Abstract
Involvement of the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) in kidney physiology has been explored recently. Synthetic PPARγ ligands can ameliorate the diabetic kidney disease through different mechanisms, involving inhibition of mesangial cell growth, reduction of mesangial matrix, and cytokine production of glomerular cells as well as promoting endothelial cell survival within the kidney glomeruli. Activation of PPARγ has additional profibrotic consequences, which can contribute to wound healing in diabetic glomerulonephritis. Beside many beneficial effects, PPARγ activation, however, can lead to severe water retention, a common side effect of thiazolidinedione therapy. This unwanted effect is due to the activation of PPARγ in the mesonephric distal collecting system, where PPARγ positively regulates sodium and water resorbtion leading to the expansion of interstitial fluid volume. Recent studies indicate that PPARγ is also involved in the normal kidney development, renal lipid metabolism, and activation of the renin-angiotensin system. In this paper, we give a synopsis of the current knowledge on PPARγ functions in kidney phyisology and pathophysiology.
1. INTRODUCTION
The nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) regulates transcription of various genes involved in lipid uptake, fatty acid metabolism, and glucose homeostasis [1], therefore, the modulation of PPARγ action is of intense interests in the medication of insulin resistance and related metabolic disorders [2–7]. Pharmacological activation of PPARγ facilitates the glucose and free fatty acid flux from striated muscle fibers to adipocytes and reduces liver gluconeogenesis by which PPARγ exerts antidiabetic benefits [1, 5, 6]. PPARγ signaling can also influence the expression of insulin-dependent glucose transport (GLUT) proteins [8], and can induce the production of hormone-like substances in adipose cells (e.g., resistin and adipokines) promoting insulin responsiveness [1]. Recent studies indicate that impaired insulin sensitivity of skeletal muscle and white adipose tissue can be a consequence of a chronic subclinical inflammation [2–6]. Activation of PPARγ in macrophages has anti-inflammatory effects, by which PPARγ ligands can reduce the local low-grade inflammation and consequent insulin resistance of muscle and adipose tissues [5, 6]. Thiazolidinediones (TZDs), synthetic ligands of PPARγ are clinically proven insulin sensitizers with antiinflammatory benefits. Nowadays, TZD therapy is a widely used medication strategy of type 2 diabetes and related diseases [5, 6].
Beside beneficial effects of TZD therapy in insulin resistance, edema and water retention also frequently occurs as secondary effects of PPARγ activation [9, 10]. The understanding of TZD side effects highly facilitated the basic research on PPARγ and kidney physiology. As a result, several fundamental findings on the involvement of PPARγ in fluid homeostasis have been explored in the recent years [1, 10–18]. These findings indicate that PPARγ is involved in the regulation of sodium and water resorbtion of the distal collecting ducts of the kidney which explains the unwanted TZD effects on interstitial fluid volume regulation [9, 17, 18]. Due to the anti-inflammatory roles of PPARγ activation, the receptor is involved in the attenuation of glomerulonephritis, which is also a potent therapeutic value of TZDs [10–16].
Many other roles are also attributed to PPARγ in normal kidney development, lipid metabolism, and endocrine functions [19]. In this paper, we give a synopsis of PPARγ actions as well as the PPARγ-independent effects of synthetic PPARγ ligands in kidney phyisology and pathophysiology.
2. PPARγ IN THE FILTRATION UNITS OF THE KIDNEY
2.1. Diabetic kidney disease is coupled to impaired mesangial cell functions
In the latest years, several articles explored the conneciton of PPARγ and the impaired function of the kidney filtration units in diabetic kidney disease [10, 12–15]. More than 30% of patients with juvenile or maturity onset diabetes mellitus develop clinically evident diabetic glomerulopathy within 10–20 years of the diabetes onset [16, 20]. After years of poor glycemic control, the structure of the glomerular walls get scarred and permeability changes can develop which are core features of the diabetic glomerulosclerosis or glomerulonephritis [20]. The disease is characterized by the strong accumulation of extracellular matrix proteins (Figure 1) and deposition of type IV collagen in the glomerular mesangium leading to the expansion of mesangial matrix and glomerular size [10, 12–16, 21, 22]. Elevated glomerular size can manifest in kidney hypertrophy [20]. Alterations of the glomerular morphology lead to fluid filtration deficits, albuminuria, glucosuria, and finally reduction of glomerular filtration [21–30].
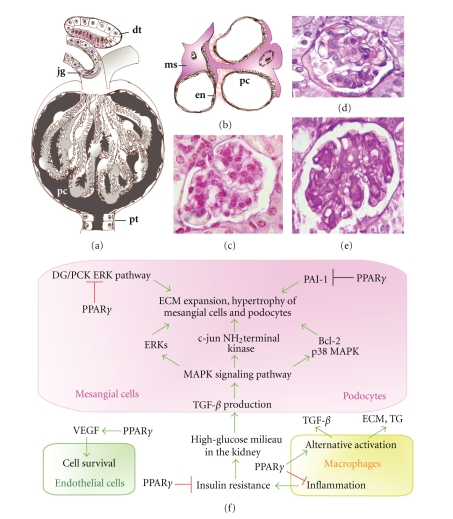
Figure 1.
