Abstract
Recent studies have shown that great ape species possess patterns of macrostructural neocortical asymmetries that are similar to those found in humans. However, little is known about the asymmetry of subcortical structures in great apes. To address this lack of data, the authors assessed left–right asymmetry of the anterior and posterior aspects of cerebellum from MRI brain scans of 53 chimpanzees (Pan troglodytes). No population-level bias was found for either the anterior or the posterior region of the cerebellum. However, a significant inverse association was found in the asymmetry quotients of the anterior and posterior regions, indicating that the cerebellum was torqued at the individual level. Additionally, handedness for tool use but not other measures was associated with variation in cerebellar asymmetries. Last, older chimpanzees had a smaller cerebellum after brain volume was adjusted for. The results are discussed in the context of brain changes in primate evolution related to tool use.
Keywords: cerebellum, torque, tool use, handedness, chimpanzee
Over the last 5 years, an increasing body of evidence has shown the presence of macrostructural asymmetries in the great ape brain that closely resemble well-known patterns of asymmetry in the human brain (Hopkins, Pilcher, & Cantalupo, 2003; Pilcher, Hammock, & Hopkins, 2001). Most of these studies have focused on the neocortex, particularly on regions believed to be homologous to cortical areas of the human brain involved in linguistic functions. For example, in great apes, as in humans, the sylvian fissure has been reported to be longer in the left hemisphere, particularly in its postcentral region (Cantalupo, Pilcher, & Hopkins, 2003; Hopkins, Pilcher, & MacGregor, 2000; Yeni-Komshian & Benson, 1976). Similarly, leftward cortical asymmetries in great apes and humans have been reported for the planum temporale, an area located on the superior temporal gyrus, which coincides with part of Wernicke’s area (Cantalupo et al., 2003; Galaburda, 1995; Gannon, Holloway, Broadfield, & Braun, 1998; Gilissen, 2001; Hopkins, Marino, Rilling, & MacGregor, 1998). A number of studies have also reported leftward asymmetries in the frontal operculum in the inferior frontal lobe, commonly believed to contain part of Broca’s area (Amunts et al., 1999; Cantalupo & Hopkins, 2001; Foundas, Eure, Luevano, & Weinberger, 1998), although the scope of this finding remains to be fully assessed (Sherwood, Broadfield, Holloway, Gannon, & Hof, 2003; Watkins et al., 2001). Some studies have revealed converging asymmetry patterns also in other regions of the great ape and human brain. For example, the great ape brain has been found to show the same frontal (right > left) and occipital (left > right) petalias, also known as developmental torque, consistently reported in the human literature (Holloway & De La Coste-Lareymondie, 1982; Hopkins & Marino, 2000; LeMay, 1976, 1982, 1985; Pilcher et al., 2001). Finally, a recent study of the primary motor cortex of great apes reported a leftward asymmetry for a region of the precentral gyrus believed to be involved in the control of the hand, a finding similar to one previously reported in humans (Hopkins & Pilcher, 2001).
Overall, this evidence clearly shows that left–right macrostructural asymmetries at the cortical level are not an exclusively human phenomenon. This finding is relevant for theories on the origin of structural asymmetries in the human brain, bringing into question the long-held assumption that the appearance of cortical asymmetries was specifically linked to the acquisition of human-like linguistic abilities (Galaburda, 1995).
The study of macrostructural asymmetries in the human brain has not been limited to the areas of the neocortex but has also included a number of subcortical regions, such as the basal ganglia (Bradley et al., 1993; Ifthikharuddin et al., 2000; Kooistra & Heilman, 1988; Szabo, Lancaster, Xiong, Cook, & Fox, 2003; Szabo, Xiong, Lancaster, Rainey, & Fox, 2001; Watkins et al., 2001) as well as the hippocampus and the amygdala (Bhati, Bookheimer, Gaillard, & Theodore, 1993; Bilir et al., 1998; Brierley, Shaw, & David, 2002; Hasboun et al., 1996; Szabo et al., 2001; Watson et al., 1992). To date, the pattern of asymmetries that has emerged from this literature is somewhat unclear (see Brierley et al., 2002, for discussion). Nonetheless, expanding the study of left–right asymmetries from the neocortex to structures at different levels of the neuraxis is important to further our understanding of the evolutionary emergence of brain asymmetry. Some studies on macrostructural asymmetries in humans have been conducted also at the level of the metencephalon, namely, the cerebellum. Although no consistent population-level asymmetry has emerged from studies that compared the total volume of the left and right cerebellar hemispheres (Levitt et al., 1999; Loebert, Clinton, & Yurgelum-Todd, 2001; Szabo et al., 2003), other studies have found in the cerebellum the same torque pattern (anterior: right > left; posterior: left > right) usually found at the cortical level (Snyder, Bilder, Wu, Bogerts, & Lieberman, 1995; Szeszko et al., 2003). Of note, Szeszko et al. (2003) observed reversed cerebellar torque in men with first-episode schizophrenia, and the severity of the symptoms increased with higher degrees of inversed torque. One aim of this study was to examine whether chimpanzees show any evidence of a torque asymmetry in the cerebellum, such as that described by Snyder et al. (1995). A preliminary comparative study between 16 capuchin monkeys and chimpanzees showed that apes had a rightward asymmetry in the anterior but not posterior region of the cerebellum (Phillips & Hopkins, 2007), and the goal of the present study was to more fully explore the potential influence of sex, rearing history, and age on cerebellar volume and lateralization.
