Abstract
Phagocytosis, which is essential for the immune response to pathogens, is initiated by specific interactions between pathogens and cell surface receptors expressed by phagocytes. This study identifies triggering receptor expressed on myeloid cells 2 (TREM-2) and its signaling counterpart DAP12 as a molecular complex that promotes phagocytosis of bacteria. Expression of TREM-2–DAP12 enables nonphagocytic Chinese hamster ovary cells to internalize bacteria. This function depends on actin cytoskeleton dynamics and the activity of the small guanosine triphosphatases Rac and Cdc42. Internalization also requires src kinase activity and tyrosine phosphorylation. In bone marrow–derived macrophages, phagocytosis is decreased in the absence of DAP12 and can be restored by expression of TREM-2–DAP12. Depletion of TREM-2 inhibits both binding and uptake of bacteria. Finally, TREM-2–dependent phagocytosis is impaired in Syk-deficient macrophages. This study highlights a novel role for TREM-2–DAP12 in the immune response to bacterial pathogens.
Introduction
Phagocytosis is an essential element of the innate immune response to infectious agents. Professional phagocytes engulf invading microorganisms, subjecting them to intracellular processes that typically lead to their degradation (Brown, 1995; Aderem and Underhill, 1999; Jutras and Desjardins, 2005). Internalized pathogens can be further exposed to pattern recognition receptors (e.g., Toll-like receptors), thereby activating additional pathways of the innate immune response (Underhill et al., 1999). Phagocytosis also contributes to antigen processing for presentation to T cells (Jutras and Desjardins, 2005).
Phagocytosis is initiated upon interaction between microorganisms and receptors expressed at the surface of phagocytes. These interactions are highly specific, and phagocytes express an elaborate arsenal of receptors that enables recognition of a wide variety of microorganisms. Once receptors are engaged, membrane remodeling at the site of interaction leads to the complete wrapping of the particle and its subsequent release in the cytoplasm within a membrane-bound compartment, the phagosome (Aderem and Underhill, 1999; Jutras and Desjardins, 2005). Membrane dynamics during phagocytosis are driven by a controlled rearrangement of the actin cytoskeleton (Groves et al., 2008).
Triggering receptors expressed on myeloid cells (TREMs) are type I membrane proteins with an extracellular Ig-like domain and a short cytoplasmic tail that has no intrinsic signaling capacity (Klesney-Tait et al., 2006). TREM signaling relies on association with DAP12, a cytosolic adapter that also associates with other receptors. DAP12 contains an immunoreceptor tyrosine-based activation motif (ITAM), which becomes phosphorylated upon activation of DAP12-associated receptors. The phosphorylated ITAM in turn recruits and activates Syk tyrosine kinase, leading to cellular responses such as regulation of cytokine production (Lanier and Bakker, 2000; Takaki et al., 2006).
Previous work has shown that TREM-2 promotes phagocytosis of apoptotic neurons by microglia (Takahashi et al., 2005), although the underlying mechanisms remain unclear. Our prior work showed that TREM-2 binds a wide variety of bacteria (Daws et al., 2003). However, receptor ligation alone does not trigger phagocytosis by default. For example, the complement receptor 3 binds C3bi-coated particles but does not promote their internalization in nonactivated cells (Wright and Silverstein, 1982, 1983). Therefore, we examined whether TREM-2 binding to bacteria promotes their phagocytosis. This study demonstrates that TREM-2–DAP12 but not TREM-1–DAP12 functions as a phagocytic receptor for bacteria.
Results and discussion
Expression of TREM-2 with DAP12 promotes phagocytosis of bacteria by CHO cells
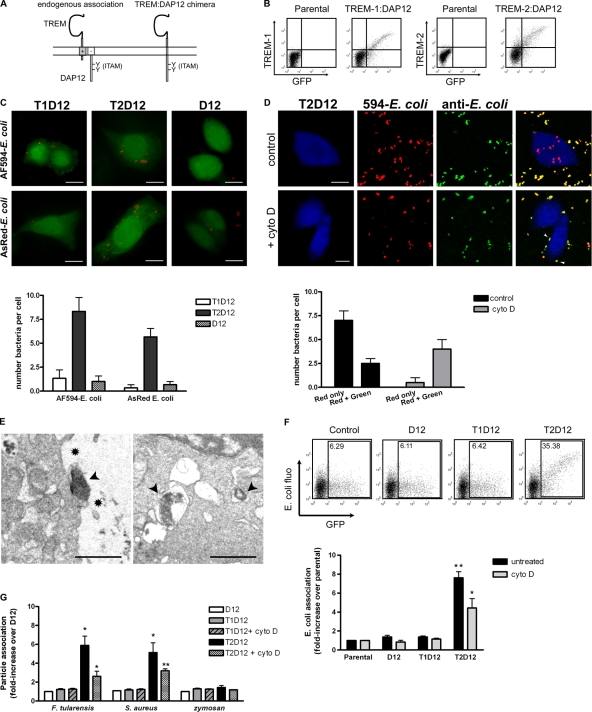
To examine the role of TREMs in phagocytosis, we individually expressed TREM-1 and TREM-2 in a nonphagocytic cell line, CHO. This approach was used by others to characterize the binding and/or phagocytosis-promoting functions of receptors, including Fc (Downey et al., 1999) and complement (Cywes et al., 1996) receptors. We anticipated that TREM function would require DAP12, the only adapter known to mediate TREM signaling (Takaki et al., 2006; Turnbull and Colonna, 2007). To ensure this, however, we expressed a chimeric molecule in which the TREM extracellular domain was covalently linked to DAP12 cytoplasmic tail (Fig. 1 A). These constructs recapitulate the functions of the endogenous TREM–DAP12 complexes (Hamerman et al., 2006). TREM–DAP12 cDNAs were expressed from a GFP bicistronic vector, allowing the use of GFP fluorescence as an indicator of their expression (Fig. 1 B).
