Abstract
Purpose
Conformal radiation therapy (CRT) aims to limit the highest radiation dose to the tissue volume at risk while sparing surrounding normal tissues. This study investigated whether treatment of childhood ependymoma with CRT would preserve cognitive function. Academic competence was chosen as the primary outcome measure given it is a measure of applied cognitive abilities in a child's natural setting.
Patients and Methods
Eighty-seven pediatric patients diagnosed with ependymoma received CRT in which doses ranging from 54.0 to 59.4 Gy were prescribed to the postoperative tumor bed with a 10-mm clinical target volume margin. Cognitive testing was conducted at the start of CRT, 6 months, and annually after the start of CRT. The median length of follow-up was 59.6 months. Academic testing included subtests from the Wechsler Individual Achievement Test (WIAT) and the Achenbach Child Behavior Checklist.
Results
Linear mixed models with random coefficients revealed a modest but significant decline in reading scores during follow-up (WIAT slope estimate −0.064 ± 0.028 points/month; P = .026). Math and spelling performance remained stable. Supratentorial tumor location and multiple surgeries were predictive of worse reading performance at CRT baseline. Male sex, longer symptomatic interval, pre-CRT chemotherapy, pre-existing endocrine deficiencies, hydrocephalus, and younger age at CRT (< 5 years) were predictive of a significant decline in reading scores over time.
Conclusion
CRT may result in better long-term cognitive outcomes when compared to conventional radiation therapy approaches. Reading appears more vulnerable than other academic skills and may decline over time despite stable intellectual functioning.
INTRODUCTION
Ependymoma is the third most common CNS tumor of childhood, affecting approximately 300 individuals under 19 years of age in the United States annually.1 Successful management typically includes surgery and postoperative radiation therapy. Treatment advances have lead to improved survival rates with 3-year progression-free survival estimates as high as 75%.2 As survival rates improve, an increased understanding of the long-term functional impact of treatment becomes imperative.
Children who receive radiation for ependymoma are at increased risk for impairments in endocrine, neurologic, and cognitive functioning.3 Findings of adverse cognitive outcomes typically come from studies investigating whole-brain irradiation at 18, 24, and 36 Gy, doses customarily used in the treatment of medulloblastoma and acute lymphoblastic leukemia.4 There is evidence to suggest total radiation dose and volume of irradiated brain play a significant role in neurocognitive outcome, with lower doses of cranial radiation associated with less intellectual impairment.5,6 Thus, there is strong rationale for reduction in radiation dose and volume when appropriate tumor control can be maintained for localized pediatric brain tumors.
Conformal radiation therapy (CRT) encompasses planning and delivery techniques developed to limit the highest radiation dose to volumes at risk for tumor while sparing surrounding normal tissues. The planning process incorporates three-dimensional imaging and sophisticated software to delineate treatment volume and important normal tissue structures to optimize dose distributions.2 A preliminary report of CRT in children with localized ependymoma revealed a high rate of disease control and stable neurocognitive performance at a median of 38 months post-treatment.2 Radiation dosimetry was predictive of intellectual functioning (IQ) after CRT in the same group of patients.4
A progressive decline in intellectual functioning after irradiation of larger brain volumes has been well-established.7-9 Academic competence has received less research attention despite being a measure of applied cognitive functioning in a child's natural setting. While IQ and academic performance are correlated in typically developing children, a dissociation in these abilities has been found in certain clinical populations,10-13 including cancer survivors.14 It is well established that utilization of special education services is increased among children treated with conventional radiation therapy for brain tumors6,15,16 and cranial irradiation has been associated with poor academic performance on standardized measures in childhood brain tumor survivors.14,15,17 Studies have also found declines in academic functioning for children 2 to 5 years after irradiation.18,19
For this study, academic testing was conducted at the start of CRT, 6 months, and annually after the start of CRT in the context of a prospective, longitudinal phase II trial of CRT. The overarching premise for the trial is that treatment with smaller-than-conventional irradiation volumes may reduce adverse effects without affecting tumor control. Accordingly, we hypothesized there would be no significant decline in academic performance after CRT. A secondary goal was to investigate the impact of demographic and clinical variables on long-term academic abilities, which has not previously been reported for CRT in childhood brain tumor survivors. We hypothesized that variables corresponding to more aggressive disease (eg, endocrine dysfunction at presentation) and more aggressive treatment (eg, pre-CRT chemotherapy) would be associated with poorer academic outcomes.
