Abstract
The anterior–posterior axis of the mouse embryo is established by formation of distal visceral endoderm (DVE) and its subsequent migration. The precise mechanism of DVE formation has remained unknown, however. Here we show that bone morphogenetic protein (BMP) signaling plays dual roles in DVE formation. BMP signaling is required at an early stage for differentiation of the primitive endoderm into the embryonic visceral endoderm (VE), whereas it inhibits DVE formation, restricting it to the distal region, at a later stage. A Smad2-activating factor such as Activin also contributes to DVE formation by generating a region of VE positive for the Smad2 signal and negative for Smad1 signal. DVE is thus formed at the distal end of the embryo, the only region of VE negative for the Smad1 signal and positive for Smad2 signal. An inverse relation between the level of phosphorylated Smad1 and that of phosphorylated Smad2 in VE suggests an involvement of antagonism between Smad1- and Smad2-mediated signaling.
Introduction
The anterior–posterior (A-P) axis is established early in mouse development. In this process, distal visceral endoderm (DVE) located at the distal tip of the embryo migrates toward the future anterior side and becomes anterior visceral endoderm (AVE; Beddington and Robertson, 1998, 1999). Several signals are necessary for A-P axis formation. For example, Nodal signaling from the epiblast induces DVE formation at embryonic day (E) 5.5 (Lu and Robertson, 2004). Removal of the extraembryonic ectoderm (ExE) leads to expansion of DVE at the pregastrulation stage (Rodriguez et al., 2005; Mesnard et al., 2006). Asymmetrical expression of Lefty1 and Cerl in DVE along the future A-P axis results in asymmetrical inhibition of Nodal signaling and thus determines the future anterior side (Yamamoto et al., 2004). Inhibition of Wnt signaling by Dkk1 is also necessary for the anterior shift of DVE (Kimura-Yoshida et al., 2005). In addition, signaling from AVE has been proposed to induce anterior and suppress posterior identity in the epiblast (Kimura et al., 2000; Perea-Gomez et al., 2002). However, the molecular mechanism of DVE formation has remained unknown.
Nodal, a secreted member of the TGF-β superfamily of ligands (Zhou et al., 1993), is required for DVE formation. ALK4 and ALK7 function as type 1 receptors for Nodal, whereas ActR2A and ActR2B function as type 2 receptors for this ligand. Nodal signaling is modulated by members of the EGF-CFC protein family and it is transduced by intracellular molecules including Smad2 and Smad3. With regard to formation of the A-P axis, Nodal−/− embryos manifest a failure of DVE formation at E5.5 (Brennan et al., 2001). However, a recent study (Mesnard et al., 2006) showed that the visceral endoderm (VE) of Nodal−/− embryos is abnormally specified before DVE formation. The primary role of Nodal in DVE formation is therefore to define an embryonic VE compartment before DVE formation.
The ExE is the source of another signal that regulates DVE formation (Rodriguez et al., 2005). Embryonic explants that lack ExE generate ectopic DVE, suggesting that a signal derived from ExE inhibits DVE formation (Rodriguez et al., 2005; Mesnard et al., 2006).
Bone morphogenetic proteins (BMPs) are also secreted ligands of the TGF-β superfamily and potential regulators of A-P patterning. BMPs function by binding to ALK1, ALK2, ALK3, or ALK6 as a type 1 receptor or to BMPR2, ActR2A, or ActR2B as a type 2 receptor (Zhao, 2003; Kishigami and Mishina, 2005). Gene targeting in the mouse has shown that BMP signaling is required for mesoderm formation (Mishina et al., 1995, 1999; Gu et al., 1999; Beppu et al., 2000; Lechleider et al., 2001; Tremblay et al., 2001). In the mouse embryo, anterior identity is established in the extraembryonic endoderm before formation of the primitive streak (Thomas et al., 1998; Beddington and Robertson, 1999). Some mutants that show a defect in mesoderm formation also manifest defective anterior patterning (Conlon et al., 1994; Brennan et al., 2001; Mesnard et al., 2006). Indeed, depletion of BMP4 has been shown to affect AVE formation (Soares et al., 2005). These various observations thus suggest that BMP signaling might regulate formation of the A-P axis, and that of DVE in particular.
To investigate the mechanism of DVE formation, we have now examined mutant mice that lack BMPR2, ActR2B, Lefty1, or Nodal and performed experiments with embryonic explants. Our data demonstrate that DVE formation is regulated by an antagonism between BMP-Smad1 and Activin/Nodal-Smad2 signaling. The DVE is thus formed at the distal region of the embryo where Smad2-mediated signal is present and Smad1-mediated signal is absent.
Results
DVE formation is impaired in Bmpr2−/− embryos
Formation of the primitive streak is impaired in Bmpr2−/− embryos (Beppu et al., 2000). To determine whether formation of the A-P axis occurs normally in these mutant embryos, we first examined the expression of AVE or DVE marker genes at E6.5 and E5.5, respectively. In wild-type embryos at E5.5, five DVE marker genes, Lefty1, Cerl, Dkk1, Lim1, and Hex, are expressed in VE at the distal tip (Fig. 1, A–E). Expression of Hex, Hesx1, and Cerl is absent at E5.2 but is apparent at E5.5 (Fig. S1, A–C and E–G, available at http://www.jcb.org/cgi/content/full/jcb.200808044/DC1), whereas Lefty1 expression is maintained between E4.0 and E5.5 (Takaoka et al., 2006; Fig. S1, D and H), indicating that cells positive for a full range of DVE markers are formed between E5.2 and E5.5. In Bmpr2−/− embryos, however, expression of AVE marker genes at E6.5 was absent or reduced compared with that in wild-type embryos (Fig. S2, A–D, A'–D', and A''–D''). Dkk1 expression was lost (Fig. S2 C') or remained relatively normal (Fig. S2 C''). At E5.5, expression of Lefty1, Cerl, Dkk1, and Lim1 was absent (4/7, 3/7, 3/7, and 3/6 embryos, respectively) or markedly reduced (3/7, 4/7, 4/7, and 3/6 embryos, respectively), and that of Hex was also lost (3/3 embryos; Fig. 1, A'–E' and N; and Fig. S2, I and I').
Figure 1.
DVE formation requires BMP signaling in the extraembryonic region. Expression of Lefty1 (A and A'), Cerl (B and B'), Dkk1 (C and C'), Lim1 (D and D'), Hex (E, E', I, and I'), Hnf4 (J and J'), Pem (K and K'), Bmp2 (L and L'), and Fgf8 (M, M', and M”) was examined by in situ hybridization in wild-type (Bmpr2+/+) and Bmpr2−/− mouse embryos at E5.5 or the indicated stages. The DVE was absent (A'–E') in the mutant embryos. The primitive endoderm (I') and extraembryonic VE (J' and K') were formed, whereas embryonic VE was absent (L' and M') or impaired (M”). Arrowheads in J' indicate the junction between the extraembryonic and embryonic regions. Green ES FM260 cell (Bmpr2+/+)←→Bmpr2+/+ tetraploid (F–H) and green ES FM260 cell←→Bmpr2−/− tetraploid (F'–H') chimeric embryos were recovered at E6.5 and examined for EGFP fluorescence (F and F') or for expression of Hex (G and G') and Lefty1 (H and H'). The expression of Hex and Lefty1 was absent in the green ES FM260 cell←→Bmpr2−/− tetraploid chimeric embryos. (N) The level of p-Smad1 staining and the level of expression of DVE markers are compared between Bmpr2−/− and Bmpr2−/−,Actr2b+/− embryos. Dark blue, light blue, orange, and red bars indicate normal expression, expression at a moderately reduced level, expression at a severely reduced level, and no expression, respectively. The numbers of embryos showing each expression pattern are indicated. Bars, 50 µm.
