + M2 for 2 h, and lysates were analyzed by immunoblotting for Grx1 expression. The bottom panel shows β-actin as a loading control. (B) Assessment of S-glutathionylation of Fas after overexpression of Grx1. pcDNA3 or Flag-Grx1–transfected cells were stimulated with FasL + M2, and after 2 h, S-glutathionylated proteins were immunoprecipitated using an antibody directed against glutathione (IP: PSSG). +DTT samples were used as reagent controls. The bottom panel represents Fas content in whole cell lysates (WCL). (C) Assessment of caspase-8 and -3 activities in cells after overexpression of Grx1. C10 cells were transfected with pcDNA3 or Flag-Grx1 plasmids and stimulated with FasL + M2. At the indicated times, cells were harvested, and lysates were prepared for evaluation of caspase activity. Results are expressed as mean ± SEM relative luminescence units (RLU)/20,000 cells. The graph represents triplicate values obtained from two independent experiments. *, P < 0.05 compared with FasL-treated pcDNA3-treated control groups (ANOVA). (D) Assessment of FasL-induced death in cells overexpressing Grx1. Cells were transfected with the indicated amounts of pcDNA3 or Flag-Grx1 plasmid (total DNA content 1.25 µg/4 × 105 cells) and stimulated with FasL + M2. After 2 h, cell survival was assessed using the MTT assay. Results represent triplicate values (mean ± SEM) from two independent experiments. *, P < 0.05 compared with the pcDNA3-transfected group (Student's t test).
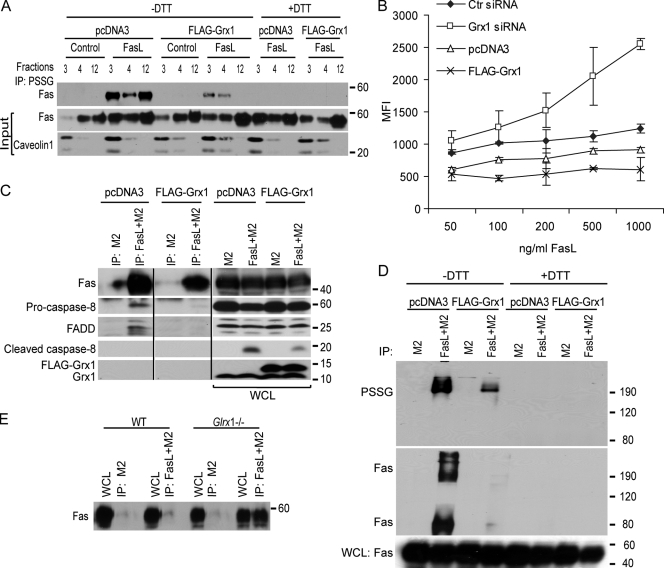
S-glutathionylation of Fas promotes binding of FasL, recruitment to lipid rafts, formation of SDS-stable high MW complexes, and enhances formation of the death-inducing signaling complex
The localization of Fas in lipid rafts is essential for binding of FasL, assembly of DISC, and subsequently the induction of apoptosis (Hueber et al., 2002; Muppidi and Siegel, 2004). Therefore, we determined whether the extent of Fas-SSG affected these parameters. As expected, after stimulation of cells with FasL, an increase in the amount of Fas localized to the lipid rafts occurred based on its colocalization with raft marker caveolin1 (Fig. 5 A and Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). IP of S-glutathionylated proteins revealed that in response to FasL ligation, Fas-SSG was present within the lipid raft fractions as well as soluble fractions (Fig. 5 A), whereas Grx1 was absent in the lipid raft fraction (Fig. S3 A). In cells overexpressing Grx1, the overall content of Fas and its S-glutathionylated state were decreased in lipid rafts compared with mock-transfected cells stimulated with FasL (Fig. 5 A and Fig. S3 A). Assessment of FasL binding demonstrated dose-dependent increases in WT cells. However, in cells overexpressing Grx1, no clear increases in FasL binding occurred at a range of concentrations (Fig. 5 B and Fig. S3 B). Furthermore, IP of the DISC revealed clear associations between FasL, Fas, FADD, and procaspase-8, which were diminished in cells overexpressing Grx1 (Fig. 5 C). Grx1 was absent in DISC in all conditions evaluated (Fig. 5 C and not depicted), which may sustain the presence of Fas-SSG in these signaling platforms. IP of FasL resulted in high MW SDS-stable PSSG complexes that were attenuated in cells overexpressing Grx1 and absent in samples treated with DTT (Fig. 5 D). Evaluation of Fas in these samples confirmed its presence in the high MW complex (∼190 kD) after IP with FasL. This high MW Fas complex was sensitive to decomposition by DTT and markedly decreased in Grx1-overexpressing cells (Fig. 5 D), demonstrating that PSSG contributes to the formation of high MW Fas complexes that are known to be required for the induction of apoptosis (Feig et al., 2007). In cells lacking Grx1, a marked increase in binding of FasL occurred (Fig. 5 B and Fig. S3 C) in association with more IP of Fas (Fig. 5 E). Collectively, these observations demonstrate that the status of S-glutathionylation of Fas regulates binding of FasL, the ability of Fas to move into lipid rafts, formation of high MW Fas complexes, and assembly of DISC.
