Abstract
Purpose
Treatment of childhood relapsed acute lymphoblastic leukemia (ALL) remains a significant challenge. The goal of the Children's Oncology Group (COG) AALL01P2 study was to develop a safe and active chemotherapy reinduction platform, which could be used to evaluate novel agents in future trials.
Patients and Methods
One hundred twenty-four patients with ALL and first marrow relapse received three, 35-day blocks of reinduction chemotherapy: 69 with early relapse (ER; < 36 months from initial diagnosis) and 55 with late relapse (LR). Minimal residual disease (MRD) was measured by flow cytometry after each treatment block.
Results
Second complete remission (CR2) rates at the end of block 1 in 117 assessable patients were 68% ± 6% for ER (n = 63) and 96% ± 3% for LR (n = 54; P < .0001). Five of seven patients with T-cell ALL (T-ALL) failed to achieve CR2. Among patients in CR2, MRD greater than 0.01% was detected at the end of block 1 in 75% ± 7% of ER (n = 36) versus 51% ± 8% of LR (n = 43; P = .0375) and 12-month event-free survival was 80% ± 7% versus 58% ± 7% in MRD-negative versus positive patients (P < .0005). Blocks 2 and 3 of therapy resulted in reduction of MRD burden in 40 of 56 patients who were MRD positive after block 1. Toxicity was acceptable during all three blocks with five deaths (4%) from infections.
Conclusion
The AALL01P2 regimen is a tolerable and active reinduction platform, suitable for testing in combination with novel agents in B-precursor ALL. Alternative strategies are needed for T-ALL. Serial MRD measurements were feasible and prognostic of outcome.
INTRODUCTION
Treatment of marrow relapse of acute lymphoblastic leukemia (ALL) presents a great challenge: only one third of children survive long-term despite intensive retrieval strategies.1 While much debate has centered on optimal postremission therapy including stem cell transplantation, many patients experience treatment failure before they reach that point. Results from the Children's Cancer Group1941 marrow relapse study showed that 50% of patients failed to enter remission, died from toxicity, or relapsed again after achieving a brief second remission.2 These data highlight the need for more effective initial reinduction strategies as a first step to improving outcomes for marrow relapse.
Given the relative lack of success in induction of durable second remissions with conventional chemotherapy combinations,2-15 the Children's Oncology Group (COG) conducted the AALL01P2 phase II pilot study with the primary objective of developing a safe and active reinduction regimen that could serve as a platform for evaluating the addition of promising new agents in future trials. At the time this study was developed, data were emerging showing that minimal residual disease (MRD) status before allogeneic stem cell transplantation (alloSCT) for marrow relapse was predictive of outcome, with patients who entered transplant without detectable MRD faring much better than those with residual disease.16,17 Given this, another objective of this study was to improve the depth of second remission, using three intensive blocks of therapy derived from combinations that were previously shown to be effective in the management of recurrent ALL.3,14,18,19 This study also sought to determine the feasibility of measuring MRD in a single COG Central Reference Laboratory at the completion of each block to monitor the kinetics of response.
PATIENTS AND METHODS
Study Population
Between January 2003 and December 2005, AALL01P2 accrued patients ages 1 to 21 years with first isolated or combined marrow relapse. Patients with both B-precursor and T-cell ALL (T-ALL) relapse were eligible. Patients with mature B-ALL and Down syndrome were excluded. Institutional review boards at participating institutions approved the study. Informed consent was obtained from patients or from parents/legal guardians.
Definitions
Marrow relapse was defined as a bone marrow showing greater than 25% blasts (M3) by conventional morphology. CNS relapse was defined as CSF WBC greater than 5/μL and a cytocentrifuge preparation demonstrating leukemic blasts, or clinical signs of CNS disease. Early marrow relapses (ERs) and late (LRs) marrow relapses were defined as recurrence less than 36 months, or ≥ 36 months after initial diagnosis, respectively.
Trial Design and Therapy
Treatment consisted of three blocks of reinduction chemotherapy for all patients, with an upfront randomization in block order (arm A = blocks 1, 2, 3; arm B = blocks 1, 3, 2), with the exception that patients with CNS leukemia were nonrandomly assigned to arm B to allow earlier introduction of high-dose cytarabine (Table 1). When the study first opened, idarubicin and dexamethasone were used in block 1. However, toxicity was unacceptable and protocol therapy was subsequently modified after the first 21 patients enrolled. Prednisone and doxorubicin replaced dexamethasone and idarubicin, respectively, such that block 1 therapy was identical to that which had been successfully administered on the Pediatric Oncology Group 9310 study.3 Patients with Philadelphia chromosome positive (Ph+) ALL received imatinib mesylate in combination with all three blocks of chemotherapy. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0). Protocol therapy consisted only of the three blocks of reinduction chemotherapy. Treatment with chemotherapy or alloSCT after the three-block reinduction was at the discretion of the treating physicians.
Table 1.