To determine the region of the embryo in which BMP signaling exerts the observed effects, we examined expression of DVE marker genes in green embryonic stem (ES) FM260 cell←→Bmpr2−/− tetraploid chimeric embryos, which were generated by aggregation of ES cells expressing EGFP with Bmpr2−/− tetraploid embryos. In such chimeras, expression of Hex (n = 3) and Lefty1 (n = 3) was absent at E6.5 (Fig. 1, F–H and F'–H'). This phenotype was indistinguishable from that of Bmpr2−/− embryos, suggesting that BMPR2 in the extraembryonic region is required for DVE formation.
We next examined whether VE is formed normally in Bmpr2−/− embryos. VE, which is composed of embryonic VE and extraembryonic VE at E5.5, is derived from the primitive endoderm of the E4.0–4.5 embryo. Expression of Hex, which is a marker of the primitive endoderm, was maintained in Bmpr2−/− embryos at E4.5 (n = 3; Fig. 1, I and I'). Expression of Hnf4 (n = 13), Gata4 (n = 4), and Pem (n = 7) was maintained in the extraembryonic VE of Bmpr2−/− embryos at E5.5 (Fig. 1, J' and K'; and Fig. S2 O'). However, expression of Bmp2 (n = 4), Fgf8 (n = 7), and Hnf4 (n = 13) in the embryonic VE was down-regulated in the mutant embryos at E5.2 and E5.5 (Fig. 1, J, J', L, L', M, M', and M”). Staining for phosphorylated ERK and expression of the ExE marker genes Eomes, Bmp4, and Mash2 were normal, whereas that of Wnt7b was slightly decreased, in the mutant embryos (Fig. S2, Q–W and Q'–W'). These results suggested that BMPR2 is not essential for formation of the primitive endoderm or extraembryonic VE, but rather is specifically required for specification of embryonic VE. The failure of DVE formation in Bmpr2−/− embryos is thus likely caused by the impaired differentiation of the primitive endoderm into embryonic VE.
Smad1-mediated signaling is reduced in Bmpr2−/− embryos
To evaluate how the lack of BMPR2 affects BMP signaling in the embryo, we examined the distribution of phosphorylated Smad1/5 (p-Smad1) in Bmpr2−/− embryos by immunohistofluorescence staining. In wild-type embryos, p-Smad1 was found in the nuclei of epiblast and primitive endoderm cells at E4.5 and of epiblast and VE cells at E5.2 (Fig. 1 N and Fig. 2, A and B). The distribution of p-Smad1 changed quickly between E5.2 and E5.5 and had shifted to the proximal epiblast and VE, excluding DVE, at E5.5 (Fig. 2 C and Fig. S3, A–J, available at http://www.jcb.org/cgi/content/full/jcb.200808044/DC1). In Bmpr2−/− embryos, the distribution of p-Smad1 was similar to that in wild-type embryos at E4.5 but showed two distinct patterns at later stages (Fig. 1 N and Fig. 2, A'–C'). In severely affected mutant embryos (8/14 embryos at E5.2 and 8/15 embryos at E5.5), p-Smad1 was apparent only in the proximal VE at E5.2 (8/8 embryos; Fig. 2 B') and was barely detected at E5.5 (8/8 embryos; Fig. 2 C'). In mildly affected embryos (6/14 embryos at E5.5 and 7/15 embryos at E5.5), p-Smad1 was found in the same regions as in wild-type embryos at E5.2, but its abundance was lower than that in the wild type (6/6 embryos; Fig. 1 N and Fig. S2). It was not detected in the epiblast and there were fewer positive cells in the VE of the mildly affected embryos at E5.5 (7/7 embryos; Fig. S2). These data suggested that BMP signaling is not completely lost but is reduced in Bmpr2−/− embryos. Other type 2 receptors may thus play a redundant role in this mutant. Variability in the phenotype of Bmpr2−/− embryos is most likely caused by the variable level of BMP signaling that remains.
Figure 2.
p-Smad1 in wild-type and Bmpr2−/− embryos. Wild-type (A–C) or Bmpr2−/− (A'–C') embryos at the indicated stages of development were subjected to immunohistofluorescence staining with antibodies to p-Smad1 (pS1; green); merged images with staining of nuclei by YOYO-1 (Nuc; red) are also shown. Staining for p-Smad1 was decreased in Bmpr2−/− embryos. Bars, 50 µm.
BMP signals via BMPR2 and ActR2B in the mouse embryo
The residual level of p-Smad1 in Bmpr2−/− embryos suggested that additional type 2 receptors may compensate for the lack of BMPR2. ActR2A and ActR2B were potential candidates for such receptors because they transduce the BMP signal as well as that of other TGF-β superfamily members (Massague and Chen, 2000; Zhao, 2003). Expression of Bmpr2 was detected in both embryonic and extraembryonic regions of the wild-type conceptus up to E6.5 (Fig. 3, A–C; Roelen et al., 1997; Beppu et al., 2000). Expression of Actr2b was also apparent in the same regions up to E5.5 as well as in the epiblast and overlying VE at E6.5 (Fig. 3 D–F; Beppu et al., 2000), suggesting that ActR2B and BMPR2 may play redundant roles. We therefore generated Bmpr2−/−,Actr2b+/− double mutant mice.
Figure 3.
BMP signaling is required for embryonic VE differentiation independently of Nodal signaling. Expression of Bmpr2 (A–C) and Actr2b (D–F) in wild-type (WT) embryos as well as expression of p-Smad1 (pS1; green) and merged images of p-Smad1 with nuclear staining (Nuc; red) for wild-type (G and H) and Bmpr2−/−,Actr2b+/− (G' and H') embryos at the indicated stages are shown. Expression of Lefty1 (I, I', S, and S'), Cerl (J and J'), Dkk1 (K and K'), Lim1 (L and L'), Hex (M, M', R, and R'), Hnf4 (N and N'), Pem (O and O'), Bmp2 (P and P'), Fgf8 (Q and Q'), Nodal (T and T'), Foxh1 (U and U'), and Cripto (V and V') in wild-type or Bmpr2−/−,Actr2b+/− embryos at E5.5 or the indicated stages was determined by in situ hybridization. Expression of p-Smad2 (pS2; green) and merged images of p-Smad2 with nuclear staining (Nuc; red) are shown for wild-type (W), Bmpr2−/−,Actr2b+/− (W'), or Bmpr2−/− (X) embryos at E5.5. Bars, 50 µm.
Removal of one copy of Actr2b from Bmpr2−/− mice had pronounced effects. Staining for p-Smad1 was reduced at E4.5 and was not detected at E5.2 in the double mutant embryos (n = 4; Fig. 1 N and Fig. 3, G, G', H, and H'). A similar pattern of p-Smad1 staining was observed in green ES FM260 cell←→Bmpr2−/−,Actr2b+/− tetraploid chimeric embryos (Fig. S3, K and L). These results suggested that both BMPR2 and ActR2b act as receptors for BMP in VE. We next examined DVE markers in Bmpr2−/−,Actr2b+/− embryos at E5.5 to determine whether BMP signaling is required for DVE formation. Expression of DVE markers was detected in some of the Bmpr2−/− embryos examined (Fig. S2). However, expression of Lefty1 (n = 5), Cerl (n = 4), Dkk1 (n = 3), Lim1 (n = 4), and Hex (n = 3) was always lost in Bmpr2−/−,Actr2b+/− embryos (Fig. 3, I–M and I'–M'). We also examined endoderm markers in Bmpr2−/−,Actr2b+/− embryos from E4.5 to E5.5. Hnf4 (n = 5) and Pem (n = 3) were expressed normally at E5.5 (Fig. 3, N, N', O, and O'), whereas expression of Bmp2 (n = 4) and Fgf8 (n = 4) was abolished at E5.2 (Fig. 3, P, P', Q, and Q'), in the double mutant embryos. At E4.5, expression of Hex (n = 3) and Lefty1 (n = 4) was maintained (Fig. 3, R, R', S, and S'), suggesting that the primitive endoderm is correctly formed at E4.5 in the double mutant embryos.
