Abstract
Purpose
To investigate the association between pre- and postdiagnosis physical activity (as well as change in prediagnosis to postdiagnosis physical activity) and mortality among women with breast cancer.
Patients and Methods
This was a prospective observational study of 933 women enrolled onto the Health, Eating, Activity, and Lifestyle Study who were diagnosed with local or regional breast cancer between 1995 and 1998 and observed until death or September 2004, whichever came first. The primary outcomes measured were total deaths and breast cancer deaths. The primary exposures were physical activity in the year before and 2 years after diagnosis and the pre- to postdiagnosis change in physical activity.
Results
Compared with inactive women, the multivariable hazard ratios (HRs) for total deaths for women expending at least 9 metabolic equivalent hours per week (approximately 2 to 3 h/wk of brisk walking) were 0.69 (95% CI, 0.45 to 1.06; P = .045) for those active in the year before diagnosis and 0.33 (95% CI, 0.15 to 0.73; P = .046) for those active 2 years after diagnosis. Compared with women who were inactive both before and after diagnosis, women who increased physical activity after diagnosis had a 45% lower risk of death (HR = 0.55; 95% CI, 0.22 to 1.38), and women who decreased physical activity after diagnosis had a four-fold greater risk of death (HR = 3.95; 95% CI, 1.45 to 10.50).
Conclusion
Moderate-intensity physical activity after a diagnosis of breast cancer may improve prognosis.
INTRODUCTION
More than 25 studies over the last 20 years have demonstrated that both premenopausal and postmenopausal women who are physically active have a 30% to 40% lower risk of developing breast cancer compared with sedentary women.1 Furthermore, recent publications have shown that approximately 2 to 3 h/wk of moderate-intensity physical activity after a breast cancer diagnosis is associated with a 40% to 50% lower risk of breast cancer death.2-4 Similar amounts of physical activity after a diagnosis of colon cancer have been associated with an approximate 60% lower risk of death.5,6 The beneficial effects of physical activity may be mediated through a reduction in body fat and beneficial changes in metabolic and sex hormones, growth factors, adipokines, immune function, or inflammation.7,8
In an effort to quantify the amount and timing of physical activity necessary for improving breast cancer prognosis, we examined the association between physical activity, assessed in the year before and 2 years after diagnosis, and mortality in an ethnically diverse sample of breast cancer survivors enrolled onto the Health, Eating, Activity, and Lifestyle (HEAL) Study.
PATIENTS AND METHODS
Study Participants
The HEAL Study is a population-based, multicenter, multiethnic prospective cohort study that has enrolled 1,183 breast cancer survivors to determine whether lifestyle, hormones, and other exposures affect breast cancer prognosis.9-11 Women were recruited through Surveillance, Epidemiology, and End Results (SEER) registries in New Mexico, Los Angeles County, and Western Washington. Women with first primary breast cancer were contacted to determine eligibility. Details of the study have been published previously.9-11
Briefly, in New Mexico, we recruited 615 women, age 18 years or older, diagnosed with in situ to regional breast cancer between July 1996 and March 1999, and living in Bernalillo, Sante Fe, Sandoval, Valencia, or Taos Counties. In Western Washington, we recruited 202 women, between the ages of 40 and 64 years, diagnosed with in situ to regional breast cancer between September 1997 and September 1998, and living in King, Pierce, or Snohomish Counties. In Los Angeles County, 366 black women with in situ to regional breast cancer, who had participated in the Los Angeles portion of the Women's Contraceptive and Reproductive Experiences Study (a case-control study of invasive breast cancer) or who had participated in a parallel case-control study of in situ breast cancer, were recruited for the HEAL Study. Eligible participants from these two studies were the subset of black women who were diagnosed with breast cancer between May 1995 and May 1998. Both Washington and Los Angeles restricted eligibility to women age 35 to 64 years at diagnosis because of competing studies and by design of the parent study.
