Abstract
Understanding the evolutionary origins of hemispheric specialization remains a topic of considerable interest in a variety of scientific disciplines. Whether nonhuman primates exhibit population-level limb preferences continues to be a controversial topic. In this study, limb preferences for ascending and descending locomotion were assessed as a means of examining the hypothesis that asymmetries in forelimb bones might be attributed to asymmetries in posture. The results indicated that captive chimpanzees showed a population-level leftward asymmetry in descending locomotion but no group bias for ascending locomotion. The results are consistent with previous behavioral studies in captive chimpanzees as well as studies on skeletal asymmetries of the forelimbs of chimpanzees.
Keywords: laterality, posture, locomotion, chimpanzees
One defining trait of the human species is population-level right handedness. Although there is some cultural variation, a significant majority of individuals in every human population studied to date prefers to use their right hand for various motor actions (Porac and Coren, 1981; Annett, 2002; Raymond and Pontier, 2004). Moreover, archeological data and analysis of stone artifacts and cave drawings suggest that population-level handedness can be dated back at least 2 mya (Bradshaw and Rogers, 1993). Whether nonhuman primates, particularly our closest-living relatives the great apes, show population-level handedness remains a topic of intense scientific debate (McGrew and Marchant, 1993; McGrew and Marchant, 1997; Hopkins, 1999; Hopkins, 2006). Data on hand preference and other forms of behavioral asymmetry in great apes are important for understanding the evolution of hemispheric specialization, particularly as it relates to handedness and language, as many have historically proposed that these two traits evolved together and are perhaps unique to hominid evolution (Warren, 1980; Corballis, 1992; Crow, 2004).
Recent studies in captive chimpanzees have shown evidence of population-level handedness for several behavioral measures such coordinated bimanual actions, bi-manual feeding, manual gestures, and throwing (Hopkins, 2007). In contrast, data from wild chimpanzees have not revealed consistent results. Studies of spontaneous hand use during daily activities have not revealed any evidence of individual or population-level handedness (Marchant and McGrew, 1996; McGrew and Marchant, 2001). When considering hand preferences for tool use, population-level left hand biases have been reported for termite fishing while population-level right hand biases have been found for leaf sponging (Boesch, 1991; Biro et al., 2003, 2006; Hopkins and Cantalupo, 2005; Lonsdorf and Hopkins, 2005). Borderline significant right hand preferences have also been reported for nutcracking (Boesch, 1991; Biro et al., 2003). The alleged discrepancy in findings on handedness in captive and wild chimpanzees have been attributed to rearing artifacts or, alternatively, to issues of tasks measurement and quantification of hand use. Whatever the case, additional data on lateralization of function for different tasks and behaviors are needed in order to provide a better framework for reconciling differences between wild and captive settings.
In addition, there is clearly additional behavioral research needed to help explain the presence of morphological and neuroanatomical asymmetries seen in nonhuman primates, and particularly, chimpanzees. For example, population-level leftward asymmetries have been found for several neuroanatomical regions and sulci including the planum tempoale, inferior frontal gyrus, and sylvian fissure (Yeni-Komshian and Benson, 1976; Gannon et al., 1998; Hopkins et al., 2000; Cantalupo and Hopkins, 2001; Cantalupo et al., 2003). Recent studies have also shown that hand use for coordinated bimanual actions and gestures correlate with asymmetries in different cortical areas (Hopkins and Cantalupo, 2004; Taglialatela et al., 2006). Less clear from the existing literature are the functional or behavioral correlates of asymmetries in morphological features, notably the forelimbs.
Recently, Sarringhaus et al. (2005) reported that chimpanzees show a population-level leftward asymmetry in the total subperiosteal area of the diaphyses in the humeri. There was also a trend toward population-level rightward asymmetries in the second metacarpal. Sarringhaus et al. (2005) suggested that the asymmetries in the forelimbs might be linked to lateralized limb use but no specific behaviors were identified. Sarringhaus et al. (2005) did suggest that the behaviors that would influence bone asymmetries would likely be postural or locomotor; in essence, behaviors that would induce a strong gravitational force on the bones of the preferred limbs.