The second aim of this study was to assess the potential association between handedness and lateralization in the cerebellum. Phillips and Hopkins (2007) failed to find an association between handedness for coordinated bimanual actions and cerebellar asymmetries in their smaller sample of chimpanzees, although the association was significant in the capuchin monkeys. In this study, we sought to expand the analysis between cerebellar asymmetry and handedness by including measures of hand preference for tool use. Our focus on testing whether handedness for tool use is linked to cerebellar asymmetries stems from recent studies that have shown that apes have a relatively larger cerebellum than would be predicted for primates of their brain size, compared with other primates (MacLeod, Zilles, Schleicher, & Gibson, 2001; Rilling & Insel, 1998). The functional significance of the larger cerebellum in great apes is unknown, but apes, and particularly chimpanzees, are very well known for their tool-using abilities in the wild and in captivity (Whiten et al., 2001), and there is at least some evidence of population-level asymmetries for tool use in wild chimpanzees (Hopkins, 2006; Lonsdorf & Hopkins, 2005). One hypothesis might be that the larger cerebellum in apes may have been selected for to support complex cognitive and motor actions underlying the evolution of tool use. If this hypothesis is correct, it might be predicted that lateralization in the cerebellum might be linked specifically to hand preference for tool use but not for other measures of hand use. We tested this hypothesis in the current study by assessing the association between cerebellar asymmetries and hand preference for multiple measures of hand use in chimpanzees.
Method
Subjects
Magnetic resonance images of the brain were collected in a sample of 53 chimpanzees (Pan troglodytes) including 33 females and 20 males ranging in age from 6 to 45 years (M = 19.83, SD = 10.27). All of the apes were housed at the Yerkes National Primate Research Center (YNPRC) of Emory University. Among the 53 apes, 17 had been raised by their mothers, whereas 34 had been raised by a human and 2 were wild caught before 1973 and had lived in captivity since that time.
Procedure
Prior to scanning, the living subjects were immobilized with a Telazol injection (2–6 mg/kg) and subsequently anesthetized with propofol (10 mg/kg/hr) following standard veterinary procedures used at the YNPRC. The subjects remained sedated for the duration of the scans as well as the time needed for transport between YNPRC and scanner location (total time, approximately 1 to 1.5 hr). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min as a function of brain size. The majority of the subjects (n = 36) were scanned using a 1.5-tesla (T) scanner (Model 51; Philips, Andover, MA). The remaining chimpanzees (n = 17) were scanned using a 3.0-T scanner (Siemens Trio, Siemens Medical Solutions USA, Malvern, PA) at the YNPRC.
For all chimpanzees scanned using the 1.5-T machine (n = 36), T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, mean number of signals = 8, and matrix size = 256 × 256). For the 17 chimpanzees that were scanned using a 3.0 T scanner, T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2,300 ms, echo time = 4.4 ms, mean number of signals = 3, matrix size = 320 × 320).
After completing MRI procedures, the subjects were returned to the YNPRC and temporarily housed in a single cage for 6–12 hr to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. The archived MRI data were transferred to a PC running Analyze 6.0 software (Mayo Foundation, Rochester, MN) for postimage processing.
Delineation of Regions of Interest and Volume Measurements
To assess the volume of the regions of interest, we aligned the MRI scans in the sagittal, coronal, and axial planes using standard anatomical landmarks and cut into 1-mm coronal slices using multiplanar reformatting software (Analyze). The region-of-interest areas in each coronal slice were measured (in mm2) using a mouse-driven computer-guided area tool available in Analyze. Two independent raters, blind to the handedness of the apes, measured the cerebellum in 10% of the sample, and the interrater reliability coefficient was .995.
Delineation of Left Versus Right Cerebellar Hemispheres
The left and right cerebellar hemispheres were delineated following the procedure used by Snyder and colleagues (1995) with human subjects. A line was traced from the dorsal to the ventral aspect of the vermis and fourth ventricle (when visible), bisecting them. The cerebellar peduncles and fourth ventricle were excluded, whereas the vermis, the hemispheric gray matter, cerebellar tonsils, vellum, and corpus medullare were included in the tracing of each cerebellar hemisphere (see Figure 1A). Detecting where the cerebellar peduncles ended and the corpus medullare began was difficult in many instances. Therefore, to limit the inclusion of white matter potentially belonging to the peduncles, we followed the procedure used by Raz and colleagues (Raz, Dupuis, Briggs, McGavran, & Acker, 1998; Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000) in studies with human subjects by starting inclusion of cerebellar white matter at the most anterior slice in which the anterior vermis could be seen.
Figure 1.