Figure 1.
Expression of TREM-2–DAP12 promotes binding and internalization of bacteria by nonphagocytic cells. (A) Schematic of TREM association with DAP12 in trans (endogenous conformation, left) or through covalent linkage (chimeric construct, right). (B) CHO cells were transfected with bicistronic constructs containing GFP together with TREM-1–DAP12 (T1D12) or TREM-2–DAP12 (T2D12) or DAP12 only (D12). Surface expression of TREM–DAP12 chimeras in GFP-positive cells was verified by flow cytometry using antibodies to TREM-1 or TREM-2. (C) T1D12, T2D12, or D12 cells were challenged with 594–E. coli (top) or AsRed–E. coli (bottom). Association of transfected cells with bacteria was analyzed by fluorescence microscopy and quantified (graph). (D) T2D12 cells challenged with 594–E. coli were stained with E. coli antibodies and analyzed by microscopy. Pseudocolors: blue, T2D12 cells; red, 594–E. coli; green, anti–E. coli staining. Internal bacteria are red, whereas extracellular bacteria are both red and green (yellow on merge). Both internalized and extracellular cell-bound bacteria were scored (graph). Arrowheads represent cell-bound bacteria. (E) T2D12 cells challenged with live, unlabeled E. coli were analyzed by electron microscopy. Both binding (left) and internalization of bacteria within phagosomes (right) were observed. Arrowheads, bacteria; asterisks, pseudopods. (F) Association of T2D12 cells with 594–E. coli was analyzed by flow cytometry and quantified by measuring the fluorescence intensity of bacteria associated with GFP-positive cells. Representative plots are shown. Internalization of bacteria (but not binding) was blocked with cyto D. Fluorescence intensities were normalized to values of parental cells. The graph represents the mean of four experiments ± SEM. *, P < 0.05; **, P < 0.01 relative to parental values. (G) T1D12, T2D12, or D12 cells were challenged with F. tularensis, S. aureus, or zymosan, and their association was quantified as in F. Fluorescence intensities were normalized to D12 values in each set. The graph represents the mean of three experiments ± SEM. *, P < 0.05; **, P < 0.01 relative to D12 values. Bars: (C and D) 10 µm; (E) 1 µm.
CHO cells expressing TREM-1–DAP12 (T1D12), TREM-2–DAP12 (T2D12), or DAP12 only (D12) were challenged with Escherichia coli coupled to Alexa Fluor 594 (594–E. coli), and the interaction was monitored by microscopy. T2D12 cells showed substantial association with 594–E. coli, whereas T1D12 or D12 cells did not (Fig. 1 C, top). A live E. coli strain internally expressing the fluorescent protein AsRed also showed increased association with T2D12 cells but not with T1D12 or D12 cells, ruling out an interaction caused by the Alexa Fluor dye (Fig. 1 C).
To determine whether the bacteria associated with T2D12 cells were extracellular or internalized, cells challenged with 594–E. coli were fixed using a nonpermeant fixative and stained with E. coli antibodies (Fig. 1 D). Both single- and double-stained bacteria colocalized with T2D12 cells, indicating that TREM-2–DAP12 promoted both binding and uptake of E. coli. Pretreatment of T2D12 cells with cytochalasin D (cyto D), which inhibits actin polymerization and blocks phagocytosis (Groves et al., 2008), abolished colocalization of single-stained bacteria with T2D12 cells, whereas double-stained bacteria bound to cells could still be detected (Fig. 1 D). Electron micrographs showed internalized bacteria found in phagosomes (Fig. 1 E, right) and cell-bound bacteria surrounded by pseudopods (Fig. 1 E, left). Thus, TREM-2–DAP12 mediates both binding and actin-dependent uptake of E. coli by CHO cells.
To strengthen and quantify these results, phagocytosis was also analyzed by flow cytometry, measuring the fluorescence of 594–E. coli associated with T2D12 cells. Consistent with the microscopy results, expression of TREM-2–DAP12 promoted E. coli association with CHO cells (Fig. 1 F). Cyto D–treated T2D12 cells showed stronger association with E. coli than did T1D12 or D12 cells, confirming that E. coli still bound T2D12 cells in the absence of phagocytosis.
To examine the generality of TREM-2–DAP12 function, association of T2D12 cells with a variety of particles was examined. Francisella tularensis (a Gram negative) and Staphylococcus aureus (a Gram positive) were each internalized through TREM-2 (Fig. 1 G), as was Pseudomonas aeruginosa (another Gram-negative pathogen; not depicted), suggesting the existence of a broad range of phagocytic substrates for TREM-2. In contrast, zymosan particles (derived from the cell wall of Saccharomyces cerevisiae, a nonpathogenic yeast) did not interact with T2D12 cells (Fig. 1 G), indicating a degree of ligand specificity. Previously, TREM-2 binding to the yeast Candida albicans was found to be minimal (Daws et al., 2003). Because the cell wall composition of C. albicans is very similar to that of S. cerevisiae (Firon et al., 2004), it is not unexpected that TREM-2 would not bind zymosan. In contrast to T2D12 cells, T1D12 cells did not support binding or uptake of any of the particles tested.