Protocol Therapy
| Drug and Dosage | Day No. |
|---|---|
| Block 1 | |
| Vincristine, 1.5 mg/m2 IV | 1, 8, 15, and 22 |
| Prednisone, 40 mg/m2/d PO* | 1-29 |
| PEG-asparaginase, 2,500 U/m2 IM | 2, 9, 16, and 23 |
| Doxorubicin, 60 mg/m2 IV* | 1 |
| Imatinib mesylate, 340 mg/m2 PO† | 1-14 (Ph+) |
| Intrathecal cytarabine | 1 |
| Intrathecal methotrexate | 8 and 29 (CNS−) |
| Triple intrathecal therapy‡ | 8, 15, 22, and 29 (CNS+) |
| Block 2 | |
| Cyclophosphamide, 440 mg/m2 IV | 1-5 |
| Etoposide, 100 mg/m2 IV | 1-5 |
| Methotrexate, 5 g/m2 IV | 22 (pending blood count recovery) |
| Imatinib mesylate, 340 mg/m2 PO† | 1-14 (Ph+) |
| Intrathecal methotrexate | 1 and 22 (CNS−) |
| Triple intrathecal therapy | 1 and 22 (CNS+) |
| G-CSF, 5 mcg/kg SQ | 6 until ANC > 1,500/μL × 2 days |
| Block 3 | |
| Cytarabine, 3 g/m2 IV every 12 hours | 1, 2, 8, and 9 (Ph−) |
| L-asparaginase, 6,000 U/m2 IM | 2 and 9 at hour 42 after cytarabine (Ph−) |
| Cytarabine, 3 g/m2 IV every 12 hours† | 1, 2 (Ph+) |
| L-asparaginase, 6,000 U/m2 IM† | 2 (Ph+) |
| Imatinib mesylate, 340 mg/m2 PO† | 1-14 (Ph+) |
| G-CSF, 5 mcg/kg SQ | 10 until ANC > 1,500/μL × 2 days |
Abbreviations: IV, intravenous; PO, oral; IM, intramuscular; SQ, subcutaneous; Ph, Philadelphia chromosome; G-CSF, granulocyte colony-stimulating factor; LP, lumbar puncture.
The first 21 patients received idarubicin 10 mg/m2 IV every day on days 1 and 2, and dexamethasone 10 mg/m2 PO on days 1 to 14 (taper days 11 to 14) during block 1. The protocol was subsequently amended for toxicity and all remaining patients received doxorubicin and prednisone as shown above.
Ph+ only. Ph+ patients did not receive day 8 or 9 cytarabine or day 9 L-asparaginase during block 3.
Triple intrathecal therapy (methotrexate, cytarabine, and hydrocortisone) was continued weekly beyond four doses until two successive LPs were free of blasts. All intrathecal medications were dosed based on age.
Assessment of Response
Bone marrow aspirates for morphology were required on days 8, 15 (if not M1 at day 8), and day 36 of block 1, and on day 36 of blocks 2 and 3. Patients with M2 (5% to 25% blasts) or M3 marrows at the end of block 1 proceeded to the next treatment block regardless of blood counts and remained on study provided they achieved an M1 (< 5% blasts) marrow by day 15 of the second administered block. Second complete remission (CR2) was defined as an M1 marrow (< 5% blasts) with no evidence of circulating blasts or extramedullary disease and with peripheral count recovery, defined as absolute neutrophil count higher than 750/μL and platelet count higher than 75,000/μL.
Participation in the MRD studies was optional. MRD was measured serially in marrow samples at the completion of each of the three blocks of therapy by flow cytometry using previously described methods.20 Sensitivity of detection of MRD was 1/10,000 in the great majority of cases. MRD at a level of greater than 1/10,000 cells (> 0.01%) was designated positive.
Statistical Methods
All data analyses were performed using the SAS software, version 9.1 (SAS Institute, CARY, NC) and R (R Development Core Team, Vienna, Austria; http://www.R-project.org).The Kaplan- Meier21 method was used to obtain estimates of event-free survival (EFS), and SEs of estimates were obtained using the method of Peto.22 Fisher's exact test was used to compare rates, and the log-rank test was used to compare survivor functions.
RESULTS
Patients
Between January 2003 and December 2005, 145 patients enrolled on AALL01P2. After the first 21 patients, block 1 therapy was modified due to unacceptable toxicity, which included three early deaths from infections. The 124 patients who received the modified regimen from August 2003 onward are the subject of this report. These initial 21 patients did not differ significantly in age, sex, disease characteristics, or block 1 response from the 124 patients that are the subject of this report. Demographic and clinical characteristics are summarized in Table 2.
Table 2.