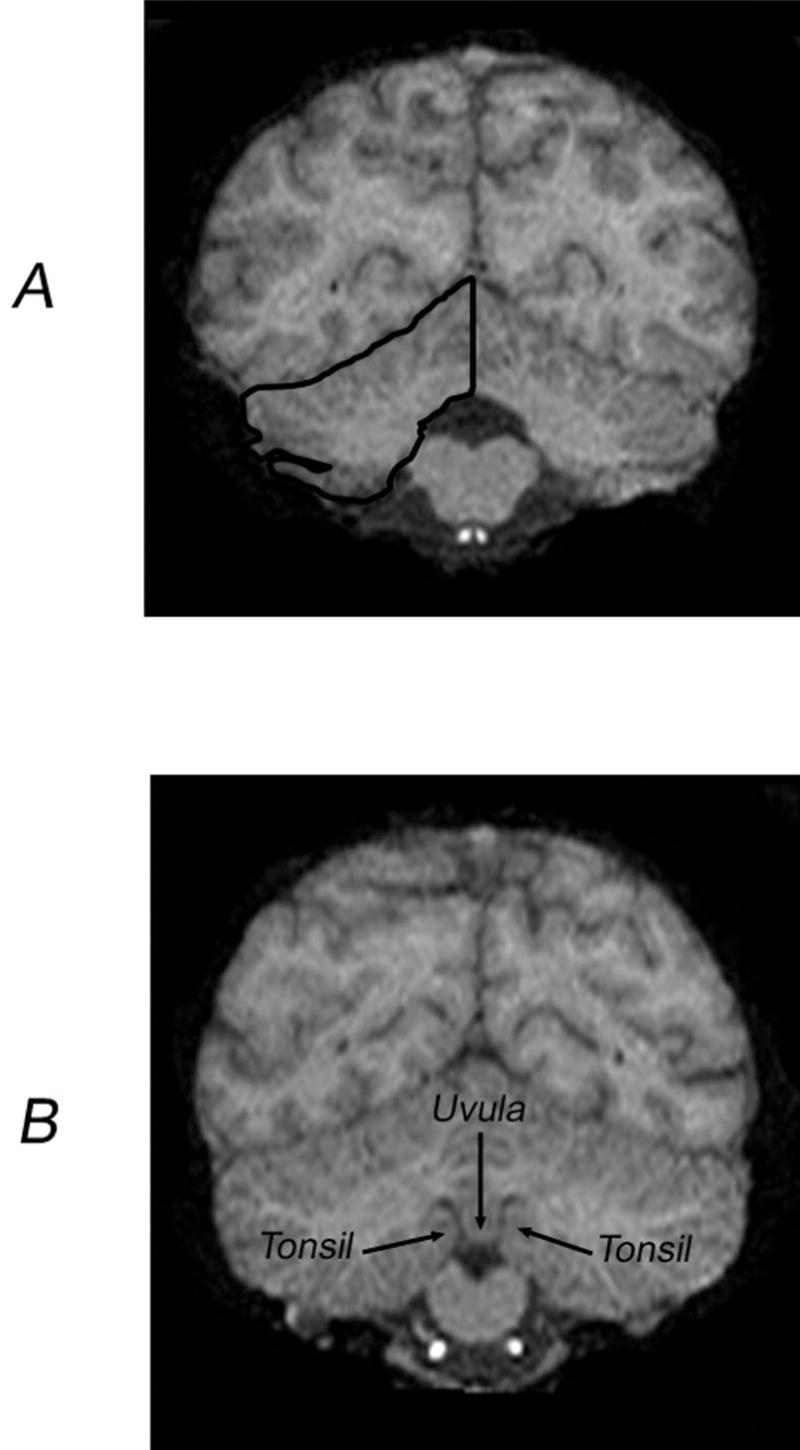
A: Bisection of the cerebellum and tracing of the right cerebellar hemisphere. B: Illustration of the coronal slice containing the uvula–tonsil landmark.
Delineation of Anterior Versus Posterior Cerebellar Portions
To subdivide the cerebellum into anterior and posterior portions we used a procedure previously developed in a study with human subjects. Snyder and colleagues (1995) identified a reliable mid-line landmark in the human cerebellum, which they called the “uvula–tonsils” landmark. This is the point at which the uvula of the vermis becomes narrower and/or the fissures between the uvula and the cerebellar tonsils become clearly visible (see Figure 1B). The same anatomical landmark was identifiable in our great ape sample and was usually found between the 23rd and 22nd slices from the posterior pole of the cerebellum (M = 22.3, SE = 0.406). Similarly, on average 23.6 slices (SE = 0.320) containing cerebellar tissue were found anterior to this landmark. Thus, similar to humans, the uvula–tonsils landmark offers a basis for bisecting the cerebellum along the anterior–posterior axis in great apes.
The anterior border of the anterior portion of the cerebellum was defined by the slice in which tissue belonging to the anterior pole of the cerebellum may be first seen (possibly on different slices for the left and right hemispheres). The most posterior slice of the anterior portion was the slice just anterior to the uvula–tonsil landmark. The anterior border of the posterior portion of the cerebellum was defined by the first slice showing the uvula–tonsil landmark, whereas the posterior border was the most posterior slice showing tissue belonging to the posterior pole of the cerebellum. This posterior landmark was often found on different slices for left and right cerebellar hemispheres.