Figure 5.
Assessment of FasL binding, presence of Fas in lipid rafts, and DISC formation after manipulation of Grx1. (A) Evaluation of S-glutathionylation of Fas in lipid rafts in cells stimulated with FasL + M2 and the impact of overexpression of Grx1. Cells were transfected with pcDNA3 or Flag-Grx1 and stimulated with FasL + M2 for 20 min. Lipid raft fractions (3 and 4) and soluble fraction (12) were subjected to IP with antiglutathione antibody and analyzed by immunoblotting for Fas. PSSG was decomposed with 50 mM DTT as a reagent control before IP. The middle and bottom panels reflect immunoblot assays of Fas and the raft marker caveolin1 present in the input samples. Complete fractionation is shown in Fig. S3 A (available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). (B) Assessment of FasL binding to cells after manipulation of Grx1. Cells were subjected to control (Ctr) and Grx1 siRNA transfection. In separate experiments, cells were transfected with pcDNA3 or Grx1 plasmids. After 48 h, cells were trypsinized and incubated with ascending doses of FasL + M2 for 20 min. Binding of FasL to cells was evaluated after incubation with FITC-conjugated anti–mouse antibody and evaluation of 10,000 events via flow cytometry. Binding of FasL to cells is reflected as mean fluorescence intensity (MFI), and absolute values are plotted on the y-axis. The x-axis depicts ascending concentrations of FasL. Note that differences absolute fluorescence intensities between pcDNA3 and control siRNA–transfected cells may be a result of the different transfection procedures. Confirmation of Grx1 overexpression and knockdown is shown in Fig. S3 B and Fig. S3 C, respectively. (C) Assessment of FasL-interacting proteins in cells overexpressing Grx1. pcDNA3 or Grx1-transfected C10 cells were treated with M2 alone or FasL + M2. Cells were lysed, and 700 µg of protein was subjected to IP using protein G agarose beads to isolate DISC proteins. After SDS-PAGE, samples were analyzed by immunoblotting for Fas, FADD, procaspase 8, cleaved caspase-8, and Grx1. IP, M2 represents control IP in the absence of FasL. Note that all samples were run on the same gel. Black lines indicate that intervening lanes have been spliced out. (D) Evaluation of PSSG and Fas content in high MW complexes after IP of FasL + M2 or M2 alone via nonreducing SDS PAGE. As a control, samples were treated with DTT before electrophoresis. (E) Assessment of interaction between FasL and Fas in WT primary tracheal epithelial cells or cells lacking Glrx1. Cells were exposed to M2 alone or FasL + M2 for 30 min, lysed, and 700 µg of protein was subjected to IP using protein G agarose beads. After SDS-PAGE, samples were analyzed by immunoblotting for Fas. WCL, whole cell lysate.
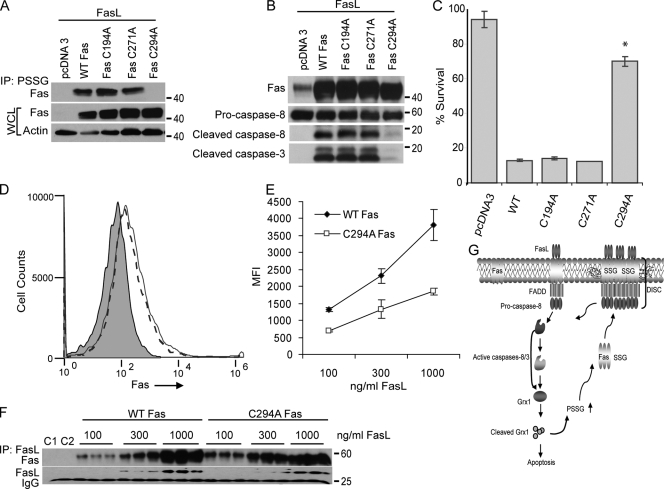
DD cysteine 294 is essential for FasL-induced S-glutathionylation of Fas, FasL binding, cleavage of caspase-8 and -3, and cell death
Murine Fas contains a total of 24 cysteines, out of which 20 are present in the ectodomain, and four are located in the DD (GenBank accession no. ABI24113). The ectodomain cysteines are known to form intramolecular disulfide bonds and therefore do not represent likely targets for S-glutathionylation. This prompted us to search for potential cysteine targets for S-glutathionylation within the DD of Fas through the generation of constructs in which cysteines were mutated to alanines. Fas mutants, C194A, C271A, C294A, or WT Fas was transfected into fibroblasts derived from lpr mice that lack Fas (Drappa et al., 1993). After stimulation with FasL, Fas-SSG was apparent in cells transfected with WT, C194A, or C271A mutant constructs. In contrast, Fas-SSG was not apparent in cells expressing C294A mutant Fas (Fig. 6 A). In lpr fibroblasts or lung epithelial cells, expression of WT, C194A, or C271A Fas constructs resulted in enhanced formation of active caspase-8 and -3 fragments after ligation of Fas, which was not apparent in cells expressing comparable levels of C294A mutant Fas (Fig. 6 B and not depicted). Cell death in response to FasL was largely abrogated in lpr fibroblasts expressing C294A mutant Fas in comparison with cells expressing WT, C194A, or C271A mutant versions of Fas (Fig. 6 C and Fig. S4 A, available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). Surface expression of C294A mutant Fas was indistinguishable from cells expressing WT Fas (Fig. 6 D and Fig. S4 B). However, binding of FasL was markedly decreased in cells expressing C294A Fas in comparison with cells expressing WT Fas (Fig. 6 E and Fig. S4 B), which is consistent with earlier observations, demonstrating that the extent of S-glutathionylation of Fas correlated directly with FasL binding (Fig. 5 B). To address potential intrinsic differences in binding affinities for FasL between WT or C294A mutant Fas, cells were transfected with WT or C294A mutant Fas (Fig. S4 C), lysed, and incubated with increasing amounts of FasL before IP with M2 and blotting for Fas. Results shown in Fig. 6 F demonstrate relatively comparable concentration-dependent increases in Fas associated with FasL and suggest that C294A mutant Fas is not intrinsically defective in binding to FasL. In aggregate, these findings demonstrate that S-glutathionylation of Fas at cysteine 294 is a critical regulatory event that promotes binding of FasL and subsequently strengthens apoptotic signaling.