In this study, asymmetries in limb use during ascending and descending locomotion were studied in a sample of captive chimpanzees. Recently, Moricello et al. (2006) reported a trend toward left-side biases in postural and locomotor behaviors of 10 captive chimpanzees. Specifically, Moricello et al. (2006) reported a trend for chimpanzees to lead with the left arm when descending in locomotion and slight right side bias in leading limb when locomoting in an ascending direction. Moricello et al. (2006) also reported a borderline significant population-level left side bias for the posture of unilateral hanging. Although asymmetries in leading limb in great apes, including chimpanzees, have been reported by others (Heestand, 1986; Cunningham et al., 1989; Marchant and McGrew, 1996; Hopkins et al., 1997; McGrew and Marchant, 2001; Peters, 2005; Harrison and Nystrom, 2008), the observation of increased left hand use when locomoting in a descending fashion has only been examined and reported in one study, with a relatively small sample size. However, such a pattern of behavioral asymmetry might explain the lateralization in limb bones reported by Sarringhaus et al. (2005), because the force of the entire body weight would fall on the preferred limb when descending in locomotion. The goal of this study was to examine forelimb leading limb asymmetries during ascending and descending locomotion in a larger sample of chimpanzees to assess whether asymmetries are present at the population-level. Furthermore, whether the degree of asymmetry was comparable to those reported in limb bones was examined and it was hypothesized that they should be comparable if forelimb asymmetries in locomotion potentially explain the morphological data reported by Sarringhaus et al. (2005).
METHOD
Subjects
The subjects were 40 captive chimpanzees including 11 males and 29 females housed at the Yerkes National Primate Research Center of Emory University. The chimpanzees ranged in age from 11 to 49 years of age (Mean = 25.48 years, s.d. 11.41).
Procedure
Limb use was recorded when the chimpanzees were observed to locomote in an ascending or descending manner, and these observations were restricted to two specific contexts. Specifically, in many of the home outdoor enclosures of the chimpanzees, bed boards are symmetrically located on the walls ~1 m above the floor. Limb use in locomotion was recorded when the chimpanzees would locomote down from the bed board onto the floor (see Fig. 1). The chimpanzees would lean forward and place an arm/hand on the floor and subsequently lead with the opposite hand. The hand/arm placed on the floor, which supported their entire body weight, was recorded as left or right during each locomotor bout. For ascending locomotion, data collection was slightly more systematic. A single piece of food was placed on a small horizontal metal ledge positioned ~3 m above the floor. The chimpanzees approached the vertical front of the cage and climbed up to retrieve the food positioned above them. The hand (and foot) used to initially support their ascending upward climb was recorded as left or right. The outdoor enclosures are 20 × 20 m in diameter and the apes had to be centrally situated in the back of the home cage at the time the food was baited in order to be considered a test trial. This was done to assure that they would have to locomote some distance before initiating their climbing behavior.
Fig. 1.

Photograph of a chimpanzees leading with the left arm during descending locomotion. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Observations were made over a 12-month period. Observations were made between 11:00 and 6:00 p.m., and all occurrences of leading limb biases were recorded within a given observation period. To assess the consistency in limb use across time, the data was split into two halves and a split-half test-retest correlation was performed to assess consistency in limb use across the entire period of time during which the data were collected. Limb use during the first half of data collection was positively and significantly correlated with data collected during the second half of data collection (r = 0.88, df = 37, P < 0.01). Subjects were only considered in the analysis if a minimum of six observations were made.
Data analysis
Handedness for each subject and measure was classified two ways. First, a handedness index (HI) was calculated based on the total number of left and right hand responses. The number of left hand responses was subtracted from the number of right hand responses and divided by the total number of responses (R − L/R + L). HI values ranged from −1.0 to 1.0 with negative values indicating left hand preferences and positive values indicating right hand preferences. The absolute value of the HI score (ABS-HI) indicates the strength of bias. Second, for each subject, binomial z-scores were derived based on the frequencies of hand use and these values were used to classify subjects as left-handed, ambiguously-handed or right-handed. Subjects with z-scores that were either below −1.95 or above 1.95 were classified as left- or right-handed, respectively. Subjects with z-scores between −1.96 and 1.96 were classified as ambiguously-handed.