Clinical and Demographic Characteristics
| Characteristic | Risk Group
|
Total
|
||||
|---|---|---|---|---|---|---|
| Early Relapse
|
Late Relapse
|
|||||
| No. | % | No. | % | No. | % | |
| Median age, years | 9.5 | 10.4 | 10.0 | |||
| Range | 1.6-21.4 | 4.3-21.1 | 1.6-21.4 | |||
| Sex | ||||||
| Male | 43 | 62 | 37 | 67 | 80 | 65 |
| Female | 26 | 38 | 18 | 33 | 44 | 35 |
| Immunophenotype | ||||||
| B lineage | 63 | 91 | 54 | 98 | 117 | 94 |
| T lineage | 6 | 9 | 1 | 2 | 7 | 6 |
| Ph+ | ||||||
| No | 67 | 97 | 53 | 96 | 120 | 97 |
| Yes | 2 | 3 | 2 | 4 | 4 | 3 |
| CNS disease | ||||||
| Negative | 63 | 91 | 47 | 85 | 110 | 89 |
| Positive | 6 | 9 | 8 | 15 | 14 | 11 |
| Ph+ and CNS | ||||||
| No | 69 | 100 | 54 | 98 | 123 | 99 |
| Yes | 0 | 0 | 1 | 2 | 1 | 1 |
| Total | 69 | 55 | 124 | |||
Abbreviation: Ph, Philadelphia chromosome.
Toxicity
Expected toxicities were observed with protocol therapy. Grade 3 or 4 toxicities which occurred with a higher than 10% incidence in each block are summarized in Table A1 (online only). Infections were the most frequent toxicity and occurred with a higher incidence during blocks 1 and 3 than during block 2. The spectrum and severity of toxicities were similar irrespective of block order (data not shown). The median duration for each block for all patients, including those who went off protocol therapy, was 37 (range, 7 to 61), 39 (range, 14 to 66), and 36 days (range, 10 to 112) for blocks 1, 2 and 3, respectively. The duration of blocks 1, 2 and 3 was shorter than 41, 45 and 41 days, respectively, in 75% of patients.
Five toxic deaths occurred among 124 patients (4.0%). These deaths all resulted from sepsis attributed to the following organisms: Staphylococcus aureus, Clostridium species, Pseudomonas aeruginosa and alpha hemolytic streptococci. Three toxic deaths occurred during block 1, and two occurred during block 3 therapy (both on arm B).
Block 1 Response
Among 117 patients assessable for response in block 1, 81.2% achieved a CR2 at the completion of the first block (day 36). CR2 rates were 68% ± 6% for ER (n = 63) and 96% ± 3% for LR (n = 54; P < .0001). CR2 rates at the end of block 1 in patients with very early recurrences (< 18 months from initial diagnosis) were 45% ± 11%, and 79% ± 6% (P = .0098) for recurrences between 18 and 36 months from initial diagnosis. CR2 rates at the end of block 1, according to time of relapse are summarized in Table 3.
Table 3.
Block 1 Remission Reinduction Rates: 4- and 12-Month EFS
| Parameter | Early Relapse (%)
|
Late Relapse (%; ≥ 36 months)
|
||||||
|---|---|---|---|---|---|---|---|---|
| < 18 Months
|
18-36 Months
|
< 36 Months
|
||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| All patients | ||||||||
| No. of patients | 24 | 45 | 69 | 55 | ||||
| CR2 rate | 45 | 11 | 79 | 6 | 68 | 6 | 96 | 3 |
| 4-month EFS | 38 | 10 | 75 | 7 | 62 | 6 | 93 | 4 |
| 12-month EFS | 13 | 7 | 48 | 8 | 35 | 6 | 80 | 5 |
| B-lineage, CNS−, Ph− | ||||||||
| No. of patients | 16 | 39 | 55 | 45 | ||||
| CR2 rate | 46 | 14 | 81 | 6 | 72 | 6 | 95 | 3 |
| 4-month EFS | 38 | 12 | 76 | 7 | 65 | 7 | 93 | 4 |
| 12-month EFS | 13 | 8 | 45 | 8 | 35 | 7 | 84 | 5 |
Abbreviations: EFS, event-free survival; CR2, second complete remission; Ph, Philadelphia chromosome.
Morphological response was tracked during block 1. Among 111 patients with day 8 marrows, the distribution of M1, M2, and M3 marrows was: 30%, 24%, and 46%, respectively. There was poor compliance with day 15 marrow assessments. Among 114 patients completing block 1 therapy and assessable for response on day 36, marrow responses were as follows: 84% M1; 7% M2; and 9% M3. All patients with M2 or M3 marrows at the end of block 1 proceeded directly to the next block of therapy, regardless of blood counts. However, only five of 18 patients with M2 or M3 marrows at the end of block 1 entered remission with ongoing therapy, and four of these five patients ultimately relapsed within 12 months. The remaining 13 patients all did not achieve CR2.