Figure 6.
S-glutathionylation of cysteine 294 of Fas promotes binding to FasL, activation of caspase-8 and -3, and cell death. (A) lpr lung fibroblasts were transfected with pcDNA3, WT Fas, or Fas mutant constructs C194A, C271A, or C294A. Cells were exposed to FasL + M2 for 2 h, and lysates were subjected to IP using antiglutathione antibody. Immunoprecipitates were subjected to SDS-PAGE, and Fas content was evaluated via immunoblotting. The middle and bottom panels show total content of Fas and β-actin in whole cell lysates (WCL) as controls. (B) Attenuation of the formation of cleaved caspase-8 and -3 fragments in cells expressing Fas C294A. Cells were transfected with pcDNA3, WT, or Fas mutant constructs before incubation with FasL + M2. Lysates were subjected to immunoblotting using anti–caspase-8 and -3 antibodies. Fas immunoblotting (top) confirms equal expression of Fas constructs. (C) Protection against death in cells expressing C294A mutant Fas. lpr lung fibroblasts were transfected and treated as described in A, and cell survival was assessed using the MTT assay. Percent survival was calculated from two independent experiments run in triplicates. Results are expressed as mean values ± SEM. *, P < 0.05 as compared with cells transfected with WT Fas (Student's t test). Confirmation that all constructs are expressed equally is demonstrated in Fig. S4 A (available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). (D) S-glutathionylation of Fas does not affect surface expression. lpr lung fibroblasts were transfected with WT Fas or C294A mutant construct, and surface expression of Fas was evaluated 48 h after transfection using anti-Fas antibody (JO2) via flow cytometry. Log Fas immunofluorescence is shown on the x-axis, and cell counts are shown on the y-axis. pcDNA3 control (gray) reflects nonspecific background fluorescence. The dashed line represents C294A mutant Fas, and the solid line indicates WT Fas. Confirmation that both constructs are expressed equally is included in Fig. S4 B. (E) Assessment of FasL binding to lpr lung fibroblasts that express WT or C294A mutant Fas constructs. 48 h after transfection, cells were trypsinized and incubated with ascending doses of FasL + M2 for 20 min. Binding of FasL to cells was evaluated after incubation with FITC-conjugated anti–mouse antibody and evaluation of 10,000 events via flow cytometry. Binding of FasL to cells is reflected as mean fluorescence intensity (MFI), and absolute values are plotted on the y-axis. The x-axis depicts ascending concentrations of FasL. Confirmation that both constructs are expressed equally is included in Fig. S4 B. Error bars indicate ± SEM. (F) Assessment of binding of WT Fas or C294A mutant Fas in the absence of PSSG. lpr lung fibroblasts were transfected with WT or C294A mutant Fas constructs (Fig. S4 C). 48 h after transfection, whole cell lysates were prepared and incubated with 100, 300, or 1,000 ng/ml FasL + 2 µg/ml M2 at 4°C for 12–16 h. Samples were subsequently incubated with protein G agarose beads and washed several times before assessment of Fas content via Western Blot analysis. C1 represents sample from WT Fas–expressing cells subjected to IP with M2 antibody alone, whereas C2 represents sample from lpr cells transfected with pcDNA3 subjected to IP with 100 ng/ml FasL + 2 µg/ml M2. The bottom panel shows content of FasL. IgG, nonspecific reactivity. (G) Proposed model that incorporates S-glutathionylation in Fas-dependent apoptosis. In response to Fas ligation, activated caspase-8 and/or -3 degrade Grx1 either directly or potentially via indirect mechanisms. Caspase-initiated decreases in Grx1 content causes S-glutathionylation of Fas at cysteine 294 to increase. This promotes binding of FasL and enhances aggregation of Fas and its accumulation in lipid rafts and formation of the DISC, thereby further enhancing caspase activities and apoptosis. S-glutathionylation of Fas provides a mechanism whereby the extent of cell death is amplified in a feed-forward regulatory loop.