Handedness Assessment
Hand use was assessed for three measures, including simple reaching, the tube task, and a tool use task designed to simulate termite or ant fishing in wild chimpanzees (Lonsdorf & Hopkins, 2005; Marchant & McGrew, 2007; McGrew & Marchant, 1992). The procedures have been described in detail elsewhere (Hopkins, 1995; Hopkins, Russell, & Cantalupo, 2007; Hopkins, Russell, Hook, Braccini, & Schapiro, 2005), and brief descriptions of each task are provided below.
Simple reaching
For the simple reaching measure, raisins were thrown one at a time into the subject’s home cage. The subjects had to locomote to a spot near the food, reach, and pick up the food. Hand use was recorded as right or left. A minimum of 50 responses was recorded from each subject. To ensure that hand choice on each trial was not influenced by the hand used on the previous trial, subjects had to reposition themselves between trials.
Tube
For the tube task, peanut butter was smeared on the inside edges of polyvinyl chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter was smeared on both ends of the PVC pipe and was placed far enough down the tube such that the subjects could not lick the contents completely off with their mouths but rather had to use one hand to hold the tube and the other hand to remove the substrate. The PVC tubes were handed to the subjects in their home cages, and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter, and brought it to their mouth, the hand used was recorded as left or right.
Simulated termite fishing
A task was designed to simulate the termite-fishing behavior of wild chimpanzees (see Figure 2). Testing was conducted using a device consisting of three PVC pipes (15 cm long, 4 cm in diameter) glued at 45° angles into three holes (4 cm in diameter) placed horizontally 15 cm apart on a rectangular plastic board (50 cm long by 20 cm wide). The end of each tube that was glued to the rectangular plastic board was open to allow access to the honey at the other end (bottom) of the tube. The bottom end of the tube consisted of a removable PVC cap. During testing, each PVC tube in the apparatus was first filled with a preferred food that had some adhesive qualities (honey or applesauce) to about one third of the whole length of the tube, which made it impossible for the subjects to reach the food directly with their fingers. After the device was placed on the cage, chimpanzees were handed small sticks or bamboo skewers (about 0.5 cm in diameter), which they then had to insert into the pipe to extract the food. Each time the chimpanzees inserted the stick, a left- or right-hand response was recorded. A minimum of 100 dipping responses summed between at least two test sessions were obtained from each subject, and their hand preferences were classified on the basis of z scores derived from the frequency of left- and right-hand use.
Figure 2.
Photograph of a chimpanzees performing the simulated termite-fishing tool use task used in this study to assess handedness.
Data Analysis
For the anterior and posterior cerebellar volumetric measures (both separately and combined), a left–right asymmetry quotient (AQ) was derived for each subject using the formula (R − L)/[(R + L) * 0.5]. The sign of the resulting value indicated the direction of asymmetry (positive value = right hemisphere bias; negative value = left hemisphere bias), and the absolute value reflected the magnitude of asymmetry. Following a common procedure in studies with human subjects, apes with AQ scores above 0.025 were categorized as having a rightward asymmetry, whereas apes with values below −0.025 were categorized as having a leftward asymmetry. Apes whose scores were between −0.025 and 0.025 were classified as having no consistent bias.
For the two handedness tasks, binomial z scores were calculated for each subject based on the frequency of left and right hand use for all assessments of hand use. Subjects with z scores greater than 1.95 or less than −1.95 were classified as right- and left-handed, respectively. All other subjects were classified as ambiguously handed. In addition, a handedness index (HI) was derived for each subject and task by subtracting the number of right-hand responses from the number of left-hand responses and dividing by the total number of responses: HI = (Nr − N1)/(Nr+ N1). Positive values reflected right-hand preferences, and negative values reflected left-hand preferences. All analyses adopted an alpha of .05 as the level of significance. Post hoc tests, when necessary, were conducted using Tukey’s honestly significant difference tests, with p < .05.
Results
Descriptive Statistics
Because different magnets were used in the data collection, we initially compared the anterior and posterior AQ values, as well as the volume of the anterior and posterior regions, between the apes scanned at 1.50 T compared with 3.0 T. No significant differences were found for any of the measures. We also tested for sex and rearing differences in the volume of the cerebellum as well as the AQ measures for the anterior and posterior cerebellum. For the comparisons in cerebellum volume, brain volume served as a covariate, to adjust for possible differences in overall brain size. No significant main effects or interactions between sex and rearing history were found on any of the cerebellar measures (see Table 1).
Table 1.
Overall Volume and Asymmetry Quotients (AQs) for the Anterior and Posterior Aspects of the Cerebellum of the Chimpanzees
| Sex | Adjusted cerebellar volume | Anterior AQ | Posterior AQ |
|---|---|---|---|
| Males | |||
| M | 46,702.48 mm3 | −.017 | .021 |
| SE | 1,944.54 | .019 | .014 |
| Females | |||
| M | 47,333.87 mm3 | −.026 | .027 |
| SE | 1,109.77 | .034 | .026 |
Age varied considerably in our sample. Thus, we correlated the anterior and posterior AQ values as well as the total cerebellum volume with age. Neither of the AQ values correlated with age, but age was significantly negatively associated with total cerebellar volume after brain volume was adjusted for: partial r(51) = −.339, p < .02. Thus, older chimpanzees had relatively smaller cerebellums.