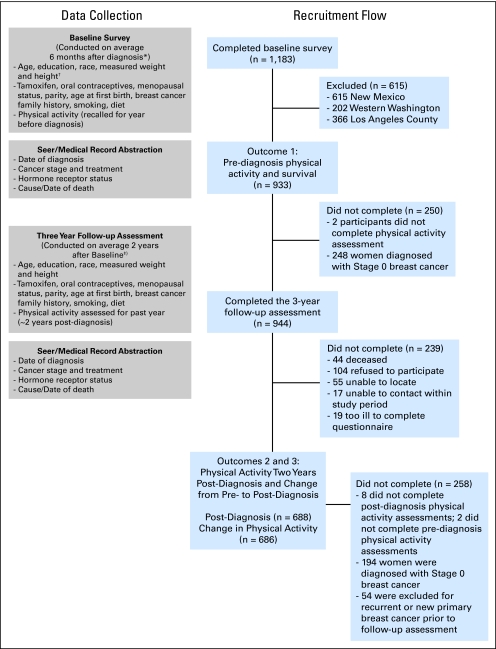
A total of 1,183 women completed in-person baseline interviews, which were conducted 6 ± 2 months (median, 5 months) after diagnosis. Among these women, 933 were diagnosed with local and regional breast cancer. A total of 944 women completed the follow-up interview conducted 3 years after diagnosis (median, 2.5 years). In the interval between the baseline and 3-year interview, 54 of these women experienced a recurrence or new breast primary. These women contributed to analyses regarding physical activity before diagnosis but not to analyses of physical activity after diagnosis. Also, 202 women were excluded from postdiagnosis analyses because of missing physical activity data (n = 8) and a diagnosis of in situ breast cancer (n = 194). Thus, our prediagnosis and postdiagnosis physical activity analyses are based on a sample of 933 and 688 breast cancer survivors, respectively (Fig 1). The study was performed with the approval of the institutional review boards of participating centers, in accord with an assurance filed with and approved by the US Department of Health and Human Services.
Fig 1.
Participant recruitment and timing of data collection. SEER, Surveillance, Epidemiology, and End Results.
Data Collection
Physical activity.
We collected information on all types of physical activity, including recreational, occupational, and household activities, using the interview-administered Modifiable Activity Questionnaire developed by Kriska.12 This questionnaire has been shown to have high validity and reliability (r = 0.73 with doubly labeled water and r = 0.92 for 3-week test-retest).12 Participants recalled the type, duration, and frequency of physical activities for the year before diagnosis at the baseline interview (Washington and New Mexico) and at the 3-year postdiagnosis interview (all three sites). Data from Los Angeles for prediagnosis physical activity were collected using a lifetime physical activity questionnaire that provided sufficient detail to classify activity according to the Modifiable Activity Questionnaire.13 Each activity was categorized as light (< 3 metabolic equivalents of energy expenditure [MET]), moderate (3 to 6 MET), or vigorous (> 6 MET).14
Outcome assessment.
Follow-up time ranged from 5 to 8 years from diagnosis, with a median follow-up time of 6 years. Women were observed until death or September 30, 2004, whichever occurred first. SEER records were used to determine the vital status of participants. Prior literature indicates that breast cancer death is accurately reported on death certificates, with confirmation rates that are greater than 93% when comparing death certificates with medical records.15
At the 3-year follow-up data collection point, women were asked whether they had any disease recurrences or new breast cancers diagnosed, breast biopsy or surgery, new adjuvant treatment, bone or computed tomography scan, or hospitalizations. A positive response triggered a request for medical records to confirm details of each recurrence or second primary breast cancer. New breast cancer primaries were also identified through linkage with SEER records. Six women self-reported recurrence or a new breast primary that was not confirmed via medical records.
Covariates.
Trained staff measured weight and height at the baseline (Washington and New Mexico) and 3-year postdiagnosis assessments (all three sites). Measurements of weight and height were made to the nearest 0.1 kg and 0.1 cm, respectively, with women wearing light indoor clothing and no shoes. All measurements were performed twice and averaged for a final value.
Medical history, demographic, and lifestyle information was collected at the baseline and 3-year visits. Information on disease stage, hormone receptor status, adjuvant therapy, and hormonal therapy was abstracted from SEER records and from physician and hospital medical records. Information on diet was collected at the 3-year follow-up assessment using a 120-item self-administered food frequency questionnaire.16
Statistical Analyses
Cox proportional hazards models were fit to our data using time since the baseline or 3-year interview as the underlying time variable. We calculated the hazard ratio (HR) of death in one exposure group and compared it with the HR in a referent exposure group adjusting for potential confounding factors. Two outcomes were defined; these were total deaths and breast cancer deaths (with deaths from other causes censored).