Discussion
The regulation of biological processes via redox-based signaling events is becoming increasingly apparent, and a role for reversible and dynamic cysteine oxidations that encompass PSSG therein is also emerging (Janssen-Heininger et al., 2008). However, the relevance of these events and the precise molecular targets in apoptotic signaling via death receptors remain unclear. The findings from this study define a new redox-based regulatory system that controls apoptosis after engagement of Fas. We demonstrate in this study that initial activation of caspase-8 and/or -3 causes degradation of Grx1, resulting in S-glutathionylation of Fas at cysteine 294, which subsequently enhances binding of FasL, aggregation of Fas, accumulation of Fas in lipid rafts, DISC assembly, and further activation of caspases, causing a propagation of apoptotic cell death (Fig. 6 G).
Activation of signaling after stimulation of growth factor receptors requires the reversible cysteine oxidation of protein tyrosine phosphatases, which occurs after the activation of NADPH oxidases and resultant increases in levels of hydrogen peroxide (Rhee et al., 2000). Our findings illuminate a new paradigm in oxidant-dependent signal transduction, as we demonstrate that redox-dependent apoptotic signaling can be initiated in an NADPH oxidase–independent manner. Instead, we have identified a caspase-initiated mechanism of oxidative signaling through direct or indirect degradation of the thiol repair enzyme Grx1. It is of relevance to note that FasL-induced caspase activity was recently shown to cleave and inactivate the mitochondrially localized antioxidant enzyme, manganese superoxide dismutase (Pardo et al., 2006), creating a redox imbalance in mitochondria associated with enhancement of apoptosis, which is in line with our current observations.
Although numerous studies exist demonstrating that steady-state levels of PSSG are increased in cells after exposure to H2O2 (for review see Forman et al., 2004), our current observations demonstrate that FasL effectuated marked increases in levels of PSSG with notable specificity based on the observed restricted patterns of S-glutathionylation (Fig. 1 A). These findings support the concept that redox-based signaling has a high degree of specificity, is compartmentalized to limited targets, and therefore has the ability to modulate selective pathways (Janssen-Heininger et al., 2008). However, it is important to highlight that increases in PSSG are not the only redox changes that occur after ligation of Fas. Denitrosylation of active-site cysteines of caspases has been reported after ligation of Fas in association with enhancement of their activity and apoptosis (Mannick et al., 1999, 2001), and recently, a role of Trx2 has been suggested herein (Benhar et al., 2008). Moreover, we demonstrate for the first time that overoxidation of Prx also occurred after ligation of Fas, likely as a result of activation of NADPH oxidases (Fig. 1 C). Importantly, overoxidation of Prx1 occurred with delayed kinetics relative to S-glutathionylation. The relative interplay between thioredoxin-catalyzed caspase denitrosylation, Fas S-glutathionylation, and Prx1 overoxidation is unclear at this time, but it is tempting to speculate that endogenous S-nitrosylation is important in homeostatic control against cell death, whereas stimulus-coupled S-glutathionylation is important in the amplification of apoptotic signaling. Overoxidation of Prx may be linked to mitochondrial pore transition, which has been linked to increases in ROS (Sato et al., 2004). This gradation wherein distinct cysteine oxidations regulate different cellular outcomes has been incorporated in a classification scheme (Hess et al., 2005) and points to the versatility of distinct cysteine oxidations in controlling biological processes.