RESULTS
The individual limb preference data for each subject and behavior are shown in Table 1. A one-sample t-test on the ascending and descending HI values revealed a significant population-level left hand bias for descending t(39) = −3.04, P < 0.009 but not ascending locomotion t(39) = −0.869, P = 0.51 (see Table 1). A mixed-model analysis of variance with HI values serving as repeated measures and sex as the between group factor, failed to reveal significant main effects or interactions. Analysis of the categorical laterality data largely confirmed the results using the HI values. A χ2 goodness-of-fit test indicated that the distribution of limb preferences for descending locomotion differed significantly from a random distribution χ2(2, N = 40) = 10.88, P < 0.003. There were 23 left-handed, 9 right-handed, and 8 apes that did not show a lateral bias. In contrast, the distribution of lateral bias for ascending locomotion did not differ significantly from a random distribution χ2(2, N = 40) = 0.99, P = 0.607. For ascending locomotion, there were 16 left-handed, 13 right-handed, and 11 chimpanzees that did not show a lateral bias. χ2 tests of independence failed to reveal significant associations between sex and lateral bias for ascending χ2(2, N = 40) = 2.45, P = 0.290 and descending χ2(2, N = 40) = 0.152, P = 0.937 locomotion.
TABLE 1.
Individual data limb preference data for ascending and descending locomotion
| Descending |
Ascending |
|||||
|---|---|---|---|---|---|---|
| #L | #R | HI | #L | #R | HI | |
| Females | ||||||
| Abby | 4 | 18 | 0.63 | 12 | 13 | 0.04 |
| Agatha | 37 | 12 | −0.51 | 18 | 32 | 0.28 |
| Amanda | 18 | 8 | −0.57 | 22 | 24 | 0.04 |
| Barbara | 16 | 0 | −1.00 | 0 | 17 | 1.00 |
| Beleka | 9 | 0 | −1.00 | 19 | 2 | −0.81 |
| Bo | 1 | 37 | 0.95 | 46 | 6 | −0.77 |
| Brandy | 13 | 4 | −0.53 | 25 | 5 | −0.67 |
| Christa | 12 | 23 | 0.31 | 31 | 2 | −0.88 |
| Cissie | 18 | 10 | −0.29 | 21 | 10 | −0.35 |
| Dara | 53 | 4 | −0.86 | 23 | 21 | −0.05 |
| Edwina | 19 | 11 | −0.27 | 17 | 26 | 0.21 |
| Elvira | 20 | 5 | −0.60 | 45 | 24 | −0.30 |
| Fiona | 9 | 25 | 0.47 | 19 | 34 | 0.28 |
| Frannie | 12 | 0 | −1.00 | 16 | 39 | 0.42 |
| Jacqueline | 43 | 1 | −0.95 | 13 | 34 | 0.45 |
| Jenda | 6 | 0 | −1.00 | 2 | 15 | 0.76 |
| Jewelle | 24 | 25 | 0.02 | 29 | 11 | −0.45 |
| Kengee | 18 | 13 | −0.16 | 22 | 39 | 0.28 |
| Lena | 33 | 8 | −0.61 | 11 | 42 | 0.58 |
| Leslie | 46 | 2 | −0.92 | 27 | 33 | 0.10 |
| Lil’One | 22 | 1 | −0.91 | 42 | 7 | −0.71 |
| Martha | 36 | 0 | −1.00 | 27 | 2 | −0.86 |
| Maxine | 0 | 19 | 1.00 | 35 | 8 | −0.63 |
| Melinda | 3 | 33 | 0.83 | 16 | 19 | 0.09 |
| Melissa | 2 | 6 | 0.33 | 24 | 37 | 0.21 |
| Rowena | 38 | 4 | −0.81 | 24 | 20 | −0.09 |
| Samantha | 13 | 9 | −0.18 | 27 | 27 | 0.00 |
| Shirley | 13 | 4 | −0.53 | 10 | 31 | 0.51 |
| Suwanee | 1 | 17 | 0.89 | 33 | 6 | −0.69 |
| Mean | −0.28 | −0.07 | ||||
| Males | ||||||
| Arthur | 20 | 0 | −1.00 | 45 | 6 | −0.76 |
| Drew | 20 | 5 | −0.60 | 6 | 5 | 0.01 |
| Elwood | 33 | 4 | −0.78 | 28 | 7 | −0.60 |
| Fritz | 30 | 2 | −0.88 | 25 | 26 | 0.02 |
| Jolson | 1 | 29 | 0.93 | 39 | 18 | −0.38 |
| Justin | 30 | 6 | −0.67 | 6 | 43 | 0.76 |
| Lux | 0 | 34 | 1.00 | 1 | 28 | 0.93 |
| Patrick | 5 | 1 | −0.67 | 23 | 8 | −0.48 |
| Rogger | 39 | 0 | −1.00 | 41 | 3 | −0.86 |
| Scott | 7 | 0 | −1.00 | 13 | 7 | −0.30 |
| Travis | 25 | 4 | −0.67 | 2 | 14 | 0.75 |
| Mean | −0.484 | −0.09 | ||||
#L, number of left limn responses; #R, number of right limb responses; HI, handedness index (see text for description).
