Abstract
At E8.5, the LIM-homeodomain factor Lmx1a is expressed throughout the otic placode but becomes developmentally restricted to non-sensory epithelia of the ear (endolymphatic duct, ductus reuniens, cochlea lateral wall). We confirm here that the ears of newborn dreher (Lmx1adr) mutants are dysmorphic. Hair cell markers such as Atoh1 and Myo7 reveal for the first time that newborn Lmx1a mutants have only three sensory epithelia: two enlarged canal cristae and one fused epithelium comprising an amalgamation of the cochlea, saccule and utricle, a “cochlear-gravistatic” endorgan. The enlarged anterior canal crista develops by fusion of horizontal and anterior crista whereas the posterior crista fuses with an enlarged papilla neglecta that may extend into the cochlear lateral wall. In the fused endorgan the cochlear region is distinguished from the vestibular region by markers such as Gata3, the presence of a tectorial membrane and cochlea-specific innervation. The cochlea-like apex displays minor disorganization of the hair and supporting cells. This contrasts with the basal half of the cochlear region which shows a vestibular epithelium-like organization of hair cells and supporting cells. The dismorphic features of the cochlea are also reflected in altered gene expression patterns. Fgf8 expression expands from inner hair cells in the apex to most hair cells in the base. Two supporting cell marker proteins, Sox2 and Prox1, also differ in their cellular distribution between the base and the apex. Sox2 expression expands in mutant canal cristae prior to their enlargement and fusion and displays a more diffuse and widespread expression in the base of the cochlear region whereas Prox1 is not detected in the base. These changes in Sox2 and Prox1 expression suggest that Lmx1a expression restricts and sharpens Sox2 expression thereby defining non-sensory and sensory epithelium. The adult Lmx1a mutant organ of Corti showed a loss of cochlear hair cells, suggesting that long term hair cell maintenance is also disrupted in these mutants.
Keywords: dreher, Lmx1a, ear, mouse, hair cell maintenance, sensory epithelium formation
INTRODUCTION
The vertebrate ear has 3 to 9 sensory epithelia consisting of hair cells and supporting cells (Lewis, et al., 1985). Mammals have three canal cristae, two gravistatic organs (utricle, saccule), an organ of Corti in the cochlea and a papilla neglecta that varies in size (Fritzsch and Wake, 1988). The initially continuous sensory epithelia become separated as a result of unknown developmental mechanisms (Fritzsch, et al., 2002) by non-sensory epithelia that orient the sensory epithelia in space, channel fluid dynamics and maintain the endolymphatic environment (Lewis, et al., 1985). Sensory and non-sensory epithelia generate diffusible factors that govern the morphogenesis of nearby non-sensory epithelia (Chang, et al., 2004a, Chang, et al., 2008, Daudet, et al., 2002, Sienknecht and Fekete, 2008). Additional secreted factors originate from the hindbrain, ectoderm and mesenchyme (Chang, et al., 2004b, Fritzsch, et al., 2006b, Ohyama, et al., 2007, Pirvola, et al., 2004). Thus both global and local interactions of various diffusible factors regulate local transcription factors that govern the morphogenetic process of the non-sensory epithelium of the ear that ultimately channels physical stimuli to specific sensory epithelia. Likewise, differentiation of sensory epithelia into hair cells and supporting cells reflects temporal expression cascades of transcription factors (Fritzsch, et al., 2006a, Kelley, 2006, Kiernan, et al., 2005). However, no single factor has been described that is exclusively associated with the non-sensory epithelia during development and throughout the ear (Chang, et al., 2008, Kiernan, et al., 1997, Raft, et al., 2004) although the vast majority of Wnt transcripts are expressed in non-sensory domains (Sienknecht and Fekete, 2008). Thus a possible feedback loop between developing sensory and non-sensory areas of the ear could exist to fine tune the morphogenesis of the ear to the histogenesis of the sensory epithelia.
Lmx1a is one of four members of the Islet-Lim homeodomain transcription factor family (Hunter and Rhodes, 2005) that has three conserved members in triploblastic animals (Isl1, Lmx1a, Lmx1b; Drosophila orthologs: tailup, CG32105, CG4328, respectively). The Islet family belongs to a large family of Lim-homeodomain transcription factors that can bind to DNA in the form of monomers that form complexes with other transcription factors or in the form of heteromultimeres (Bhati, et al., 2008b, Hunter and Rhodes, 2005, Matthews and Visvader, 2003). GATA, bHLH and LMX factors interact with Lim-homeodomain factors during development. For example, such complexes are required during development of reticular formation in the hindbrain (Alenina, et al., 2006) and motoneuron formation in the spinal cord (Lee, et al., 2008,Matthews and Visvader, 2003). Likewise, in insect mechanosensory development, Isl and Gata/pannier antagonize each other to regulate expression of bHLH genes necessary to develop sensory and non-sensory cells (Asmar, et al., 2008, Biryukova and Heitzler, 2005), presumably through competition for binding to another Lim-homeodomain factor. Consistent with the emerging concept of molecular conservation of essential neurosensory developmental modules across phyla (Adam, et al., 1998, Caldwell and Eberl, 2002, Fritzsch, et al., 2000, Fritzsch, et al., 2007, Pierce, et al., 2008), Gata3 is necessary for neurosensory development of the vertebrate ear (Karis, et al., 2001, Lillevali, et al., 2006). Interestingly haploinsufficiency of Gata3 causes hearing loss (Van Esch and Devriendt, 2001). In the ear, the expression of Isl1 (Radde-Gallwitz, et al., 2004), Lmx1a (Failli, et al., 2002), Lhx3 (Hertzano, et al., 2007) and Lim only factors (LMOs) have been described (Deng, et al., 2006) but no functional analysis using LoF or Gof as yet exists.