Roles of PPARγ in the filtration units of the kidney. The kidney capsules (a) contain the glomerular capillaries covered with podocytes (pc). In the wall of the afferent arterioles, modified smooth muscle cells form the juxtaglomerular system (jg). The filtrated urine is guided to the proximal tubules (pt). The distal tubules (dt) can return to the cortical kidney capsules and their epithelial layers serve as a chemosensory region, the macula densa (labeled with red). (b) PPARγ activation affects either podocyte (pc), mesangial cell (ms), or endothel cell (en) functions. (c) Periodic acid-Schiff (PAS) stained sections of a normal kidney capsule in mouse. (d) Glomerulonephritis in high-fat diet fed mouse and (e) type 2 diabetic (db/db) mouse, showing intensive PAS staining of the expanded mesangial matrix, thickening of glomerular walls, and enlargement of kidney capsules. (f) Summary of PPARγ-mediated cellular events in mesangial cells, podocytes, kidney macrophages, and glomerular endothel cells.
Glomerular mesangial cells have a central role in the development of diabetic glomerulonephritis (Figure 1(b)), since these cells can overproduce the extracellular matrix proteins of the glomerular mesangium in response to chronic hyperglycemia [11–13, 21].
2.2. Effects of PPARγ activation in mesangial cells
Activation of PPARγ as well as PPARα in mesangial cells can attenuate the overproduction of the mesangial matrix (Figure 1(f)), as it has been described in animal models of diabetic nephropathy [21, 22]. Diabetes in apolipoprotein-E (ApoE)-deficient mice is associated with a significant accumulation of extracellular matrix proteins and increased immunostaining for collagen IV in the glomerular compartments (Figures 1(d), 1(e)). Treatment with rosiglitazone results in a significant reduction in collagen IV deposition [21]. In Otsuka Long-Evans Tokushima Fatty (OLETF), type 2 diabetic rats glomerular hypertrophy correlates well with the expression of large quantities of the Bcl-2 protein, an apoptosis-suppressing molecule in the mesangial cells [22]. This finding suggests that persistent proliferation and prolonged survival of the mesangial cells can also contribute to the supernormal matrix secretion in glomerulopathy. The gene encoding Bcl-2 has a PPAR response element by which PPARγ can increase Bcl-2 mRNA transcription. However, some reports have indicated that TZD treatment can decrease the level of Bcl-2 and induce apoptosis independently of PPARγ [22].
TZDs cannot only reduce glomerular cross-sectional area and the mesangial matrix size as well as collagen IV synthesis but also enhance the tumor growth factor beta-1 (TGF-β1) positive staining areas in the kidney of OLETF rats [22]. TGF-β seems to be a central molecule in the PPAR agonist action [10, 12, 22]. This growth factor activates several intracellular signal transduction systems involved in the regulation of the extracellular matrix biosynthesis (Figure 1(f)), including mitogen-activated protein kinases (MAPKs), the extracellular signal-regulated kinases (ERKs), the c-jun NH2-terminal kinases, diacylglycerol/protein kinase C extracellular signal-regulated kinase pathway, and the p38 MAPK [23–30]. PPARγ agonists besides their anti-inflammatory effect can inhibit TGF-β expression leading to a repression in glomerular proliferation [16, 22, 30]. PPARγ also has a direct effect on key extracellular matrix regulators as plasminogen activator inhibitor-1 (PAI-1). PAI-1 is a member of the serine protease inhibitor superfamily and it can inhibit proteolysis of the extracellular matrix, leading to matrix accumulation and sclerosis. PPARγ agonists may inhibit PAI-1 transcription by antagonizing the activities of activator protein-1 (AP-1) and nuclear factor κB [23–31].
The presence of TGF-β1 in the mesangial cells refers to a mechanism by which high-glucose milieu induces inflammatory and profibrotic cytokine production in glomerular cells (Figure 1(f)). In diabetic nephropathy, mesangial cells as well as podocytes and interstitial cells can secrete monocyte chemoattractant protein-1 (MCP-1) and TGF-β1 which may initiate macrophage infiltration into the kidney [10, 12–15]. The number of infiltrated machrophages is being increased both in the glomeruli and the renal interstitium with the development of diabetic kidney disease in OLETF rats. TZDs have an anti-inflammatory effect in the peripheral tissues, therefore treatment with pioglitazone or rosiglitazone decreases macrophage infiltration of the kidney [19, 22, 30]. MCP-1 can also influence the alternative macrophage activation. Alternatively activated macrophage release factors such as IL-1ra/IL-1F3, IL-10, and TGF-β [31–33]. TGF-β functions indirectly to promote extracellular matrix building by inducing nearby kidney fibroblasts to produce matrix components [34]. The alternatively activated macrophages themselves produce extracellular matrix components, as fibronectin and a cross-linking enzyme transglutaminase (Figure 1(f)), as well as osteopontin, which is involved in cell adhesion to the matrix [32, 35]. The molecules secreted by the alternatively activated macrophages can promote wound repair due to their anti-inflammatory, fibrotic, proliferative, and angiogenic activities [32–35].