Analysis of Direction of Asymmetries
Cerebellar measures
When AQ scores for the whole cerebellum (i.e., anterior and posterior aspects combined) were considered, 16 chimpanzees showed an asymmetry (7 leftward, 9 rightward), whereas 37 did not. A chi-square goodness-of-fit test revealed that there were significantly more symmetrical than asymmetrical cerebella, χ2(1, N = 53) = 8.32, p < .01. The absence of an overall asymmetry for the whole cerebellum in this subject population was confirmed also at the continuous level by performing a one-sample t test on the AQ scores, t(52) = 1.079, ns.
A different pattern of results emerged when the cerebellum was subdivided into anterior and posterior aspects. In both cases, a significant majority of the apes showed either a leftward or a rightward asymmetry compared with no bias for both the anterior (47 vs. 6), χ2(1, N = 53) = 31.72, p < .01, and posterior regions (39 vs. 14), χ2(1, N = 53) = 11.79, p < .01. The proportions of individuals with leftward or rightward biases were comparable for the anterior (leftward, n = 26; rightward, n = 21) and posterior (leftward, n = 16; rightward, n = 23) regions of the cerebellum.
Overall, the absence of a consistent population-level directional asymmetry for the anterior and posterior aspects of the cerebellum in spite of the clear presence of asymmetries at the individual level was further confirmed by one-sample t tests on the AQ scores: anterior cerebellum, t(52) = −0.825, ns; posterior cerebellum, t(52) = 1.483, ns.
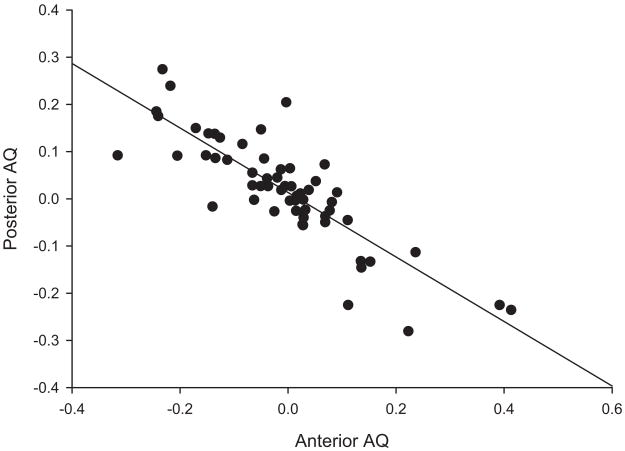
Also, the mean AQ score for the anterior cerebellum (−0.012, SE = 0.014) did not differ statistically from that of the posterior one (0.016, SE = 0.011), paired-samples t(52) = −1.207, ns), further confirming the absence of group-level cerebellar torque. To thoroughly explore the possible presence of some systematic changes in cerebellar asymmetry along the anterior–posterior axis, we computed a correlation between the anterior and posterior AQ scores. As shown in Figure 3, this analysis revealed a significant negative association between the anterior and posterior AQ measures (r = −.676, N = 53, p < .001). Thus, the larger the bias was in one direction (either left or right) for the anterior aspect of the cerebellum, the larger was the bias in the opposite direction for the posterior aspect of the cerebellum. Clearly, this shows the presence of cerebellar torque at the individual level in our subject population.
Figure 3.
Scatter plot and regression line of individual asymmetry quotients (AQs) for the anterior and posterior aspects of the cerebellum (r =−.676, p < .001).
Relationship between cerebellar asymmetries and handedness
Rather than perform a series of correlations between the HI scores of each measure and cerebellar region, we initially assessed which, if any, of the three handedness measures best predicted variation in the AQ values for the anterior and posterior cerebellar regions. This was accomplished by performing two multiple regression analyses with the individual HI values for the reach, tube, and simulated termite-fishing tasks serving as predictor variables. The AQ scores for the anterior and posterior cerebellar regions served as the outcome measure for each regression analysis. For the anterior AQ values, none of the HI values were significant predictors (R = .224), F(3, 46) = 0.81, ns; however, for the AQ values for the posterior region, the handedness measures did significantly predict variation (R = .472), F(3, 46) = 4.38, p < .009. The partial correlation coefficient indicated that the HI values for the tool use measure were the only significant predictor of variation in the AQ values (β = −.460, p < .001) (see Table 2).
Table 2.
Partial Correlation Coefficients Between Anterior and Posterior Asymmetry Quotient (AQ) Values and the Handedness Index Values for Simple Reaching, Tube, and Simulated Tool Use Handedness Tasks
| AQ | Simple reaching | Tube | Simulated termite fishing |
|---|---|---|---|
| Anterior | −.012 | .023 | .223 |
| Posterior | −.074 | .047 | −.460* |
p < .01.