The dreherJ (Lmx1adr) point mutation is one of 13 known spontaneous mutations in the Lmx1a gene causing neurological, skeletal and otic abnormalities (Chizhikov, et al., 2006, Millonig, et al., 2000). The morphology of the dreherJ mutant ear was initially described (Deol, 1964, Deol, 1983) and thought to be a consequence of malformations in the hindbrain (Manzanares, et al., 2000). More recent in situ expression show, however, a more robust and earlier expression of Lmx1a in the developing mouse (Failli, et al., 2002) and chicken ear compared to expression in the hindbrain (Giraldez, 1998). These in situ hybridization data raise the possibility that local, otic Lmx1a expression is required for ear development and its absence in the ear is causally linked to the ear defects. Since Lmx1a interacts with other Lim and LMO factors, it is an intriguing and likely possibility that Lmx1a and other Isl family members co-operate to regulate the sensory and non-sensory development of the ear. Their role in ear morphogenesis could thus parallel that of tailup/pannier in the fly mechanosensory development (Biryukova and Heitzler, 2005) and could display a conserved interaction of bHLH, Gata and Lim transcription factors in the regulation of mechanosensory development across phyla (Fritzsch, et al., 2007). In agreement with this hypothesis, Lmx1a is predominantly expressed in the non-sensory otic epithelium and Lmx1adr mutant mice show fusion and enlargement of sensory epithelia, dysmorphogenesis of the ear and disrupted histogenesis of sensory epithelia that eventually leads to the degeneration of hair cells. These data suggest that Lim domain factors indeed play a possibly conserved role in regulating the distinction between sensory and non-sensory epithelia in mechanosensory development across phyla. Further work is needed to unravel the details of the molecular interactions that are regulated by Lmx1a in the developing mouse ear.
Materials & Methods
Mice
Atoh1tm2Hzo mice were obtained from Dr. Huda Zoghbi (Bermingham, et al., 2001) and Lmx1adr/J mice from Jackson Labs and maintained in an AALAC approved facility under an IACUC approved protocol. Breeding and genotyping of the mice was as previously described (Bermingham, et al., 2001, Millonig, et al., 2000). Experimental animals were of mixed genetic stock. Timed breeding took place overnight, with midnight considered time 0.0; noon of the first day was considered as embryonic day 0.5 (E0.5). Postnatal day 0 (P0) was the equivalent of embryonic day 19 (E19) regardless of the actual birth date.
Detection of β-galactosidase activity
To detect β-gal activity, ears were dissected, briefly (30 min.) fixed in 4% paraformaldehyde/PBS, rinsed in phosphate buffer, and stained with X-gal. as previously described (Fritzsch, et al., 2005a). When required, we enhanced the X-gal. reaction using 2-photon photoactivation on whole mounts and sections (Matei, et al., 2006). Stained ears were mounted flat or alternatively, embedded in a soft epoxy resin, sectioned (3 µm) using a histology grade diamond knife (Dumont) , imaged using a compound light microscope (Nikon Eclipse 800) and captured using a Coolsnap camera and Metamorph software.
Immunohistochemistry & In Situ Hybridization
Primary antibodies were rat anti–mouse β-tubulin (Sigma #T6793, 1:800), Hoechst nuclear stain (Sigma), MyoVII (gift of T. Hasson, San Diego) and chicken anti BDNF (R&D Systems, 3AF248, 1:100). Whole mount in situ hybridization was carried out according to standard procedures (Pauley, et al., 2003) with digoxigenin-labeled riboprobes specific for Sox2, Fgf8, Gata3 and Fgf10. Anti-dig-AP antibody and BM Purple (Roche) colorimetric signal detection was used. Some whole mount reacted ears were subsequently embedded in epoxy resin, cut at 5–10 µm thickness, counterstained with toluidine blue and viewed with a Nikon E800 microscope using DIC.
Secondary Alexa 488, 543, and 634–conjugated antibodies (Molecular Probes) were used predominantly on whole mounted, microdissected sensory epithelia (Matei, et al., 2005a). Sections and whole mounts were imaged using a confocal system (Biorad 2000 mounted on a Nikon E800 or Zeiss LSM 510). Images were assembled into plates using CorelDraw software.
Lipophilic dye tracing
PTI lipophilic tracers (NV red, NV Maroon) were used for afferent and efferent neuronal fibers (Fritzsch, et al., 2005b). Briefly, dyes were inserted into central targets and the fibers were filled with the diffusible dye, epithelia were microdissected and viewed with a confocal system (Zeiss LSM 510).
SEM imaging
Ears were microdissected, osmicated, dehydrated and critical point dried as previously described (Ma, et al., 2000). Ears were mounted on stubs and imaged with a Hitachi SEM.
RESULTS
Lmx1a expression is concentrated in certain non-sensory epithelia patches
Lmx1a expression was shown to be rather widespread throughout the ear between E8.5 and E10.5 (Failli, et al., 2002), but these expression analyses were limited to only those early embryonic ages. We therefore extended these investigations of Lmx1a expression using in situ hybridization. At E10.5 virtually the entire otocyst was positive for Lmx1a (Fig. 1A) except for a small anteroventral quadrant, the area of prosensory formation (Farinas, et al., 2001, Fekete and Wu, 2002, Ma, et al., 1998). Over the next two days, Lmx1a expression became focused in the developing endolymphatic duct (Fig. 1C, F, G) and the lateral margin of the cochlea duct (Fig. 1G). Strong expression also persisted in the saccular roof (Fig. 1H,I, J), the ductus reuniens (Fig. 1H,I) and near pigment cells in the utricular roof and the canal cristae (Fig. 1H,J). In the cochlea, Lmx1a was immediately lateral to the developing organ of Corti and medial to the pigment cells of the stria vascularis (Fig. 1K). These data suggest that Lmx1a outlines certain non-sensory epithelia of the ear and may be involved in specifying sharp boundaries between sensory and non-sensory epithelia.
Fig. 1. Lmx1a expression undergoes dynamic changes in wildtype and mutants.