Age proved to be a significant variable in relation to strength but not direction of asymmetry. Significant positive associations were found between age and strength of asymmetry for ascending (r = 0.450, df = 38, P < 0.001) and descending (r = 0.511, df = 38, P < 0.001) locomotion. Thus, older subjects were more lateralized than young apes. Because age correlated with strength of handedness, we subsequently compared males and females on the ABS-HI values for the ascending and descending locomotion using a mixed-model analysis of covariance. Age served as the covariate while sex was the between group factor. The ABS-HI values for ascending and descending locomotion were the repeated measures. A significant main effect for sex was found F(1, 37) = 11.36, P < 0.002 (see Fig. 2). For both ascending and descending locomotion, males were significantly more lateralized than females. The main effect for lateral bias measure approached conventional levels of statistical significance F(1, 37) = 3.18, P < 0.09 with ABS-HI values for descending higher than ascending locomotion (see Fig. 2). Lastly, Pearson product-moment correlations among the different measures indicated that the HI values for ascending and descending did not significantly correlate with each other (r = −0.114, df = 38, P = 0.495) but the ABS-HI scores did significantly correlate (r = 0.360, df = 38, P < 0.03). Apes that were more lateralized for descending locomotion were similarly more lateralized for ascending locomotion but not necessarily in the same direction.
Fig. 2.
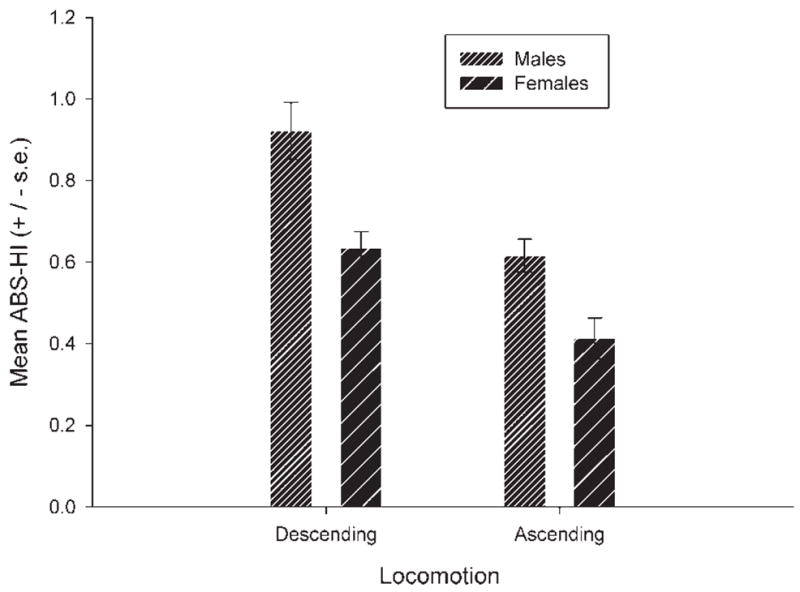
Adjusted mean ABS-HI (±s.e.) for ascending and descending locomotion in male and female chimpanzees.
DISCUSSION
The results of this study are relatively straightforward. Chimpanzees showed a population-level leftward leading limb asymmetry in descending locomotion and no bias for ascending locomotion. In addition, older subjects were more lateralized in locomotor asymmetries than younger apes and males were more lateralized than females, after statistically controlling for age.