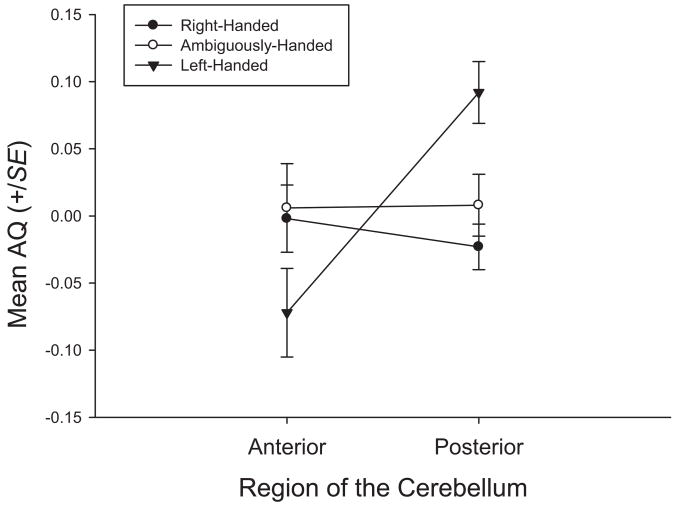
In addition to the regression analyses, we conducted several analyses of variance (ANOVAs) comparing the anterior and posterior AQ values as a function of sex and handedness, when considered as a dichotomous trait. The anterior and posterior cerebellar AQ values were compared using a mixed-model ANOVA. Cerebellar region served as a repeated measure and sex and handedness served as between-groups factors. For simple reaching and the tube task, no significant main effects or interactions were found; however, for the tool use measure, a significant two-way interaction was found between handedness and cerebellar region, F(2, 47) = 4.50, p < .02. The mean AQ values for the anterior and posterior regions of the cerebellum in left-, right-, and ambiguously handed chimpanzees are shown in Figure 4. Post hoc analysis indicated that no significant differences between the three handedness groups were found for the anterior AQ values. For the posterior cerebellum AQ values, right- and ambiguously handed chimpanzees had significantly greater leftward asymmetries compared with the left-handed chimpanzees. Moreover, in left-handed chimpanzees, the anterior AQ values were significantly more leftward compared with the posterior AQ values.
Figure 4.
Average asymmetry quotients (AQs) for the anterior and posterior sections of the cerebral hemispheres in left-handed, ambiguously handed, and right-handed chimpanzees.
Discussion
Several significant findings emerged from this study. First, chimpanzees as a whole showed no evidence of population-level asymmetries in the cerebellum. Second, chimpanzees did show a torque asymmetry in the cerebellum, and this form of lateralization was correlated with handedness for tool use but not other measures of hand use. Third, age-related changes in the volume of the cerebellum were evidenced, with older chimpanzees having a relatively smaller cerebellum after brain volume was adjusted for. Last, neither sex nor rearing history had a significant influence on either cerebellar volume or asymmetry.
The cerebellum of great apes clearly showed a reversal of left–right asymmetry along the anterior–posterior axis. However, there was no prevalence in the population of one directional torque pattern over the other, a result that is inconsistent with at least one report on human brains (Snyder et al., 1995). In addition, the lack of population-level torque asymmetry for the cerebellum stands in contrast to evidence of right-frontal, left-occipital torque asymmetries at the cortical level in apes and humans (LeMay, 1985; Pilcher et al., 2001). Thus, different patterns of cerebro–cerebellar torque are revealed when comparing humans and great apes. Humans show the population-level rightward-anterior/leftward-posterior torque originally described by LeMay (1976) at both the neocortical and cerebellar levels, suggesting the presence of a direct association between the two patterns (Snyder et al., 1995). In contrast, great apes show a humanlike population-level torque for the neocortex but only an individual-level torque for the cerebellum, with no apparent association between the two.
The difference between human and great ape findings offers a comparative basis for assessing the tenability of some ideas on the relationship between lateral asymmetries at different levels of the neuraxis. In particular, the idea that the typical pattern of neocortical developmental torque might reflect an earlier pattern of growth at the level of the metencephalon (see Snyder et al., 1995) would be applicable to humans but not great apes. This would suggest the possible presence of species-specific differences in the mechanisms responsible for the expression of asymmetry patterns across different levels of the neuraxis. In humans, neocortical and cerebellar torque patterns might very well be directly related through common developmental growth gradients during encephalization of the neural tube, as suggested by Snyder and colleagues (1995). In great apes, however, cerebellar torque appears to be independent from the neocortical one, suggesting that a different mechanism might account for the torque pattern at the cerebellar level. What that mechanism might be is unclear, and additional research is needed. In particular, the potential species difference should be interpreted with caution because the results in humans have not been consistent across studies, in terms of directional asymmetries of the cerebellum when divided or not divided along an anterior–posterior gradient (Snyder et al., 1995; Szeszko et al., 2003).