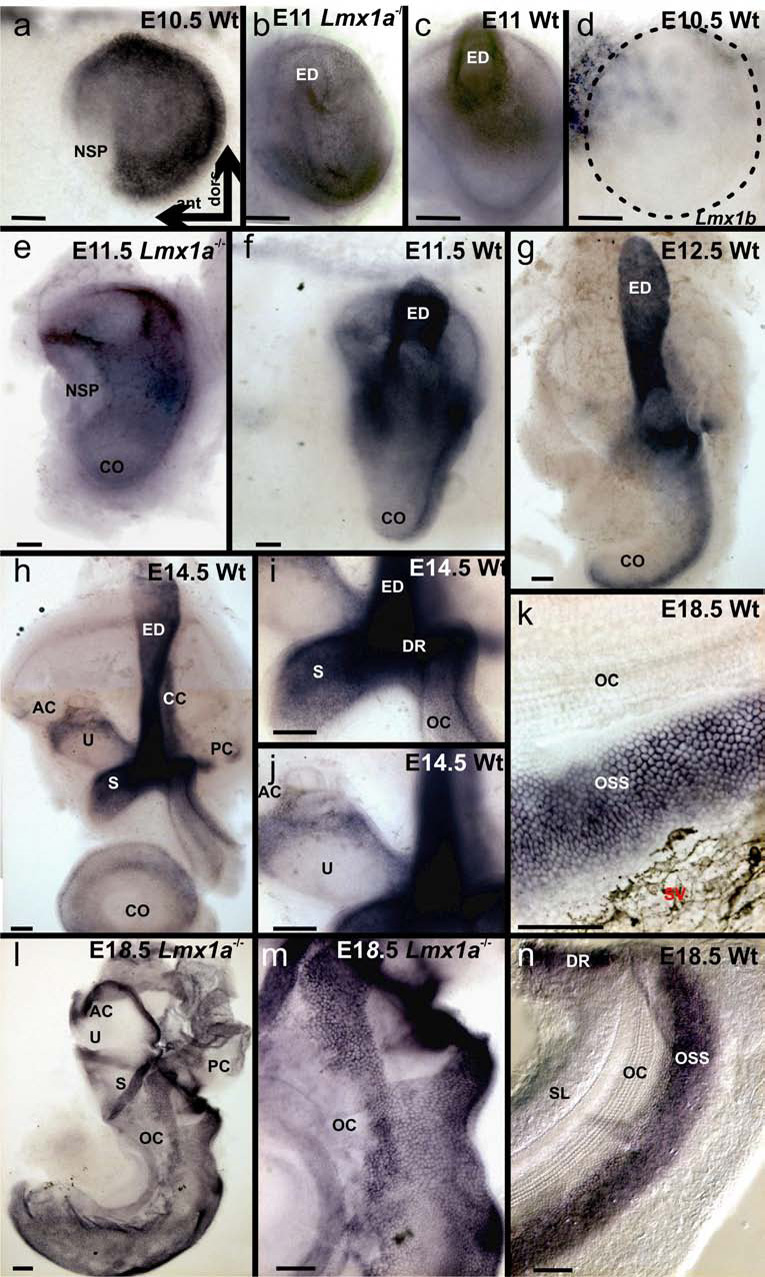
A. In the E10.5 wildtype, expression is in all but the neurosensory precursor epithelium (NSP). B., C. Compare the wildtype endolymphatic duct (ED) with its vestigial mutant counterpart. D. Lmx1b is not expressed in the E10.5 ear (dotted line), but is strongly expressed in the hindbrain (in and out of focus labeling top left). E., F. In the E11.5 mutant, Lmx1a expression is incompletely segregated to the lateral/posterior cochlea (CO) and no trace of an endolymphatic duct is apparent. G. At E12.5, expression is becoming confined to the ED, lateral cochlea and the non-sensory saccule. H.-J. By E14.5, expression centers on the ED and radiates to more-or-less constricted spacer epithelia between sensory epithelial territories. Most conspicuous of these is the ductus reunions (DR). K. At E18.5, cochlear expression is localized to the outer spiral sulcus (OSS). Melanocytes are conspicuous in the adjacent stria vascularis (SV). L.-N. In wildtype mice, the organ of Corti (OCin N.) is entirely free of Lmx1a expression. However, mutant Lmx1a mRNA is broadly expressed throughout the basal turn (L,M). All whole ears are oriented as in A. Scale bars are 100 µm.
Since Lmxa1dr is a nonsense mutation (Millonig et al., 2000), the presence of the mutated Lmx1a mRNA permits detection of alterations in Lmx1a expression patterns in these functional null mutants. Alteration of normal Lmx1a expression became evident at E11 in the mutant ears. Unlike the obvious concentrated endolymphatic duct expression in the wildtype, Lmx1a expression remained widespread in the mutant (Fig, 1B,C). The endolymphatic duct in Lmx1a mutants never developed beyond a rudimentary structure (Fig. 1E,F). The pattern of Lmx1a distribution in the mutants suggests a loss of segregation to non-sensory epithelia as evidenced by overlapping distribution of expression within the basal sensory region instead of clear segregation to the outer spiral sulcus (Fig. 1L–N). Although Lmx1a and Lmx1b diverged before the split of protostomia and deuterostomia, they both still share large areas of expression in the mammalian brain (Chizhikov et al., 2006). In order to determine if a similar overlapping expression pattern exists in the inner ear, we investigated the expression of Lmx1b. The well characterized expression of Lmx1b in the hindbrain and isthmus region was replicated, but no significant expression of Lmx1b was observed in the otocyst (Fig. 1D) or in later stages of the ear formation in wildtype or Lmx1a mutant mice. Based on these observations, Lmx1b could be eliminated as having a direct role in inner ear development. However, the strong and early expression in the adjacent hindbrain could indicate that Lmx1b expression might indirectly affect ear development.
We next investigated the distribution of Lmx1a expression in near radial sections of epoxy resin embedded, E18.5 Lmx1a ISH reacted ears (Fig. 2). These data show that in the mutant there is a clear medial to lateral organization of the cochlea. However, the cellular organization of the organ of Corti did not show the clear 1 row of inner and 3–4 rows of outer hair cells found in wildtype (Fig. 2 A–D). Consistent with our data on whole mounted ears, we find a considerable expansion of the expression area of the mutated Lmx1a mRNA beyond the lateral wall area of the wildtype. Expression could expand to the stria vascularis and even to Reissner’s membrane (Fig. 2A’–D’). It appears that in the absence of functional Lmx1a protein mechanisms that help focus the Lmx1a expression onto the narrow part of the lateral wall between Claudius cells and the stria vascularis do not work properly.
Fig. 2. Lmx1a expression is less restricted in mutants.