TREM-2–dependent phagocytosis in CHO cells requires tyrosine phosphorylation and src kinase activity and is enhanced by Syk
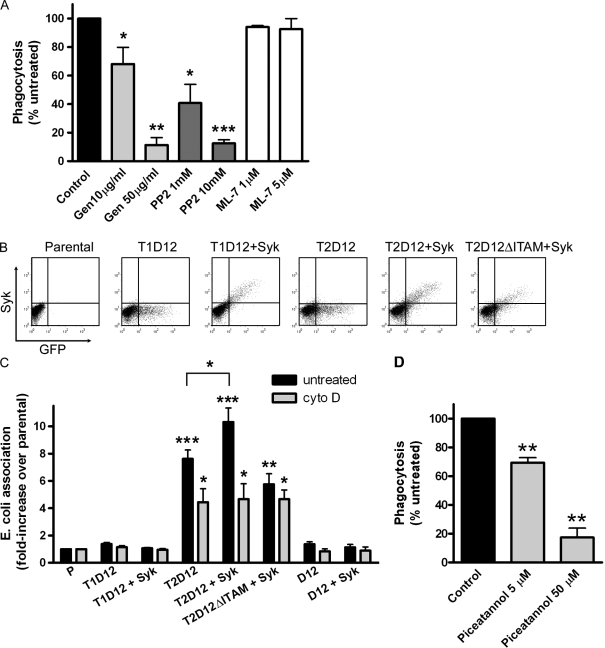
To initiate a signaling cascade, TREM receptors depend on their association with the ITAM-containing adapter DAP12 (Klesney-Tait et al., 2006). Upon TREM binding, phosphorylation of the ITAM by src kinases allows the recruitment of Syk tyrosine kinase (Lanier and Bakker, 2000; Takaki et al., 2006). We examined the role of these signaling components. Pretreatment of T2D12 cells with genistein (tyrosine kinase inhibitor) or PP2 (src family kinase inhibitor) reduced bacterial uptake, whereas inhibition of myosin light chain kinase did not (Fig. 2 A). Furthermore, a T2D12 construct in which a mutation in the ITAM prevented its phosphorylation (Hamerman et al., 2006) allowed binding of bacteria but failed to mediate phagocytosis (Fig. 2 C). Thus, TREM-2–DAP12 signaling depends on src-mediated tyrosine phosphorylation, likely within the ITAM domain of DAP12. Interestingly, uptake of E. coli was increased by Syk transfection in T2D12 cells but not in T1D12 or in T2D12ΔITAM cells (Fig. 2, B and C). Thus, enhancement of phagocytosis by Syk requires both TREM-2 and phosphorylation of the DAP12 ITAM. A prominent role for Syk in DAP12 signaling through its ITAM has been described previously (Takaki et al., 2006). However, our results indicate that significant TREM-2–dependent phagocytosis occurs in the absence of exogenous Syk (Fig. 1), which raises the possibility that Syk might be endogenously expressed in CHO cells or that a related kinase might be involved. Similar observations were made in CHO cells expressing the Fc receptor FcγRIIa: substantial phagocytosis was observed in the absence of exogenous Syk (Downey et al., 1999). However, other studies in leukocytes revealed an essential role for Syk in FcγRIIa-dependent uptake (Crowley et al., 1997; Kiefer et al., 1998). Syk is primarily expressed in myeloid cells (Berton et al., 2005) but is also found in nonmyeloid tissues (Yanagi et al., 2001). Indeed, phagocytosis by T2D12 cells was inhibited by the Syk inhibitor piceatannol (Fig. 2 D), but because this inhibitor may act on other kinases, any conclusion about the identity of the kinase involved in CHO cells requires further investigation. Our results nonetheless indicate that Syk positively regulates TREM-2 function in phagocytosis by CHO cells.
Figure 2.
TREM-2–DAP12-mediated phagocytosis requires tyrosine phosphorylation and src kinase activity, and it is enhanced by Syk. (A) T2D12 cells were treated with genistein (tyrosine kinase inhibitor), PP2 (src kinase inhibitor), or ML-7 (myosin light chain kinase inhibitor) for 2 h at the indicated concentrations before addition of 594–E. coli. Controls for binding were treated with cyto D. Bacteria association was assessed by flow cytometry as in Fig 1. To quantify uptake, values obtained with cyto D were subtracted from that of untreated samples. The graph shows values normalized to controls (no inhibitor). *, P ≤ 0.05; **, P < 0.01; ***, P < 0.001 relative to controls. (B) CHO cells were transfected with T1D12, T2D12, D12, or a variant of T2D12 with a mutated ITAM (T2D12ΔITAM). Where indicated, cells were cotransfected with Syk. (C) 594–E. coli association with transfected cells was quantified as in Fig. 1 F. *, P ≤ 0.05; **, P < 0.01; ***, P < 0.001 relative to parental values. (D) T2D12 cells treated with the indicated concentrations of piceatannol were challenged with E. coli, and uptake was analyzed as in Fig. 2 A. **, P < 0.01. The graphs represent the mean of three experiments ± SEM.