2.3. Role of PPARγ in podocytes and capillaries in glomerulonephritis
Podocyte injury is also among the primary events in early development of the glomerulosclerosis [33, 36]. A decrease in podocyte number in type 2 diabetic Pima Indians correlates closely with those patients who have microalbuminuria, the earliest manifestation of diabetic nephropathy [37]. High-glucose treatment or the epithelial cell toxin puromycin aminonucleosid (PAN) supplementation induces podocyte injury and PPARγ upregulation in podocyte culture [37]. This increase of PPARγ is counterregulatory and might promote podocyte healing and repair. Pioglitazone treatment of podocytes can inhibit expression or phosphorylation of cell proliferation and antiapoptotic proteins (e.g., p27Kip1, p42 MAPK, Bcl-2) which can be one major molecular mechanism behind the therapeutic potential of TZDs on high glucose-induced hypertrophy of podocytes [22, 23].
Microangiopathy of glomerular capillaries is also a hallmark of the diabetic nephropathy [10, 12–15, 33]. Endothelial growth and survival are regulated by two factors, vascular endothelial growth factor (VEGF) and angioprotein which are also expressed by podocytes (Figure 1(f)). PPARγ agonists can protect glomerular capillaries against injury both by increasing podocyte VEGF expression and by decreasing Aglp4 [38]. TZDs, therefore, can prevent angiopathy of the capillaries in the glomeruli, one causing event of progressive kidney disease.
3. PPARγ IN THE DISTAL COLLECTING SYSTEM
3.1. Expression of PPARγ in the nephron ducts
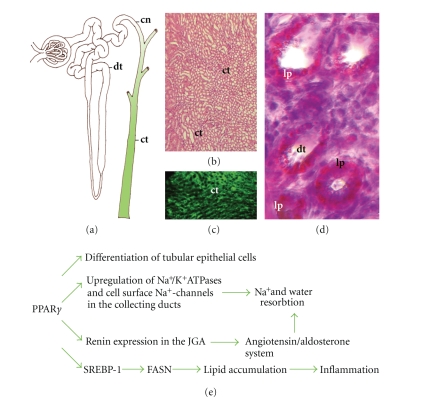
Under physiological conditions, PPARγ is dominantly expressed in the collecting system of the mammalian urinary tract, including connective renal tubules and collecting ducts (Figure 2(a)). PPARγ is abundant in the inner renal medulla (Figures 2(b), 2(c)) and localized to the epithelial layer starting from medullary collecting ducts to the urothelium of the ureter and the bladder [39–41]. PPARγ also occurs in renal medullary interstitial cells [39]. The PPARγ partner RXRα has a complimentary distribution in the collecting ducts [42]. The connective tubules and collective ducts are parts of the distal collecting system, where hormone-regulated ion exchange and water resorbtion takes place and provides the balance of interstitial fluid volume (Figure 2(e)). If aldosterone is present, sodium is resorbed and potassium is secreted. Sodium transport is followed by passive water resorbtion, therefore, this mechanism regulates the total electrolite and water volume in the body [43]. The epithelium of the collecting ducts is responsive to antidiuretic hormone. If the hormone is present, the epithelia becomes permeable to water. The distal collecting system is, therefore, a major site of fluid volume regulation.
Figure 2.
Roles of PPARγ in the collecting system of the kidney. (a) Expression of PPARγ is confined to the distal collecting system (labeled with green) including connective tubules (cn) and collective ducts (ct). (b) Hematoxylin and esoin stained cross-sections of the kidney medulla showing numerous collective ducts (ct). (c) Fluorescent PPARγ immunostaining in the same region of the kidney. (d) Oil red-O stained sections of the distal tubules (dt) showing severe lipid accumulation in type 2 diabetic (db/db) mice. (e) Summary of PPARγ functions in the collective system.
3.2. Embryology and phylogenetic homologies of PPARγ expressing collecting ducts
In mammals, the development of the kidney collecting system differs from the other excretory parts of the kidney [44]. Collecting ducts and tubules are formed by the ureteric bud, which is an outgrowth of the dorsomedial wall of the mesonephric duct. The proliferating mesonephric bud penetrates the developing metanephric tissues and dilates forming the primitive renal pelvis and calyces. The further subdivisions of the calyces form the presumptive collecting ducts [44]. According to the recent literature, PPARγ expression is mainly confined to the collecting system of the kidney [39, 41, 45–55], which has a mesonephric origin (Figure 2(a)). A lower expression of PPARγ1 in the proximal tubules, which are derived from the metanephric tissue, has been indicated in the rat kidney [56] while in mesangial cells and podocytes of the kidney capsules PPARγ is upregulated only under pathological conditions as chronic hyperglycemia or glomerulonephritis [10, 12–15]. The distribution pattern of PPARγ suggests that PPARγ may have been coupled to the mesonpehros in the vertebrate phylogeny. Supporting this possibility, the kidney of teleost fishes, which is a functioning mesonephros and a phylogenic homolog of the mammalian collecting system, contains all of the three PPAR isoforms [57–59]. Like their mammalian homologs, fish PPARs bind to a variety of natural PPAR response elements (PPREs) present in the promoters of mammalian or piscine genes.