Outcome
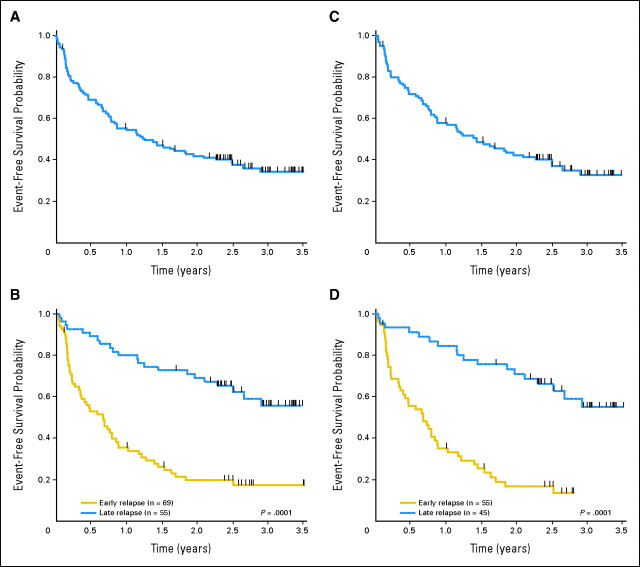
Early response was determined at the completion of the three blocks of protocol therapy, a time point which serves as a surrogate for disease status before stem cell transplantation. The overall EFS rate at 4 months was 76% ± 4% for all patients: 62% ± 6% and 93% ± 4% for ER and LR (P < .0001), respectively (Fig 1). In the subset of patients with very early marrow relapses (< 18 months from diagnosis), 4 month EFS was 38% ± 10%, compared with 75% ± 7% in those with relapses 18 to 36 months from diagnosis. Responses were also determined separately in B-precursor, CNS-negative, and Ph-negative patients (Table 3) and according to treatment arm. There were no significant differences in outcome between treatment arms (order of blocks delivered; data not shown).
Fig 1.
Outcomes based on site and timing of recurrence. Outcomes after first isolated or combined marrow relapse. (A) Overall event-free survival (EFS) of all patients enrolled after the study was amended for initial toxicity. (B) EFS according to timing of relapse in all patients. (C) Overall EFS of B-precursor, CNS-negative, Philadelphia chromosome-negative patients. (D) EFS according to timing of relapse in B-precursor, CNS-negative, Philadelphia chromosome-negative patients. (B, D) Solid line = early relapse (< 36 months from initial diagnosis), dashed line = late marrow relapse (≥ 36 months from initial diagnosis).
MRD Response
MRD was measured by flow cytometry at the completion of each block of therapy (Table A2 and Fig A1, online only). For patients who attained CR2, MRD greater than 0.01% was present at the end of block 1 in 62% ± 5% of patients overall, and in 75% ± 7% of patients with ER compared with 51% ± 8% of patients with LR (P = .038). In this same group of patients, 43% ± 11% of ER and 25% ± 8% of LR patients were still MRD-positive at the completion of the third treatment block.
The 12-month EFS rate for all patients in morphological remission and MRD negative at the end of block 1 was 80% ± 7%, compared with 58% ± 7% in those who were MRD positive (P = .0005; Fig 2). The 12-month EFS rate for patients that were MRD negative versus positive at the end of block 1 was 67% ± 16% versus 42% ± 10% (P = .012) for ER and 86% ± 8% versus 77% ± 9% (P = .0904) for LR patients.
Fig 2.
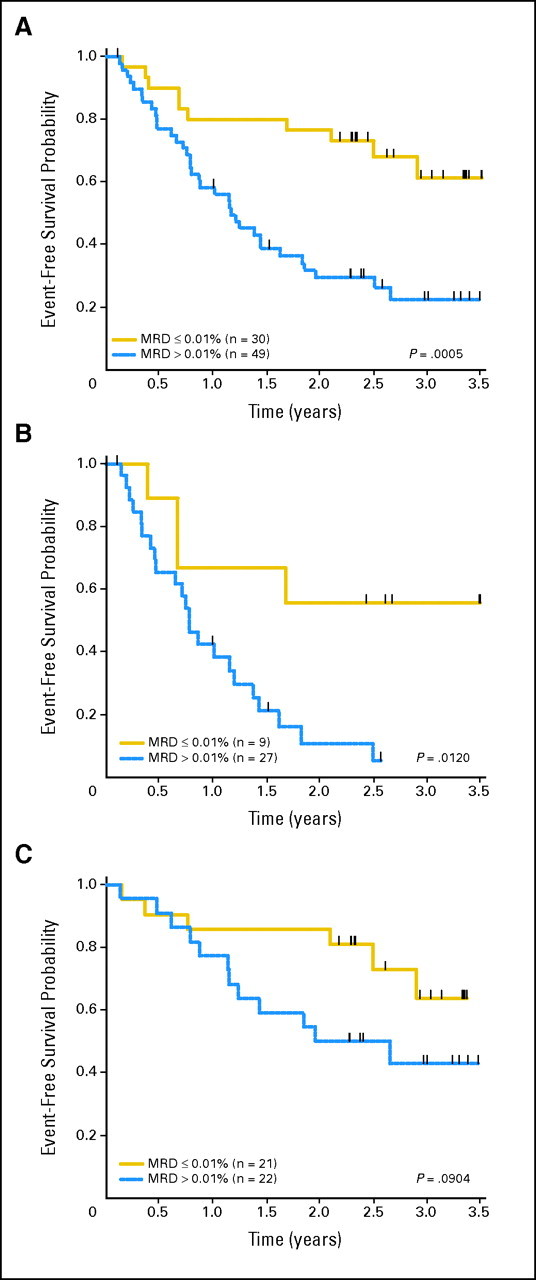
Outcomes according to block 1 minimal residual disease (MRD) response. Event-free survival (EFS) probabilities for patients in morphological CR2 who were MRD negative versus positive at the end of block 1. (A) All patients: 12-month EFS 80% ± 7% versus 58% ± 7% in MRD-negative versus MRD-positive patients. (B) Early marrow relapse: 12-month EFS 67% ± 16% versus 42% ± 10% for MRD-negative versus -positive patients. (C) Late marrow relapse: 12-month EFS 86% ± 8% versus 77% ± 9% in MRD-negative versus -positive patients.