The results are largely consistent with the previous behavioral data reported by Moricello et al. (2006) on asymmetries in descending locomotion, albeit in a larger sample in which a greater number of observations were obtained on each subject. Indeed, the mean percentage right hand use value for descending locomotion reported here (Mean = 37%) is close to that reported by Moricello et al. (2006) (Mean = 39%) and do not differ statistically when the two sets of data are compared directly t(38) = 0.46, n.s. Thus, the results appear to be consistent between these two samples of captive chimpanzees.
At face value, the results differ from previous reports of leading limb asymmetries in captive and wild chimpanzees. Heestand (1986) and Harrison and Nystrom (2008) have reported rightward asymmetries in leading limb in great apes, including chimpanzees. In wild chimpanzees, Marchant and McGrew (1996) found no evidence of population-level leading limb asymmetries. None of the authors in these studies, however, distinguished between ascending and descending locomotion, and therefore the results might differ because of this distinction.
The apparent differences among reports of leading limb asymmetries in great apes may also reside in how “leading limb” is interpreted, here and in other studies. In the studies by Heestand (1986) and Harrison and Nystrom (2008) in apes, leading limb in locomotion was defined as the limb that led out in locomotion from a quadrupedal posture. Note that in descending locomotion, the left arm is planted so as to support the animal’s posture while the opposite arm, in essence, “leads out” in locomotion. From the previous reports of leading limb asymmetries, the authors reported which limb “led” which could be construed as either a) which hand was placed to support the posture or b) which arm was used to physically lead. Unfortunately, the operational definitions for leading limb provided in these manuscripts are not entirely clear but this is an important issue that needs to be resolved. Perhaps, measuring the weight-bearing limb might be the most parsimonious means of defining locomotor asymmetries. Heestand (1986) also reported limb preferences for climbing, which was defined as the arm used when beginning to ascend or descend from a vertical substrate. Although far fewer apes showed asymmetries in locomotion for climbing comparing to leading limb, there were significantly more right- compared to left-handed gorillas and chimpanzees for this behavior. Again, it is unclear from the paper by Heestand (1986) what exactly constituted the leading limb in these measures and unfortunately, there was no distinction made between ascending and descending locomotion. Notwithstanding these limitations, the results of Heestand (1986) and Harrison and Nystrom (2008) do reinforce the view that asymmetries can be seen in postural and locomotor behaviors of apes. This conclusion is further supported by the recent studies reporting population-level rightward asymmetries in hand-over-hand locomotion in wild orangutans (Stafford et al., 1990; Redmond and Lamperez, 2004; Peters and Rogers, 2007), suggesting that the type of locomotion might also influence lateralization in leading limb, as has been suggested for other vertebrates (Malashichev, 2006).
The results reported here are also consistent with the prediction of the findings on skeletal asymmetries reported by Sarringhaus et al. (2005). That is, when descending in their locomotion, captive chimpanzees led with their left arm, thereby placing the majority of their body weight on the bones and muscles of this limb. This differential use of the left side of the body in the placement of body weight during descending locomotion would presumably explain the greater leftward asymmetries in the subperiosteal area of the diaphyses in the humeri. More direct evidence of the relation between locomotor asymmetries and bone asymmetries are needed to infer a causal ink between the two variables but, based on the collective results, the prediction would be for a strong relationship to be evident in chimpanzees (and perhaps other monkeys and apes).
The results on limb asymmetries reported by Sarringhaus et al. (2005) differ from reports of limb asymmetries in humans (see Auerbach and Ruff, 2006 for review) and may reflect inherent differences in limbs during weight-bearing locomotion. Humans show rightward asymmetries in the forelimbs while they show smaller but significant leftward biases for the lower limb bones (Auerbach and Ruff, 2006). The rightward forelimb asymmetries are consistent with the evidence that most humans are right-handed but the hind limb data are more difficult to interpret because most humans also report themselves to be right-footed (Porac and Coren, 1981). Auerbach and Ruff (2006) suggest that when measuring footedness, investigators are not typically quantifying the weight-bearing limb but the active foot. Thus, for example, footedness is measured by recording which foot is used to kick the ball, not which foot is planted on the ground when kicking a ball, which would be the weight-bearing limb. This definitional issue in the human literature is not unlike the question of how leading limb is defined in the measurement of hand use in nonhuman primates, as discussed earlier. With respect to both apes and humans, the argument here is that bone asymmetries are going to be most strongly linked with behavioral measures that reflect inherent differences in weight-bearing actions that have continuous use. Moreover, whether the asymmetries are present in the forelimbs or hind limbs will, to some extent, be contingent upon the primary mode of locomotion of a specific species in relation to developmental processes (Hallgrimsson, 1999).