Studies investigating neurocognitive outcomes in brain tumor survivors have been limited by small, heterogeneous samples. Even studies of posterior fossa tumors have typically combined patients with medulloblastoma and ependymoma despite different treatment approaches and functional outcomes.5,9,18 To our knowledge, this is the largest study to date of children treated for localized ependymoma. The longitudinal design and sample size allows for a thorough investigation of predictors of academic performance not previously reported in the literature.
PATIENTS AND METHODS
Patients
From July 1997 through June of 2007, 131 patients with intracranial ependymoma were enrolled on a phase II trial of CRT. Criteria for study enrollment included age between 1 and 25 years at the time of irradiation, histologic confirmation of intracranial ependymoma, no evidence of dissemination, no prior irradiation, no ongoing chemotherapy, and adequate performance status (Eastern Cooperative Oncology Group performance grade 0 to 2).20 The study was approved by the institutional review board and written informed consent was required before participation.
While all children underwent neurocognitive assessments (including intellectual testing) at the start of CRT, only children older than 5 years of age during their post-CRT follow-up time period (n = 87) were able to participate in academic testing due to the age range of the academic measure. No patients had significant sensory loss or motor impairment that would preclude valid psychometric testing. All patients were primary English speakers. Clinical and demographic characteristics are summarized in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics (N = 87)
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Male | 46 | 52.9 |
| Female | 41 | 47.1 |
| Tumor location | ||
| Infratentorial | 65 | 74.7 |
| Supratentorial | 22 | 25.3 |
| Hydrocephalus | ||
| No | 32 | 36.8 |
| Yes | 55 | 63.2 |
| CSF shunting | ||
| No | 60 | 69.0 |
| Yes | 27 | 31.0 |
| Pre-CRT chemotherapy | ||
| No | 69 | 79.3 |
| Yes | 18 | 20.7 |
| Extent of resection | ||
| Gross total | 71 | 81.6 |
| Near total | 9 | 10.3 |
| Subtotal | 7 | 8.1 |
| No. of pre-CRT surgeries | ||
| 1 | 55 | 63.2 |
| 2-4 | 32 | 36.8 |
| Mean age at CRT, years | 5.99 | |
| SD | 4.46 | |
| Range | 1.06-18.87 | |
| Mean time from diagnosis to CRT, months | 5.76 | |
| SD | 11.76 | |
| Range | 0.59-69.68 | |
| Mean growth hormone at CRT,* ng/dL | 21.54 | |
| SD | 19.48 | |
| Range | 2.00-120.00 | |
Abbreviations: CRT, conformal radiation therapy; Gross-total resection, a resection after which the only remaining tumor cells were visible with the use of the operating microscope; Near-total resection, a resection after which only residual tumor < 5 mm thick was visible on postoperative neuroimaging; Subtotal resection, a resection after which > 5 mm thick of residual tumor was visible on postoperative neuroimaging; SD, standard deviation.
Sixty-five of 87 had growth hormone measurements.
CRT
Patients received CRT, including intensity-modulated radiation therapy, over 6 to 7 weeks using conventional fractionation (1.8 Gy per day) with a prescribed dose of 59.4 Gy. Children younger than 18 months received 54.0 Gy. The irradiated clinical target volume included a 10-mm margin surrounding the tumor, tumor bed, or both, in order to treat subclinical microscopic disease. An additional 3 to 5 mm, expanded in three dimensions, was included to account for uncertainty in patient positioning and image registration. Target volume definitions and treatment parameters has been previously reported.3
Clinical Variables
All patients underwent resection before CRT. Additional surgery was initiated to maximize extent of resection before irradiation. Eighteen patients received chemotherapy before irradiation; most received multiagent chemotherapy including cyclophosphamide and cisplatin or carboplatin, etoposide, and vincrinstine. Hydrocephalus was categorized as present or not present based on neuroimaging scans at the time of diagnosis. Peak growth hormone levels were determined using provocative testing.21
Academic Testing
Patients underwent serial neurocognitive testing at baseline (start of CRT), 6 months, and annually after the start of CRT. If beginning CRT was given logistic priority, baseline testing was delayed slightly. Academic testing consisted of three subtests from the Wechsler Individual Achievement Test (WIAT; Word Reading, Spelling and Math Reasoning).22 These subtests are content representative, reliable, and have good convergent/discriminant validity. Performance on each subtest was converted to an age-standardized score with a mean of 100 and standard deviation of 15.