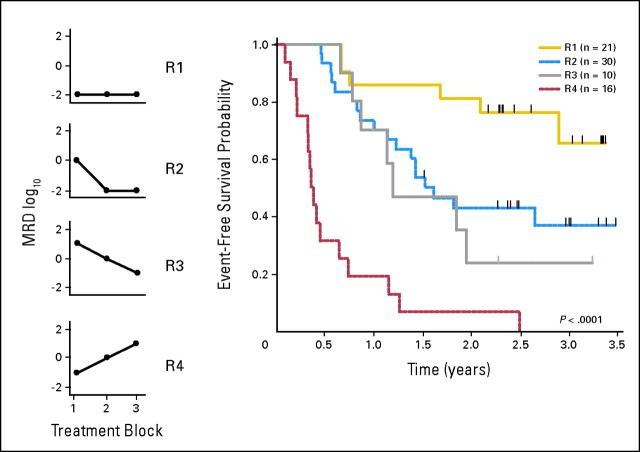
Kinetic patterns of the MRD response demonstrated four patterns. One group of patients was negative after all three blocks of therapy (R1). A second group was MRD positive at the end of block 1, but became negative with further treatment (R2). A third group was MRD-positive at all three time points, but showed a higher than 1 log reduction in disease burden (R3) and a fourth showed persistently positive or rising MRD (R4). There were 21 R1, 30 R2, 10 R3, and 16 R4 patients. The 12-month EFS probabilities for the four groups were 86% ± 8%, 73% ± 8%, 70% ± 16%, and 19% ± 10%, respectively (P < .0001; Fig 3).
Fig 3.
Kinetics of minimal residual disease (MRD). MRD was measured after each block of therapy and four general response patterns were observed among 77 patients with MRD data for multiple time points. R1 patients included those negative at all time points tested; R2 included those who became negative after either block 2 or block 3; R3 included those patients who decreased but still had detectable disease at the end of the final block of therapy; and R4 included patients showed no decline or rising MRD. The 12-month EFS probabilities for the four groups were 86% ± 8%, 73% ± 8%, 70% ± 16%, and 19% ± 10%, respectively.
Outcomes of CNS-Positive, T-Cell, and Ph+ ALL
The outcomes of the seven T-ALL, four Ph+, and 14 combined marrow and CNS relapse patients were analyzed separately. Six of seven T-ALL relapses occurred early. Five of the seven patients with T-ALL failed to achieve a second remission and no T-ALL relapse patient survived longer than 10 months. All four Ph+ patients achieved CR2 at the end of block 1 but two relapsed and died. Thirteen of 14 patients (n = six ER; n = eight LR) with combined marrow and CNS relapses achieved CR2 with a 4-month EFS of 86% ± 9% and a 12-month EFS of 64% ± 13%.
DISCUSSION
Despite the success in treating the majority of children with newly diagnosed ALL, effective treatment strategies for marrow relapse have been elusive. The challenges begin early in therapy with a significant number of reinduction failures, particularly when disease recurs early. Moreover, when remissions are achieved, they are less durable as evidenced by reports of subsequent relapses in up to one third of patients within the median time to alloSCT.2
The primary objective of the AALL01P2 study was to develop a reinduction regimen for marrow relapse that was safe and feasible with the intention of later using this regimen as a platform to combine with promising new agents. Because second remissions are frequently short lived, the study used three nonoverlapping blocks of chemotherapy in an effort to achieve deeper remissions. The chemotherapy combinations used in these blocks had previously been defined to be active in the relapse setting.14,18,19 This study was also designed to monitor MRD sequentially after each treatment block to help assess the relative efficacy of individual blocks.
Patients on this study were randomly assigned upfront to two different block orders to determine if the sequence of therapy influenced outcomes. There were no significant differences in outcome between arms A and B and because of greater toxicity associated with blocks 1 and 3 compared with block 2, we have elected to use the 1, 2, 3 block order going forward. Protocol therapy consisted of the three-block reinduction only. Postinduction therapy varied and was at the discretion of the treating physician, precluding any comparisons between ongoing chemotherapy and alloSCT.
When this study first opened, toxicity in block 1 was too high, prompting substitution of doxorubicin and prednisone for idarubicin and dexamethasone (as in the POG-9310 regimen).3 After this modification, the toxic death rate of 4.0% was similar to the 3% to 8% rates seen with other regimens.1,3,5,12,23 Other toxicities were manageable with infectious complications occurring most commonly. The incidence of fever and neutropenia and documented infections was highest during blocks 1 and 3, and similar to the incidence observed with other regimens of similar intensity.12 Despite the toxicities, the median time for completion of each block of therapy was very close to the scheduled 36 days.