Physical activity was measured as activity in the year before diagnosis, activity in the second year after diagnosis, and change in physical activity between these two reference points. We conducted analyses with physical activity classified in tertiles, as the recommended amount of physical activity (ie, 0, > 0 to < 9, and ≥ 9 MET-h/wk, with 9 MET-h/wk equal to approximately 150 min/wk of moderate-intensity physical activity),17 and in physical activity categories similar to the Holmes et al2 publication (ie, 0, > 0 to < 9, ≥ 9 to < 15, ≥ 15 to < 24, and ≥ 24 MET-h/wk). Results were similar for each of the classification approaches used and for different types of physical activity; thus, we present our results using categories of recommended recreational physical activity. Categories for change in physical activity from before to after diagnosis were classified as inactive (ie, 0 MET-h/wk before and after diagnosis), a decrease in activity of more than 3 MET-h/wk, maintenance of activity within 3 MET-h/wk, or an increase in activity of at least 3 MET-h/wk. For all three physical activity analyses, the 0 MET-h/wk category was the reference group.
We evaluated the relationship between physical activity and death using an age-adjusted model and a multivariable model with adjustments for age, race, disease stage, treatment, tamoxifen use at the baseline or 3-year interview, body mass index at the 3-year follow-up interview, fruit/vegetable servings per day at the 3-year follow-up interview, and prediagnosis physical activity for analysis of change in physical activity. Other covariates considered were study site, education, parity, age at first full-term pregnancy, family history of breast cancer, estrogen receptor (ER) status, menopausal status, history of smoking, history of oral contraceptive use, and diet. However, because none of these covariates altered the HR by more than 10% and because some of the covariates were strongly associated with covariates already included in the analyses (eg, race and study site), they were not included in the multivariable-adjusted models.
To minimize the possible influence of recurrent disease on postdiagnosis physical activity, we did a subgroup analysis excluding women who had an adverse event (recurrence, new primary, or death) within the 2 years after completing the postdiagnosis physical activity questionnaire. We also considered whether the association between postdiagnosis physical activity and death varied according to certain demographic and prognostic variables.
We tested for heterogeneity of trends in risk using a 1-df χ2 test. In addition, a Kaplan-Meier survival analysis examining 5-year survival, stratified by physical activity category, was conducted. All analyses were performed using SAS Version 8.2 (SAS Institute, Cary, NC).
RESULTS
Among the 933 women included in this analysis, there were 164 deaths (115 from breast cancer), 56 breast cancer recurrences, and 40 new breast primaries. The distribution of covariates according to category of physical activity in the year before diagnosis is shown in Table 1.
Table 1.
Characteristics of HEAL Breast Cancer Survivors According to Physical Activity Category in the Year Before Diagnosis (N = 933)
| Characteristic | Sports/Recreational Physical Activity (% of patients)
|
||
|---|---|---|---|
| 0 MET-h/wk (n = 397) | > 0-8.9 MET-h/wk (n = 237) | ≥ 9 MET-h/wk (n = 299) | |
| Age, years* | |||
| Mean | 55.6 | 56.1 | 54.9 |
| SD | 11.8 | 11.4 | 11.3 |
| BMI at baseline, kg/m2† | |||
| Mean | 28.0 | 27.0 | 25.1ठ|
| SD | 6.3 | 5.9 | 4.6 |
| BMI at 3 years after diagnosis, kg/m2‖ | |||
| Mean | 29.7 | 28.4 | 26.0ठ|
| SD | 7.6 | 6.7 | 5.5 |
| Age at first birth, years | |||
| Mean | 21.9 | 23.8 | 23.7 |
| SD | 5.2 | 5.2 | 5.0 |
| Postmenopausal* | 59 | 65¶ | 56§ |
| High school graduate | 88 | 97¶ | 97‡ |
| Race | |||
| African American | 57 | 9¶ | 17‡ |
| Non-Hispanic white | 32 | 68¶ | 67‡ |
| Hispanic white | 10 | 19¶ | 13§ |
| Other | 1 | 4 | 3 |
| Disease stage | |||
| Local | 64 | 72 | 72 |
| Regional | 36 | 28 | 28 |
| Treatment | |||
| Surgery only | 28 | 21 | 23 |
| Surgery + radiation | 30 | 36 | 37 |
| Surgery + any chemotherapy | 42 | 43 | 40 |
| ER status | |||
| Positive | 63 | 71 | 68 |
| Negative | 28 | 21 | 19‡ |
| Unknown | 10 | 8 | 13 |
| Tamoxifen use | 41 | 59¶ | 53‡ |
| Current smoker* | 19 | 15 | 7ठ|
NOTE. Physical activity during the year before diagnosis was assessed during baseline interview (approximately 6 months after diagnosis).