As was highlighted earlier, it has been demonstrated previously that oxidants promote ligand-independent clustering of Fas and increase its tyrosine nitration (Shrivastava et al., 2004), but detailed analysis of the nature of oxidative modification or the location of these modifications was not available. Evidence for posttranslational modifications of cysteines of Fas has been limited to the discovery of palmitoylation of the membrane proximal cysteine 194 residue, which is critical for targeting Fas to lipid rafts, receptor internalization, formation of very high MW DISC complexes, and subsequent apoptosis (Chakrabandhu et al., 2007; Feig et al., 2007). In contrast to those observations, expression of mutant Fas lacking cysteine 194 did protect against FasL-induced caspase activation or apoptosis in this study (Fig. 6, B and C) and suggests possible cell type differences in the regulation of Fas-induced apoptosis. Instead, we have identified a novel posttranslational modification of Fas that involves S-glutathionylation of cysteine 294. S-glutathionylation of Fas was detected in a variety of primary cell types and cell lines and was demonstrated to be functionally significant based on our observations, demonstrating that S-glutathionylation of Fas promotes FasL binding and enhances trafficking of Fas into lipid rafts and assembly of DISC, thereby amplifying the strength of the apoptotic signal. Cysteine 294 is located in the carboxyterminal end of DD of mouse Fas and is conserved in the DDs of rat and human Fas (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). Analogous to conditions favoring S-nitrosylation (Hess et al., 2005), the flanking of cysteine 294 of Fas by acidic and basic amino acids, its localization within the carboxyterminal tail, and the hydrophobic compartment formed by lipid rafts are plausible factors that favor its susceptibility for S-glutathionylation or sustain this cysteine oxidation. This scenario highlights the possibility that other DD-containing receptors may also be regulated through S-glutathionylation, although this remains to be formally tested.
These findings, demonstrating that modulation of Grx1 greatly impacts Fas-dependent proapoptotic signaling, identify Grx1 as a survival factor that protects cells against apoptosis. Indeed, a role of Grx1 as a survival factor is supported by findings that demonstrate its ability to enhance the activation of nuclear factor κB in association with deglutathionylation of cysteine 179 of IKK-β (Reynaert et al., 2006). Furthermore, a role for Grx2 in the protection against dopamine-induced apoptosis has been reported via its ability to induce activation of nuclear factor κB (Daily et al., 2001). Antiapoptotic effects of Grx have also been linked to its regulation of the redox state of Akt (Murata et al., 2003) and activation of Ras-phosphoinositide 3-kinase and c-Jun N-terminal kinase pathways (Daily et al., 2001). In contrast, a proapoptotic role for Grx1 has been identified in endothelial cells stimulated with tumor necrosis factor α. In the latter study, Grx activity increased in response to tumor necrosis factor α, and Grx1 associated with S-glutathionylated caspase-3, caused its deglutathionylation, and enhanced its enzymatic activity (Pan and Berk, 2007). These conflicting data demonstrate that the outcome of S-glutathionylation is clearly controlled by ligand-dependent modulation of Grx activity and the molecular targets of Grx1.
In summary, our study reveals a new dimension in regulation of the Fas signaling pathway that is redox based in nature. Caspase-initiated degradation of Grx1 and subsequent S-glutathionylation of Fas represents a feed-forward amplification loop to enhance apoptosis (Fig. 6 G). This study identifies Grx1 as an attractive target to modulate death receptor–induced apoptosis.
Materials and methods
Reagents and antibodies
The following antibodies were used: Fas (Millipore), FADD (MBL International), caspase-8 (Enzo Biochem, Inc.), caspase-3 (Cell Signaling Technology), glutathione (Virogen), biotin (Jackson ImmunoResearch Laboratories), Prx1 and Prx-SO2H (Lab Frontier), Grx1 (American Diagnostica Inc.), Trx1 (Santa Cruz Biotechnology, Inc.), caveolin1 and JO2 (BD), and β-actin and Flag (M2; Sigma-Aldrich). Secondary antibodies were obtained from GE Healthcare, Jackson ImmunoResearch Laboratories, or Invitrogen.
Cell culture
A line of murine alveolar type II epithelial cells (C10), NIH 3T3 cells (provided by A. Howe, University of Vermont, Burlington, VT), primary lung fibroblasts, tracheal epithelial cells, CD4+ T lymphocytes from WT and Glrx1−/− mice, or primary lung fibroblasts derived from lpr mice were used. Cells were isolated and propagated as described previously (Shrivastava et al., 2004; Reynaert et al., 2006; Hinshaw-Makepeace et al., 2008). NIH 3T3 cells were grown in Dulbecco's minimum essential medium containing 10% FBS, 100 U/ml penicillin-streptomycin, 2.5 mg/ml glucose, and 10 µg/ml pyruvate. Before treatment with FasL, cells were starved in serum-free medium for 2 h.
FasL treatment and assessment of cell death
C10 cells were treated with 200 ng/ml Flag-FasL (Enzo Biochem, Inc.) + 0.5 µg/ml anti-Flag cross-linking antibody. Fibroblasts or CD4+ T lymphocytes were treated with 500 ng/ml FasL + 1 µg/ml M2. As reagent controls, cells were treated with M2 alone. Cell death was assessed using the MTT assay (Promega). Activation of caspase-8 and -3 was measured using reagents (Caspase-Glo 8 and Caspase-Glo 3/7; Promega).