Remission reinduction rates with this regimen were 68% for ER and 96% for LR. Patients with very early relapse (< 18 months) fared exceptionally poorly with CR2 rates of only 45%. These results are very similar to what has been consistently reported in the literature: 66% to 82% for ER and 90% to 95% for LR. 2-8,10,11,15,23-25 Even when intensive salvage strategies including alloSCT are employed, longer-term EFS rates for ER are only 10% to 20%, compared with 40% to 50% for LR.2,5 It is striking that these outcomes have been remarkably consistent over recent decades, irrespective of differences in the components of salvage regimens.
In addition to timing of relapse, blast immunophenotype and site of relapse are both important prognostic variables. Historically, patients with T-cell relapse have fared poorly.2,5,26,27 In a recent report by St Jude Children's Research Hospital, CR2 rates for this population were 60%, with a 5-year EFS of only 5%.25 Although the number of T-cell patients on this study was very small, this therapy was ineffective for this group: five of seven patients experience reinduction failure and there were no survivors.
Isolated marrow relapses are the most challenging to treat, whereas isolated extramedullary relapses have more favorable outcomes and combined relapses have an intermediate prognosis.24,28 Patients with combined relapses on this study had CR2 rates and a 12-month EFS probability which were similar to patients with isolated marrow relapses. However, there were only 14 patients with combined relapses on this study, limiting comparisons in outcomes.
Our results highlight several important points about MRD in trials of relapsed ALL. First, the rates of MRD positivity are much higher than observed in first-line ALL clinical trials. Using the same methodologies, 26% of children with newly diagnosed high-risk ALL that received a four-drug induction regimen were MRD positive at end induction, versus 62% of relapsed ALL patients in this trial.29 Second, as previously demonstrated in the relapse setting,30,31 early MRD response was a strong predictor of outcome. The absence of MRD at the end of the first month of reinduction therapy portended better outcomes in all patients, and separately in ER and LR patients. The combination of timing of relapse and MRD appeared to identify three groups of patients. ER patients who were MRD positive had a dismal outcome, while LR patients who were MRD negative had an excellent outcome, approaching that seen in newly diagnosed patients. MRD-negative ER patients and MRD-positive LR patients appeared to form an intermediate group. These data suggest that MRD may be helpful in stratifying salvage therapy in the near future.
The kinetic pattern of MRD in patients showed continued regression in disease burden with subsequent blocks of therapy in 40 (71%) of 56 of patients who had measurable disease at the end of the first block. These results suggest that the additional blocks of therapy are effective and contribute to the durability of the remission. Also supporting this contention is the finding that 4-month EFS probabilities on this study, which is a time point approximating time to SCT, were very similar to the CR2 rates observed at the end of block 1. Despite further reduction in MRD burden with ongoing block therapy in the majority of patients, and more durable responses during the initial 4 months, later failures occurred, suggesting that alternative postinduction therapy may be needed. Subsequent blocks of therapy appeared less effective for patients who did not achieve a morphologic CR after block 1, as those patients had dismal outcomes. These findings agree with those recently reported by Gaynon and colleagues2 where none of the nine patients with M2 marrows at the end of reinduction survived.
Taken together, several conclusions about early response and MRD can be drawn. First, three-block reinduction chemotherapy appears effective for those patients who achieve a morphological remission and are MRD-negative at the end of the first month of treatment. Second, approximately 70% of patients who achieve CR2, but have detectable MRD at the end of block 1, will have sustained remissions and further reductions in MRD with ongoing chemotherapy. Finally, patients who fail to achieve CR2 at the end of block 1, or who have persistent MRD at the end of three blocks, have exceptionally poor outcomes and may benefit from novel treatment strategies.