Abbreviations: HEAL, Health, Eating, Activity, and Lifestyle Study; MET, metabolic equivalent; SD, standard deviation; BMI, body mass index; ER, estrogen receptor.
Determined at baseline interview (approximately 6 months after diagnosis).
Baseline BMI was only measured in Washington and New Mexico.
≥ 9 MET-h/wk significantly different from 0 MET-h/wk, P < .05.
≥ 9 MET-h/wk significantly different from > 0-8.9 MET-h/wk, P < .05.
Postdiagnosis BMI was measured at all three sites approximately 3 years after diagnosis.
> 0-8.9 MET-h/wk significantly different from 0 MET-h/wk, P < .05.
Table 2 lists the age- and multivariable-adjusted HRs for total deaths and breast cancer deaths by recreational physical activity in the year before diagnosis. Women who engaged in at least 9 MET-h/wk of physical activity had a 31% lower risk of death (HR = 0.69; 95% CI, 0.45 to 1.06; P for trend = .045) compared with women who were inactive in the year before diagnosis. Results were essentially unchanged after excluding women who had an adverse event (ie, recurrence, new primary, or death) within 2 years of diagnosis (n = 49).
Table 2.
Associations Between Breast Cancer Outcomes and Physical Activity in the Year Before Diagnosis
| Outcome | Total Patients (N = 933) | Physical Activity During Year Before Diagnosis
|
|||
|---|---|---|---|---|---|
| 0 MET-h/wk (n = 397) | > 0-8.9 MET-h/wk (n = 237) | ≥ 9 MET-h/wk (n = 299) | P for Trend | ||
| Total No. of deaths | 164 | 88 | 43 | 33 | |
| Age-adjusted HR | 1.00 | 0.84 | 0.49 | .0005 | |
| 95% CI | 0.58 to 1.21 | 0.33 to 0.75 | |||
| Multivariable-adjusted HR* | 1.00 | 1.14 | 0.69 | .045 | |
| 95% CI | 0.75 to 1.74 | 0.45 to 1.06 | |||
| No. of breast cancer deaths | 115 | 62 | 29 | 24 | |
| Age-adjusted HR | 1.00 | 0.80 | 0.50 | .0042 | |
| 95% CI | 0.52 to 1.25 | 0.31 to 0.79 | |||
| Multivariable-adjusted HR* | 1.00 | 1.31 | 0.83 | .27 | |
| 95% CI | 0.80 to 2.21 | 0.49 to 1.38 | |||
NOTE. Physical activity during the year before diagnosis was assessed during baseline interview (approximately 6 months after diagnosis).
Abbreviations: MET, metabolic equivalent; HR, hazard ratio.
Adjusted for age, race, disease stage, initial treatment, and tamoxifen use.
Results for analyses examining the association between physical activity during the second year after diagnosis and adverse outcomes are listed in Table 3. Women who reported participating in any recreational physical activity or at least 9 MET-h/wk 2 years after diagnosis had 64% and 67% lower risk of death than inactive women (HR = 0.36; 95% CI, 0.17 to 0.73; and HR = 0.33; 95% CI, 0.15 to 0.73; P for trend = .0046), respectively. Results were essentially unchanged even after excluding women who experienced a recurrence, new primary, or death within 2 years after completing the postdiagnosis physical activity questionnaire (n = 24). Furthermore, results were similar when we used the five physical activity categories (similar to the Nurses’ Health Study categories) of less than 3, 3 to 8.9, 9 to 14.9, 15 to 23.9, and more than 24 MET-h/wk. Compared with women who engaged in less than 3 MET-h/wk of physical activity, the multivariable adjusted HRs of total deaths were 0.39 (95% CI, 0.16 to 0.95) for 3 to 8.9 MET-h/wk, 0.38 (95% CI, 0.11 to 1.28) for 9 to 14.9 MET-h/wk, 0.78 (95% CI, 0.33 to 1.84) for 15 to 23.9 MET-h/wk, and 0.27 (95% CI, 0.08 to 0.94) for 24 or more MET-h/wk (P for trend = .038).
Table 3.