The evidence of an association between cerebellar torque asymmetries and handedness for tool use but not other measures of hand preference warrants some discussion. The results of a task-specific association between handedness and cerebellar asymmetries are consistent with previous studies that have demonstrated task-specific association between hand preference and asymmetries in the motor-hand area of the precentral gyrus (Hopkins & Cantalupo, 2004), inferior frontal gyrus (Taglialatela, Cantalupo, & Hopkins, 2006), and planum temporale (Hopkins et al., 2007). With specific reference to handedness for tool use, Hopkins et al. (2007) found that for the simulated termite-fishing task, right-handed chimpanzees had significantly greater leftward asymmetries in the planum temporale and inferior frontal gyrus than left- and ambiguously handed chimpanzees. Combining these results with those reported in this study indicates that handedness for the simulated termite-fishing task is associated with asymmetries in the language homologues as well as the cerebellum in chimpanzees. The results reported here are also consistent with the hypothesis that changes in the organization of the cerebellum in great apes, as well as the language homologues, may be associated with the evolution of tool-using abilities and that this may have served as the neurological foundation for the emergence of language and speech, as suggested by some (Greenfield, 1991; Leiner & Leiner, 1987; Leiner, Leiner, & Dow, 1993; Schmahmann & Pandya, 1997).
Increasing age was significantly associated with a decrease in the volume of the cerebellum, even after brain size was adjusted for. Brain volume was not significantly negatively associated with increasing age (r =−.233, p <.10), although the results did approach conventional levels of significance. Evidence of selective regional shrinkage of the cerebellum associated with normal aging has been reported in humans (Raz et al., 1998) and might be associated with changes in motor performance or skill, but this remains to be tested in chimpanzees. In two studies examining grasping skill in chimpanzees, no age-related changes in performance were noted (Hopkins, Cantalupo, Wesley, Hostetter, & Pilcher, 2002; Hopkins & Russell, 2004).
Two other observations are of note. First, contrary to the initial study by Phillips and Hopkins (2007), we did not find significant population-level asymmetries in either the anterior or the posterior region of the cerebellum, as was reported in the initial study on 16 chimpanzees. Thus, with a larger sample size, these results were not replicable. Second, no significant association was found between handedness for the tube task and either the anterior or the posterior cerebellum in this study. This result is consistent with the previous report by Phillips and Hopkins (2007) on chimpanzees and suggests that handedness for the tube task is not a good predictor of variation in the torque asymmetry of the cerebellum.
In summary, the results presented here indicate that variation in the torque asymmetry of the cerebellum is associated with handedness for tool use but not other motor tasks. The selective association between cerebellar asymmetry and handedness for tool use is consistent with the general view that the cerebellum is functionally involved in the coordination of motor movements. Collectively, the results are consistent with a growing body of data showing associations between behavioral and brain asymmetries in nonhuman animals (Hopkins, in press). Although this study examined cerebellar asymmetries for the entire structure, further analysis focusing on gray–white distinction between hemispheres and structures within the cerebellum would be of interest (MacLeod, Zilles, Schleicher, Rilling, & Gibson, 2003).
Acknowledgments
This work was supported in part by National Institutes of Health Grants RR-00165, NS-42867, NS-36605, and HD-38051. Some of the MRI scans were collected during many hours of dedication by Jim Rilling and Tom Insel. Special thanks are also directed to Elizabeth Strobert, Jack Orkin, and Brent Swenson and the rest of the veterinarian staff for assisting in the care of the animals during scanning.
Contributor Information
Claudio Cantalupo, Department of Psychology, Clemson University, Clemson, South Carolina, and Division of Psychobiology, Yerkes National Primate Research Center, Atlanta, Georgia.
Hani Freeman, Division of Psychobiology, Yerkes National Primate Research Center.
William Rodes, Department of Psychology, Clemson University, Clemson, South Carolina.
William Hopkins, Division of Psychobiology, Yerkes National Primate Research Center and Department of Psychology, Agnes Scott College, Decatur, Georgia.
References
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: Cytoarchitecture and inter-subject variability. Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bhati S, Bookheimer SY, Gaillard WD, Theodore WH. Measurement of whole temporal lobe and hippocampus for MR volumetry: Normative data. Neurology. 1993;43:2006–2010. doi: 10.1212/wnl.43.10.2006. [DOI] [PubMed] [Google Scholar]
- Bilir E, Craven W, Hugg J, Gilliam F, Martin R, Faught E, Kuzniecky R. Volumetric MRI of the limbic system: Anatomic determinants. Neuroradiology. 1998;40:138–144. doi: 10.1007/s002340050554. [DOI] [PubMed] [Google Scholar]
- Bradley SP, Riddle MA, Cohen DJ, Katz LD, Smith C, Leckman JF. Human basal ganglia volume asymmetries on magnetic resonance images. Magnetic Resonance Imaging. 1993;11:493–498. doi: 10.1016/0730-725x(93)90468-s. [DOI] [PubMed] [Google Scholar]
- Brierley B, Shaw P, David AS. The human amygdala: A systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Research Reviews. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001 November 29;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca’s area: The pars triangularis and pars opercularis. Brain and Language. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Anatomic basis of cerebral dominance. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge, MA: MIT Press; 1995. pp. 51–73. [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998 January 9;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gilissen E. Structural symmetries and asymmetries in human and chimpanzee brains. In: Falk D, Gibson KR, editors. Evolutionary anatomy of the primate cerebral cortex. Cambridge, England: Cambridge University Press; 2001. pp. 187–215. [Google Scholar]
- Greenfield PM. Language, tools, and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behavioral and Brain Sciences. 1991;14:531–550. [Google Scholar]