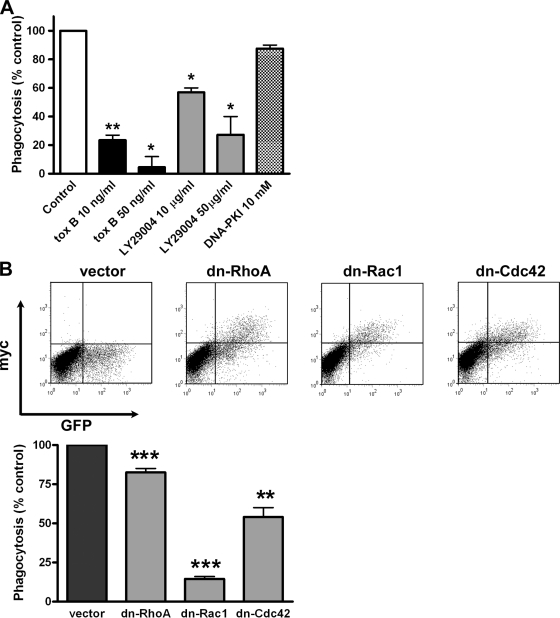
TREM-2–DAP12-dependent phagocytosis in CHO cells requires Rac1 and Cdc42
GTPases of the Rho family play essential roles in phagocytosis: rearrangement of actin-rich formations in the vicinity of the particle leads to its internalization by the surrounding membrane (Niedergang and Chavrier, 2005). Pretreatment of T2D12 cells with Clostridium difficile toxin B, an inhibitor of the Rho family of GTPases, reduced uptake of E. coli (Fig. 3 A). Consistent with this, phagocytosis was abrogated by inhibition of PI-3 kinase (Fig. 3 A), an activator of GTPases (Andrews et al., 2007) required for pseudopod extension during phagocytosis (Cox et al., 1999), but not by inhibition of DNA–protein kinase (Fig. 3 A). During phagocytosis, pseudopod formation and membrane expansion around the particle relies on Rac and Cdc42 (Caron and Hall, 1998), whereas formation of stress fibers beneath the bound particle and subsequent membrane retraction depend on Rho activity (Olazabal et al., 2002). The contribution of each GTPase to phagocytosis through TREM-2 was assessed through expression of dominant-negative (dn) constructs (Fig. 3 B). dn Rac1 and dn Cdc42 inhibited E. coli uptake, whereas dn RhoA had a marginal effect (Fig. 3 B). This shows a major role for Rac1 and Cdc42 and is consistent with electron micrographs showing pseudopod formation around bacteria (Fig. 1 D; Caron and Hall, 1998).
Figure 3.
TREM-2–DAP12-mediated phagocytosis requires Rac1 and Cdc42 activity. (A) T2D12 cells were treated with C. difficile toxin B (Rho GTPase family inhibitor), LY29004 (PI-3 kinase inhibitor), or DNA–protein kinase inhibitor before phagocytosis of 594–E. coli. *, P < 0.05; **, P < 0.01 relative to untreated conditions. (B) Expression of dn Rho, Rac, or Cdc42 into T2D12 cells was verified by flow cytometry. Phagocytosis was analyzed as in A. **, P < 0.01; ***, P < 0.001 relative to vector-transfected cells. The graphs represent the mean of three experiments ± SEM.
TREM-2 contributes to phagocytosis of bacteria by macrophages
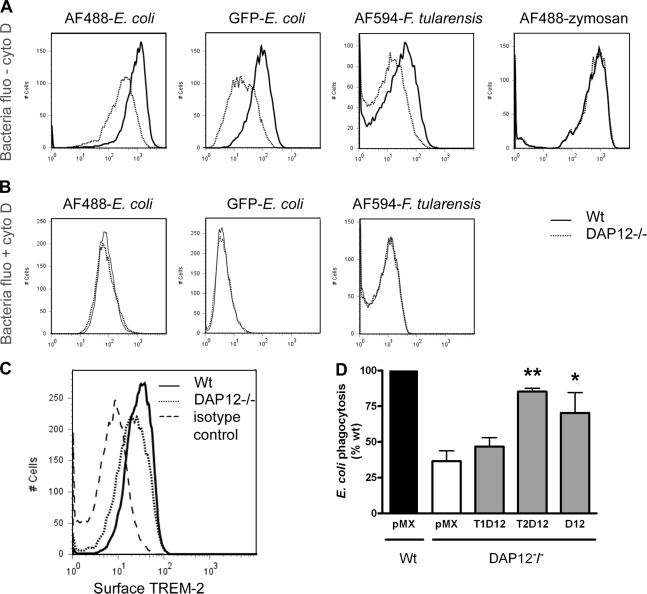
Results obtained in CHO cells identified TREM-2–DAP12 as a molecular complex that promotes phagocytosis of bacteria. To validate this observation in professional phagocytes, we first focused on DAP12-deficient (DAP12−/−) macrophages. Association of both E. coli and F. tularensis with DAP12−/− bone marrow–derived macrophages (BMDMs) was reduced compared with wild-type (wt) BMDMs, whereas that of zymosan was comparable (Fig. 4 A). In contrast, no difference was found in binding (Fig. 4 B), indicating that the reduced association of bacteria with DAP12−/− cells reflects a decreased uptake but not a reduced binding. A possible increased bacterial degradation in DAP12−/− BMDMs was ruled out by the use of bafilomycin A1, an inhibitor of lysosomal fusion. Furthermore, we verified that TREM-2 was still expressed on DAP12−/− BMDMs (Fig. 4 C). Importantly, reintroduction of either DAP12 alone or TREM-2–DAP12 into DAP12−/− BMDMs restored phagocytosis, whereas TREM-1–DAP12 did not (Fig. 4 D). Thus, the decreased uptake by DAP12−/− BMDMs can be attributed, at least in part, to an impaired TREM-2 signaling. However, because DAP12 is a signaling adapter for various receptors (Takaki et al., 2006), other DAP12-associated molecules in addition to TREM-2 (but excluding TREM-1) might also participate in phagocytosis.