Associations Between Breast Cancer Outcomes and Physical Activity 2 Years After Diagnosis
| Outcome | Total Patients (n = 688) | Physical Activity 2 Years After Diagnosis
|
|||
|---|---|---|---|---|---|
| 0 MET-h/wk (n = 114) | > 0-8.9 MET-h/wk (n = 297) | ≥ 9 MET-h/wk (n = 277) | P for Trend | ||
| Total No. of deaths | 53 | 22 | 18 | 13 | |
| Age-adjusted HR | 1.00 | 0.41 | 0.30 | .014 | |
| 95% CI | 0.21 to 0.78 | 0.15 to 0.61 | |||
| Multivariable-adjusted HR* | 1.00 | 0.36 | 0.33 | .046 | |
| 95% CI | 0.17 to 0.73 | 0.15 to 0.73 | |||
| No. of breast cancer deaths | 30 | 8 | 13 | 9 | |
| Age-adjusted HR | 1.00 | 0.66 | 0.48 | .21 | |
| 95% CI | 0.27 to 1.64 | 0.18 to 1.26 | |||
| Multivariable-adjusted HR* | 1.00 | 0.72 | 0.65 | .46 | |
| 95% CI | 0.28 to 1.85 | 0.23 to 1.87 | |||
NOTE. Physical activity 2 years after diagnosis was assessed during follow-up interview (approximately 3 years after diagnosis).
Abbreviations: MET, metabolic equivalent; HR, hazard ratio.
Adjusted for age, race, disease stage, initial treatment, tamoxifen use, body mass index, and fruit/vegetable servings per day.
Table 4 lists the HRs of adverse outcomes according to change in physical activity from before to after breast cancer diagnosis. Women who decreased their physical activity were at an approximate four-fold increased risk of experiencing a death than inactive women (HR = 3.95; 95% CI, 1.45 to 10.50). Results were similar after excluding women who had an adverse event (ie, recurrence, new primary, or death) within the 2 years after completing the postdiagnosis physical activity questionnaire (n = 24). African American race and prediagnosis physical activity were statistically significant covariates.
Table 4.
Associations Between Breast Cancer Outcomes and Change in Physical Activity From Before to After Diagnosis
| Outcome | Total Patients (n = 688) | Change in Physical Activity
|
|||
|---|---|---|---|---|---|
| Inactive (n = 175) | Decrease (n = 153) | Maintain (n = 163) | Increase (n = 195) | ||
| Total No. of deaths | 53 | 16 | 19 | 11 | 7 |
| Age-adjusted HR | 1.00 | 1.37 | 0.86 | 0.41 | |
| 95% CI | 0.71 to 2.67 | 0.40 to 1.85 | 0.17 to 1.00 | ||
| Multivariable-adjusted HR* | 1.00 | 3.95 | 1.55 | 0.55 | |
| 95% CI | 1.45 to 10.50 | 0.64 to 3.80 | 0.22 to 1.38 | ||
| No. of breast cancer deaths | 30 | 10 | 7 | 7 | 6 |
| Age-adjusted HR | 1.00 | 0.82 | 0.80 | 0.53 | |
| 95% CI | 0.31 to 2.15 | 0.31 to 2.12 | 0.19 to 1.47 | ||
| Multivariable-adjusted HR* | 1.00 | 3.69 | 2.47 | 0.82 | |
| 95% CI | 0.88 to 15.92 | 0.78 to 7.78 | 0.29 to 2.34 | ||
NOTE. Change in physical activity from the year before diagnosis to 2 years after diagnosis: inactive = 0 MET-h/wk before and after diagnosis; decrease = < −3 MET-h/wk (mean ± standard deviation [SD] = −18.1 ± 16.6 MET-h/wk); maintain = ± 3 MET-h/wk (mean ± SD = 0.0 ± 2.4 MET-h/wk); and increase > 3 MET-h/wk (mean ± SD = 18.3 ± 16.7 MET-h/wk).
Abbreviations: HR, hazard ratio; MET, metabolic equivalent.
Adjusted for age, race, disease stage, initial treatment, tamoxifen use, and prediagnosis physical activity (MET-h/wk).
The protective benefit of physical activity seemed to be stronger in certain subgroups, including women diagnosed with a higher stage of disease and women with tumors that were ER positive (Table 5). However, we did not have the power to demonstrate statistically significant differences in these stratified analyses because of the small number of events in some of the subgroups. The unadjusted 5-year survival rates for women who engaged in 9 or more MET-h/wk, more than 0 but less than 9 MET-h/wk, and 0 MET-h/wk after diagnosis were 94%, 89%, and 71%.
Table 5.