We then examined the expression of genes for Nodal signaling components and the distribution of phosphorylated Smad2/3 (p-Smad2) in Bmpr2−/−,Actr2b+/− embryos (Whitman, 2001). Nodal, Foxh1, and Cripto (n = 7, 4, and 4, respectively) were all expressed normally in the double mutant embryos at E5.5 (Fig. 3, T–V and T'–V'). The distribution of p-Smad2 in the double mutant embryos remained similar to that in wild-type or Bmpr2−/− embryos at E5.5 (Fig. 3, W, W', and X). Unlike Bmpr2−/−,Actr2b+/− embryos in which expression of DVE markers was always absent, it was detected in some of the Bmpr2−/−,Nodal+/− embryos examined (Fig. S4, A–M, available at http://www.jcb.org/cgi/content/full/jcb.200808044/DC1). Furthermore, p-Smad1 and p-Smad2 staining patterns of the Bmpr2−/−,Nodal+/− embryos were similar to those of the Bmpr2−/− embryos (Fig. S4, N–P). These results (summarized in Fig. 1 N) thus indicated that both BMPR2 and ActR2B function as type 2 receptors for BMP signaling during formation of embryonic VE and DVE.
Smad1 signaling is absent when and where DVE is newly formed
Staining for p-Smad1 was apparent throughout the primitive endoderm and VE until E5.2 (Fig. 2, A and B). At E5.5, however, p-Smad1 had disappeared from DVE while it was still apparent in the proximal portion of VE (Fig. 2 C and Fig. S3). In Lefty1−/− embryos (n = 5), the region of VE negative for p-Smad1 expanded (Fig. 4, A and C) along with the expansion of the region positive for DVE markers (Fig. 4, E–H). Thus, p-Smad1 is lost when and where DVE is formed.
Figure 4.
Expansion of the region positive for DVE markers and negative for p-Smad1 in Lefty1 mutant embryos. Wild-type (A and B) or Lefty1−/− (C and D) embryos at E5.5 were subjected to immunohistofluorescence staining with antibodies to p-Smad1 (pS1; A and C, green) or to p-Smad2 (pS2; B and D, green). Merged images with nuclear staining (Nuc; red) are also shown. Green color in merged images indicates increased level of p-Smad1 or p-Smad2 staining. Bracket in D indicates the region (the epiblast and VE in the distal region) with increased p-Smad2 staining. Wild-type (L1+/+) or Lefty1−/− (L1−/−) embryos at E5.5 were also examined for expression of Hex (E and F) and Cerl (G and H). Bars, 50 µm.
We next examined the distribution of p-Smad1 in embryo explants lacking ExE. An inhibitory signal derived from ExE has been shown to restrict induction of DVE to the distal tip of the E5.5 embryo (Rodriguez et al., 2005). Ectopic expression of a Hex-Venus transgene was thus induced in VE when ExE was removed at E5.5 (Fig. 5, A–E and K; Rodriguez et al., 2005). Removal of ExE also induced the complete loss of p-Smad1 (Fig. 5, A–G and L) and up-regulation of p-Smad2 (Fig. 5, I and J). Several observations suggested that the BMP signal disappears from the prospective DVE before, not after, DVE formation (between E5.2 and E5.5 in the wild-type embryo). First, loss of the BMP signal was not caused by expression of the DVE-specific gene Cerl, whose product can inhibit BMP signaling (Belo et al., 2000), because the distribution of p-Smad1 appeared normal in Cerl−/− embryos (Fig. 5 H). Second, in explants lacking ExE, expansion of DVE begins ∼90 min after removal of ExE, whereas p-Smad1 staining is lost from VE much earlier, ∼30 min after ExE removal (Fig. 5, C–E, K, and L). These results suggested that loss of the BMP signal from prospective DVE, which coincides with the onset of DVE formation, may be required for DVE formation.
Figure 5.
BMP signaling is lost when and where DVE is newly formed. The DVE region was monitored by detection of the expression of a Hex-Venus transgene (blue) before (A) or at the indicated times after (C–E) removal of ExE. Wild-type (A, F, and I) or Cerl−/− (H) embryos or wild-type embryo explants stripped of ExE (C–E, G, and J) at E5.5 were subjected to immunohistofluorescence staining with antibodies to p-Smad1 (pS1; A and C–H, green) or to p-Smad2 (pS2; I and J, green). Merged images with nuclear staining (Nuc; red) are also shown. Green color in merged images indicates increased level of p-Smad1 or p-Smad2 staining. The explants shown in G and J were examined after culture for 6 h. Arrowheads in C–E indicate the boundaries of Hex-Venus expression. The experimental strategy for explant experiments is shown in B. The ExE was separated from the epiblast and embryonic VE by a cut along the embryonic–extraembryonic junction at E5.5. The epiblast and embryonic VE were then cultured alone. The effects of ExE removal on expression of Hex-Venus (K) and p-Smad1 staining (L) at the indicated times of culture are summarized. Blue and orange bars indicate no change and expansion of the Venus positive domain (K) or no change and down-regulation of p-Smad1 (L), respectively. The numbers of embryos showing each pattern are indicated. Bars, 50 µm.
BMP is the ExE-derived inhibitory signal that restricts DVE formation
The loss of p-Smad1 immediately before DVE formation suggests that the ExE-derived signal that inhibits DVE formation may be the BMP signal itself. Previous observations support this notion. First, expression of Bmp4 and Bmp8b is apparent in ExE (Coucouvanis and Martin, 1999; Ying et al., 2000). Second, the DVE region expands in response to removal of ExE (Rodriguez et al., 2005; Mesnard et al., 2006), although such DVE expansion was not observed in a third study (Georgiades and Rossant, 2006). The knockout serum replacement used for culture in this latter study is known to contain a high level of BMP signaling activity (Xu et al., 2005), however, which may explain why DVE failed to expand.
To examine whether the ExE-derived inhibitory signal is indeed the BMP signal, we examined the response of DVE to BMP or a BMP inhibitor. When wild-type embryos were cultured from E5.2 to E5.5 in the presence of BMP4, expression of Hex, Lefty1, and Cerl was lost (Fig. S5, A–F, available at http://www.jcb.org/cgi/content/full/jcb.200808044/DC1). Whereas staining for p-Smad1 was lost in VE in response to removal of ExE at E5.5 (Fig. 6 A), p-Smad1 staining was maintained in VE if the explants lacking the ExE were cultured with BMP4 (Fig. 6 C). Moreover, expansion of the DVE region in such explants was inhibited by BMP4 (Fig. 6, B and D; and Fig. S5, T–W). In contrast, the region positive for DVE markers expanded and the level of expression of DVE markers increased when whole embryos at E5.2 were cultured with Noggin (Fig. 6, E–J), which induced down-regulation of p-Smad1 (Fig. 6, K and L).
Figure 6.