Figure 4.
Expression of TREM-2–DAP12 restores phagocytosis in DAP12−/− macrophages. (A and B) wt and DAP12−/− BMDMs were challenged with E. coli (surface or internally labeled), F. tularensis, and zymosan. Particle association was assessed by flow cytometry in the absence (A) or in the presence of cyto D or at 4°C (B). Representative histograms are shown. (C) Surface expression of TREM-2 was analyzed on wt and DAP12−/− BMDMs. (D) DAP12−/− BMDMs were transduced with DAP12, TREM-1–DAP12, TREM-2–DAP12, or empty vector (pMX). Phagocytosis of 594–E. coli by transduced cells was compared with that of wt BMDMs transduced with empty vector (100%). The graph represents the mean of four experiments ± SEM. *, P ≤ 0.05; **, P < 0.01 relative to DAP12−/−/pMX values.
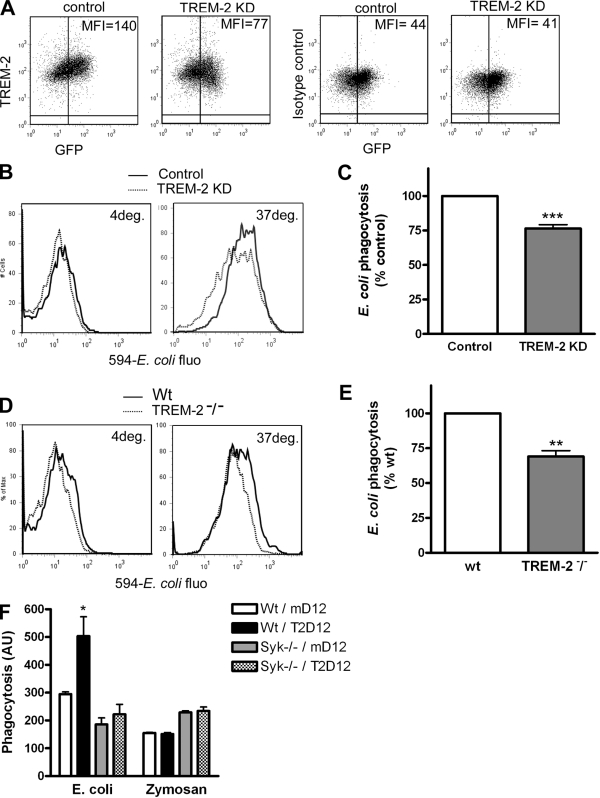
Finally, TREM-2 levels on BMDMs were reduced by the introduction of a short hairpin RNA (Fig. 5 A; Hamerman et al., 2006). TREM-2 knockdown (KD) cells showed both reduced binding and uptake of bacteria (Fig. 5 B), which is consistent with a role for TREM-2 in promoting both functions (Fig. 1; Daws et al., 2003). Quantification revealed a 25% reduction in internalization (Fig. 5 C). Binding and phagocytosis were also examined in TREM-2−/− BMDMs, which allows a direct assessment of TREM-2 relative contribution to phagocytosis. As with TREM-2 KD, both binding and uptake of E. coli were decreased in the absence of TREM-2 (Fig. 5 D). Under these conditions, ∼30% of phagocytosis could be attributed to TREM-2 (Fig. 5 E). Altogether, these data demonstrate a role for TREM-2 in the nonopsonic uptake of E. coli by macrophages.
Figure 5.
TREM-2 promotes phagocytosis of bacteria by macrophages in a Syk-dependent manner. (A) Cellular levels of TREM-2 on BMDMs were decreased by the expression of a short hairpin RNA (TREM-2 KD) and measured by flow cytometry. An isotype antibody was used as a negative control. MFI, mean fluorescence intensity. (B) E. coli association with control or TREM-2 KD BMDMs at 4 or 37°C was assessed by flow cytometry. (C) Bacteria uptake was quantified by subtracting 4°C from 37°C fluorescence values. The graph represents the mean of five experiments ± SEM. ***, P ≤ 0.001. (D and E) E. coli association with wt or TREM-2−/− BMDMs was assessed as in B, and bacteria uptake was quantified in E as described in C. The graph represents the mean of three experiments + SEM. **, P ≤ 0.01. (F) TREM-2–D12 or DAP12 alone (mutated in its transmembrane domain) was expressed in wt or Syk−/− BMDMs, and phagocytosis of E. coli or zymosan was quantified by subtracting 4°C from 37°C fluorescence values. The graph represents the mean of three experiments ± SEM. *, P ≤ 0.05 between wt/mutated DAP12 and wt/TREM-2–DAP12. AU, arbitrary unit.