Associations Between Total Deaths and Physical Activity 2 Years After Diagnosis Stratified by Demographic and Prognostic Variables
| Variable | 2 Years After Diagnosis Physical Activity
|
P for Interaction | |
|---|---|---|---|
| 0 MET-h/wk | > 0 MET-h/wk | ||
| Age at diagnosis < 55 years | |||
| Total No. of women | 32 | 294 | |
| Total No. of deaths | 5 | 10 | |
| Multivariable HR* | 1.00 | 0.18 | |
| 95% CI | 0.05 to 0.60 | ||
| Age at diagnosis ≥ 55 years | |||
| Total No. of women | 82 | 280 | |
| Total No. of deaths | 17 | 21 | |
| Multivariable HR | 1.00 | 0.29 | .93 |
| 95% CI | 0.14 to 0.60 | ||
| African American women | |||
| Total No. of women | 42 | 149 | |
| Total No. of deaths | 6 | 12 | |
| Multivariable HR | 1.00 | 0.47 | |
| 95% CI | 0.16 to 1.35 | ||
| Hispanic white women | |||
| Total No. of women | 8 | 74 | |
| Total No. of deaths | 2 | 6 | |
| Multivariable HR | 1.00 | 0.67 | |
| 95% CI | 0.05 to 9.62 | ||
| Non-Hispanic white women | |||
| Total No. of women | 61 | 336 | |
| Total No. of deaths | 13 | 12 | |
| Multivariable HR | 1.00 | 0.26 | .078 |
| 95% CI | 0.10 to 0.65 | ||
| BMI < 25 kg/m2 | |||
| Total No. of women | 37 | 263 | |
| Total No. of deaths | 9 | 19 | |
| Multivariable HR | 1.00 | 0.47 | |
| 95% CI | 0.19 to 1.14 | ||
| BMI ≥ 25 kg/m2 | |||
| Total No. of women | 77 | 311 | |
| Total No. of deaths | 13 | 12 | |
| Multivariable HR | 1.00 | 0.31 | .40 |
| 95% CI | 0.13 to 0.74 | ||
| Stage I disease | |||
| Total No. of women | 85 | 407 | |
| Total No. of deaths | 13 | 19 | |
| Multivariable HR | 1.00 | 0.53 | |
| 95% CI | 0.23 to 1.20 | ||
| Stage II-IIIA disease | |||
| Total No. of women | 29 | 167 | |
| Total No. of deaths | 9 | 12 | |
| Multivariable HR | 1.00 | 0.17 | .17 |
| 95% CI | 0.06 to 0.48 | ||
| Surgery only | |||
| Total No. of women | 35 | 131 | |
| Total No. of deaths | 9 | 8 | |
| Multivariable HR | 1.00 | 0.28 | |
| 95% CI | 0.07 to 1.06 | ||
| Radiation | |||
| Total No. of women | 41 | 206 | |
| Total No. of deaths | 7 | 8 | |
| Multivariable HR | 1.00 | 0.61 | |
| 95% CI | 0.15 to 2.40 | ||
| Any chemotherapy | |||
| Total No. of women | 38 | 237 | |
| Total No. of deaths | 6 | 15 | |
| Multivariable HR | 1.00 | 0.44 | .22 |
| 95% CI | 0.15 to 1.30 | ||
| ER negative | |||
| Total No. of women | 15 | 120 | |
| Total No. of deaths | 1 | 10 | |
| Multivariable HR | 1.00 | 1.26 | |
| 95% CI | 0.15 to 11.00 | ||
| ER positive | |||
| Total No. of women | 86 | 393 | |
| Total No. of deaths | 18 | 16 | |
| Multivariable HR | 1.00 | 0.20 | .27 |
| 95% CI | 0.09 to 0.46 | ||
NOTE. Physical activity 2 years after diagnosis was assessed during follow-up interview (approximately 3 years after diagnosis).
Abbreviations: MET, metabolic equivalent; HR, hazard ratio; BMI, body mass index; ER, estrogen receptor.
Adjusted for age, race, disease stage, initial treatment, tamoxifen use, BMI, and fruit/vegetable servings per day.
Fast walking was the most common postdiagnosis recreational activity performed by HEAL participants. Bicycling and yoga were the second and third most common recreational activities reported, respectively.