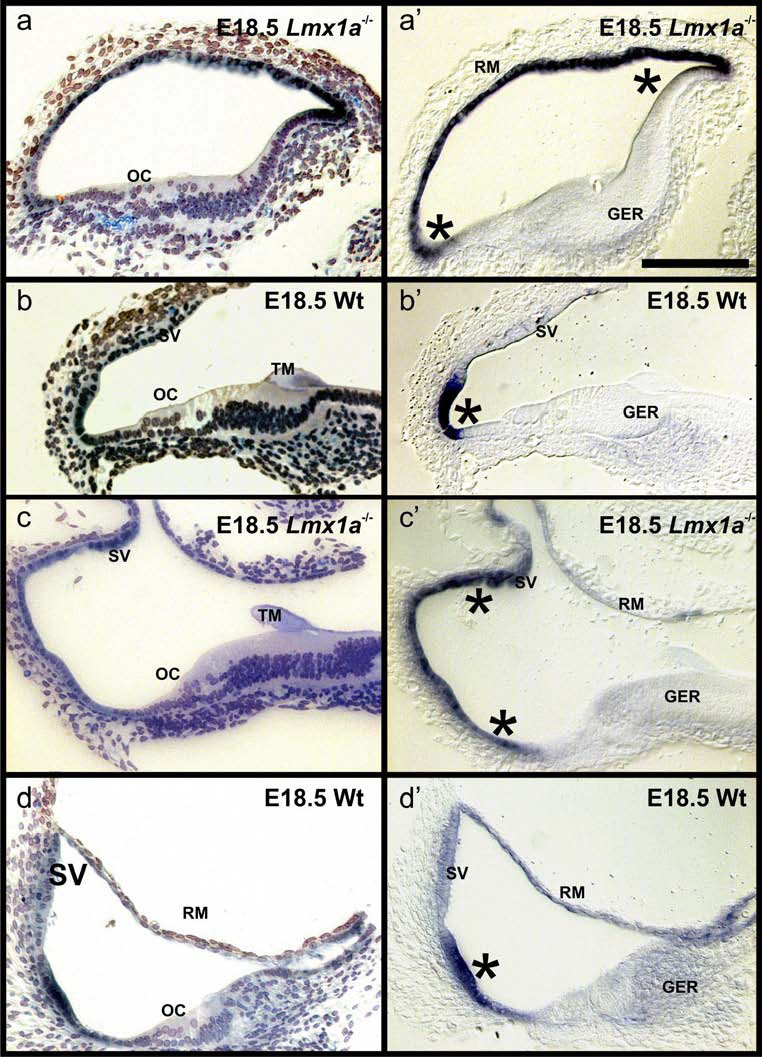
These sections show the distribution of Lmx1a ISH reaction product in wildtype (B,D) and Lmx1a mutant mice of embryonic day 18.5. The ears were reacted for whole mount in situ hybridization (see Fig. 1), embedded in soft epoxy resin and sectioned at 5–10 µm. These radial sections show a well organized organ of Corti (OC) with one inner and 3–4 rows of outer hair cells in the wildtype (B,D). In contrast, hair cells are disorganized in the Lmx1a mutant mice (A,C). Nevertheless, the main medial-to-lateral areas of the cochlea such as greater epithelial ridge (GER) with a tectorial membrane (TM), OC and lateral wall are distinct. In wildtype mice the Lmx1a in situ signal is in the lateral wall (asterisk) adjacent to the stria vascularis (SV; B’, D’). In contrast, in the Lmx1a mutant mice the strong Lmx1a in situ signal expands to include the stria vascularis (asterisks) or even Reissner’s membrane (RM; A’,C’). Bar in A’ indicates 100 µm for all images.
Mutation of the Lmx1a gene produces a unique inner ear phenotype
The altered Lmx1a expression pattern in dr mutants suggests a basis for the observed disruption of sensory epithelium segregation cues. Since the previous studies described only an inner ear dysmorphogensis in the Lmx1a mutant lines (Deol, 1964, Deol, 1983); http://www.informatics.jax._org/Lmx1a_alleles), we extended these preliminary observations to define the extent of this interference in sensory and non-sensory epithelium formation. The Atoh1tm2Hzo allele carrying the targeted LacZ reporter (Atoh1LacZ) (Fritzsch, et al., 2005a) was used to identify both differentiated and undifferentiated hair cell precursors and in turn assisted in defining the regions of the developing sensory epithelium.
Postnatal one and two week old (P7 and P14) wildtype mice had six discrete AtohLacZ positive sensory patches located in a complex three dimensional labyrinth of ducts and recesses (Fig. 3a). The papilla neglecta was barely detectable and consisted of 5–8 hair cells (data not shown). In contrast, P7 and P14 Lmx1adr/dr mutants had only a single, undivided sac that was wide in the region of the canal cristae and continuously tapered toward the apical tip of the cochlea. While each sensory epithelium of the wildtype ears resided within its own recess (three ampullae for the canal cristae, the utricular and saccular recesses separated by the constricted utriculo-saccular foramen and the cochlear duct separated from the saccule by the ductus reuniens), none of these non-sensory constrictions were found in Lmx1a mutants. Nor was the constriction that normally separates the posterior ampulla from the cochlea. The data suggest that Lmx1a is, directly or indirectly, involved in the morphogenesis of the specific constrictions and ducts that separate the individual sensory epithelia. These mutant ears also lacked an endolymphatic duct/sac (Fig. 1E, L, 3B).
Fig. 3. Postnatal Lmx1a mutant ears reveal disorganized sensory epithelia.