Results in CHO cells suggested the involvement of Syk or of a related kinase in phagocytosis through TREM-2 (Fig. 2). In BMDMs, overexpression of TREM-2–DAP12, which increased E. coli uptake by wt cells, had no effect on phagocytosis of E. coli by Syk−/− cells (Fig. 5 F). In contrast, deletion of Syk did not inhibit zymosan uptake as previously reported (Herre et al., 2004; Underhill et al., 2005). This indicates that Syk is required for TREM-2 function in phagocytosis by BMDMs.
We have identified a novel phagocytosis pathway initiated by ligation of TREM-2, which involves DAP12 phosphorylation and Syk activation. The expression of TREM-2 was previously shown to promote uptake of apoptotic neurons by microglia (Takahashi et al., 2005), although the underlying mechanisms and associated signaling events are unknown. Recent work by Ziegenfuss et al. (2008) shows that Draper, a Drosophila melanogaster phagocytic receptor, promotes uptake of apoptotic bodies by microglia through activation of its intrinsic ITAM and recruitment of the Syk orthologue, Shark. Thus, the association of phagocytosis with this signaling cascade has been preserved throughout evolution.
TREM-2–DAP12 has also been shown to regulate the production of reactive oxygen species in response to Salmonella typhimurium by macrophages, although TREM-2 binding to S. typhimurium could not be demonstrated (Charles et al., 2008). Thus, TREM-2 may perform additional innate immune functions without binding to pathogens, possibly by engaging endogenous ligands. This study is the first demonstration that the TREM-2–DAP12 complex is fully competent to promote internalization of bacteria by both nonphagocytic cells and professional phagocytes. TREM-2 is also a negative regulator of Toll-like receptor signaling initiated by microbial components (Hamerman et al., 2006). Therefore, TREM-2 function in both pathogen uptake and Toll-like receptor pathways might allow a fine-tuning of the macrophage response to infection.
Materials and methods
Cell culture and transfection
CHO cells were maintained in RPMI 1640 + 10% FCS, transfected using Lipofectamine 2000 (Invitrogen), and used 24 h after transfection. BMDMs were derived from femurs of sex- and age-matched wt or knockout mice (C57BL/6 background) as described previously (Roach et al., 1997). DAP12-deficient mice (provided by L. Lanier, University of California, San Francisco, San Francisco, CA) and TREM-2–deficient mice were previously described (Bakker et al., 2000; Turnbull et al., 2006). Syk-deficient cells were obtained as described previously (Mocsai et al., 2006). Cells were maintained in DME with 10% FCS and 10% CMG14-12 cell culture supernatant as a source of macrophage colony-stimulating factor.
Retrovirus-mediated transduction in BMDMs
cDNAs (D12, T1D12, and T2D12) were introduced into BMDMs by using retroviral infection as described previously (Hamerman et al., 2006). The packaging cell line Plat-E (provided by T. Kitamura, University of Tokyo, Tokyo, Japan; Morita et al., 2000) was transfected with retroviral constructs using Lipofectamine 2000. Virus-containing supernatants were collected 48 h later, added onto 3-d marrow cells in the presence of 4 µg/ml polybrene, and incubated for at least 3 d before assays. Retrovirus-mediated depletion of endogenous TREM-2 was obtained as previously described (Hamerman et al., 2006).
Reagents
TREM antibodies were purchased from R&D Systems. Syk antibody was purchased from Cell Signaling Technology. Cyto D and bafilomycin A1 were obtained from Sigma-Aldrich. Genistein, PP2, C. difficile toxin, LY29004, ML-7, and DNA–protein kinase inhibitor were purchased from EMD. Alexa Fluor particles and dye and E. coli antibodies were obtained from Invitrogen.
DNA constructs
TREM–DAP12 and DAP12 cDNAs were previously described (Hamerman et al., 2006), and Flag-Syk cDNA (provided by I. Frasier, California Institute of Technology, Pasadena, CA) was previously described (Zavzavadjian et al., 2007). Myc-tagged dn RhoA, Rac1, and Cdc42 were generated in the laboratory of G. Bokoch (The Scripps Research Institute, LA Jolla, CA) and distributed by Addgene.
Bacteria
Alexa Fluor 594 or 488 particles (zymosan, E. coli, and S. aureus) were purchased from Invitrogen. AsRed– and GFP–E. coli were provided by the Sandia National Laboratories. F. tularensis strain U112 was provided by D. Monack (Stanford University, Stanford, CA). All cultures were grown to midlog phase. Alexa Fluor 594 staining of F. tularensis was performed according to the manufacturer's instructions. Bacterial viability after staining was assessed by dilution plating and found to be comparable with that of unstained bacteria.
Phagocytosis assays
Cells were transferred into HBSS + 0.1% BSA for 1 h. 1 µM bafilomycin A1 was added to prevent lysosomal degradation of internalized particles. To block phagocytosis and to assess bacteria binding, 2 µM cyto D was added in control wells for 10 min. Alternatively, cells were transferred to 4°C to allow particle binding but not internalization. Particles at an MOI of 10:1 (BMDM) or 50:1 (CHO) were spun onto cells at 500 g for 3 min, and phagocytosis assays were performed for 60 min in a 37°C incubation chamber. For analysis by microscopy, cells challenged with 594–E. coli were fixed with 3.7% PFA and stained with an E. coli antibody (Invitrogen). The number of extracellular cell-bound (double stained) and intracellular (single stained) bacteria was scored on 50 cells. For analysis by flow cytometry (FACSCalibur; BD), the mean fluorescence intensity of cell-associated bacteria was determined. Analysis was performed on the whole population with parental cells or was restricted to transduced BMDMs or transfected CHO cells (expressing GFP in each case) as indicated. A quantitative estimation of phagocytosis was obtained by subtracting extracellular fluorescence from total bacterial fluorescence.