3.3. Role of PPARγ in the balance of fluid homeostasis
As its distribution pattern suggests, the clinically most relevant function of PPARγ is the modulation of electrolyte and water resorbtion [17, 18, 41, 60]. Edema and fluid retention are common and serious side effects of TZD therapy, which are due to supernormal sodium resorbtion and consequent interstitial fluid volume expansion [9, 32]. Since PPARγ is a significant target for TZDs and it is predominantly expressed in the collecting ducts, critical sites for the control of fluid metabolism, its possible involvement in fluid metabolism has been recently elucidated. PPARγ activation can modulate sodium resorbtion through the stimulation of epthelial sodium channels and the Na+/K+-ATPase system [41, 60]. Additionally, TZDs can ditsurb the renin-angiotensin-aldosterone system also (Figure 2(e)). In human collecting duct cell culture PPARγ activation enhances the expression of cell surface epithelial sodium channels which can facilitate the sodium resorbtion ability of the tubular cells [41]. The role of PPARγ in the regulation of sodium resorbtion has been also confirmed by studies carried out on mice with collecting duct-specific ablation of PPARγ [17, 18]. These studies show a critical role for PPARγ in systemic fluid retention through the regulation of renal sodium transport, and that the adverse effects of TZD in fluid metabolism are indeed PPARγ-dependent. A gene encoding for the gamma subunit of the epithelial sodium channel has been identified as a critical PPARγ target gene in the control of electrolyte and water resorbtion of the collecting ducts (Figure 2(e)).
3.4. Proliferation and metabolism of kidney epithelia and effects of PPARγ
PPARγ has some additional functions in the collecting system of the kideny. During embryogenesis, the expression of PPARγ in urothelium [41, 46, 55] suggests its possible involvement in the urothelial proliferation and differentiation. In cultured rat kidney epithelial cells, both troglitazone and 15d-PGJ2 significantly inhibit cell proliferation and dramatically alter cell shape by induction of cell process formation [19, 41]. TZDs or PPARγ overexpression induces the Klotho gene expression in mouse kidneys and renal epithelial cell culture promoting insulin sensitivity and reducing cellular aging [46].
The PPARγ ligand TZDs alter not only cellular growth and survival but also metabolic processes of the kidney collecting duct epithelia including carbohydrate, lipid metabolism, and albumine transport [19, 47]. TZDs can activate PPARγ-regulated genes as well as P-ERK and AMP-activated protein kinase pathways which modulate gluconeogenesis, cellular acidosis, glutamine metabolism, and ammoniagenesis of porcine tubular cells [19]. It is possible that modulation of kidney carbohydrate metabolism by TZDs has a beneficial role in the glycemic control [19]. Interestingly some in vitro studies with kidney epithelial cells of the opossum have revealed that TZD affects protein handling of tubular epithelia also [47]. Rosiglitazone, ciglitazone, and troglitazone can inhibit the uptake of FITC-labeled albumin by tubular epithelial cells in a dose-dependent manner without any cytotoxic effect. Unexpectedly, in tubular cells overexpressing PPARγ or in cells treated with the PPARγ antagonist GW9662, albumin handling cannot be affected. Similarly, the PPARγ ligand 15d-PGJ2, which is structurally unrelated to TZDs, has no effect on albumin uptake [47]. Albumin handling of tubular cells can be, therefore, affected by TZDs independently from PPARγ. Effects of TZDs on tubular protein uptake, however, can be physiologically less relevant than the benefits of TZD administration on glomerular functions which conseqeuntly reduce albuminuria.
PPARγ is also involved in the renal lipid metabolism (Figures 2(d), 2(e)). Abnormal renal lipid synthesis plays a role in the pathogenesis of diabetic nepropathy [48]. Renal lipid deposits in glomerulosclerosis have been mentioned even in the first description of the diabetic kidney alterations by Kimmelstiel and Wilson in 1936 [49]. Lipid deposits are present in the kidney of diabetic humans as well as of diabetes model rodents [48–55, 61, 62]. In diabetic animals upregulation of kidney SREBP-1, the key enzyme of fatty acid synthesis can lead to the renal accumulation of lipids as well as mesangial matrix expansion and kidney hypertrophy [51, 52]. Elevated levels of plasma lipids also can contribute to renal fat deposition and facilitate the development of glomerulosclerosis [53]. High glucose concentration can also increase SREBP-1 expression in cultured rat mesangial cells, suggesting that impaired glycemic control can disturb renal lipid metabolism through altered SREBP-1 gene expression, which is regulated by PPARγ [51]. It is possible that the transcriptional activity of PPARγ in the duct cells is upregulated by insulin and C-protein, a protein fragment of proinsulin [54]. Both insulin and C-peptide can induce a concentration-dependent activation of PPARγ and both agents can augment the TZD-stimulated PPARγ activity giving the possibility that hyperinsulinemia in type 2 diabetes can augment PPARγ as well as PPARγ-regulated SREBP-1 gene functions.
Renal lipid accumulation, however, not only is a consequence of the hyperglycemia or dyslipidemia but also can predispose or provoke glomerulonephritis. Recent in vitro studies suggest that low-density lipoproteins and very low-density lipoproteins induce upregulation of growth factors, TGF-β, and matrix proteins in cultured renal mesangial and tubular cells [55, 61]. This direct effect of lipids on gene expression of kidney cells can initiate the development of mesangial matrix expansion which is a hallmark of glomerulonephritic syndrome. In mice with upregulated SREBP-1 expression, the signs of glomerulonephritis as albuminuria, renal cholesterol, and triglyceride deposits occur without changes in glucose homeostasis or serum lipid levels [51]. In these SREBP-1 transgenic mice, the elevated renal lipid content is coupled with increased TGF-β and vascular endothelial growth factor (VEGF) expression [51]. VEGF plays a pivotal role in the pathogenesis of glomerulosclerosis [63]. PPARγ haploinsufficiency as well as Pro12Ala (P12A) allele polymorphism of PPARγ has a protective role in the development of diabetic nephropathy [64]. In mice with heterozygous PPARγ mutation, high-fat diet results in a less severe nephropathy and lipid depositions than in wild type animals [45].