Identification of BMP as the inhibitory signal from ExE that restricts DVE formation. The DVE region was monitored by detection of the expression of a Hex-Venus transgene (B, D, Q, R, T, U, W, and X, green) at the indicated times after removal of ExE (B and D) or after ExE attachment (Q, R, T, U, W, and X) at E5.5. Embryo explants stripped of ExE (A and C) or normal embryos (K–N) at E5.5 were subjected to immunohistofluorescence staining with antibodies to p-Smad1 (pS1; green) or to p-Smad2 (pS2; green), as indicated. Merged images with nuclear staining (Nuc; red) are also shown. Green color in merged images indicates increased level of p-Smad1 or p-Smad2 staining. The explants or embryos were cultured with BSA (A, B, K, and M), BMP4 (C and D), or Noggin (Nog; L and N) for 6 h or the indicated times. Expression of Lefty1 (L1), Cerl, and Lim1 was determined in E5.2 embryos cultured for 8 h with either BSA (E, G, and I) or Noggin (F, H, and J). The experimental strategy for the explant experiments involving removal and attachment of ExE (P–X) is shown in O. Chimeric explants were composed of an embryonic portion harboring the Hex-Venus transgene and an extraembryonic portion treated with BSA or Noggin, as indicated. Bright-field images alone are shown in P, S, and V. Fluorescence images superimposed on bright-field images (B, D, Q, R, T, U, W, and X) are also shown. Bars, 50 µm.
Finally, we generated chimeric explants composed of an embryonic portion harboring the Hex-Venus transgene and an extraembryonic portion treated with Noggin or with BSA as a control (Fig. 6, O–X). The extraembryonic portion treated with BSA inhibited expansion of the DVE region (Fig. 6, P–R), showing typical ExE activity. However, the extraembryonic portion treated with Noggin allowed expansion of DVE, indicating that the inhibitory ExE activity was abolished by Noggin (Fig. 6, S–U). If the Noggin-treated ExE was attached to the side of a whole embryo harboring the Hex-Venus transgene (Fig. 6 O), DVE formation was not affected (Fig. 6, V–X). These results indicate that the BMP signal is the inhibitory signal derived from ExE. We therefore conclude that DVE is formed in a portion of embryonic VE that is negative for BMP signaling and positive for Activin signaling.
Antagonism between Smad2- and Smad1-mediated signaling in VE
A recent study showed that induction of DVE depends on Nodal and proprotein convertase activities (Mesnard et al., 2006). To detect Nodal activity in DVE, we first examined the distribution of p-Smad2 in wild-type embryos at E5.5. Staining for p-Smad2 was apparent in the epiblast (especially on the distal side) and VE including DVE (Fig. 3 W, Fig. 4 B, and Fig. 7 A). We also examined the distribution of p-Smad2 in Nodal−/− embryos at E5.5. Unexpectedly, however, staining for p-Smad2 was still apparent in the VE, including DVE, of these mutant embryos (Fig. 7, B and C), suggesting that the appearance of p-Smad2 in DVE is induced by another TGF-β–related factor. Activin is the most likely candidate because Activin-βA and Activin-βB are expressed in the decidual zone of the dam, and recombinant Activin is able to induce ectopic DVE (Jones et al., 2006; Mesnard et al., 2006). GDF1 and GDF3 may be other candidates that trigger Smad2-mediated signaling because DVE formation is impaired in a portion of the mutant mice lacking Gdf3 or Gdf3 and Gdf1 (Chen et al., 2006; Andersson et al., 2007). However, recent evidence (Andersson et al., 2007; Tanaka et al., 2007) has shown that the native form of GDF1 and GDF3 is incapable of inducing downstream signaling. GDF1 and GDF3 act as coligands of Nodal, which interact with Nodal and stimulate Nodal activity (Tanaka et al., 2007).
Figure 7.
Smad2 signaling in VE is essential for specification of DVE. (A–L) Expression of p-Smad2 (pS2; green) or p-Smad1 (pS1; green), and merged images with nuclear staining (Nuc; red), for wild-type (WT; A and J) or Nodal−/− (B, C, and K) embryos at E5.5 as well as for E5.5 wild-type embryos treated with BSA (D and G), follistatin (Fol; E, H, and I), or Activin (Act; F and L) for 7 h. Arrowheads indicate loss of p-Smad2 fluorescence from DVE of the follistatin-treated embryo (E). Green color in merged images indicates increased level of p-Smad1 or p-Smad2 staining. Bracket in F indicates the region with increased p-Smad2 staining. (M–U) Expression of Hex (M, P, and S), Cerl (N, Q, and T), and Lefty1 (L1; O, R, and U) in E5.5 wild-type embryos treated with BSA, follistatin, or Activin, as indicated, for 7 h. (V) Summary of the effects of treatment with BSA, follistatin, or Activin on the expression of p-Smad1 in DVE, p-Smad2 in DVE, and the indicated genes. Light blue, dark blue, orange, and red bars indicate normal expression, expanded expression, no expression, and expanded no expression portion, respectively. The numbers of embryos showing each expression pattern are indicated. Bars, 50 µm.
To examine further whether Activin signaling contributes to the appearance of p-Smad2 in DVE, we cultured wild-type embryos at E5.5 with follistatin, which inhibits Activin signaling but not Nodal signaling. Staining for p-Smad2 was maintained in the epiblast, but was lost in most part of the VE of follistatin-treated embryos (7/10 embryos; Fig. 7, D and E), indicating that Activin activates Smad2 in DVE. Moreover, expression of the DVE marker genes Hex, Cerl, and Lefty1 was reduced in the follistatin-treated embryos compared with that in control embryos (Fig. 7, M–R and V). Fgf8 expression was maintained in follistatin-treated embryos, suggesting that follistatin does not impair embryonic VE formation (Fig. S5, G and H). Although follistatin has been shown to inhibit BMP signaling (Yamashita et al., 1995), p-Smad1 staining in follistatin-treated embryos was increased or normal in DVE (Fig. 7, G–I). Conversely, treatment of embryos with Activin resulted in up-regulation of p-Smad2 and down-regulation of p-Smad1 on the distal side (Fig. 7, D, F, G, and L). Moreover, expression of DVE maker genes was markedly expanded and/or up-regulated in the Activin-treated embryos (Fig. 7, S–V). Treatment of E5.5 embryos with SB431542, a drug that inhibits Activin and Nodal signaling, resulted not only in loss of p-Smad2 and up-regulation of p-Smad1 in DVE but also in down-regulation of the expression of DVE markers (Fig. S5, K–R; Mesnard et al., 2006). These results suggested that Smad2 signaling, most likely triggered by Activin, plays an essential role in DVE formation. Furthermore, an inverse relation between the level of p-Smad1 and that of p-Smad2 suggests that antagonism between Smad1 and Smad2 signaling operates in VE.
Antagonism between Smad1 and Smad2 signaling is also apparent in the mutant embryo lacking Lefty1, an inhibitor of Nodal signaling. The distribution of p-Smad2 in Lefty1+/− embryos at E5.5 was similar to that in the wild type (unpublished data). However, the level of p-Smad2 was increased on the distal side of Lefty1−/− embryos at this stage (n = 5; Fig. 4, B and D). In contrast, the level of p-Smad1 was decreased on the distal side of Lefty1−/− embryos (n = 5; Fig. 4, A and C). In addition, the expression domains of Hex (n = 5) and Cerl (n = 5) were markedly expanded in the distal region of Lefty1−/− embryos (Fig. 4, E–H).