Microscopy imaging
Bacteria colocalization with cells (Fig. 1 C) was analyzed with a fluorescence microscope (Eclipse TE300; Nikon) with a 60× objective. Images were acquired with a camera (CoolSNAP HQ2; Photometrics) using Simple PCI software (Compix). Triple-staining experiments (Fig. 1 D) were analyzed with a fluorescence confocal microscope (LSM510; Carl Zeiss, Inc.) using a 63× objective.
TREM surface staining
CHOs were fixed with PFA and incubated with 5 µg/ml TREM-1 or TREM-2 antibodies for 30 min at 4°C followed by 2 µg/ml Alexa Fluor 647 secondary antibodies for 30 min at 4°C. TREM surface staining was analyzed by flow cytometry.
Syk and myc intracellular staining
CHO cells transfected with Flag-Syk or myc-tagged RhoA, Rac1, and Cdc42 were detached, fixed in PFA, permeabilized with −20°C methanol, incubated with 5 µg/ml anti-Syk or 2.5 µg/ml anti-myc for 30 min at 4°C, and then incubated with 2 µg/ml Alexa Fluor 647 secondary antibodies. Intracellular staining was measured by flow cytometry.
Electron microscopy
T2D12 cells incubated with E. coli for 60 min were fixed in Karnovsky's fixative, postfixed in reduced OsO4, and stained with uranyl-acetate. After ethanol dehydration and clearing in propylene oxide, cells were embedded in eponate 12 (Ted Pella Co.). Thin sections were examined under an electron microscope (Tecnal 10; Philips).
Statistical analysis
All samples were analyzed in duplicates. Experiments were repeated at least three times, and statistical significance was determined by using the two-tailed paired Student's t test. Results were considered significant when P ≤ 0.05.
Acknowledgments
We thank T. Kitamura for providing Plat-E cells, I. Frasier for the Syk cDNA and for scientific discussions, and D. Monack for F. tularensis. We thank L. Lanier for the DAP12−/− mice, M. Nakamura, E. Niemi, and Clare Abram (University of California, San Francisco, San Francisco, CA) for reagents, and I. Hsieh (Cell Imaging Laboratory, San Francisco VA Medical Center, San Francisco, CA) for help with electron microscopy.
This work was supported by the Laboratory Directed Research and Development program at Sandia National Laboratories. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the U.S. Department of Energy's National Nuclear Security Administration under contract DE-AC04-94AL85000. Additional support was provided by National Institutes of Health grant RO1 CA87922 (to W.E. Seaman), by National Institutes of Health grant GM062114 to the Alliance for Cellular Signaling, and by the Veterans Administration.
Footnotes
Abbreviations used in this paper: BMDM, bone marrow–derived macrophage; cyto D, cytochalasin D; dn, dominant negative; ITAM, immunoreceptor tyrosine-based activation motif; KD, knockdown; TREM, triggering receptor expressed on myeloid cells; wt, wild type.
References
- Aderem A., Underhill D.M. 1999. Mechanisms of phagocytosis in macrophages.Annu. Rev. Immunol. 17:593–623 [DOI] [PubMed] [Google Scholar]
- Andrews S., Stephens L.R., Hawkins P.T. 2007. PI3K class IB pathway in neutrophils.Sci. STKE. doi:10.1126/stke.4072007cm3 [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Hoek R.M., Cerwenka A., Blom B., Lucian L., McNeil T., Murray R., Phillips L.H., Sedgwick J.D., Lanier L.L. 2000. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming.Immunity. 13:345–353 [DOI] [PubMed] [Google Scholar]
- Berton G., Mocsai A., Lowell C.A. 2005. Src and Syk kinases: key regulators of phagocytic cell activation.Trends Immunol. 26:208–214 [DOI] [PubMed] [Google Scholar]
- Brown E.J. 1995. Phagocytosis.Bioessays. 17:109–117 [DOI] [PubMed] [Google Scholar]
- Caron E., Hall A. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases.Science. 282:1717–1721 [DOI] [PubMed] [Google Scholar]
- Charles J.F., Humphrey M.B., Zhao X., Quarles E., Nakamura M.C., Aderem A., Seaman W.E., Smith K.D. 2008. The innate immune response to Salmonella by macrophages is dependent on TREM2-DAP12.Infect. Immun. 76:2439–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Tseng C.C., Bjekic G., Greenberg S. 1999. A requirement for phosphatidylinositol 3-kinase in pseudopod extension.J. Biol. Chem. 274:1240–1247 [DOI] [PubMed] [Google Scholar]
- Crowley M.T., Costello P.S., Fitzer-Attas C.J., Turner M., Meng F., Lowell C., Tybulewicz V.L., DeFranco A.L. 1997. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages.J. Exp. Med. 186:1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cywes C., Godenir N.L., Hoppe H.C., Scholle R.R., Steyn L.M., Kirsch R.E., Ehlers M.R. 1996. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in Chinese hamster ovary cells.Infect. Immun. 64:5373–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws M.R., Sullam P.M., Niemi E.C., Chen T.T., Tchao N.K., Seaman W.E. 2003. Pattern recognition by TREM-2: binding of anionic ligands.J. Immunol. 171:594–599 [DOI] [PubMed] [Google Scholar]