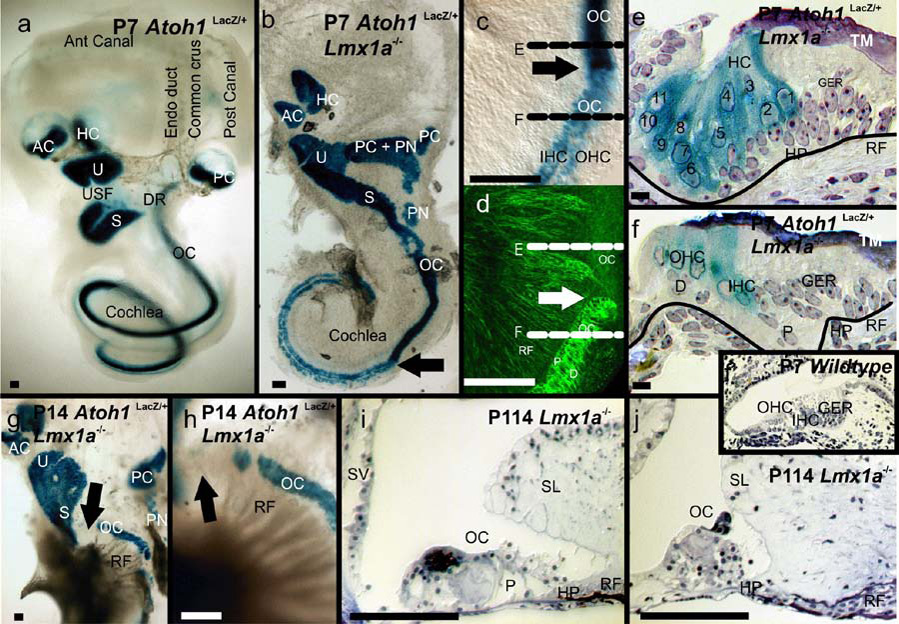
A. In the wildtype, six discrete sensory epithelia are separated from one another by constricted, non-sensory epithelial spacers. Two of three semicircular canals and an endolymphatic duct can be identified as shadows. B. In the Lmx1a mutant, the anterior and horizontal cristae are separated by a common cruciate eminence, while the posterior crista is grossly enlarged and extended by the presence of both embedded and detached papilla neglecta-like sensory epithelia (PN). The utricle, saccule and cochlear sensory epithelia appear continuous with one another. The basal turn of the organ of Corti appears as a uniform band of hair cells that is discretely separated (arrow) from an apex in which inner and outer hair cells can be identified. C, Higher magnification view of the arrowed transition in B. The densely packed hair cells of the basal cochlea are above the arrow and the apex below it. The black lines indicate the planes of section in E and F. D. Same tissue as C, but stained for beta-tubulin to reveal nerve fibers and pillar and Deiter’s cells. Note the absence of tubulin-containing pillar and Deiter’s cells in the base and their conspicuous appearance at the transition to the apex. E. A medio-lateral near radial section across the base of the mutant cochlear duct as indicated by the dotted line in C. Note the presence of a tectorial membrane (TM). Up to 11 rows of hair cells are marked by the blue Atoh1LacZ reaction product, unlabelled supporting cells are present below the hair cells. F. A recognizable organ of Corti with inner and outer hair cells is present in the apex (compare with wildtype insert). G,H Beginning around P14, hair cells disappear, starting in the base (arrow). In contrast, nerve fibers continue to mature as indicated by the osmium tetroxide stained myelin (black fibers). I., J. By P114 the organ of Corti is grossly disorganized, lacks identifiable hair cells and shows massive aberrations in almost all associated epithelia such as the spiral limbus (SL) and the stria vascularis (SV). These data suggest that absence of Lmx1a is ultimately incompatible with hair cell maintenance though that its absence does not interfere with their initial formation. Scale bars are 100µm.
Fusion of inner ear sensory endorgans
At P7/14, only three distinct sensory epithelia instead of the usual six were found in Lmx1a mutants (Fig. 3A,B). Near the anterodorsal pole was a single crista consisting of two unequal sized hemicristae separated by a non-sensory cruciate eminence, suggesting that the horizontal crista had fused with one hemicrista of the anterior crista. Likewise, the posterior crista consisted of two asymmetric hemicrista separated by a cruciated eminence. The enlarged hemicrista sometimes had a distinct extension or patches extending into the cochlear duct (Fig. 3B), which might represent a fusion of the posterior canal hemicrista with the papilla neglecta. The papilla neglecta generally consists of only of a few hair cells in mammals but it can be very large in elasmobranches and becomes the comparatively large amphibian papilla in frogs (Fritzsch and Wake, 1988, Lewis, et al., 1985).
In addition to these two enlarged cristae, the Lmx1adr ear contained one large continuous band of hair cells, with a very large patch adjacent to the anterior crista that we tentatively identified as a utricle-like region based on its topology (Fig. 3B; 5A). This utricular area blended into a somewhat smaller, elongated patch that we identified as a putative saccule. The saccular region continued into a progressively tapering band of hair cells that stretched to the apex of what appeared to be a shortened cochlear duct with the organ of Corti. While the hair cells of this patch were continuous, the pattern of innervation indicated four distinct regions in this epithelium (Fig. 3B,G; 4C,). The utricle region was innervated by fibers that were accompanied by others that continued on to the anterior crista, as in wildtype animals. Likewise, the saccular portion received fibers from both the superior and inferior vestibular ganglion as in wildtype (Fig. 4C). In contrast, the cochlea organ of Corti, showed two distinct regions with respect to its innervation: The base received only a patchy and somewhat reduced density of nerve fibers whereas the apex was densely innervated with radial fibers like wildtype (Fig. 3D; 4A,B,C). These fibers did, however, arise from a typical spiral ganglion.
Fig. 5. Atoh1LacZ staining and Prox1 immunodetection show disorganization of hair and supporting cells.
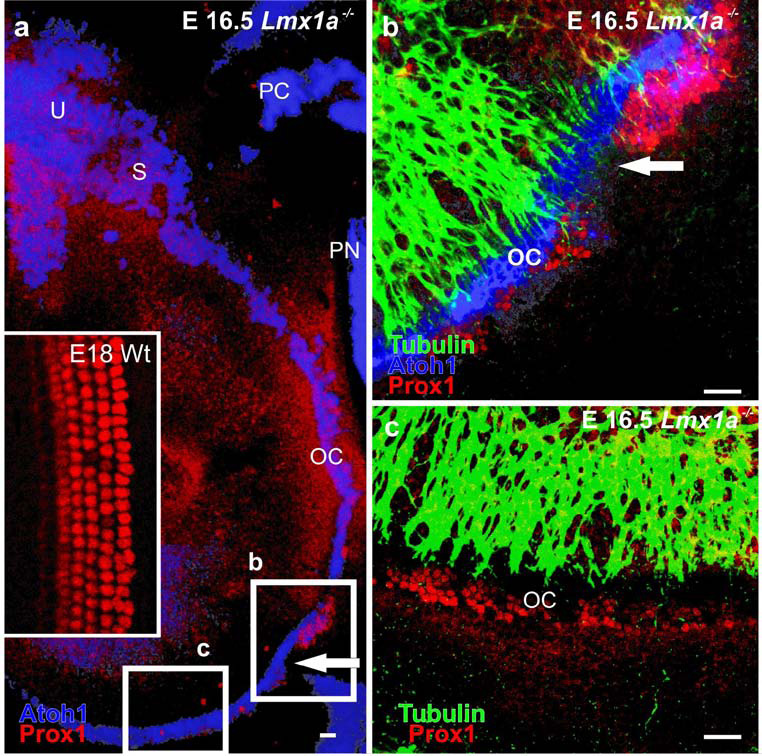
In these E16.5 Lmx1a null confocal micrographs, Atoh1lacZ is UV activated and false colored blue. Prox1 is immuno-stained red. A. The fields of B and C are boxed. The inset shows a wildtype Prox1 supporting cell staining pattern. Note that the supporting (pillar, Deiter's) cells are precisely organized. B. The basal-apical transition is arrowed. The basal OC is to the upper right of the arrow. Note that an unorganized mass of Prox1 stained cells is located just basal to the transition. Blue hair cells are located medial to the Prox1 positive cells. C. The mutant E16.5 cochlear apex demonstrates a disorganized, but otherwise continuous band of supporting cells in the OC. Scale bars are 100µm.