The Achenbach Child Behavior Checklist (CBCL)23 is a parent questionnaire that assesses a child's level of behavioral and emotional adjustment. One scale, School Problems, was used as an external criterion for measuring academic changes revealed on the WIAT. This scale contains items that assess level of academic performance, intensity of academic services, repeated grades and school problems. The CBCL was standardized on a large sample representative of the United States’ population. Age- and sex-based T-scores are derived for each with a mean of 50 and a standard deviation of 10. Lower scores indicate greater problems.
IQ Assessment
IQ was estimated based on either the mental index of the Bayley scales24 or the Information, Similarities, and Block Design subtests from the age-appropriate Wechsler scale (Wechsler Preschool and Primary Scales of Intelligence, Revised [WPPSI-R],25 Wechsler Intelligence Scale for Children, Third Edition [WISC-III]26 and Wechsler Adult Intelligence Scale, Revised [WAIS-Revised])27 using a formula presented in Sattler.28 This method for estimating IQ correlates highly with IQs derived from full administration (r = 0.93). Age-based scaled scores, with a mean of 100 and standard deviation of 15, were derived using each standardization sample.
Statistical Analyses
Longitudinal changes in academic scores were investigated using linear mixed-effects models with random intercepts and slopes. Separate models were created for each WIAT subtest score. The impact of demographic and clinical variables on long-term academic achievement was estimated using both univariate and multivariate analyses. Continuous covariates were divided at their median value or an established clinical cut point (eg, 10 ng/mL for peak growth hormone). For multivariate analyses, a backward selection method was used; all variables with P < .05 for the slope term were retained in the model as both an intercept and slope term. Interaction terms were not considered given the ratio of number of covariates to patients.
To investigate the impact of age at CRT on academic outcomes, the simple linear mixed-effects models described earlier were adjusted. The typical baseline (intercept) estimate in these linear models is the start of CRT. Given academic assessment is only conducted for children at least 5 years of age, children younger than 5 years of age at CRT (n = 44) do not have true baseline values using the simple model. Therefore, the model was adjusted to take the time of first eligible academic testing as the baseline (online-only Appendix). This adjusted model was used for analyzing the effect of age at CRT on academic testing after initiation of testing.
To investigate the association of change in academic ability with change in IQ and parent report of school problems, Pearson correlations were calculated among the slope of academic ability scores with the slopes of IQ and the CBCL School Problems scale generated from separate models covering the same time period. The significance level was set for α = .05; P values were not adjusted for multiple testing to reduce type II errors. All analyses were performed by the biostatistical coauthors using SAS, version 9.1(SAS Institute, Cary, NC).
RESULTS
Academic Outcomes
A total of 309 evaluations were conducted for the 87 patients in this study with a median follow-up time of 59.6 months (range, 0.5 to 99.7 months). Group mean scores for all three academic scores were within the average range at the start of CRT. A significant decline was revealed in reading scores during the follow-up period (−0.064 ± 0.028 points/month). In contrast, math and spelling remained stable without significant change (Table 2). Given only reading showed a significant decline, only reading performance was considered for the remainder of analyses.
Table 2.
Longitudinal Change in WIAT Academic Scores
| WIAT Measure | No. | Baseline (intercept)*
|
Slope†
|
||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | ||
| Reading | 87 | 100.14 | 1.49 | .93 | −0.064 | 0.028 | .026‡ |
| Math | 86 | 97.35 | 1.68 | .12 | 0.037 | 0.030 | .228 |
| Spelling | 86 | 99.18 | 1.63 | .62 | −0.043 | 0.029 | .147 |
Abbreviation: WIAT, Wechsler Individual Achievement Test.
Intercept scores represent academic performance at CRT baseline. Scores are reported as standard scores, which have a mean of 100 and standard deviation of 15. None of the baseline scores differ significantly from 100.
The slope represents change in academic performance in standard points per month.