Age positively correlated with strength of laterality in locomotion. These results are also consistent with the view that habitual use of the preferred limbs becomes more canalized with age and this, in turn, might lead or contribute to the expression of asymmetries in the bones. Similarly, males were more lateralized than females. The male chimpanzees are heavier than the females and the increased weight in males might induce greater locomotor asymmetries and potentially differences in asymmetries in the bones, as has been suggested for results found in human bones (Auerbach and Ruff, 2006). Sarringhaus et al. (2005) did not report the sexes of the specimens in their sample (presumably they were not known) but, based on the results reported here, sex differences in lateralization in bilateral forearm bones would be predicted in chimpanzees. It is important to note that age is confounded with body weight; thus, whether it is age per se or body weight that explains the differences in lateralization is not clear. The fact that males differed from females, after adjusting for age, supports the view that body weight is the key factor but more direct evidence is necessary to resolve this issue.
Previously, Falk et al. (1988) reported population-level biases for a number of bones in the rhesus monkey, including a right side bias for humeri, a finding that differs from those reported by Sarriunghaus et al. (2005). If it is assumed that the behavioral results reported here also apply to asymmetries in humeri reported by Sarringhaus et al. (2005), then it can be hypothesized that rhesus monkeys might show a rightward bias in descending locomotion, opposite that observed in chimpanzees. At present, there are no data on leading limb in locomotion in Old or New World monkeys that have distinguished between ascending and descending movements.
Lastly, based on the results reported here, it can be asked why chimpanzees show a leftward asymmetry in descending locomotion. In other words, why is postural support on the left side? One interpretation might be that postural support with the left arm may allow the apes to lead with the right arm in locomotion or, to perhaps keep the right hand free for manipulation or other manual actions. This interpretation is largely consistent with the postural origins (PO) theory of language originally proposed by MacNeilage et al. (1987), although some have questioned the empirical support for this theory (Papademetriou et al., 2005). The PO theory postulates that in more distantly related primates, such as prosimians, there was a right side bias in postural control due to a left hand bias in ballistic motor movements associated with insectivorous predation. In primates more closely related to humans, particularly those inhabiting terrestrial environments, the postural asymmetries would have shifted to the left side of the body due to increasing selection for fine motor manipulation by the right hand.
In summary, captive chimpanzees showed a population-level leftward bias in leading limb for descending but not ascending locomotion. The asymmetries were more pronounced in older apes and in males compared to females. The extent to which these observations might generalize to wild chimpanzees is unclear but seems like a fruitful avenue for future investigations as a means of bridging methods and results between these two contexts. Similarly, additional studies on postural and locomotor asymmetries would provide a larger framework for evaluating positional asymmetries in primates and how they might relate to different biomechanical and ecological factors. Lastly, the extent to which individual differences in positional and locomotor asymmetries correlate with morphological and neuroanatomical asymmetries remains to be evaluated but would provide important results relevant to interpreting structural–functional relationships in primates.
Acknowledgments
The author appreciates the assistance of Marco Dadda in some of the data collection. Guidelines for the ethical treatment of animals were adhered to during all aspects of this research.
Grant sponsor: NIH; Grant numbers: RR-00165, NS-36605, NS-42867, HD-38105, HD-56232.