ActR2A and ActR2B are rate-limiting factors involved in the antagonistic balance
We next examined the molecular basis of the antagonism between BMP and Activin–Nodal signaling. Activin–Nodal and BMP share common signaling components, extracellular components such as ActR2A and ActR2B as well as intracellular components including Smad4. If the amount of a common component is limited, an increase in one signal would result in a decrease in the other. To test this possibility, we introduced a Smad4 or Actr2a+Actr2b expression vector into a p-Smad1–positive cell in the distal region of E5.2 embryo and examined if such cells would maintain or lose p-Smad1 12 h later (equivalent to E5.7; Fig. 8). Normally, such p-Smad1–positive cells at E5.2 (Fig. 2 B) lose p-Smad1 by E5.5 (Fig. 2 C). However, if a limiting factor commonly used for both signaling is overexpressed into such a cell, it would prevent the antagonism and the cell would remain positive for p-Smad1 at E5.5. This is what we observed when Actr2 was overexpressed (Fig. 8 A).
Figure 8.
A limited level of type II Activin receptors is responsible for antagonistic balance between Smad1 and Smad2 signaling in the mouse embryo. (A) Experimental strategy. An effector gene, together with a GFP expression vector, was introduced into epiblast cells on the distal side of E5.2 mouse embryos. The embryos were cultured for 12 h and were examined for the distribution of p-Smad1 by immunohistofluorescence staining. (B–E) Localization of p-Smad1 (pS1; green) and merged images of p-Smad1 with GFP-positive cells (GFP; red). Arrowhead indicates the GFP-positive cell. S4, Smad4; Actr2, Actr2a plus Actr2b. Bars, 50 µm.
Expression of EGFP alone (10/10 embryos) or EGFP+Smad4 (8/8 embryos) did not influence the level of p-Smad1. Thus, the cell in the distal region of the E5.7 embryo that received EGFP or EGFP+Smad4 lost p-Smad1 (Fig. 8, B and C). In contrast, the cells that received Actr2a+Actr2b expression vectors remained positive for p-Smad1 (25/25 embryos; Fig. 8, D and E). These results suggest that the limited level of ActR2A and ActR2B is responsible for the antagonistic balance between Smad2 and Smad1 signaling.
Discussion
Dual roles of BMP signaling in DVE formation
Four different types of endoderm are formed from the primitive endoderm before gastrulation: parietal endoderm, extraembryonic VE, embryonic VE, and DVE (Fig. 9). Primitive endoderm cells that maintain contact with the ectoderm differentiate into VE, whereas those that migrate away from the ectoderm along the inner surface of the trophectoderm form parietal endoderm (Nadijcka and Hillman, 1974; Enders et al., 1978). VE cells give rise to both extraembryonic VE and embryonic VE, and the distal portion of embryonic VE becomes DVE. We have now shown that BMP signaling plays dual roles in DVE formation.
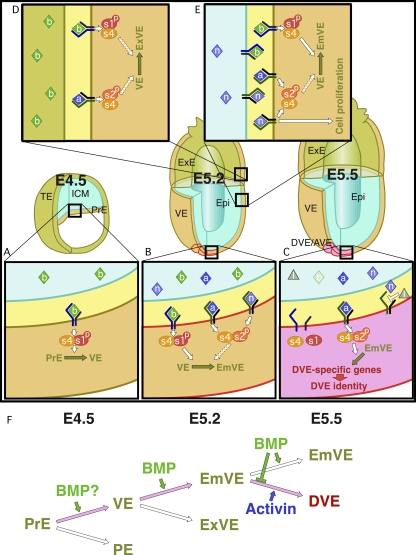
Figure 9.
The location of DVE formation is determined by the concerted action of Smad1- and Smad2-mediated signals. (A) BMP signaling promotes the differentiation of primitive endoderm (PrE) into VE until E4.5. (B) VE differentiates into embryonic VE as a result of the concerted action of BMP and other signals until E5.2. (C) DVE arises from a region of embryonic VE in which Activin signaling is present and BMP signaling is absent at E5.5. (D and E) VE gives rise to extraembryonic and embryonic VE until E5.2. (F) Summary of the relation between endoderm development and signaling by multiple TGF-β superfamily members from E4.5 to E5.5. a, Activin; b, BMP; Epi, epiblast; ICM, inner cell mass; l, Lefty1; n, Nodal; PE, parietal endoderm; PrE, primitive endoderm; s1p, phosphorylated Smad1/5; s2p, phosphorylated Smad2/3; s4, Smad4; TE, trophectoderm. Broken arrows indicate the mechanism is obscure.
At early stages of development (until E5.2), BMP signaling is required for formation of embryonic VE. BMP signaling, together with Nodal signaling, thus promotes the differentiation of VE into embryonic VE rather than into extraembryonic VE. This conclusion is supported by previous observations (Mesnard et al., 2006) as well as by our present data. First, extraembryonic VE markers remain ectopically expressed at high levels in the embryonic region and embryonic VE markers fail to be induced in Nodal−/− embryos (Mesnard et al., 2006). In addition, given that the p-Smad1 staining observed in wild-type embryos at E5.5 is lost in Nodal−/− embryos (Fig. 7), Nodal signaling is necessary for the appearance of BMP signaling. Nodal signaling may therefore induce the BMP signal in VE. Second, embryonic VE markers also failed to be induced in Bmpr2−/−,Actr2b+/− embryos, in which p-Smad1 staining was absent at E5.2 (Fig. 3). However, extraembryonic VE markers were maintained in such embryos at E5.5. Embryonic VE markers were also down-regulated in Bmpr2−/− embryos, in which p-Smad1 staining was reduced at E5.2 (Fig. 2). These results indicate that BMP signaling is essential until E5.2 for formation of embryonic VE, whereas it is dispensable for formation of extraembryonic VE.
At E5.5, DVE cells arise from a portion of embryonic VE that lacks the BMP signal. Whereas embryonic VE cells that have lost the BMP signal appear to be induced to differentiate into DVE, extraembryonic VE is not competent to become DVE even if it loses the BMP signal. First, in wild-type embryos at E5.2, before DVE formation, the entire embryonic VE is positive for the BMP signal. At E5.5, however, the BMP signal is lost specifically from DVE, with the remainder of the embryonic VE remaining positive for this signal (Fig. 2). Second, in Lefty1−/− embryos, the BMP-negative region of embryonic VE expands to the proximal side, toward which DVE formation also expands (Fig. 4). Third, the portion of embryonic VE that is converted to DVE was found to be increased in Noggin-treated embryos (Fig. 6). Although the BMP signal disappeared in both embryonic VE and extraembryonic VE of such embryos, extraembryonic VE was not converted to DVE. Fourth, in embryo explants stripped of ExE, in which Bmp4 and Bmp8b are expressed (Coucouvanis and Martin, 1999; Ying et al., 2000), the loss of BMP signaling precedes DVE expansion, suggesting that the former may be responsible for the latter. Expansion of Hex expression thus begins ∼90 min after the removal of ExE, whereas the BMP signal is lost from VE much earlier, ∼30 min after ExE removal (Fig. 5). Finally, in ExE-stripped embryos treated with BMP4 (Fig. 6) or with knockout serum replacement that contains BMP activity (Georgiades and Rossant, 2006), the Hex-expressing domain fails to expand. These results indicate that loss of the BMP signal from embryonic VE results in its conversion to DVE.
How is the BMP/Smad1 signal down-regulated in the region of DVE formation?