P < .05.
Demographic and Clinical Predictors of Reading Performance
Univariate.
Table 3 presents the results of univariate analyses investigating the effects of demographic and clinical variables on reading scores. Supratentorial tumor location was significantly associated with worse reading performance at CRT baseline Further, male sex, longer symptomatic interval, hydrocephalus, pre-CRT chemotherapy, and pre-existing endocrine deficiencies were associated with a statistically significant decline in reading scores over time. Children with a single surgical resection experienced a significant decline over time; this finding suggests a reading vulnerability associated with multiple surgeries at baseline with individuals receiving single surgeries showing a later decline. Infratentorial tumor location was also statistically predictive of a reading decline; however, this finding resulted from differences in SE rather than a change in slope.
Table 3.
Demographic and Clinical Predictors of WIAT Reading (N = 87)
| Variable | No. | Baseline (intercept)*
|
Slope†
|
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P‡ | Estimate | SE | P§ | P¶ | ||
| Sex | ||||||||
| Female | 41 | 100.21 | 2.25 | .944 | −0.042 | 0.042 | .326 | .484 |
| Male | 46 | 99.99 | 2.01 | −0.082 | 0.038 | .033‖ | ||
| Tumor location | ||||||||
| Infratentorial | 65 | 102.83 | 1.75 | .005‖ | −0.074 | 0.033 | .028‖ | .977 |
| Supratentorial | 22 | 94.07 | 2.50 | −0.076 | 0.055 | .170 | ||
| Diagnosis to treatment, months | ||||||||
| > 1.5 | 50 | 99.61 | 1.95 | .672 | −0.088 | 0.037 | .019‖ | .335 |
| ≤ 1.5 | 37 | 100.89 | 1.98 | −0.034 | 0.042 | .428 | ||
| Hydrocephalus | ||||||||
| Yes | 55 | 101.12 | 1.98 | .444 | −0.071 | 0.036 | .047‖ | .813 |
| No | 32 | 98.80 | 2.28 | −0.057 | 0.049 | .243 | ||
| CSF shunting | ||||||||
| Yes | 27 | 97.40 | 2.71 | .229 | −0.062 | 0.051 | .225 | .989 |
| No | 60 | 101.32 | 1.78 | −0.063 | 0.034 | .067 | ||
| Pre-CRT chemotherapy | ||||||||
| Yes | 18 | 99.70 | 3.54 | .854 | −0.153 | 0.070 | .029‖ | .155 |
| No | 69 | 100.42 | 1.67 | −0.045 | 0.031 | .149 | ||
| No. of pre-CRT surgeries | ||||||||
| 1 | 55 | 102.25 | 1.90 | .082** | −0.086 | 0.034 | .013‖ | .352 |
| 2-4 | 32 | 96.98 | 2.34 | −0.033 | 0.045 | .465 | ||
| GH at diagnosis,†† ng/mL | ||||||||
| > 10 | 47 | 100.89 | 1.84 | .854 | −0.038 | 0.036 | .295 | .125 |
| ≤ 10 | 18 | 100.19 | 3.32 | −0.147 | 0.061 | .017‖ | ||
Abbreviations: WIAT, Wechsler Individual Achievement Test; CRT, conformal radiation therapy; GH, growth hormone; Diagnosis to treatment, the time duration between diagnosis and CRT.
Intercept scores represent academic performance at baseline. Scores are reported as standard scores, which have a mean of 100 and standard deviation of 15.
The slope represents the change in academic performance in standard points per month.
Indicates whether the difference between subgroup means at baseline is statistically significant.
Indicates whether there is a statistically significant change in the subgroup mean performance over time.
Indicates whether there is a significant difference in the rate of change between the two groups over time.
P < .05.
P ≤ .10.
Sixty-five of 87 had growth hormone measurements at CRT.
Multivariate.
Table 4 displays results of the multivariate models. Tumor location and number of surgeries were significantly predictive of baseline reading scores; patients with an infratentorial tumor had on average a score 11.21 points higher than patients with supratentorial tumors and patients with one surgical resection had on average a score 12.05 higher than patients with multiple surgeries. Further, patients with a longer symptomatic interval, lower growth hormone levels, and a single surgery experienced a steeper decline over time. Number of surgeries and symptomatic interval were highly correlated (r = 0.56; P < .0001) and thus likely redundant in this model; no other variables in these models correlated significantly.