4. PPARγ FUNCTION IN THE JUXTAGLOMERULAR APPARATUS
Kidney is not only an excretory organ but also serves endocrine functions by the secretion of renin, a 37 kDa protein hormone produced by the juxtaglomerular cells. Juxtaglomerular cells are modified smooth muscle cells in the media of the afferent arteriole adjacent to the renal capsule (Figure 1(a)). Renin acts on a plasma protein called angiotensinogen, producing an inactive decapeptide, the angiotensin I. This substance as a result of the action of a converting enzyme present in high concentration in lung endothelial cells, becoming an octapeptide called angiotensin II. Angiotensin II enhances the secretion of aldosterone in the adrenal gland [65, 66]. The main targets of aldosterone are the distal tubules, where it can regulate sodium reabsorption (Figure 2(e)).
Human renin gene enhancer is modulated by PPARγ activation [67, 68]. In human renin-producing cell line CaLu-6, endogenous or pharmacological PPARγ agonists (unsaturated fatty acids and TZDs) can stimulate renin mRNA transcription [67, 68].
Although renin production is facilitated by PPARγ activation, the hypertensive effects of angiotensin II can be attenuated by TZDs [69–71]. In addition to its role in controlling water and salt homeostasis, the inhibition of the renin-angiotensin system reduces the incidence of type 2 diabetes in patients with hypertension or congestive heart failure and also reduces the risk of nephropathy in diabetic patients [71]. The mechanisms underlying these protective effects appear to be complex and may involve an improvement of both insulin sensitivity and insulin secretion. Recent works suggest that aldosterone and mineralocorticoid receptors regulate PPARγ expression [72, 73]. Aldosterone as well as angiotensin receptor blockers appear to induce PPARγ activity in the adipose tissue, which could explain the protective effect of the renin-angiotensin system inhibition against the development of type 2 diabetes [71]. It is unlikely, however, that the favorable effects of TZDs on diabetic nephropathy would be related to a dierct effect on the renin-angiotensin system [74].
5. CYTOTOXIC EFFECTS OF PPARγ LIGANDS ON TUBULAR EPITHELIAL CELLS
Synthetic PPARγ ligands are widely used drugs for the treatment of insulin resistance. There is an evidence that these drugs have beneficial effects on the improvement of metabolic parameters as proteinuria in type 2 diabetes, however, some severe metabolic secondary effects have been recognized [75–77].
Increasing number of synthetic PPARγ ligands is commercially available today (e.g., troglitazone, rosiglitazone, pioglitazone, ciglitazone, muraglitazar) for treatment of type 2 diabetes complications. Many reports have described the side effects of them including antiproliferative and apoptotic actions in cultures of renal proximal tubular cells [78], mesangial cells [79], and interstitial fibroblasts [80]. Ciglitazone has a direct necrotic effect on renal proximal tubular cells at a concentration range similar to its therapeutical plasma levels. Interestingly, these cytotoxic effects are not universal for all PPARγ agonists because pioglitazone is not cytotoxic in the same cell lines [81]. Although renoprotective effects of dual PPARα and PPARγ activation have been reported in type 2 diabetic animals [82], muraglitazar (a PPARα/γ dual agonist) can induce multifocal urothelial necrosis and proliferation in young male rats which is thought to be provoked by muraglitazar-associated changes in urine composition [83].
6. SUMMARY
PPARγ agonists have many beneficial effects combined with their independent antiatherosclerotic actions and their important effects on dyslipidemia and insulin resistance in the medication of kidney disease coupled to diabetes [10, 12–15, 21, 82]. Activation of PPARγ attenuates diabetic glomerulonephritis due to its anti-inflammatory and profibrotic effects [32–35]. PPARγ and PPARα have similar antidiabetic and renoprotective effects, therefore administration of PPARα or PPARα/γ dual agonists may be also useful for the prevention of kidney complications of type 1 as well as type 2 diabetes mellitus [13, 21, 82]. On the other hand, PPARγ signaling can facilitate lipid accumulation or induce a direct necrotic cell death of tubular epithelial cells, therefore synthetic PPARγ ligands, especially TZDs should be used with a great foresight in the medication of insulin-resistant diabetes mellitus [1, 48–54].
The most recently discovered role of PPARγ in the positive regulation of salt and water resorbtion have elucidated the pathomechanism of water retention and edema in patients treated with TZDs, the widely used PPARγ agonists [17, 18]. Edema and fluid retention can be fatal side effects of TZDs, which can be attenuated by the combination of TZD therapy with diuretics [9]. The selective PPAR modulator (SPPARM) approach has also been proposed as a method to avoid unwanted complications of PPARγ ligands [9].
Some comparative data suggest that PPARγ is coupled to the mesonephric parts of the vertebrate kidney, therefore the involvement of PPARγ in the intesrtitial fluid volume regulation can be an ancient and evolutionarily conserved role [56–58]. Some other components of renal PPARγ activation, including the function of PPARγ in the moduation of renal endocrine functions, are still undefined [67, 74] indicating the timeliness of future research in the field of PPARγ and kidney physiology.