In summary, the AALL01P2 study established a reinduction regimen for initial marrow relapse which was feasible to administer, with acceptable toxicity and comparable remission reinduction rates to other contemporary salvage regimens. Extending the duration of reinduction to three blocks appeared to be beneficial for the group of patients with initial favorable morphologic responses. The inferior reinduction rates and the persistence of MRD at the end of block 1 in the majority of patients highlight the urgent need for the integration of new agents into salvage regimens to reduce early disease burden more effectively. A COG phase I/II study using the anti-CD22 monoclonal antibody epratuzumab with this platform is presently underway.32 Ongoing laboratory initiatives are also seeking to define mechanisms of relapse and targeted agents of promise for incorporation into future salvage treatment strategies for this challenging group of patients.33
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Paul S. Gaynon, Genzyme, Sanofi-aventis, Enzyme Research Funding: Paul S. Gaynon, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth A. Raetz, Michael J. Borowitz, Meenakshi Devidas, Stephen P. Hunger, Naomi J. Winick, Bruce M. Camitta, Paul S. Gaynon, William L. Carroll
Administrative support: Elizabeth A. Raetz
Provision of study materials or patients: Elizabeth A. Raetz, Naomi J. Winick, Bruce M. Camitta, Paul S. Gaynon, William L. Carroll
Collection and assembly of data: Elizabeth A. Raetz, Michael J. Borowitz, Meenakshi Devidas, Stephen B. Linda
Data analysis and interpretation: Elizabeth A. Raetz, Michael J. Borowitz, Meenakshi Devidas, Stephen B. Linda, Stephen P. Hunger, Naomi J. Winick, Bruce M. Camitta, Paul S. Gaynon, William L. Carroll
Manuscript writing: Elizabeth A. Raetz, Michael J. Borowitz, Meenakshi Devidas, Stephen P. Hunger, Naomi J. Winick, Bruce M. Camitta, Paul S. Gaynon, William L. Carroll
Final approval of manuscript: Elizabeth A. Raetz, Michael J. Borowitz, Meenakshi Devidas, Stephen B. Linda, Stephen P. Hunger, Naomi J. Winick, Bruce M. Camitta, Paul S. Gaynon, William L. Carroll
Supplementary Material
Appendix
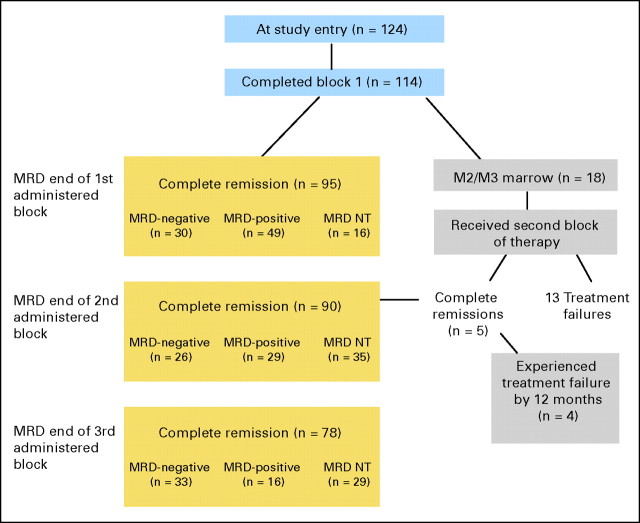
Fig A1.
Minimal residual disease (MRD) response rates according to timing of relapse (online only). MRD responses after the second and third blocks of therapy are shown in this flow diagram in those patients achieving CR2 at the end of block 1. At the end of three blocks of therapy, 16 (33%) of 49 of patients overall, remained MRD positive. Note that one patient with an M1 marrow at the end of block 1 (day 36) was determined not to be in CR2 due to absence of count recovery on day 36 and an M3 marrow 1 week later, and was excluded from this analysis. Among 18 patients with M2 or M3 marrows at the end of block 1, 13 did not achieve remission despite the administration of the second block of chemotherapy. Five entered remission with ongoing chemotherapy; however, four of these five patients developed disease recurrence within 1 year. NT, not tested.
Table A1.
Grade 3 and Higher Toxicities That Occurred With ≥ 10% Incidence Within a Block
| Toxicity | Block 1 (n = 124)
|
Block 2 (n = 91)
|
Block 3 (n = 102)
|
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection) | 50 | 40.3 | 26 | 28.6 | 45 | 44.1 |
| Fibrinogen | 40 | 32.3 | 0 | 0 | 0 | 0 |
| Hemoglobin | 78 | 62.9 | 59 | 64.8 | 79 | 77.5 |
| Hyperglycemia | 15 | 12.1 | 0 | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 0 | 0 | 17 | 16.7 |
| Infection (documented clinically or microbiologically) with grade 3 or 4 neutropenia | 24 | 19.4 | 10 | 11.0 | 36 | 35.3 |
| Leukocytes (total WBC) | 30 | 24.2 | 13 | 14.3 | 20 | 19.6 |
| Neutrophils/granulocytes (ANC/AGC) | 121 | 97.6 | 88 | 96.7 | 101 | 99.0 |
| Platelets | 99 | 79.8 | 59 | 64.8 | 98 | 96.1 |
| ALT | 18 | 14.5 | 0 | 0 | 0 | 0 |
| Transfusion | ||||||
| Platelets | 93 | 75.0 | 41 | 45.1 | 98 | 96.1 |
| pRBCs | 95 | 76.6 | 68 | 74.7 | 92 | 90.2 |
Abbreviations: ANC, absolute neutrophil count; AGC, absolute granulocyte count; pRBCs, packed red blood cells.
Table A2.
Minimal Residual Disease Response Rates According to Timing of Relapse
| Administered Block | Minimal Residual Disease Positive Patients* (%)
|
|
|---|---|---|
| Early Relapse | Late Relapse | |
| 1 | 75 ± 7 (n = 36) | 51 ± 8 (n = 43) |
| 2 | 70 ± 10 (n = 23) | 41 ± 4 (n = 32) |
| 3 | 43 ± 11 (n = 21) | 25 ± 8 (n = 28) |
All patients in complete remission at the end of block 1.
Supported in part by Children's Oncology Group Grants No. U10 CA98543 and R21CA110344 (M.J.B.) from the National Cancer Institute.
Presented in part at the Annual Meeting of the American Society of Hematology, Orlando, FL, December 9-12, 2006.