Table 4.
Multivariate Models of WIAT Reading (n = 65)
| Covariate* | Parameter | Estimate | SE | P |
|---|---|---|---|---|
| Intercept | α0 | 84.26 | 4.33 | .0006†‡ |
| No. of surgeries (1 v > 1) | α1 | 12.05 | 3.52 | .0008†§ |
| Diagnosis to treatment (> 1.5 v ≤ 1.5 months) | α2 | 5.16 | 3.33 | .12§ |
| GH at diagnosis (> 10 v ≤ 10 ng/mL) | α3 | −2.16 | 3.32 | .52§ |
| Tumor (infratentorial v supratentorial) | α4 | 11.21 | 2.56 | < .0001†§ |
| Slope | β0 | 0.37 | 0.069 | .60§ |
| No. of surgeries (1 v > 1) | β1 | −0.23 | 0.066 | .0006†§ |
| Diagnosis to treatment (> 1.5 v ≤ 1.5 months) | β2 | −0.22 | 0.061 | .0004†§ |
| GH at diagnosis (> 10 v ≤ 10 ng/mL) | β3 | 0.20 | 0.060 | .0012†§ |
Abbreviations: WIAT, Wechsler Individual Achievement Test; CRT, conformal radiation therapy; GH, growth hormone; Diagnosis to treatment, the time duration between diagnosis and CRT.
Parameters are for the model: WIAT reading = α0 + α1X1 + α2X2 + α3X3 + α4X4 + (β0 + β1X1 + β2X2 + β3X3) × time where X1 is the number of surgeries (= 1 for 1 surgery and 0 for > 1 surgery), X2 is the months from diagnosis to treatment (= 1 if > 1.5 months and 0 if ≤ 1.5 months), X3 is growth hormone at CRT diagnosis (= 1 if > 10 and 0 if ≤ 10), and X4 is tumor location (= 1 if infratentorial and 0 if supratentorial).
P < .05.
Compared with a normative mean of 100.
Group comparisons based on the categorical split for the covariate.
Age at CRT.
The linear model adjusted to investigate the effect of age at CRT on academic abilities was fitted separately for children under 5 (3a) and at least 5 (3b) at the time of CRT. For children younger than 5, average WIAT Reading at 5 years of age was 95.18, adjusted by an increase of 1.93 per year (P = .40) from CRT start to turning 5. WIAT reading scores decreased significantly by 4.01 points per year (P = .023) adjusted by an increase of 0.096 points per year from CRT start to turning 5 (P = .10). For children 5 years or older at CRT start, average WIAT Reading at CRT was 99.50. WIAT reading scores decreased by 0.83 per year (P = .066). Neither group differed significantly from the normative mean of 100 at respective baseline and the decline in reading over time only reached significance for the younger age group.
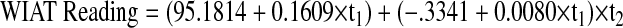 |
(3a) |
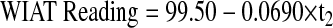 |
(3b) |
Relationship between reading and IQ.
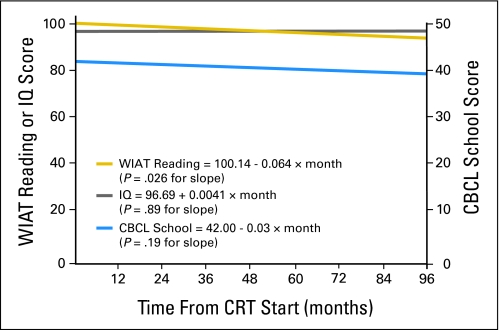
A linear model examining IQ as a function of time since CRT indicates that IQ did not significantly decline in this group of patients during the follow-up time period (P = .89).
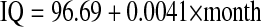 |
The Pearson correlation between model slopes for WIAT reading and IQ was not statistically significant (r = 0.06; P = .59). Taken together, these findings provide evidence that reading performance declined despite stable IQ. A linear model examining parent reported school problems from the CBCL did not reveal a significant increase in school problems during the follow-up time period (P = .19).
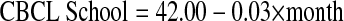 |
However, the Pearson correlation between the slopes for WIAT reading and CBCL school problems was statistically significant (r = −.32; P = .005), indicating a decline in reading correlated with increased school problems (Fig 1).