ACKNOWLEDGMENT
This contribution was supported by the Hungarian Scientific Research Fund (OTKA) Grant (no.76091 to T. Rőszer).
References
- 1.Heikkinen S, Auwerx J, Argmann CA. PPARγ in human and mouse physiology. Biochimica et Biophysica Acta. 2007;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of Clinical Investigation. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of Clinical Investigation. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan SZ, Ivashchenko CY, Whitesall SE, et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARγ-deficient mice rescued from embryonic lethality. The Journal of Clinical Investigation. 2007;117(3):812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-γ and FOXO1 to FFA and inflammation. Trends in Endocrinology and Metabolism. 2007;18(3):100–107. doi: 10.1016/j.tem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JG. Should we use PPAR agonists to reduce cardiovascular risk? PPAR Research. 2008;2008:13 pages. doi: 10.1155/2008/891425. Article ID 891425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrian AD. The complex role of PPARγ in renal dysfunction in obesity: managing a Janus-faced receptor. Vascular Pharmacology. 2006;45(1):36–45. doi: 10.1016/j.vph.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Ruan X, Zheng F, Guan Y. PPARs and the kidney in metabolic syndrome. American Journal of Physiology. 2008;294(5):F1032–F1047. doi: 10.1152/ajprenal.00152.2007. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Nagai Y, Takamura T, Nohara E, Kobayashi K-I. Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism. 2002;51(4):403–408. doi: 10.1053/meta.2002.30953. [DOI] [PubMed] [Google Scholar]
- 13.Ko GJ, Kang YS, Han SY, et al. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrology Dialysis Transplantation. 2008;23(9):2750–2760. doi: 10.1093/ndt/gfn157. [DOI] [PubMed] [Google Scholar]
- 14.Westerweel PE, den Ouden K, Nguyen TQ, Goldschmeding R, Joles JA, Verhaar MC. Amelioration of anti-Thy1-glomerulonephritis by PPAR-γ agonism without increase of endothelial progenitor cell homing. American Journal of Physiology. 2008;294(2):F379–F384. doi: 10.1152/ajprenal.00019.2007. [DOI] [PubMed] [Google Scholar]
- 15.Ohga S, Shikata K, Yozai K, et al. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. American Journal of Physiology. 2007;292(4):F1141–F1150. doi: 10.1152/ajprenal.00288.2005. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Yanagida H, Kuwajima H, Takemura T. Antiproliferative effect of fluvastatin and thiazolidinedione in mesangial cells of diabetic rats. Pediatric Nephrology. 2004;19(1):26–32. doi: 10.1007/s00467-003-1306-y. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor γ blocks thiazolidinedione-induced fluid retention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan Y, Hao C, Cha DR, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nature Medicine. 2005;11(8):861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 19.Turturro F, Oliver R, III, Friday E, Nissim I, Welbourne T. Troglitazone and pioglitazone interactions via PPAR-γ-independent and -dependent pathways in regulating physiological responses in renal tubule-derived cell lines. American Journal of Physiology. 2007;292(3):C1137–C1146. doi: 10.1152/ajpcell.00396.2006. [DOI] [PubMed] [Google Scholar]
- 20.Fine LG, Norman J. Cellular events in renal hypertrophy. Annual Review of Physiology. 1989;51:19–32. doi: 10.1146/annurev.ph.51.030189.000315. [DOI] [PubMed] [Google Scholar]
- 21.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrology Dialysis Transplantation. 2006;21(9):2399–2405. doi: 10.1093/ndt/gfl212. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Wada J, Hida K, et al. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes. 2006;55(6):1666–1677. doi: 10.2337/db05-1285. [DOI] [PubMed] [Google Scholar]
- 23.Vojtek AB, Cooper JA. Rho family members: activators of MAP kinase cascades. Cell. 1995;82(4):527–529. doi: 10.1016/0092-8674(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 24.Choi ME, Ballermann BJ. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-β receptors. The Journal of Biological Chemistry. 1995;270(36):21144–21150. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- 25.Hartsough MT, Mulder KM. Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. The Journal of Biological Chemistry. 1995;270(13):7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- 26.Atfi A, Djelloul SH, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. The Journal of Biological Chemistry. 1997;272(3):1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 27.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. The Journal of Biological Chemistry. 1999;274(38):27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 28.Hayashida T, Poncelet A-C, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney International. 1999;56(5):1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 29.Inoki K, Haneda M, Ishida T, et al. Role of mitogen-activated protein kinases as downstream effectors of transforming growth factor-β in mesangial cells. Kidney International. 2000;58(77):S76–S80. doi: 10.1046/j.1523-1755.2000.07712.x. [DOI] [PubMed] [Google Scholar]