E.A.R. and M.J.B. contributed equally to this article.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al: Pathways through relapses and deaths of children with acute lymphoblastic leukemia: Role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol 24:5750-5762, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Gaynon PS, Harris RE, Altman AJ, et al: Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol 24:3150-3156, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Abshire TC, Pollock BH, Billett AL, et al: Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood 96:1709-1715, 2000 [PubMed] [Google Scholar]
- 4.Buchanan GR, Rivera GK, Boyett JM, et al: Reinduction therapy in 297 children with acute lymphoblastic leukemia in first bone marrow relapse: A Pediatric Oncology Group study. Blood 72:1286-1292, 1988 [PubMed] [Google Scholar]
- 5.Einsiedel HG, von Stackelberg A, Hartmann R, et al: Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: Results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol 23:7942-7950, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Feig SA, Ames MM, Sather HN, et al: Comparison of idarubicin to daunomycin in a randomized multidrug treatment of childhood acute lymphoblastic leukemia at first bone marrow relapse: A report from the Children's Cancer Group. Med Pediatr Oncol 27:505-514, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Giona F, Testi AM, Rondelli R, et al: ALL R-87 protocol in the treatment of children with acute lymphoblastic leukaemia in early bone marrow relapse. Br J Haematol 99:671-677, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Lawson SE, Harrison G, Richards S, et al: The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: A report on the medical research council UKALLR1 study. Br J Haematol 108:531-543, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Leahey AM, Bunin NJ, Belasco JB, et al: Novel multiagent chemotherapy for bone marrow relapse of pediatric acute lymphoblastic leukemia. Med Pediatr Oncol 34:313-318, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Rivera GK, Hudson MM, Liu Q, et al: Effectiveness of intensified rotational combination chemotherapy for late hematologic relapse of childhood acute lymphoblastic leukemia. Blood 88:831-837, 1996 [PubMed] [Google Scholar]
- 11.Sadowitz PD, Smith SD, Shuster J, et al: Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood 81:602-609, 1993 [PubMed] [Google Scholar]
- 12.Thomson B, Park JR, Felgenhauer J, et al: Toxicity and efficacy of intensive chemotherapy for children with acute lymphoblastic leukemia (ALL) after first bone marrow or extramedullary relapse. Pediatr Blood Cancer 43:571-579, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Uderzo C, Dini G, Locatelli F, et al: Treatment of childhood acute lymphoblastic leukemia after the first relapse: Curative strategies. Haematologica 85:47-53, 2000 [PubMed] [Google Scholar]
- 14.Reaman GH, Ladisch S, Echelberger C, et al: Improved treatment results in the management of single and multiple relapses of acute lymphoblastic leukemia. Cancer 45:3090-3094, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Testi AM, Del Giudice I, Arcese W, et al: A single high dose of idarubicin combined with high-dose ARA-C for treatment of first relapse in childhood ‘high-risk’ acute lymphoblastic leukaemia: A study of the AIEOP group. Br J Haematol 118:741-747, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Bader P, Hancock J, Kreyenberg H, et al: Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia 16:1668-1672, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Knechtli CJ, Goulden NJ, Hancock JP, et al: Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood 92:4072-4079, 1998 [PubMed] [Google Scholar]
- 18.Crooks GM, Sato JK: Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 17:34-38, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Wells RJ, Feusner J, Devney R, et al: Sequential high-dose cytosine arabinoside-asparaginase treatment in advanced childhood leukemia. J Clin Oncol 3:998-1004, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Borowitz MJ, Pullen DJ, Shuster JJ, et al: Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: Relation to other risk factors: A Children's Oncology Group study. Leukemia 17:1566-1572, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53:457-481, 1958 [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, et al: Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples Br J Cancer 35:1-39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy A, Cargill A, Love S, et al: Outcome after first relapse in childhood acute lymphoblastic leukaemia-lessons from the United Kingdom R2 trial. Br J Haematol 130:67-75, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Henze G, Fengler R, Hartmann R, et al: Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood 78:1166-1172, 1991 [PubMed] [Google Scholar]
- 25.Rivera GK, Zhou Y, Hancock ML, et al: Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer 103:368-376, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Chessells JM, Veys P, Kempski H, et al: Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol 123:396-405, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Schroeder H, Garwicz S, Kristinsson J, et al: Outcome after first relapse in children with acute lymphoblastic leukemia: A population-based study of 315 patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Med Pediatr Oncol 25:372-378, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Buhrer C, Hartmann R, Fengler R, et al: Superior prognosis in combined compared to isolated bone marrow relapses in salvage therapy of childhood acute lymphoblastic leukemia. Med Pediatr Oncol 21:470-476, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Borowitz MJ, Devidas M, Hunger SP, et al: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children's Oncology Group study. Blood 111:5477-5485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coustan-Smith E, Gajjar A, Hijiya N, et al: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia 18:499-504, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Eckert C, Biondi A, Seeger K, et al: Prognostic value of minimal residual disease in relapsed child-hood acute lymphoblastic leukaemia. Lancet 358:1239-1241, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Raetz E, Cairo M, Borowitz M, et al: Chemoimmunotherapy re-induction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: Children's Oncology Group pilot study ADVL04P2. J Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- 33.Bhojwani D, Kang H, Moskowitz NP, et al: Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: A Children's Oncology Group study. Blood 108:711-717, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.