Fig 1.
Relationships among reading, intellectual functioning (IQ), and parent reported school problems. Linear models of Wechsler Individual Achievement Test (WIAT) reading, IQ, and Achenbach Child Behavior Checklist (CBCL) School Problems. The WIAT reading and IQ are on the primary vertical axis, and CBCL School Problems is on the secondary axis.
DISCUSSION
Children treated with CRT for localized ependymoma perform well academically many years post-treatment. On average, the group lost 3.84 standard points on the reading measure over 5 years. This magnitude of loss is clinically meaningful but relatively small. Further, math and spelling performance remained stable over time, indicating that the group was gaining academic skills in these areas at a rate commensurate with their peers. Taken together, these findings suggest that CRT results in better long-term academic outcomes compared with conventional radiation therapy approaches.15,18
Increased vulnerability of reading among academic skills contrasts with prior findings of greater math impairment.15,19,29 This discrepancy may relate to greater specificity in radiation delivery, the selected math measure (applied math rather than rote computation used in prior studies) and/or the younger age of diagnosis for ependymoma versus other irradiated tumors. In fact, it has been shown that reading is more adversely affected in patients irradiated at a younger age.18 A younger age at irradiation may incur a greater risk to reading due to missed early reading instruction and/or the impact of treatment on early developing brain systems. Current results are consistent with findings of an earlier and steeper decline in reading, among academic skills, in children with medulloblastoma also treated with CRT directed at the posterior fossa.30 It has been proposed by one author (R.J.O.) that posterior fossa–directed CRT disrupts the left hemispheric lateralization of function in the ventral visual pathway that occurs during typical reading development,31 but not in dyslexia.32 We have direct evidence from functional neuroimaging to support this theory; when performing orthographic processing,33 survivors of posterior fossa brain tumor (medulloblastoma and ependymoma) demonstrate less left lateralization of brain activation in the ventral visual pathway than age matched healthy siblings (unpublished data).
Supratentorial tumors and multiple surgeries predicted worse reading performance at CRT start. There is increasing recognition that hemispheric tumors and multiple resections result in greater neuropsychological disruption.34 It has also been demonstrated that there can be a significant level of cognitive impairment with surgery alone.35 These findings may suggest a benefit of maximal resection on first surgical approach to optimize preservation of cognitive abilities or perhaps indicate that less skilled neurosurgeons (those leaving further resectable tumor requiring additional surgery) produce more residual injury.
Younger age at CRT was predictive of a significant decline in reading over time. This finding is consistent with studies of conventional radiation therapy15,18,30,36 and indicates that a longer period of undisturbed neurodevelopment is protective with respect to cognitive outcome. Male sex as a significant predictor of reading decline contradicts findings of female sex conferring greater cognitive vulnerability.34 While this finding requires replication, it may be that some abilities (particularly language-based abilities) are more vulnerable in males. Longer symptomatic interval predicted worse reading outcome. Shorter symptom duration, as an indicator of more aggressive disease, has been shown to confer greater cognitive risk.37 Given the existing literature and the correlation between multiple surgeries and symptomatic interval in this study, it is likely this finding reflects greater risk with increased surgeries rather than longer symptomatic interval. Similarly, pre-CRT chemotherapy, hydrocephalus at presentation, and endocrine dysfunction, indices of more aggressive treatment or greater neurological disruption, all predicted a significant decline in reading over time.
It is important to note that the reading decline was independent of a decline in intellectual functioning, which remained stable throughout the study period. This change in reading corresponded with parent report of increased school problems. Therefore, the common practice of studying IQ as a sole cognitive outcome may fail to identify problems brain tumor survivors experience in their day-to-day lives.
Our results should be interpreted in the context of common limitations. First, a frequent constraint to clinical studies is reliance on normative populations rather than a comparison group. The academic measures included here are gold standards; the standardization samples are large and representative. Further, mean IQ for the study sample is comparable to standardization samples and score distributions are normally distributed. Second, while new versions of some of the cognitive measures were released during the study time period, the same versions were used throughout the study thus choosing potential cohort effects over difficulty combining data from different test versions for longitudinal analyses. Third, the academic tests did not extend below an age of 5 given these academic skills do not typically emerge before 5. It is possible to assess preacademic skills such as phonological awareness and early number concepts, which is recommended for future studies.