Although it is not clear how BMP signaling is down-regulated in the DVE region at E5.5, several mechanisms are possible. First, a BMP antagonist (or antagonists), such as Cerl, may be responsible. However, p-Smad1 was also absent in DVE of Cerl−/− embryos (Fig. 5 H), suggesting that Cerl is either not involved in or alone is not sufficient for the down-regulation of BMP signaling. Second, Bmp4 and Bmp8b, which encode the major BMP ligands at this stage, are expressed in ExE (Coucouvanis and Martin, 1999; Ying et al., 2000); elongation of the embryo at the egg cylinder stage along the proximodistal axis between E5.2 and E5.5 may therefore prevent BMPs from reaching the distal end of the embryo. Also, a low level of Bmp2 expression that can be detected in the distal region of the E5.2 embryo disappears by E5.5 (Fig. S5, I and J). Finally, an antagonism between Smad2 and Smad1 signaling may play the major role in down-regulation of the BMP signal. An inverse relation between the level of p-Smad1 and p-Smad2 was thus repeatedly observed in E5.2–5.5 embryos. In the wild-type embryo at E5.5, for instance, BMP signaling via p-Smad1 was detected predominantly in the proximal epiblast, a distribution opposite to that of p-Smad2 (Fig. 2 C vs. Fig. 4 B; and Fig. 7, A vs. J and D vs. G). Furthermore, p-Smad1 was lost from the distal half of VE in E5.5 Lefty1−/− embryos (Fig. 4 C) and Activin-treated wild-type embryos (Fig. 7 L), whereas the level of p-Smad2 was increased in this region. Staining for p-Smad1 was also lost from the entire VE, whereas that for p-Smad2 was increased in embryo explants stripped of ExE (Fig. 5) and in Noggin-treated embryos (Fig. 6, L and N). Staining for p-Smad2 was completely lost, whereas that for p-Smad1 was increased in embryos treated with SB431542 (Fig. S5, K–N). The antagonism between Smad1 and Smad2 signaling may convert a small shift of the balance to a robust difference.
Antagonism between Smad1 and Smad2 signaling
Our data suggest a previously unknown role for Activin in mouse embryonic patterning. Inhibition of Activin action by follistatin (Fig. 7, P–R) or SB431542 (Fig. S5, P and R) thus abolished DVE formation. Such treatment not only down-regulated p-Smad2 in DVE but also slightly up-regulated p-Smad1 on the distal side of the embryo. Conversely, treatment with Activin expanded the site of DVE formation by up-regulating p-Smad2 and down-regulating p-Smad1 on the distal side of the embryo (Fig. 7). These observations are again indicative of an antagonism between Smad1 and Smad2 signaling during DVE formation. Treatment of explants lacking ExE with SB431542 induced the loss of both p-Smad1 and p-Smad2. DVE formation was abolished in such explants (Mesnard et al., 2006; this study), suggesting that DVE formation requires both the absence of Smad1 signal and the presence of Smad2 signal. The balance between the ratio of p-Smad1 and p-Smad2 may determine whether or not a cell in the VE will become DVE. To test this, it would be necessary to quantify the amounts of p-Smad1 and p-Smad2 in individual cells and to monitor their interaction with Smad4, such as by FRET analysis.
Mutant embryos lacking Activin establish a normal A-P axis, most likely because Activin is provided by the decidual zone of the dam, not by the embryo (Albano et al., 1994; Jones et al., 2006). Maternally derived molecules of a large molecular weight can cross Reichert membrane and be transferred to the embryo. For example, maternal immunoglobulins (Bernard et al., 1977) and maternal and DiI-labeled high density lipoprotein intravenously injected into a pregnant mother (Smith et al., 2006) have been found in the embryo. In fact, maternally derived Activin proteins have been detected within the embryo including the VE between E3.5 and E6.5 (Jones et al., 2006).
Antagonism between Smad2 and Smad1 signaling may play a role in vertebrate development more generally. For example, BMP4 blocks the dorsal mesoderm-inducing activity of Activin in Xenopus laevis (Dale et al., 1992; Jones et al., 1992; Piccolo et al., 1996; Zimmerman and Mathews, 1996). Also in Xenopus, Chordin and Noggin prevent BMPs from interacting with their cognate receptors and thus allow underlying Activin signaling in the dorsal region of the embryo to induce dorsal mesoderm (Piccolo et al., 1996; Zimmerman and Mathews, 1996). Expression of Nodal is observed in the deep region of the involuting marginal zone, which is equivalent to AVE in mouse, before the onset of gastrulation in Xenopus embryos (Jones et al., 1995). Whereas p-Smad2 is present in these cells, p-Smad1 is absent (Schohl and Fagotto, 2002). In the mouse, Chordin and Noggin, in addition to Nodal, are expressed in the future node region (Conlon et al., 1994; Bachiller et al., 2000), and they may similarly shift the balance toward Activin–Nodal signaling. Indeed, the reduction in the level of Nodal signaling in Foxh1−/− embryos prevents node formation (Hoodless et al., 2001; Yamamoto et al., 2001). These observations suggest that the antagonism between Activin–Nodal and BMP signaling may play a role in organizer formation in vertebrates.
What is the molecular basis of the antagonism between BMP and Activin–Nodal signaling? Our result suggests that common signaling components, ActR2a and ActR2B, are rate-limiting factors in the E5.5 mouse embryo (Fig. 8). However, these results obtained by overexpression experiments need to be confirmed by other approaches because overexpressed type II receptor molecules may bind to type I receptor easily, which is often sufficient to activate signaling, or may interact with nonphysiological type I receptors. In Xenopus animal caps, Activin and BMP signaling have been shown to antagonize each other through the intracellular component Smad4 (Candia et al., 1997). However, the level of Smad4 was not low enough to cause the antagonism at least in the E5.5 mouse embryo. Different mechanisms may underlie the antagonism between BMP and Activin–Nodal depending on the cell type.
Materials and methods
In situ hybridization and immunohistofluorescence
Embryos were carefully staged on the basis of their morphology. Whole-mount in situ hybridization was performed according to a standard procedure or a method designed for peri-implantation embryos (Strumpf et al., 2005; protocol is available at http://www.sickkids.ca/rossant/protocols/doublefluor.asp). Plasmids to generate probes for in situ hybridization were provided by R. Behringer (University of Texas, Houston, TX), R.S. Beddington (National Institute for Medical Research, London, England, UK), J.E. Darnell Jr. (The Rockefeller University, New York, NY), B. Hogan (Duke University Medical Center, Durham, NC), C.L. MacLeod (University of California, San Diego, La Jolla, CA), A. McMahon (Harvard University, Cambridge, MA), C. Nihers (Deutsches Krebsforschungszentrum, Heidelberg, Germany), M.S. Parmacek (University of Pennsylvania, Philadelphia, PA), J. Rossant (The Hospital for Sick Children, Toronto, Canada), and E. De Robertis (University of California, Los Angeles, CA). Immunostaining was performed with rabbit polyclonal antibodies to p-Smad1 generated in response to the phosphorylated C-terminal region of mouse Smad1 (Persson et al., 1998; a gift from P. ten Dijke, Leiden University Medical Center, Leiden, Netherlands), rabbit monoclonal antibodies to p-Smad2 (Cell Signaling Technology), and Alexa Fluor 568–conjugated goat antibodies to rabbit immunoglobulin G (Invitrogen) used at 1:200, 1:25, and 1:2,000 dilutions, respectively. Nuclei were stained with YOYO-1 (Invitrogen). For immunostaining, embryos recovered in DME or PBS including phosphatase inhibitor cocktail containing 10 mM sodium fluoride, 2 mM sodium orthovanadate, and 50 mM β-glycerophosphate were fixed in 4% PFA for 10 min and were blocked in TSA blocking reagent (PerkinElmer). Methanol series is necessary for p-Smad1 staining before the blocking process. Washing solution is PBS including 0.1% Triton X-100. Confocal images were obtained with a laser scanning confocal microscope (LSM 510; Carl Zeiss, Inc.) mounted on an inverted microscope (Axiovert 100M; Carl Zeiss, Inc.), using Plan Apochromat 20×/0.75 NA and C-Apochromat 40×/1.2 NA objectives (Carl Zeiss, Inc.). Images were processed with Photoshop CS software (Adobe). Genomic DNA isolated from whole embryos was used for genotype analysis. Second antibody alone did not give rise to detectable staining of p-Smad1 and p-Smad2.