Grx1 activity assay
Cells were lysed in 137 mM Tris-HCl, pH 8.0, 130 mM NaCl, and 1% NP-40. Lysates were cleared by centrifugation, equalized for protein content, and incubated with reaction buffer (137 mM Tris-HCl, pH 8.0, 0.5 mM glutathione, 1.2 U glutathione disulfide reductase [Roche], 0.35 mM NADPH, 1.5 mM EDTA, pH 8.0, and 2.5 mM cysteine-SO3) at 30°C. Consumption of NAPDH was followed spectrophotometrically at 340 nm. Data are expressed in units, in which 1 U equals the oxidation of 1 µmol NADPH/min/mg protein.
Cleavage of human Grx1 (hGrx1) by caspases
hGrx1 (American Diagnostica Inc.) was resuspended in 50 mM Hepes, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol, and 400 µM DTT. Recombinant human caspase-3 or -8 (EMD) was incubated with hGrx1 for the indicated time period at 37°C. Reaction mixtures were analyzed by immunoblot analysis for Grx1.
IP of Grx1 and interacting caspases
C10 cells were treated with FasL and M2. Lysates were prepared (20 mM Tris, pH 7.4, 150 mM NaCL, 10% glycerol, and 0.5% NP-40 with protease inhibitor cocktail), and Grx1 was immunoprecipitated from 500 µg of protein using 1 µg/ml anti-Grx1 antibody using protein G agarose beads. The samples were analyzed via SDS-PAGE using antibodies that detect cleaved p18 and pro p55 forms of caspase-8 and p17 and p19 fragments of caspase-3, respectively. Immunoprecipitated Grx1 was detected using anti-Grx1 antibody. As a control, lysates were incubated with isotype control IgG.
IP of S-glutathionylated proteins
Cells were exposed to FasL and M2 as indicated. Lysates were prepared (50 mM Tris, pH 7.4, 150 mM NaCl, 0.25% SDS, 1% NP-40, 0.5% CHAPS, and 20 mM N-ethylmaleimide with protease inhibitor cocktail [Sigma-Aldrich]), and protein content was equalized. 2 µg/ml antiglutathione antibody was added to immunoprecipitate glutathionylated proteins using protein G agarose beads. Samples were analyzed by immunoblotting using anti-Fas antibody. Fractions from sucrose gradients were treated with 0.25% SDS and 10 mM N-ethylmaleimide for 1 h before IP. As a control, a portion of the lysate was treated with 50 mM DTT to reduce glutathionylated proteins, and these samples were purified through columns (Micro-BioSpin; Bio-Rad Laboratories) to remove DTT before subsequent IP. Alternatively, cells were pretreated with biotinylated glutathione ethyl ester as described previously (Reynaert et al., 2006), and lysates were immunoprecipitated using antibiotin antibody. The samples were subsequently analyzed by immunoblotting using anti-Fas antibody.
Lipid raft preparation
C10 cells were starved for 2 h and treated with 1 µg/ml FasL + M2 2 µg/ml for 20 min at 37°C. Subsequent steps were performed on ice. Cells were washed two times with cold PBS and lysed (Muppidi and Siegel, 2004). Cells were scraped into a grinder (Wheaton) and gently homogenized. Homogenates were placed on the bottom of a centrifuge tube (SW41; Beckman Coulter), mixed with 85% sucrose, and overlaid with 35% and 5% sucrose. The gradient was allowed to settle for 30 min on ice before centrifugation for 16 h at 200,000 g. 1-ml fractions were collected and analyzed by immunoblotting (Muppidi and Siegel, 2004).
DISC isolation and analysis
DISC isolation was performed according to Holler et al. (2003). In brief, C10 cells (n = 1 × 108 cells/60-mm dish; after transfection, n = 1 × 106 cells/60-mm dish) were starved for 2 h and treated with 1 µg/ml FasL plus cross-linking antibody and 2 µg/ml M2 for 20 min at 37°C. Subsequent steps were performed on ice. Cells were washed once with PBS, lysed for 10 min, and processed as described previously (Holler et al., 2003).
Site-directed mutagenesis
Site-directed mutagenesis of WT mouse Fas was performed using the following primers with a site-directed mutagenesis kit (QuikChange II XL; Agilent Technologies): mfasC194A, (forward) 5′-GTACCGGAAAAGAAAGGCCTGGAAAAGGAGACAGG-3′ and (reverse) 5′-CCTGTCTCCTTTTCCAGGCCTTTCTTTTCCGGTAC-3′; mfasC271A, (forward) 5′-GAAAGTCCAGCTGCTCCTGGCCTGGTACCAATCTCATGG-3′ and (reverse) 5′-CCATGAGATTGGTACCAGGCCAGGAGCAGCTGGACTTTC-3′; and mfasC294A, (forward) 5′-GGGTCTCAAAAAGCCGAAGCCCGCAGAACCTTAG-3′ and (reverse) 5′-CTAAGGTTCTGCGGGCTTCGGCTTTTTTGAGACCC-3′. Mutated constructs were verified by sequence analysis.