This study contributes to the growing literature in support of CRT approaches by revealing improved functional outcomes in the context of similar survival rates. These findings support the rationale for further reducing the clinical and planning target volumes, dose to normal tissue, and increasing dose conformity using advanced radiation delivery methods including proton-beam radiation therapy. While children treated with CRT for local ependymoma were shown to gain intellectual skills at a rate commensurate with peers, reading was shown to be vulnerable. This findings suggests that reading is an area to monitor closely and intervene early. Further, caution should be taken when IQ is used as the sole determinate of cognitive outcome. Finally, specific demographic and clinical risk factors were identified. Some provide opportunities for education of parents and providers regarding heightened risk (eg, tumor location, age at diagnosis, and sex) while others provide unique opportunities for change in clinical care that may mitigate negative cognitive sequelae (eg, careful management of hydrocephalus and eliminating preradiation chemotherapy trials).
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Xiaoping Xiong, Thomas E. Merchant
Provision of study materials or patients: Thomas E. Merchant
Collection and assembly of data: Heather M. Conklin, Thomas E. Merchant
Data analysis and interpretation: Heather M. Conklin, Chenghong Li, Xiaoping Xiong, Robert J. Ogg, Thomas E. Merchant
Manuscript writing: Heather M. Conklin, Xiaoping Xiong, Robert J. Ogg, Thomas E. Merchant
Final approval of manuscript: Heather M. Conklin, Xiaoping Xiong, Robert J. Ogg, Thomas E. Merchant
Appendix
For children 5 or older at conformal radiation therapy (CRT), the time of first testing is still the start of CRT; whereas, for children younger than 5, the time of first testing is when the patient turns 5. Therefore, the time from start of CRT to the time they turn 5 is allowed in the model as a covariate, adjusting the intercept and the slope of the typical linear mixed effects model with random coefficients. This is represented by the equation:
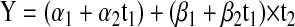 |
(1) |
where t1 is the time, in months, from start of CRT to first academic testing (t1 = 0 if age ≥ 5 at CRT and t1 = the time from start of CRT to age 5 if age < 5 at CRT) and t2 is the time from first testing to last testing (t2 = the time from CRT to last follow-up if age ≥ 5 at CRT and t2 = the time from turning age 5 to the last follow-up if age < 5 at CRT). Adding the covariate of age at CRT (X) to model (1) for both intercept and slope results in the equation:
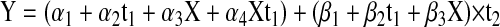 |
(2) |
If X = 1 when age at CRT ≥ 5 years and X = 0 when age at CRT younger than 5 years, model 2 becomes:
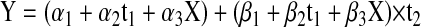 |
(3) |
Model 3 was used for analyzing the effect of age at CRT on academic testing for after actual initiation of testing.
Supported in part by Cancer Center Support Grant No. CA21765 from the National Cancer Institute, by Research Project Grant No. RPG-99-252-01-CCE from the American Cancer Society and by the American Lebanese Syrian Associated Charities (ALSAC).
Presented in part at the International Neuropsychological Society in Portland, OR, February 7-10, 2007.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on www.JCO.org.