- Downey G.P., Botelho R.J., Butler J.R., Moltyaner Y., Chien P., Schreiber A.D., Grinstein S. 1999. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors.J. Biol. Chem. 274:28436–28444 [DOI] [PubMed] [Google Scholar]
- Firon A., Lesage G., Bussey H. 2004. Integrative studies put cell wall synthesis on the yeast functional map.Curr. Opin. Microbiol. 7:617–623 [DOI] [PubMed] [Google Scholar]
- Groves E., Dart A.E., Covarelli V., Caron E. 2008. Molecular mechanisms of phagocytic uptake in mammalian cells.Cell. Mol. Life Sci. 65:1957–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman J.A., Jarjoura J.R., Humphrey M.B., Nakamura M.C., Seaman W.E., Lanier L.L. 2006. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12.J. Immunol. 177:2051–2055 [DOI] [PubMed] [Google Scholar]
- Herre J., Marshall A.S., Caron E., Edwards A.D., Williams D.L., Schweighoffer E., Tybulewicz V., Reis e Sousa C., Gordon S., Brown G.D. 2004. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages.Blood. 104:4038–4045 [DOI] [PubMed] [Google Scholar]
- Jutras I., Desjardins M. 2005. Phagocytosis: at the crossroads of innate and adaptive immunity.Annu. Rev. Cell Dev. Biol. 21:511–527 [DOI] [PubMed] [Google Scholar]
- Kiefer F., Brumell J., Al-Alawi N., Latour S., Cheng A., Veillette A., Grinstein S., Pawson T. 1998. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils.Mol. Cell. Biol. 18:4209–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesney-Tait J., Turnbull I.R., Colonna M. 2006. The TREM receptor family and signal integration.Nat. Immunol. 7:1266–1273 [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Bakker A.B. 2000. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function.Immunol. Today. 21:611–614 [DOI] [PubMed] [Google Scholar]
- Mocsai A., Abram C.L., Jakus Z., Hu Y., Lanier L.L., Lowell C.A. 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs.Nat. Immunol. 7:1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Kojima T., Kitamura T. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses.Gene Ther. 7:1063–1066 [DOI] [PubMed] [Google Scholar]
- Niedergang F., Chavrier P. 2005. Regulation of phagocytosis by Rho GTPases.Curr. Top. Microbiol. Immunol. 291:43–60 [DOI] [PubMed] [Google Scholar]
- Olazabal I.M., Caron E., May R.C., Schilling K., Knecht D.A., Machesky L.M. 2002. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis.Curr. Biol. 12:1413–1418 [DOI] [PubMed] [Google Scholar]
- Roach T., Slater S., Koval M., White L., Cahir McFarland E.D., Okumura M., Thomas M., Brown E. 1997. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion.Curr. Biol. 7:408–417 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Rochford C.D., Neumann H. 2005. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2.J. Exp. Med. 201:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki R., Watson S.R., Lanier L.L. 2006. DAP12: an adapter protein with dual functionality.Immunol. Rev. 214:118–129 [DOI] [PubMed] [Google Scholar]
- Turnbull I.R., Colonna M. 2007. Activating and inhibitory functions of DAP12.Nat. Rev. Immunol. 7:155–161 [DOI] [PubMed] [Google Scholar]
- Turnbull I.R., Gilfillan S., Cella M., Aoshi T., Miller M., Piccio L., Hernandez M., Colonna M. 2006. Cutting edge: TREM-2 attenuates macrophage activation.J. Immunol. 177:3520–3524 [DOI] [PubMed] [Google Scholar]
- Underhill D.M., Ozinsky A., Hajjar A.M., Stevens A., Wilson C.B., Bassetti M., Aderem A. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens.Nature. 401:811–815 [DOI] [PubMed] [Google Scholar]
- Underhill D.M., Rossnagle E., Lowell C.A., Simmons R.M. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production.Blood. 106:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D., Silverstein S.C. 1982. Tumor-promoting phorbol esters stimulate C3b and C3b′ receptor-mediated phagocytosis in cultured human monocytes.J. Exp. Med. 156:1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D., Silverstein S.C. 1983. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes.J. Exp. Med. 158:2016–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi S., Inatome R., Takano T., Yamamura H. 2001. Syk expression and novel function in a wide variety of tissues.Biochem. Biophys. Res. Commun. 288:495–498 [DOI] [PubMed] [Google Scholar]
- Zavzavadjian J.R., Couture S., Park W.S., Whalen J., Lyon S., Lee G., Fung E., Mi Q., Liu J., Wall E., et al. 2007. The alliance for cellular signaling plasmid collection: a flexible resource for protein localization studies and signaling pathway analysis.Mol. Cell. Proteomics. 6:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenfuss J.S., Biswas R., Avery M.A., Hong K., Sheehan A.E., Yeung Y.G., Stanley E.R., Freeman M.R. 2008. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling.Nature. 453:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]