Generation and analysis of chimeric embryos
Chimeras were generated by ES cell tetraploid embryo aggregation. Two-cell embryos were collected from intercrosses between Bmpr2+/− or Bmpr2+/−,Actr2b+/− mice and were aggregated with green ES FM260 cells expressing EGFP (provided by M. Okabe, Osaka University, Osaka, Japan). Chimeric embryos were recovered at the indicated stages, fixed, and both examined by fluorescence microscopy (MZ FLIII; Leica) using PlanApo 1.0× WILD objective (Leica) and processed for whole-mount in situ hybridization or for immunohistofluorescence analysis. The genotype of the host embryo was determined retrospectively with extraembryonic tissue. Images were obtained with a digital camera (DP11; Olympus) and processed with Photoshop CS software.
Embryo explant culture
For explant cultures, the ExE and epiblast overlying embryonic VE were removed from ICR mouse (SLC) or Hex-Venus transgenic mouse embryos dissected at E5.5 with fine forceps and a tungsten needle (Takaoka et al., 2006). Chimeric explants were generated with the use of a fine glass needle (Drummond Scientific Co.). The explants were cultured for 6 h or the indicated times under a humidified atmosphere of 5% CO2 at 37°C in dishes or in gel matrix (Mebiol) containing DME supplemented with 75% rat serum. Noggin, BMP4, follistatin, Activin (R&D Systems), and SB431542 (Sigma-Aldrich) were used at concentrations of 500 ng/ml, 50 ng/ml, 1.2 µg/ml, 50 µg/ml, and 10 µmol/l, respectively. Images were analyzed using a laser scanning confocal microscope (LSM 510) mounted on an inverted microscope (Axiovert 100M) using Plan Apochromat 20×/0.75 NA and C-Apochromat 40×/1.2 NA objectives (Carl Zeiss, Inc). Images were processed with Photoshop CS software.
Introduction of expression vectors into the epiblast
Introduction of expression vectors (a GFP expression alone or with various effectors; Mizushima and Nagata, 1990) into the epiblast was performed as described by Yamamoto et al. (2004). Embryos were cultured for 12 h under a humidified atmosphere of 5% CO2 at 37°C in dishes containing DME supplemented with 75% rat serum.
Online supplemental material
Fig. S1 shows expression of DVE markers in the wild-type mouse embryos at E5.2 and E5.5. Fig. S2 shows phenotype of AVE, VE, and ExE in Bmpr2−/− embryos. Fig. S3 shows p-Smad1 staining in wild-type E5.5 embryos and in the chimeric embryo at E5.0. Fig. S4 shows phenotype of DVE in Bmpr2−/−,Nodal+/− embryos. Fig. S5 shows effects of SB431542, follistatin, and BMP4 on DVE formation. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200808044/DC1.
Acknowledgments
We thank A. Ralston, P. Georgiades, J. Quinn, S. McMaster, and Y. Yamanaka for help with whole-mount fluorescence in situ hybridization, embryo culture, and tetraploid aggregation; M. Okabe for green ES FM260 cells; P. ten Dijke for antibodies to p-Smad1; R. Behringer, R.S. Beddington, J.E. Darnell Jr., B. Hogan, C.L. MacLeod, A. McMahon, C. Nihers, M.S. Parmacek, J. Rossant, and E. De Robertis for probe constructs for in situ hybridization; K. Yusa, Y. Wada, T. Nakamura, and Y. Yamanaka for comments on the manuscript; H. Nishimura, K. Miyama, S. Ohishi, and K. Yamashita for technical assistance; and Y. Mishina for exchanging unpublished data.
This work was supported by a grant from Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation (to H. Hamada) as well as by a Grant-in-Aid for Scientific Research on Priority Areas and a Grant-in-Aid for Young Scientists (to M. Yamamoto) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. M. Yamamoto is also a recipient of a fellowship from the Japan Society for the Promotion of Science for Japanese Junior Scientists.
Footnotes
Abbreviations used in this paper: A-P, anterior–posterior; AVE, anterior visceral endoderm; BMP, bone morphogenetic protein; DVE, distal visceral endoderm; E, embryonic day; ES, embryonic stem; ExE, extraembryonic ectoderm; p-Smad, phosphorylated Smad; VE, visceral endoderm.
References
- Andersson O., Bertolino P., Ibanez C.F. 2007. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development.Dev. Biol. 311:500–511 [DOI] [PubMed] [Google Scholar]
- Albano R.M., Arkell R., Beddington R.S., Smith J.C. 1994. Expression of inhibin subunits and follistatin during postimplantation mouse development: decidual expression of activin and expression of follistatin in primitive streak, somites and hindbrain.Development. 120:803–813 [DOI] [PubMed] [Google Scholar]
- Bachiller D., Klingensmith J., Kemp C., Belo J.A., Anderson R.M., May S.R., McMahon J.A., McMahon A.P., Harland R.M., Rossant J., De Robertis E.M. 2000. The organizer factors Chordin and Noggin are required for mouse forebrain development.Nature. 403:658–661 [DOI] [PubMed] [Google Scholar]
- Beddington R.S., Robertson E.J. 1998. Anterior patterning in mouse.Trends Genet. 14:277–284 [DOI] [PubMed] [Google Scholar]
- Beddington R.S., Robertson E.J. 1999. Axis development and early asymmetry in mammals.Cell. 96:195–209 [DOI] [PubMed] [Google Scholar]
- Belo J.A., Bachiller D., Agius E., Kemp C., Borges A.C., Marques S., Piccolo S., De Robertis E.M. 2000. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development.Genesis. 26:265–270 [PubMed] [Google Scholar]
- Beppu H., Kawabata M., Hamamoto T., Chytil A., Minowa O., Noda T., Miyazono K. 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos.Dev. Biol. 221:249–258 [DOI] [PubMed] [Google Scholar]
- Bernard O., Ripoche M.A., Bennett D. 1977. Distribution of maternal immunoglobulins in the mouse uterus and embryo in the days after implantation.J. Exp. Med. 145:58–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Lu C.C., Norris D.P., Rodriguez T.A., Beddington R.S., Robertson E.J. 2001. Nodal signalling in the epiblast patterns the early mouse embryo.Nature. 411:965–969 [DOI] [PubMed] [Google Scholar]
- Candia A.F., Watabe T., Hawley S.H., Onichtchouk D., Zhang Y., Derynck R., Niehrs C., Cho K.W. 1997. Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads.Development. 124:4467–4480 [DOI] [PubMed] [Google Scholar]
- Chen C., Ware S.M., Sato A., Houston-Hawkins D.E., Habas R., Mazuk M.M., Shen M.M., Brown C.W. 2006. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo.Development. 133:319–329 [DOI] [PubMed] [Google Scholar]
- Conlon F.L., Lyons K.M., Takaesu N., Barth K.S., Kispert A., Herrmann B., Robertson E.J. 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse.Development. 120:1919–1928 [DOI] [PubMed] [Google Scholar]
- Coucouvanis E., Martin G.R. 1999. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo.Development. 126:535–546 [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B.M., Smith J.C. 1992. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development.Development. 115:573–585 [DOI] [PubMed] [Google Scholar]
- Enders A.C., Given R.L., Schlafke S. 1978. Differentiation and migration of endoderm in the rat and mouse at implantation.Anat. Rec. 190:65–77 [DOI] [PubMed] [Google Scholar]
- Georgiades P., Rossant J. 2006. Ets2 is necessary in trophoblast for normal embryonic anteroposterior axis development.Development. 133:1059–1068 [DOI] [PubMed] [Google Scholar]