Fig. 4. Late innervation and sensory epithelia are disorganized.
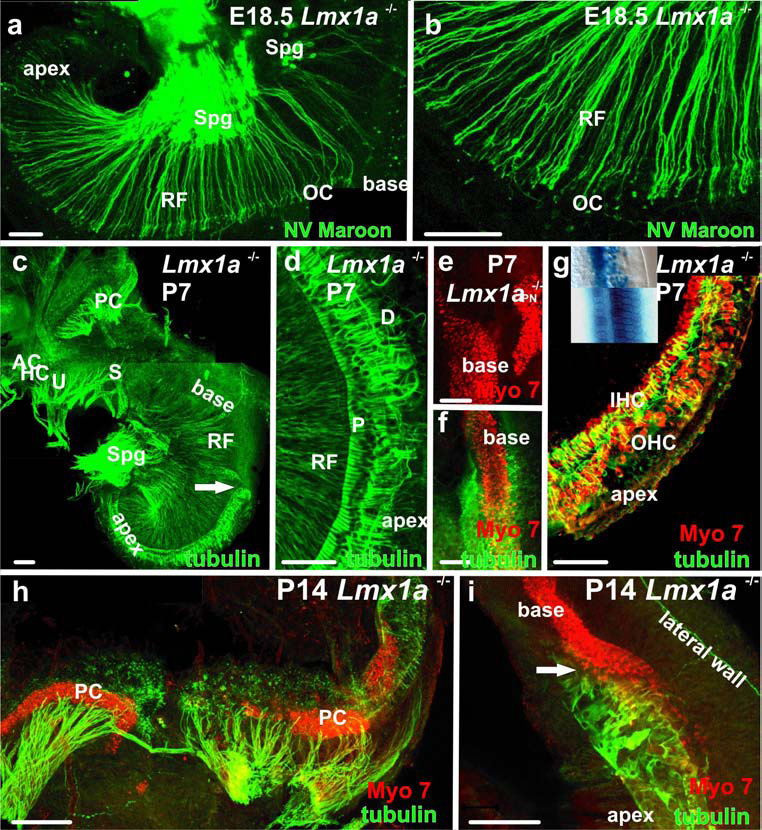
A, B Afferent radial fibers to the base of the E18.5 mutant cochlea stained with the lipophylic dye, NV Maroon (A,B) and anti-acetylated tubulin (C,D,F-I). Note that the fibers enter the organ of Corti but do not extend to the outer hair cells as they would in a comparable wildtype ear. There is a notable difference between the packing density of radial fibers in the base and the apex consistent with the reduced presence of spiral ganglia in the base. C There is a complete absence of pillar/Deiter’s cells basal to the cochlear transition (arrow) and clear distinction of innervations between the densely innervated saccule and the poorly innervated basal turn of the cochlea. D. Disorganized Deiter’s cell processes (D) in the apex of the ear in A (now stained for β-tubulin) show longitudinal extension along the cochlea. E. Myo7a staining shows close proximity of OC and papilla neglecta hair cells in the basal cochlea of a P7 mutant. F. The basal/apical cochlear transition of the ear in C., with β-tubulin stained supporting cells and Myo7a stained hair cells. Note that the packing density of hair cells is inversely related to supporting cell labeling. This pattern is maintained as long as hair cells can be labeled by Myo7a antibodies (I). G. Apical cochlea of the ear in C/D. IHC’s, and OHC’s can be recognized, but the organization is inferior to that of the wildtype (see Atoh1lacZ stained hair cells in the inset, mutant apex above, wildtype below). H. The grossly enlarged and elongated posterior crista of a P14 mutant shows fibers targeted to the Myo7a positive hair cells. Scale bars are 100 µm.
Beyond the differences in density of innervation, older Lmx1a mutant mice showed tubulin-positive pillar and Deiter’s cells only in the apex (Fig. 3D; 4D,F, G,I). The absence of these cells in the base correlated with a different distribution of hair cells. In the apex, both inner and outer hair cells could be identified and were separated from one another by unusually positioned but otherwise typical pillar cells (Fig. 3C–F, 4 E–I). It was therefore identifiable as an organ of Corti-like organization of cells. In contrast, in the base, hair cells formed multiple rows of uniform cells that defied any histological characterization as inner or outer hair cells (Fig. 3C,E; 4 F,I). Despite their unusual pattern of distribution, all were hair cells as revealed by both Atoh1LacZ histochemistry (Fig. 3B,C,G) and immunofluorescent detection of the hair cell-specific marker, myosin VIIa (Myo7a) (Fig. 4D,F,H,I). The common utriculo-sacculo-cochlear sensory epithelium (Fig. 3B,5A) of dr mutant mice is thus reminiscent of the common macula of jawless vertebrates (Lewis, et al., 1985). This common macula is then the precursor of the several sensory epithelia that segregate from one another during the course of development and evolution (Fritzsch, 2003, Fritzsch, et al., 2002). Clearly, Lmx1a is a major molecular player in this process during development and may play the same role during ear evolution.