Evaluation of binding of FasL and surface expression of Fas
After transfection, cells were trypsinized, scraped into eppendorf tubes, centrifuged briefly, and incubated on ice with 1% FBS containing PBS with anti-Fas antibody (JO2; 1 µg/ml) or with isotype control antibody. After 20 min, cells were washed and incubated with1 µg/ml FITC-conjugated secondary antibody for 20 min. Cells were fixed, and 10,000 events were analyzed by flow cytometry (BD). To assess binding of FasL, cells were harvested and incubated with the indicated concentration of FasL and M2 (1.5 µg/ml) for 20 min, washed, and incubated with 1 µg/ml FITC-conjugated anti–mouse antibody before fixation and subsequent assessment of 10,000 events via flow cytometry. To evaluate whether intrinsic differences in binding of FasL to WT or C294A Fas occurred in the absence of PSSG, lpr fibroblasts were transfected with WT or C294A mutant Fas, lysed (Holler et al., 2003), and 200 µg lysate was incubated with 100, 300, or 1,000 ng/ml FasL + 2 µg/ml M2 at 4°C for 12–16 h. Samples were subjected to IP and evaluation of Fas content via Western blot analysis.
Image processing and statistical analysis
Digital images were acquired by scanning x-ray film on a photo scanner (perfection 2450; EPSON). Photoshop (Adobe) and Illustrator (CS3; Adobe) were used to create and assemble figures. Images were obtained from samples run on the same gel. In some cases, lanes were reassembled for consistency, as is indicated by a vertical dividing line. In Fig. S3 A, the vertical black lines demarcate different gels. When required, contrast and brightness were adjusted equally in all lanes. No other manipulations were done. All experiments were performed three times. Data were analyzed by one-way analysis of variance (ANOVA) using the Tukey test to adjust for multiple comparisons or the Student's t test where appropriate (Excel; Microsoft). Data from multiple experiments were averaged and expressed as mean values ± SEM.
Online supplemental material
Fig. S1 shows the sequence of mouse Grx1 and putative caspase cleavage sites (A) and the lack of Fas-SSG in cells exposed to staurosporine (B). Fig. S2 shows confirmation of the lack of Grx1 in fibroblasts derived from Glrx1−/− mice. Fig. S3 shows Fas trafficking into lipid rafts in response to Fas ligation in cells transfected with control or Grx1 plasmid (A) and confirmation of increases and decreases in content Grx1 after overexpression and knockdown, respectively (B and C). Fig. S4 shows assessment of expression levels of WT and mutant Fas proteins. Fig. S5 shows alignment of primary sequences of mouse, rat, and human Fas proteins. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1.
Acknowledgments
We thank Jessica Reiss, Jenny Russell, Karen Fortner, Colette Charland, and Andreas Koenig for technical support, Alan Howe for providing NIH 3T3 cells, and Nicholas Heintz for critical review of the manuscript.
This work was funded by the National Institutes of Health (grants R01 HL079331 and HL60014). Y. Janssen-Heininger and N.L. Reynaert have a patent application utilizing Grx-based derivatization to detect S-glutathionylated proteins in situ.
Footnotes
Abbreviations used in this paper: ANOVA, analysis of variance; DD, death domain; DISC, death-inducing signaling complex; DPI, diphenyliodonium; FADD, Fas-associated DD; FasL, Fas ligand; Grx, glutaredoxin; IP, immunoprecipitation; MW, molecular weight; Prx, peroxiredoxin; ROS, reactive oxygen species; WT, wild type.