- Gu Z., Reynolds E.M., Song J., Lei H., Feijen A., Yu L., He W., MacLaughlin D.T., van den Eijnden-van Raaij J., Donahoe P.K., Li E. 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo.Development. 126:2551–2561 [DOI] [PubMed] [Google Scholar]
- Hoodless P.A., Pye M., Chazaud C., Labbe E., Attisano L., Rossant J., Wrana J.L. 2001. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse.Genes Dev. 15:1257–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Lyons K.M., Lapan P.M., Wright C.V., Hogan B.L. 1992. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction.Development. 115:639–647 [DOI] [PubMed] [Google Scholar]
- Jones C.M., Kuehn M.R., Hogan B.L., Smith J.C., Wright C.V. 1995. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation.Development. 121:3651–3662 [DOI] [PubMed] [Google Scholar]
- Jones R.L., Kaitu'u-Lino T.J., Nie G., Sanchez-Partida L.G., Findlay J.K., Salamonsen L.A. 2006. Complex expression patterns support potential roles for maternally derived activins in the establishment of pregnancy in mouse.Reproduction. 132:799–810 [DOI] [PubMed] [Google Scholar]
- Kimura C., Yoshinaga K., Tian E., Suzuki M., Aizawa S., Matsuo I. 2000. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals.Dev. Biol. 225:304–321 [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C., Nakano H., Okamura D., Nakao K., Yonemura S., Belo J.A., Aizawa S., Matsui Y., Matsuo I. 2005. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm.Dev. Cell. 9:639–650 [DOI] [PubMed] [Google Scholar]
- Kishigami S., Mishina Y. 2005. BMP signaling and early embryonic patterning.Cytokine Growth Factor Rev. 16:265–278 [DOI] [PubMed] [Google Scholar]
- Lechleider R.J., Ryan J.L., Garrett L., Eng C., Deng C., Wynshaw-Boris A., Roberts A.B. 2001. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion.Dev. Biol. 240:157–167 [DOI] [PubMed] [Google Scholar]
- Lu C.C., Robertson E.J. 2004. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning.Dev. Biol. 273:149–159 [DOI] [PubMed] [Google Scholar]
- Massague J., Chen Y.G. 2000. Controlling TGF-beta signaling.Genes Dev. 14:627–644 [PubMed] [Google Scholar]
- Mesnard D., Guzman-Ayala M., Constam D.B. 2006. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning.Development. 133:2497–2505 [DOI] [PubMed] [Google Scholar]
- Mishina Y., Suzuki A., Ueno N., Behringer R.R. 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis.Genes Dev. 9:3027–3037 [DOI] [PubMed] [Google Scholar]
- Mishina Y., Crombie R., Bradley A., Behringer R.R. 1999. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis.Dev. Biol. 213:314–326 [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. 1990. pEF-BOS, a powerful mammalian expression vector.Nucleic Acids Res. 18:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadijcka M., Hillman N. 1974. Ultrastructural studies of the mouse blastocyst substages.J. Embryol. Exp. Morphol. 32:675–695 [PubMed] [Google Scholar]
- Perea-Gomez A., Vella F.D., Shawlot W., Oulad-Abdelghani M., Chazaud C., Meno C., Pfister V., Chen L., Robertson E., Hamada H., et al. 2002. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks.Dev. Cell. 3:745–756 [DOI] [PubMed] [Google Scholar]
- Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engstrom U., Heldin C.H., Funa K., ten Dijke P. 1998. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation.FEBS Lett. 434:83–87 [DOI] [PubMed] [Google Scholar]
- Piccolo S., Sasai Y., Lu B., De Robertis E.M. 1996. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4.Cell. 86:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez T.A., Srinivas S., Clements M.P., Smith J.C., Beddington R.S. 2005. Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm.Development. 132:2513–2520 [DOI] [PubMed] [Google Scholar]
- Roelen B.A., Goumans M.J., van Rooijen M.A., Mummery C.L. 1997. Differential expression of BMP receptors in early mouse development.Int. J. Dev. Biol. 41:541–549 [PubMed] [Google Scholar]
- Schohl A., Fagotto F. 2002. Beta-catenin, MAPK and Smad signaling during early Xenopus development.Development. 129:37–52 [DOI] [PubMed] [Google Scholar]
- Smith B.T., Mussell J.C., Fleming P.A., Barth J.L., Spyropoulos D.D., Cooley M.A., Drake C.J., Argraves W.S. 2006. Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation.BMC Dev. Biol. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M.L., Haraguchi S., Torres-Padilla M.E., Kalmar T., Carpenter L., Bell G., Morrison A., Ring C.J., Clarke N.J., Glover D.M., Zernicka-Goetz M. 2005. Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi.BMC Dev. Biol. 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D., Mao C.A., Yamanaka Y., Ralston A., Chawengsaksophak K., Beck F., Rossant J. 2005. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst.Development. 132:2093–2102 [DOI] [PubMed] [Google Scholar]
- Takaoka K., Yamamoto M., Shiratori H., Meno C., Rossant J., Saijoh Y., Hamada H. 2006. The mouse embryo autonomously acquires anterior-posterior polarity at implantation.Dev. Cell. 10:451–459 [DOI] [PubMed] [Google Scholar]
- Tanaka C., Sakuma R., Nakamura T., Hamada H., Saijoh Y. 2007. Long-range action of Nodal requires interaction with GDF1.Genes Dev. 21:3272–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.Q., Brown A., Beddington R.S. 1998. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors.Development. 125:85–94 [DOI] [PubMed] [Google Scholar]
- Tremblay K.D., Dunn N.R., Robertson E.J. 2001. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation.Development. 128:3609–3621 [DOI] [PubMed] [Google Scholar]
- Whitman M. 2001. Nodal signaling in early vertebrate embryos: themes and variations.Dev. Cell. 1:605–617 [DOI] [PubMed] [Google Scholar]
- Xu R.H., Peck R.M., Li D.S., Feng X., Ludwig T., Thomson J.A. 2005. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells.Nat. Methods. 2:185–190 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Meno C., Sakai Y., Shiratori H., Mochida K., Ikawa Y., Saijoh Y., Hamada H. 2001. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse.Genes Dev. 15:1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Saijoh Y., Perea-Gomez A., Shawlot W., Behringer R.R., Ang S.L., Hamada H., Meno C. 2004. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo.Nature. 428:387–392 [DOI] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke P., Huylebroeck D., Sampath T.K., Andries M., Smith J.C., Heldin C.H., Miyazono K. 1995. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects.J. Cell Biol. 130:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y., Liu X.M., Marble A., Lawson K.A., Zhao G.Q. 2000. Requirement of Bmp8b for the generation of primordial germ cells in the mouse.Mol. Endocrinol. 14:1053–1063 [DOI] [PubMed] [Google Scholar]
- Zhao G.Q. 2003. Consequences of knocking out BMP signaling in the mouse.Genesis. 35:43–56 [DOI] [PubMed] [Google Scholar]
- Zhou X., Sasaki H., Lowe L., Hogan B.L., Kuehn M.R. 1993. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation.Nature. 361:543–547 [DOI] [PubMed] [Google Scholar]
- Zimmerman C.M., Mathews L.S. 1996. Activin receptors: cellular signalling by receptor serine kinases.Biochem. Soc. Symp. 62:25–38 [PubMed] [Google Scholar]