DISCUSSION
We found that women who participated in any moderate-intensity recreational physical activity, such as brisk walking, after diagnosis had an approximately 64% lower risk of death than inactive women. Furthermore, exercising at recommended amounts of 2.5 h/wk of moderate-intensity physical activity compared with no exercise was associated with a slightly higher risk reduction of 67%.17 Although our risk reductions were observed for total deaths, the majority of deaths were from breast cancer.
Our results of an inverse association between postdiagnosis physical activity and total deaths are similar to the three other reports examining this relationship2-4 and two reports examining postdiagnosis physical activity and colon cancer deaths5,6; however, the amount of exercise necessary for reducing the risk of death differed for our study compared with the other reports. We observed an association with any recreational physical activity, whereas the other studies observed associations with approximately 2 to 3 hours per week of physical activity. The HEAL Study included a more detailed assessment of physical activity than the other studies, asking about 20 different types of activities and their average duration per session, thus allowing us to more accurately categorize women as inactive, somewhat active, or active at recommended levels. Furthermore, our final results focused on recreational physical activity (eg, brisk walking for exercise) rather than any type of physical activity (eg, gardening). Additional differences between the HEAL Study and the other breast cancer studies include sample size and date of diagnosis; our cohort of women with breast cancer (N = 933) was smaller than the cohort of women enrolled onto the other breast cancer studies (N = 1,490 to 4,482), and HEAL participants were diagnosed in a more recent time period (1996 to 1999) than some of the other studies (1984 to 1998).
Because study recruitment occurred between 1995 and 1999, the majority of women with hormone receptor–positive breast cancer were taking tamoxifen, and during the study follow-up period, less than 1% of the sample reported switching from tamoxifen to aromatase inhibitors. Among women with known ER status, an inverse association between participating in physical activity and death was observed among women whose tumors were ER positive but not among women whose tumors were ER negative. Similar findings were observed for breast cancer death. The Nurses’ Health Study also observed stronger influences of physical activity on breast cancer death among women whose tumors were ER positive (relative risk = 0.50; 95% CI, 0.34 to 0.74).2 Associations between physical activity and breast cancer outcomes were hypothesized to be stronger in women with ER-positive disease because of the observed beneficial effect of exercise on estrogen levels.18 Thus, the association between physical activity and reduced risk for death may be explained by effects on sex hormones. However, physical activity may affect death via additional mechanisms including reduced insulin levels or reduced inflammation markers.8
A strength of the HEAL study is that we assessed whether change in physical activity from before to after diagnosis was associated with adverse outcomes relative to women who remained inactive before and after diagnosis. We found an increased risk of death with decreasing physical activity after adjusting for important covariates. We have previously reported that HEAL women who decreased their physical activity levels from before to after diagnosis gained more weight than women who increased their physical activity levels.10 Thus, weight gain may partly explain the increased HR observed among women who decreased their physical activity levels.19,20 These findings emphasize the importance of participating in physical activity after a diagnosis of breast cancer to gain the maximum benefits of physical activity on survival. Although one study has examined change in physical activity on colon cancer prognosis,5 no other study has examined change in physical activity on breast cancer prognosis, and few studies have examined physical activity before diagnosis and prognosis in cancer survivors.21,22 Nonsignificant associations were observed for physical activity in the year before diagnosis and survival by Abrahamson et al21 and Enger and Bernstein.22
Other strengths of the HEAL study include the quality of the physical activity data. Although we report recreational physical activity, similar associations were observed with any type of moderate-intensity physical activity. Other strengths include measured weight at the 3-year visit, a multiethnic cohort of breast cancer survivors, and results adjusted for various prognostic and lifestyle factors including dietary patterns. Compared with the Women's Healthy Eating and Living observational analysis of greater survival after breast cancer among physically active women with high vegetable/fruit intake, the protective benefit of participating in physical activity among HEAL women was similar among women eating less than five versus five or more vegetable/fruit servings per day relative to women reporting less than five vegetable/fruit servings per day and no physical activity.
In the HEAL study, physical activity was statistically significantly associated with total deaths but not breast cancer deaths. Given that the majority of deaths were from breast cancer, our lack of statistical significance is most likely a result of the fewer number of deaths, and therefore, there was insufficient power to examine the association between physical activity and breast cancer deaths. Other limitations include lack of uniform eligibility criteria, the inability to separate race and study site, and self-report of physical activity.