References
- Benhar M., Forrester M.T., Hess D.T., Stamler J.S. 2008. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins.Science. 320:1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabandhu K., Herincs Z., Huault S., Dost B., Peng L., Conchonaud F., Marguet D., He H.T., Hueber A.O. 2007. Palmitoylation is required for efficient Fas cell death signaling.EMBO J. 26:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily D., Vlamis-Gardikas A., Offen D., Mittelman L., Melamed E., Holmgren A., Barzilai A. 2001. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways.J. Biol. Chem. 276:21618–21626 [DOI] [PubMed] [Google Scholar]
- Drappa J., Brot N., Elkon K.B. 1993. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr.Proc. Natl. Acad. Sci. USA. 90:10340–10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W.C., Martins L.M., Kaufmann S.H. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis.Annu. Rev. Biochem. 68:383–424 [DOI] [PubMed] [Google Scholar]
- Feig C., Tchikov V., Schutze S., Peter M.E. 2007. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling.EMBO J. 26:221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A.P., Holmgren A. 2004. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system.Antioxid. Redox Signal. 6:63–74 [DOI] [PubMed] [Google Scholar]
- Forman H.J., Fukuto J.M., Torres M. 2004. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers.Am. J. Physiol. Cell Physiol. 287:C246–C256 [DOI] [PubMed] [Google Scholar]
- Hengartner M.O. 2000. The biochemistry of apoptosis.Nature. 407:770–776 [DOI] [PubMed] [Google Scholar]
- Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. 2005. Protein S-nitrosylation: purview and parameters.Nat. Rev. Mol. Cell Biol. 6:150–166 [DOI] [PubMed] [Google Scholar]
- Hinshaw-Makepeace J., Huston G., Fortner K.A., Russell J.Q., Holoch D., Swain S., Budd R.C. 2008. c-FLIP(S) reduces activation of caspase and NF-kappaB pathways and decreases T cell survival.Eur. J. Immunol. 38:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.S., Xiong Y., Ho D.S., Gao J., Chua B.H., Pai H., Mieyal J.J. 2007. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia.Free Radic. Biol. Med. 43:1299–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N., Tardivel A., Kovacsovics-Bankowski M., Hertig S., Gaide O., Martinon F., Tinel A., Deperthes D., Calderara S., Schulthess T., et al. 2003. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex.Mol. Cell. Biol. 23:1428–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.L., Fang L.W., Lu S.P., Chou C.K., Luh T.Y., Lai M.Z. 2003. DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation.Oncogene. 22:8168–8177 [DOI] [PubMed] [Google Scholar]
- Hueber A.O., Bernard A.M., Herincs Z., Couzinet A., He H.T. 2002. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes.EMBO Rep. 3:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. 2008. Redox-based regulation of signal transduction: principles, pitfalls, and promises.Free Radic. Biol. Med. 45:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J.D. 2004. NOX enzymes and the biology of reactive oxygen.Nat. Rev. Immunol. 4:181–189 [DOI] [PubMed] [Google Scholar]
- Mannick J.B., Hausladen A., Liu L., Hess D.T., Zeng M., Miao Q.X., Kane L.S., Gow A.J., Stamler J.S. 1999. Fas-induced caspase denitrosylation.Science. 284:651–654 [DOI] [PubMed] [Google Scholar]
- Mannick J.B., Schonhoff C., Papeta N., Ghafourifar P., Szibor M., Fang K., Gaston B. 2001. S-Nitrosylation of mitochondrial caspases.J. Cell Biol. 154:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi J.R., Siegel R.M. 2004. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death.Nat. Immunol. 5:182–189 [DOI] [PubMed] [Google Scholar]
- Murata H., Ihara Y., Nakamura H., Yodoi J., Sumikawa K., Kondo T. 2003. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt.J. Biol. Chem. 278:50226–50233 [DOI] [PubMed] [Google Scholar]
- Pan S., Berk B.C. 2007. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway.Circ. Res. 100:213–219 [DOI] [PubMed] [Google Scholar]
- Pardo M., Melendez J.A., Tirosh O. 2006. Manganese superoxide dismutase inactivation during Fas (CD95)-mediated apoptosis in Jurkat T cells.Free Radic. Biol. Med. 41:1795–1806 [DOI] [PubMed] [Google Scholar]
- Peter M.E., Budd R.C., Desbarats J., Hedrick S.M., Hueber A.O., Newell M.K., Owen L.B., Pope R.M., Tschopp J., Wajant H., et al. 2007. The CD95 receptor: apoptosis revisited.Cell. 129:447–450 [DOI] [PubMed] [Google Scholar]
- Reynaert N.L., van der Vliet A., Guala A.S., McGovern T., Hristova M., Pantano C., Heintz N.H., Heim J., Ho Y.S., Matthews D.E., et al. 2006. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta.Proc. Natl. Acad. Sci. USA. 103:13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.G., Bae Y.S., Lee S.R., Kwon J. 2000. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation.Sci. STKE. 2000: [DOI] [PubMed] [Google Scholar]
- Sato T., Machida T., Takahashi S., Iyama S., Sato Y., Kuribayashi K., Takada K., Oku T., Kawano Y., Okamoto T., et al. 2004. Fas-mediated apoptosome formation is dependent on reactive oxygen species derived from mitochondrial permeability transition in Jurkat cells.J. Immunol. 173:285–296 [DOI] [PubMed] [Google Scholar]
- Shrivastava P., Pantano C., Watkin R., McElhinney B., Guala A., Poynter M.L., Persinger R.L., Budd R., Janssen-Heininger Y. 2004. Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-Jun N-terminal kinase.Mol. Cell. Biol. 24:6763–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts M.D., Zheng L., Lenardo M.J. 2003. The death effector domain protein family: regulators of cellular homeostasis.Nat. Immunol. 4:404–409 [DOI] [PubMed] [Google Scholar]
- Wajant H. 2002. The Fas signaling pathway: more than a paradigm.Science. 296:1635–1636 [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Poole L.B., Karplus P.A. 2003. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling.Science. 300:650–653 [DOI] [PubMed] [Google Scholar]