- 30.Isshiki K, Haneda M, Koya D, Maeda S, Sugimoto T, Kikkawa R. Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes. 2000;49(6):1022–1032. doi: 10.2337/diabetes.49.6.1022. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends in Immunology. 2001;22(6):328–336. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 32.Mosser DM. The many faces of macrophage activation. Journal of Leukocyte Biology. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias P, Díez JJ. Peroxisome proliferator-activated receptor gamma agonists in renal disease. European Journal of Endocrinology. 2006;154(5):613–621. doi: 10.1530/eje.1.02134. [DOI] [PubMed] [Google Scholar]
- 34.Song E, Ouyang N, Hörbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cellular Immunology. 2000;204(1):19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. The American Journal of Pathology. 1994;145(6):1450–1462. [PMC free article] [PubMed] [Google Scholar]
- 36.Mundel P, Shankland SJ. Podocyte biology and response to injury. Journal of the American Society of Nephrology. 2002;13(12):3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 37.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. The Journal of Clinical Investigation. 1997;99(2):342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H-C, Ma L-J, Ma J, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney International. 2006;69(10):1756–1764. doi: 10.1038/sj.ki.5000336. [DOI] [PubMed] [Google Scholar]
- 39.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. American Journal of Physiology. 1997;273(6):F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 40.Broeders N, Abramowicz D. Peroxisome proliferator-activated receptors (PPARS): novel therapeutic targets in renal disease. Kidney International. 2002;61(1):354–355. doi: 10.1046/j.1523-1755.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 41.Hong G, Lockhart A, Davis B, et al. PPARγ activation enhances cell surface ENaCα via up-regulation of SGK1 in human collecting duct cells. The FASEB Journal. 2003;17(13):1966–1968. doi: 10.1096/fj.03-0181fje. [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Michele DE, Park J, et al. Expression of peroxisomal proliferator-activated receptors and retinoid X receptors in the kidney. American Journal of Physiology. 1999;277(6):F966–F973. doi: 10.1152/ajprenal.1999.277.6.F966. [DOI] [PubMed] [Google Scholar]
- 43.Marieb EN. Human Anatomy and Physiology. 3rd edition. Redwood City, Calif, USA: Benjamin-Cummings; 1995. [Google Scholar]
- 44.Sadler TW. Langman's Medical Embryology. 9th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 45.Kume S, Uzu T, Araki S-I, et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. Journal of the American Society of Nephrology. 2007;18(10):2715–2723. doi: 10.1681/ASN.2007010089. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Li Y, Fan Y, et al. Klotho is a target gene of PPAR-γ . Kidney International. 2008;74(6):732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 47.Chana RS, Brunskill NJ. Thiazolidinediones inhibit albumin uptake by proximal tubular cells through a mechanism independent of peroxisome proliferator activated receptor gamma. American Journal of Nephrology. 2006;26(1):67–74. doi: 10.1159/000091807. [DOI] [PubMed] [Google Scholar]
- 48.Keane WF. The role of lipids in renal disease: future challenges. Kidney International. 2000;57(75):S27–S31. [PubMed] [Google Scholar]
- 49.Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. The American Journal of Pathology. 1936;12(1):83–98. [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HS, Lee JS, Koh HI, Ko KW. Intraglomerular lipid deposition in routine biopsies. Clinical Nephrology. 1991;36(2):67–75. [PubMed] [Google Scholar]
- 51.Sun L, Halaihel N, Zhang W, Rogers H, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. The Journal of Biological Chemistry. 2002;277(21):18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Jiang T, Li J, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54(8):2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 53.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. American Journal of Nephrology. 2004;24(1):46–53. doi: 10.1159/000075925. [DOI] [PubMed] [Google Scholar]
- 54.Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-γ by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. The Journal of Biological Chemistry. 2004;279(48):49747–49754. doi: 10.1074/jbc.M408268200. [DOI] [PubMed] [Google Scholar]
- 55.Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-α, -β, and -γ during rat embryonic development. Endocrinology. 1998;139(6):2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 56.Sato K, Sugawara A, Kudo M, Uruno A, Ito S, Takeuchi K. Expression of peroxisome proliferator-activated receptor isoform proteins in the rat kidney. Hypertension Research. 2004;27(6):417–425. doi: 10.1291/hypres.27.417. [DOI] [PubMed] [Google Scholar]
- 57.Ibabe A, Grabenbauer M, Baumgart E, Fahimi HD, Cajaraville MP. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) Histochemistry and Cell Biology. 2002;118(3):231–239. doi: 10.1007/s00418-002-0434-y. [DOI] [PubMed] [Google Scholar]
- 58.Batista-Pinto C, Rodrigues P, Rocha E, Lobo-da-Cunha A. Identification and organ expression of peroxisome proliferator activated receptors in brown trout (Salmo trutta f. fario) Biochimica et Biophysica Acta. 2005;1731(2):88–94. doi: 10.1016/j.bbaexp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Leaver MJ, Boukouvala E, Antonopoulou E, et al. Three peroxisome proliferator-activated receptor isotypes from each of two species of marine fish. Endocrinology. 2005;146(7):3150–3162. doi: 10.1210/en.2004-1638. [DOI] [PubMed] [Google Scholar]