LITERATURE CITED
- Annett M. Handedness and brain asymmetry: the right shift theory. Hove: Psychology Press; 2002. [Google Scholar]
- Auerbach BM, Ruff CB. Limb bone bilateral asymmetry: variability and commonality among modern humans. J Hum Evol. 2006;50:203–218. doi: 10.1016/j.jhevol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal Cognit. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Biro D, Sousa C, Matsuzawa T. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In: Matsuzawa T, Tomonaga T, Tanaka M, editors. Cognitive development of chimpanzees. New York: Springer; 2006. pp. 476–507. [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. Int J Primatol. 1991;6:541–558. [Google Scholar]
- Bradshaw JL, Rogers LJ. The evolution of lateral asymmetries, language, tool use, and intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided brain: evolution of the generative mind. New York: Oxford University Press; 1992. [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): a reply to Rogers’ review of the speciation of modern Homo sapiens. Laterality: asymmetries of body, brain and cognition. 2004;9:233–242. [Google Scholar]
- Cunningham D, Forsythe C, Ward JP. A report of behavioral lateralization in an infant orangutan. Primates. 1989;30:249–253. [Google Scholar]
- Falk D, Pyne L, Helmkamp RC, DeRousseau CJ. Directional asymmetry in the forelimb of Macaca mulatta. Am J Phys Anthropol. 1988;77:1–6. doi: 10.1002/ajpa.1330770102. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B. Ontogenetic patterning of skeletal flutuating asymmetry in rhesus macaques and humans: evolutionary and developmental implications. Int J Primatol. 1999;20:121–151. [Google Scholar]
- Harrison RM, Nystrom P. Handedness in captive bonobos (Pan paniscus) Folia Primatol. 2008;79:253–268. doi: 10.1159/000113539. [DOI] [PubMed] [Google Scholar]
- Heestand J. Unpublished doctoral dissertation. University of Washington; Seattle: 1986. Behavioral lateralization in four species of ape. [Google Scholar]
- Hopkins WD. On the other hand: statistical issues in the assessment and interpretation of hand preference data in non-human primates. Int J Primatol. 1999;20:851–866. [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychol Bull. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Hemispheric specialization in chimpanzees: evolution of hand and brain. In: Shackelford T, Keenan JP, Platek SM, editors. Evolutionary cognitive neuroscience. Boston: MIT Press; 2007. pp. 99–120. [Google Scholar]
- Hopkins WD, Bard KA, Griner KM. Locomotor adaptation and leading limb asymmetries in neonatal chimpanzees (Pan troglodytes) Int J Primatol. 1997;18:104–114. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): a critical analysis and some alternative explanations. Laterality. 2005;10:65–80. doi: 10.1080/13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure length asymmetries in primates revisited: a comparative MRI study. Brain Behav Evol. 2000;56:293–299. doi: 10.1159/000047213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proc Natl Acad Sci USA. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav Brain Sci. 1987;10:247–303. [Google Scholar]
- Malashichev YB. One-sided limb preferences is linked to alternating limb locomotion in Anuran amphibians. J Comp Psychol. 2006;120:401–410. doi: 10.1037/0735-7036.120.4.401. [DOI] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: comprehensive study of spontaneous activities. J Hum Evol. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- McGrew WC, Marchant LF. Are gorillas right-handed or not? Hum Evol. 1993;8:17–23. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbk Phys Anthropol. 1997;40:201–232. [Google Scholar]
- Moricello A, Fernandez-Carriba D, Loeches A. Motor asymmetries in locomotion and postural control in chimpanzees (Pan troglodytes) Am J Primatol. 2006;68:802–811. doi: 10.1002/ajp.20280. [DOI] [PubMed] [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences for reaching and other hand-use measures. J Comp Psychol. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Peters H. Unpublished doctoral dissertation. University of New England; Australia: 2005. Behavioral lateralization in the orangutan (Pongo pygmaeus pygmaeus) [Google Scholar]
- Peters HH, Rogers LJ. Limb use and preferences in wild orangutans during feeding and locomotor behavior. Am J Primatol. 2007;69:1–15. [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer; 1981. [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Redmond JC, Lamperez A. Leading limb preference during brachiation in the gibbon family member, Hylobates syndactylus (siamangs): a study of the effects of singing on later-alisation. Laterality. 2004;9:381–396. doi: 10.1080/13576500342000211. [DOI] [PubMed] [Google Scholar]
- Sarringhaus LA, Stock JL, Marchant LF, McGrew WC. Bilateral asymmetry in the limb bones of the chimpanzee (Pan troglodytes) Am J Phys Anthropol. 2005;128:840–845. doi: 10.1002/ajpa.20190. [DOI] [PubMed] [Google Scholar]
- Stafford DK, Milliken GW, Ward JP. Lateral bias in feeding and brachiation in Hylobates. Primates. 1990;31:407–414. [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol Psychol. 1980;8:351–359. [Google Scholar]
- Yeni-Komshian G, Benson D. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 1976;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]