- Hasboun D, Chantome M, Zouaoui A, Sahel M, Deladoeuille M, Sourour N, et al. MR determination of hippocampal volume: Comparison of three methods. American Journal of Neuroradiology. 1996;17:1091–1098. [PMC free article] [PubMed] [Google Scholar]
- Holloway RL, De La Coste-Lareymondie MC. Brain endocast asymmetry in pongids and hominids: Some preliminary findings on the paleontology of cerebral dominance. American Journal of Physical Anthropology. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. The evolution of hemispheric specialization in primates. London: Elsevier; (in press) [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter AB, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere specialization in motor skill. Journal of Experimental Psychology: General. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino LM. Cerebral width asymmetries in nonhuman primates as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38:493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area with magnetic resonance imaging: The left hemisphere is larger in great apes. Behavioral Neuroscience. 2001;115:1159–1164. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher D, Cantalupo C. Neural basis of cognition, communication and handedness in non-human primates. In: Maestripieri D, editor. Primate psychology. Cambridge, MA: Harvard University Press; 2003. pp. 424–450. [Google Scholar]
- Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure asymmetries in nonhuman primates revisited. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behavioral Neuroscience. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes) Neuropsychologia. 2004;42:990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): Implication for theories on the evolution of language. Psychological Science. 2007;18:971–977. doi: 10.1111/j.1467-9280.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifthikharuddin SF, Shrier DA, Numaguchi Y, Tang X, Ning R, Shibata DK, Kurlan R. MR volumetric analysis of the human basal ganglia: Normative data. Academic Radiology. 2000;7:627–634. doi: 10.1016/s1076-6332(00)80579-6. [DOI] [PubMed] [Google Scholar]
- Kooistra CA, Heilman KM. Motor dominance and lateral asymmetry of the globus pallidus. Neurology. 1988;38:388–390. doi: 10.1212/wnl.38.3.388. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL. Cerebro–cerebellar learning loops in apes and humans. Italian Journal of Neurological Sciences. 1987;8:425–436. doi: 10.1007/BF02334599. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends in Neurosciences. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man and nonhuman primates. Annals of the New York Academy of Sciences. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological aspects of human brain asymmetry: An evolutionary perspective. Trends in Neurosciences. 1982;5:273–275. [Google Scholar]
- LeMay M. Asymmetries of the brains and skulls of nonhuman primates. In: Glick SD, editor. Cerebral lateralization in nonhuman species. New York: Academic Press; 1985. pp. 223–245. [Google Scholar]
- Levitt JJ, McCarley RW, Nestor PG, Petrescu C, Donnino R, Hirayasu Y. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: Clinical and cognitive correlates. American Journal of Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebert RT, Clinton CM, Yurgelum-Todd DA. Morphometry of individual cerebellar lobules in schizophrenia. American Journal of Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences, USA. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CE, Zilles K, Schleicher A, Rilling J, Gibson KR. Expansion of the neocerebellum in Hominoidea. Journal of Human Evolution. 2003;44:401–429. doi: 10.1016/s0047-2484(03)00028-9. [DOI] [PubMed] [Google Scholar]
- MacLeod CE, Zilles K, Schleicher A, Gibson KR. The cerebellum: An asset to hominoid cognition. In: Galdikas BMF, Briggs NE, Sheeran LK, Shapiro GL, Goodall J, editors. All apes great and small: African apes. Vol. 1. New York: Kluwer; 2001. [Google Scholar]
- Marchant LF, McGrew WC. Ant fishing by chimpanzees is not lateralised. Primates. 2007;48:22–26. doi: 10.1007/s10329-006-0020-3. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Current Anthropology. 1992;33:114–119. [Google Scholar]
- Phillips K, Hopkins WD. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella) Neuropsychologia. 2007;45:2333–2339. doi: 10.1016/j.neuropsychologia.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher D, Hammock L, Hopkins WD. Cerebral volume asymmetries in non-human primates as revealed by magnetic resonance imaging. Laterality. 2001;6:165–180. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: A prospective MRI study. American Journal of Neuroradiology. 1998;19:65–71. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual–motor skill. Microscopy Research and Technique. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Evolution of the cerebellum in primates: Differences in relative volume among monkeys, apes and humans. Brain, Behavior and Evolution. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. International Review of Neurobiology. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Sherwood CS, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca’s area homologue in African great apes: Implication for language evolution. Anatomical Record. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bilder RM, Wu H, Bogerts B, Lieberman JA. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia. 1995;33:407–419. doi: 10.1016/0028-3932(94)00125-9. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Lancaster JL, Xiong J, Cook C, Fox P. MR imaging volumetry of subcortical structures and cerebellar hemispheres in normal persons. American Journal of Neuroradiology. 2003;24:644–647. [PMC free article] [PubMed] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: Differences regarding handedness. American Journal of Neuroradiology. 2001;22:1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biological Psychiatry. 2003;53:450–459. doi: 10.1016/s0006-3223(02)01529-9. [DOI] [PubMed] [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: A voxel-based statistical analysis of 142 MRI scans. Cerebral Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Glotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew W, Nishida T, Reynolds V, Sugiyama Y, et al. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]
- Yeni-Komshian G, Benson D. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 1976 April 23;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]