Hair cells are lost in the adult Lmx1a mutant cochlea
Using Atoh1LacZ histochemistry we found gaps in the distribution of cochlear hair cells as early as P14 (Fig. 1 G, H; arrow). Notably, these gaps tended to appear at the boundaries between the utricle, saccule and basal region of the cochlea. Likewise, the apex showed a patchy distribution of hair cells in the older epithelia (Fig. 3G). Investigating the cochlear histology at 2–3 months of age or older revealed a complete loss of all hair cells and severe dysmorphogenesis of the organ of Corti, including the adjacent areas such as spiral limbus, Reissner’s membrane and stria vascularis (Fig. 3I,J). Few hair cells remained in the vestibular organs (data not shown). These data suggest that Lmx1a is not only playing a major role in early histogenesis and morphogenesis of the ear but is also essential for long term maintenance of hair cells.
The amalgamated cochlear-gravistatic endorgan boundary
In a series of experiments, we next characterized the unique morphological and histological phenotype of the fused cochlear-gravistatic endorgan. Scanning electron microscopy (SEM) was used to define the apical specializations in the area where the saccular and cochlear regions merged (Fig.6A,B). Despite our best efforts we could not mechanically remove the tectorial membrane near this transition site. Importantly, the presence of a tectorial membrane defines a molecular transition between the saccule (no tectorial membrane) and the cochlea (tectorial membrane) despite continuity of hair cells. Closer examination revealed distinct vestibular-type hair cells in the saccular and utricular regions (Fig. 6C,D,E). Near the tectorial membrane we found medial cells to display vestibular-type, long, organ pipe-like stereocilia. In contrast, more C-shaped, shorter stereocilia reminiscent of organ of Corti inner hair cells prevailed more laterally, indicating an introgression zone of vestibular and organ of Corti type of hair cell differentiation. Ventral to this zone, radial histological sections (Fig. 3E) showed that 8–11 rows of hair cells were present in the basal part the cochlear region. Interestingly, the polarity of these inner hair cell-like cells was normal in the more medial cells but the more lateral cells were rotated 90 degrees toward the base, much as in mice mutant for the Foxg1 (Pauley, et al., 2006) and Neurog1 (Ma, et al., 2000) genes.
Fig. 6. Continuity of hair cells in late embryos as revealed by Atoh1LacZ staining and SEM.
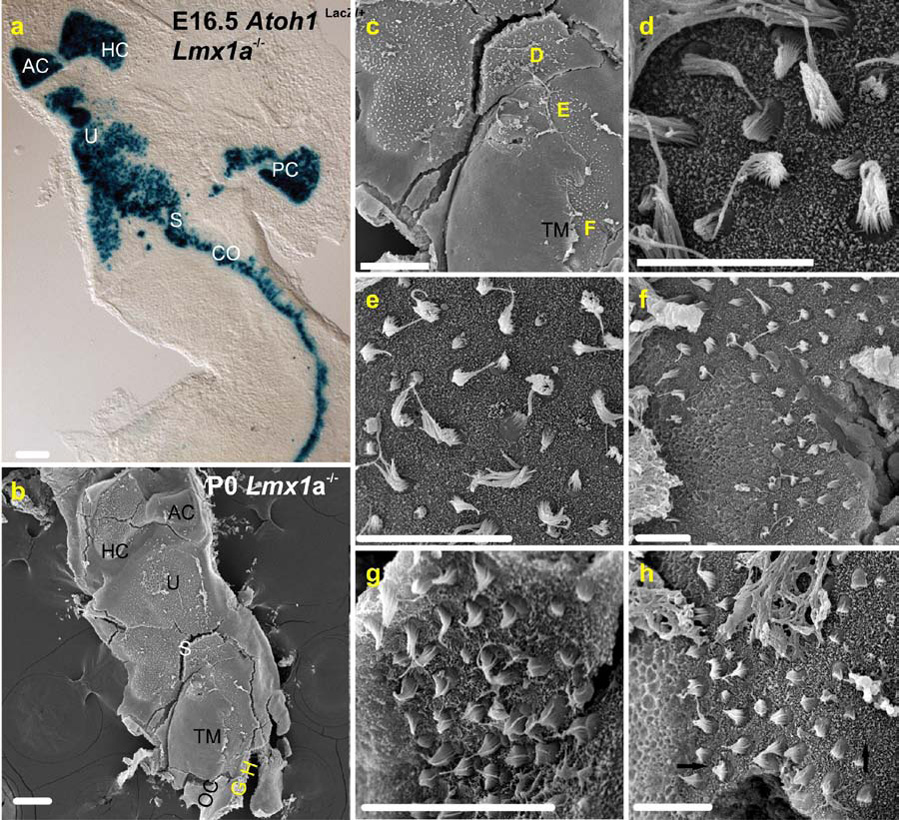
A The sensory epithelia of the E16.5 Lmx1a mutant ear revealed by Atoh1lacZ staining of hair cells. Note that the juxtaposition of sensory epithelia found at P7 in Fig. 1B is already apparent at E16.5. B. A P0 Lmx1a mutant ear oriented similar to that in A and viewed in the scanning electron microscope. The positions of the micrographs in G and H are indicated at the bottom of the micrograph. C. A higher power micrograph centered on the “S” (saccule) of B. The positions of micrographs in D–F are indicated, as is the smooth, flat surface of the tectorial membrane (TM). D. Micrograph of the saccular macula. The sterocilia come in long and immature short variations of the pipe-organ arrangement characteristic of vestibular hair cells. E. A region of the basal cochlea (note the adjacent tectorial membrane in C) close to the saccule. A mix of hair cells with long, vestibular-like and short, C-shaped cochlear inner hair cell-like sterocilia are present. F. Further toward the apex, but still in the basal cochlea, vestibular type hair cells are found adjacent to the tectorial membrane, and cochear-like hair cells further lateral. G. Still in the base, but further toward the apex, multiple “rows” of hair cells all possess short, cochlea-like sterocilia bundles. H. Adjacent to G. in the base, variously oriented cochlear hair cells are present (arrows indicate polarity). For abbreviations, see Fig.1. Medial is left and lateral right in Fig’s. 3 D–H. Scale bars are 100µm.