In conclusion, our results suggest that participating in any moderate-intensity recreational physical activity, such as brisk walking, after a diagnosis of breast cancer is associated with a lower risk of death. Encouraging women to maintain or increase their physical activity after a diagnosis of breast cancer may be beneficial to their overall health. Future studies examining the association between physical activity and breast cancer outcomes are needed, as are randomized controlled trials examining the potential effect of physical activity on prognosis in women.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Melinda L. Irwin, Anne McTiernan, Frank D. Gilliland, Richard N. Baumgartner, Leslie Bernstein
Financial support: Anne McTiernan, Frank D. Gilliland, Richard N. Baumgartner, Leslie Bernstein
Administrative support: Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Provision of study materials or patients: Melinda L. Irwin, Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Collection and assembly of data: Melinda L. Irwin, Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Data analysis and interpretation: Melinda L. Irwin, Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Kathy Cronin, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Manuscript writing: Melinda L. Irwin, Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Kathy Cronin, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Final approval of manuscript: Melinda L. Irwin, Ashley Wilder Smith, Anne McTiernan, Rachel Ballard-Barbash, Kathy Cronin, Frank D. Gilliland, Richard N. Baumgartner, Kathy B. Baumgartner, Leslie Bernstein
Acknowledgments
We thank Kristin LaCroix, Shelley Tworoger, and Lynda McVarish for their contributions to the Health, Eating, Activity, and Lifestyle (HEAL) study, as well as the HEAL participants for their ongoing dedication to this study.
Supported by National Cancer Institute Grants No. N01-CN-75036-20, NO1-CN-05228, and NO1-PC-67010 and Training Grant No. T32 CA09661. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by the National Institutes of Health Grant No. M01-RR-00037 and the University of New Mexico Grant No. NCRR M01-RR-0997. Data collection for the Women's Contraceptive and Reproductive Experiences Study at the University of Southern California was supported by Contract No. N01-HD-3-3175 from the National Institute of Child Health and Human Development, and patient identification was supported in part by Contract No. 050Q-8709-S1528 from the California Department of Health Services.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Thune I, Ferberg A: Physical activity and cancer risk: Dose-response and cancer, all sites and site specific. Med Sci Sports Exerc 33:S530-S550, 2001. (suppl) [DOI] [PubMed] [Google Scholar]
- 2.Holmes MD, Chen WY, Feskanich D, et al: Physical activity and survival after breast cancer diagnosis. JAMA 293:2479-2486, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Holick CN, Newcomb PA, Trentham-Dietz A, et al: Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 17:379-386, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Pierce JP, Stefanick ML, Flatt SW, et al: Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 25:2345-2351, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Giovannucci EL, Holmes MD, et al: Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24:3527-3534, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24:3535-3541, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Aiello E, McTiernan A: Weight loss in breast cancer patient management. J Clin Oncol 20:1128-1143, 2002 [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A, Ulrich C, Slate S, et al: Physical activity and cancer etiology: Associations and mechanisms. Cancer Causes Control 9:487-509, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Irwin ML, McTiernan A, Bernstein L, et al: Physical activity levels among breast cancer survivors. Med Sci Sports Exerc 36:1484-1491, 2004 [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin ML, McTiernan A, Baumgartner R, et al: Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic and lifestyle factors. J Clin Oncol 23:774-782, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin ML, McTiernan A, Bernstein L, et al: Relationship of obesity and physical activity with c-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 14:2881-2888, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriska A: Modifiable Activity Questionnaire. Med Sci Sports Exer 29:S73–S78, 1997. (suppl) [Google Scholar]
- 13.Bernstein L, Patel AV, Ursin G, et al: Lifetime recreational exercise activity and breast cancer risk among black and white women. J Natl Cancer Inst 97:1671-1679, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, et al: Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498–S516, 2000. (suppl 9) [DOI] [PubMed] [Google Scholar]
- 15.Percy C, Stanek E, Gloeckler L: Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health 71:242-250, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson R, Kristal A, Tinker L, et al: Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 9:178-187, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Pate R, Pratt M, Blair S, et al: Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 273:402-407, 1995 [DOI] [PubMed] [Google Scholar]
- 18.McTiernan A, Tworoger S, Ulrich C, et al: Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res 64:2923-2928, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kroenke CH, Chen WY, Rosner B, et al: Weight, weight gain and survival after breast cancer diagnosis. J Clin Oncol 23:1370-1378, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Caan BJ, Emond JA, Natarajan L, et al: Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat 99:47-57, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Abrahamson P, Gammon M, Lund M, et al: Recreational physical activity and survival among young women with breast cancer. Cancer 107:1777-1785, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Enger SM, Bernstein L: Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer 90:2138-2141, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]