REFERENCES
- 1.Central Brain Tumor Registry of the United States: Statistical Report: Primary Brain Tumors in the United States, 1995-1999. Hinsdale, IL, Central Brain Tumor Registry of the United States, 2002
- 2.Merchant TE, Mulhern RK, Krasin MJ, et al: Preliminary results from a phase II trial of conformal radiation therapy and evaluations of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 22:3156-3162, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE: Current management of childhood ependymoma. Oncology 16:629-644, 2002 [PubMed] [Google Scholar]
- 4.Merchant TE, Kiehna EN, Li C, et al: Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys 63:1546-1554, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Grill J, Renaux VK, Bultau C, et al: Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 45:137-145, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al: Intellectual outcome in children with malignant tumors of the posterior fossa: Influence of the field of irradiation and quality of surgery. Child's Nerv Syst 11:340-346, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Copeland DR, deMoor C, Moore BDI, et al: Neurocognitive development of children after cerebellar tumor in infancy: A longitudinal study. J Clin Oncol 17:3476-3486, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Palmer SL, Goloubeva O, Reddick WE, et al: Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol 19:2302-2308, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Spiegler BJ, Bouffet E, Greenberg ML, et al: Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol 22:706-713, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Marshall RM, Hynd GW, Handwerk MJ, et al: Academic underachievement in ADHD subtypes. J Learn Disabil 30:635-642, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Massetti GM, Lahey BB, Pelham WE, et al: Academic achievement over 8 years among children who met modified criteria for attention-deficit/hyperactivity disorder at 4-6 years of age. J Abnorm Child Psychol 36:399-410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell WG, Chavez JM, Lee H, et al: Academic underachievement in children with epilepsy. J Child Neurol 6:65-72, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Williams J, Phillips T, Griebel ML, et al: Factors associated with academic achievement in children with controlled epilepsy. Epilepsy Behav 2:217-223, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Johnson DL, McCabe MA, Nicholson HS, et al: Quality of long-term survival in young children with medulloblastoma. J Neurosurg 80:1004-1010, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Seaver E, Geyer R, Sulzbacher S, et al: Psychosocial adjustment in long-term survivors of childhood medulloblastoma and ependymoma treated with craniospinal irradiation. Pediatr Neurosurg 20:248-253, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Radcliffe J, Bennett D, Kazak AE, et al: Adjustment in childhood brain tumor survival: Child, mother, and teacher report. J Pediatr Psychol 21:529-539, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Carlson-Green B, Morris RD, Krawiecki N: Family and illness predictors of outcome in pediatric brain tumors. J Pediatr Psychol 20:769-784, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Mabbott DJ, Spiegler BJ, Greenberg ML, et al: Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol 23:2256-2263, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Packer RJ, Sutton LN, Atkins TE, et al: A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg 70:707-713, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, et al: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 1982 [PubMed] [Google Scholar]
- 21.Merchant TE, Williams T, Smith JM, et al: Preirradiation endocrinopathies in pediatric brain tumor patients determined by dynamic tests of endocrine function. Int J Radiat Oncol Biol Phys 54:45-50, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D: Wechsler Individual Achievement Test. New York, NY, Psychological Corporation, 1992
- 23.Achenbach TM, Edelbrock C: The Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT, University Associates in Psychiatry, 1991
- 24.Bayley N: Bayley Scales of Infant Development (ed 2). New York, NY, Psychological Corporation, 1993
- 25.Wechsler D: Wechsler Preschool and Primary Scales of Intelligence-Revised. San Antonio, TX, Psychological Corporation, 1989
- 26.Wechsler D: Wechsler Intelligence Scale for Children (ed 3). New York, NY, Psychological Corporation, 1991
- 27.Wechsler D: Wechsler Adult Intelligence Scale-Revised. New York, NY, The Psychological Corporation, 1981
- 28.Sattler JM: Assessment of Children (ed 3). San Diego, CA, Jerome M. Sattler Publisher Inc, 1992, pp 219-243
- 29.Silverman CL, Palkes H, Talent B, et al: Late effects of radiotherapy on patients with cerebellar medulloblastoma. Cancer 54:825-829, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Mulhern RK, Palmer SL, Merchant TE, et al: Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 23:5511-5519, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Turkeltaub PE, Gareau L, Flowers DL, et al: Development of neural mechanisms for reading. Nat Neurosci 6:767-773, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Shaywitz BA, Skudlarski P, Holahan JM, et al: Age-related changes in reading systems of dyslexic children. Ann Neurol 61:363-370, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ogg RJ, Zou P, Allen DN, et al: Neural correlates of a clinical continuous performance test. Magn Reson Imaging 26:504-512, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Butler RW, Haser JK: Neurocognitive effects of treatment for childhood cancer. Ment Retard Dev Disabil 12:184-191, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Carpentieri SC, Waber DP, Pomeroy SL, et al: Neuropsychological functioning after surgery in children treated for brain tumor. Neurosurg 52:1348-1356, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ellenberg L, McComb JG, Seigel SE, et al: Factors affecting intellectual outcome in pediatric brain tumor patients. Neurosurg 21:638-644, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Dennis M, Spiegler BJ, Hetherington CR: Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neuro Onc 29:91-101, 1996 [DOI] [PubMed] [Google Scholar]