- 60.Chen L, Yang B, McNulty JA, et al. GI262570, a peroxisome proliferator-activated receptor γ agonist, changes electrolytes and water reabsorption from the distal nephron in rats. The Journal of Pharmacology and Experimental Therapeutics. 2005;312(2):718–725. doi: 10.1124/jpet.104.074088. [DOI] [PubMed] [Google Scholar]
- 61.Lee HS. Oxidized LDL, glomerular mesangial cells and collagen. Diabetes Research and Clinical Practice. 1999;45(2-3):117–122. doi: 10.1016/s0168-8227(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 62.Okada M, Takemura T, Yanagida H, Yoshioka K. Response of mesangial cells to low-density lipoprotein and angiotensin II in diabetic (OLETF) rats. Kidney International. 2002;61(1):113–124. doi: 10.1046/j.1523-1755.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 63.Flyvbjerg A, Dagnæs-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51(10):3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 64.Pollex RL, Mamakeesick M, Zinman B, Harris SB, Hegele RA, Hanley AJG. Peroxisome proliferator-activated receptor γ polymorphism Pro12Ala is associated with nephropathy in type 2 diabetes. Journal of Diabetes and Its Complications. 2007;21(3):166–171. doi: 10.1016/j.jdiacomp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiological Reviews. 1990;70(4):1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 66.Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension. 2005;45(1):3–8. doi: 10.1161/01.HYP.0000149717.55920.45. [DOI] [PubMed] [Google Scholar]
- 67.Todorov VT, Desch M, Schmitt-Nilson N, Todorova A, Kurtz A. Peroxisome proliferator-activated receptor-γ is involved in the control of renin gene expression. Hypertension. 2007;50(5):939–944. doi: 10.1161/HYPERTENSIONAHA.107.092817. [DOI] [PubMed] [Google Scholar]
- 68.Todorov VT, Desch M, Schubert T, Kurtz A. The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by peroxisome proliferator-activated receptor-γ . Endocrinology. 2008;149(9):4647–4657. doi: 10.1210/en.2008-0127. [DOI] [PubMed] [Google Scholar]
- 69.Diep QN, Mabrouk ME, Cohn JS, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ . Circulation. 2002;105(19):2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 70.Takai S, Jin D, Kimura M, et al. Inhibition of vascular angiotensin-coverting enzyme by telmisartan via the peroxisome proliferator-activated receptor γ agonistic property in rats. Hypertension Research. 2007;30(12):1231–1237. doi: 10.1291/hypres.30.1231. [DOI] [PubMed] [Google Scholar]
- 71.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus—part 2: overview of physiological and biochemical mechanisms. Diabetes & Metabolism. 2004;30(6):498–505. doi: 10.1016/s1262-3636(07)70147-7. [DOI] [PubMed] [Google Scholar]
- 72.Guo C, Ricchiuti V, Lian BQ, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caprio M, Fève B, Claës A, Viengchareun S, Lombès M, Zennaro M-C. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. The FASEB Journal. 2007;21(9):2185–2194. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 74.Lansang MC, Coletti C, Ahmed S, Gordon MS, Hollenberg NK. Effects of the PPAR-γ agonist rosiglitazone on renal haemodynamics and the renin-angiotensin system in diabetes. Journal of the Renin-Angiotensin-Aldosterone System. 2006;7(3):175–180. doi: 10.3317/jraas.2006.028. [DOI] [PubMed] [Google Scholar]
- 75.Narayanan PK, Hart T, Elcock F, et al. Troglitazone-induced intracellular oxidative stress in rat hepatoma cells: a flow cytometric assessment. Cytometry Part A. 2003;52(1):28–35. doi: 10.1002/cyto.a.10011. [DOI] [PubMed] [Google Scholar]
- 76.Guo L, Zhang L, Sun Y, et al. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR γ agonists on rat primary hepatocytes and human HepG2 cells. Molecular Diversity. 2006;10(3):349–360. doi: 10.1007/s11030-006-9038-0. [DOI] [PubMed] [Google Scholar]
- 77.Jung JY, Yoo CI, Kim HT, Kwon CH, Park JY, Kim YK. Role of mitogen-activated protein kinase (MAPK) in troglitazone-induced osteoblastic cell death. Toxicology. 2007;234(1-2):73–82. doi: 10.1016/j.tox.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Arici M, Chana R, Lewington A, Brown J, Brunskill NJ. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-γ . Journal of the American Society of Nephrology. 2003;14(1):17–27. doi: 10.1097/01.asn.0000042167.66685.ea. [DOI] [PubMed] [Google Scholar]
- 79.Tsuchiya T, Shimizu H, Shimomura K, Mori M. Troglitazone inhibits isolated cell proliferation, and induces apoptosis in isolated rat mesangial cells. American Journal of Nephrology. 2003;23(4):222–228. doi: 10.1159/000072053. [DOI] [PubMed] [Google Scholar]
- 80.Parameswaran N, Hall CS, Bomberger JM, Sparks HV, Jump DB, Spielman WS. Negative growth effects of ciglitazone on kidney interstitial fibroblasts: role of PPAR-γ . Kidney & Blood Pressure Research. 2003;26(1):2–9. doi: 10.1159/000069764. [DOI] [PubMed] [Google Scholar]
- 81.Giral H, Villa-Bellosta R, Catalán J, Sorribas V. Cytotoxicity of peroxisome proliferator-activated receptor α and γ agonists in renal proximal tubular cell lines. Toxicology in Vitro. 2007;21(6):1066–1076. doi: 10.1016/j.tiv.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Cha DR, Zhang X, Zhang Y, et al. Peroxisome proliferator-activated receptor α/γ dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes. 2007;56(8):2036–2045. doi: 10.2337/db06-1134. [DOI] [PubMed] [Google Scholar]
- 83.Van Vleet TR, White MR, Sanderson TP, et al. Subchronic urinary bladder effects of muraglitazar in male rats. Toxicological Sciences. 2007;96(1):58–71. doi: 10.1093/toxsci/kfl176. [DOI] [PubMed] [Google Scholar]