This non-cochlear organization of hair cells and supporting cells in the cochlea-like basal region might result from mis-expression of transcription factors uniquely associated with the organ of Corti. One transcription factor associated with supporting cells is Prox1 (Bermingham-McDonogh, et al., 2006, Fritzsch, et al., 2008, Puligilla, et al., 2007). Prox1 distribution in Lmx1adr mice showed a marked deviation from the wildtype, even in the apex that was more typical in its supporting cell and hair cell organization (Fig. 5A–C, 7A–D). Instead of a regular pattern of supporting and hair cells, Prox1 positive cells in apex of Lmx1a mutants showed irregular distribution with an inconsistent number of rows. To obtain more information about co-localization of hair cells and Prox1 positive supporting cells, hair cells were labeled by BDNF immunodetection. In Lmx1a mutants, the innermost row of hair cells showed strong BDNF staining, much like that observed in wildtype mice. However, unlike the wildtypes, BDNF was just above background levels in the most lateral outer hair cells of the mutants (Fig. 7B”). Most interesting was the mutant distribution of Prox1 near the transition from the near normal apical organ of Corti to the more vestibular-like basal region. Prox1 could not be detected in the base, except for a large aggregate of labeled cells near the cochlear-saccular transition zone (Fig. 7C,D). We sought to determine how early this organization became apparent and found it in place as early as E16.5 using Prox1 immunodetection and Atoh1LacZ labeled hair cells (Fig. 5). As in later stages there was little to no expression of Prox1 throughout the base except at this transition zone (Fig. 5, 7). In wildtype mice, outer hair cells are regularly interspersed with Prox1 expressing Deiter’s cells (Fig. 7 A”’). However, in Lmx1a mutants at these stages, all hair cells were medial to the Prox1 expression area, in both the apical and basal regions (Fig. 7 C”’). Combined these data suggest that Prox1 and Atoh1 expressing cells partially segregate in the absence of Lmx1a expression implying that Lmx1a functions in setting up the topology for those two cell types and their specific distribution along the cochlea. A possible candidate could be diffusible factors emanating from the lateral spiral sulcus (Fig. 1K; 2 B’) where the Lmx1a expression is most prominent.
Fig. 7. Hair cells, supporting cells and nerve fibers are disorganized.
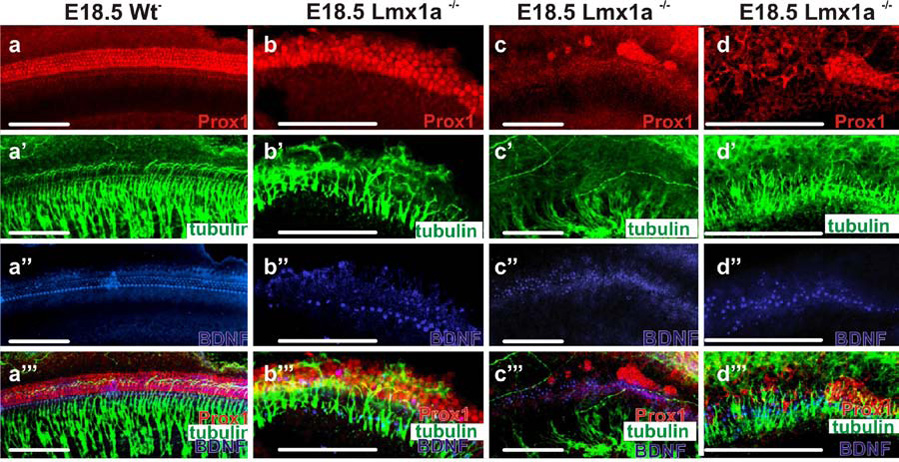
E18.5 organs of Corti immunohistochemically (ImHC) stained for Prox1, β-tubulin, BDNF, and all three combined (rows A-A’’’), in the wildtype, mutant apex, mutant base, and high mag. mutant base (columns A–D). A–D Prox1 ImHC. there is an orderly expression in supporting cells in wildtype, some organization in the apex in the mutant but clumping of PROX1 stained supporting cells in the mutant base (see also A’’’–D’’’). A’–D’ β-tubulin ImHC. Note that nerve fibers in the base stop short of the OC (see also A’’’ – D’’’). A’’–D’’’ BDNF ImHC stains IHC’s strongly and OHC’s weakly in the wildtype. A row of IHC’s can be recognized in the mutant apex, but strongly stained cells are scattered in the base. A’’’ – D’’’ The superimposed images show that hair cells and supporting cells overlap and orderly nerve fibers project between the supporting cells in wildtype whereas hair cells and supporting cells do not overlap in mutants with disorganized fibers projecting between the labeled supporting cells (apex) or reaching hair cells (base) Scale bars are 100µm.
The distinct basal cellular organization can be defined by gene expression
We next examined Gata3, Fgf8 and Fgf10 transcript distribution to further elucidate the extent of the basal region disorganization at the level of gene expression. The distribution of Gata3, a zinc finger transcription factor, is uniquely associated with the cochlea, but not observed in the vestibular (specifically, saccular) sensory epithelia (Karis, et al., 2001). It is known to interact with Lim-homeodomain and bHLH factors needed for cell fate specification (Matthews and Visvader, 2003). In addition, the two fibroblast growth factors have distinct expression patterns where Fgf8 is associated with the inner hair cells and Fgf10 is expressed in the greater epithelial ridge just adjacent to inner hair cells (Pauley, et al., 2003, Pirvola, et al., 2002). Expression of both Fgf10 and Gata3 showed that the base region gradually merged into the apex with respect to these markers, but distinct from the saccular region (Fig 8 A,B,E,G). There was also an expansion of Gata3 expression across the cochlea in Lmx1a mutants compared to wildtype littermates (Fig. 8 E,G). In Lmx1a mutants, Fgf8 expression expanded across most of the several rows of hair cells in the basal organ of Corti, whereas only inner hair cells were labeled in the apical region, as in wildtype littermates (Fig. 8 C, D ,F). Most interestingly Lmx1b, which shares a high sequence homology with Lmx1a, is a known regulator of Fgf8 in the isthmic region of mice and zebrafish (Alexandre, et al., 2006, Guo, et al., 2007, O'Hara, et al., 2005). Since our data show an expansion of Fgf8 expression in the base (Fig. 8 C) but no Lmx1b expression was detected in the ear, this suggests that wildtype Lmx1a somehow restricts Fgf8 expression in the basal cochlea to inner hair cells. This might result from, its early expression in this region during the otocyst stage, or from diffusible factors released as a result of its later expression in the outer spiral sulcus (Daudet, et al., 2002).
Fig. 8. Fgf10, Fgf8 and Gata3 mRNA expression reveal disorganization of the organ of Corti.
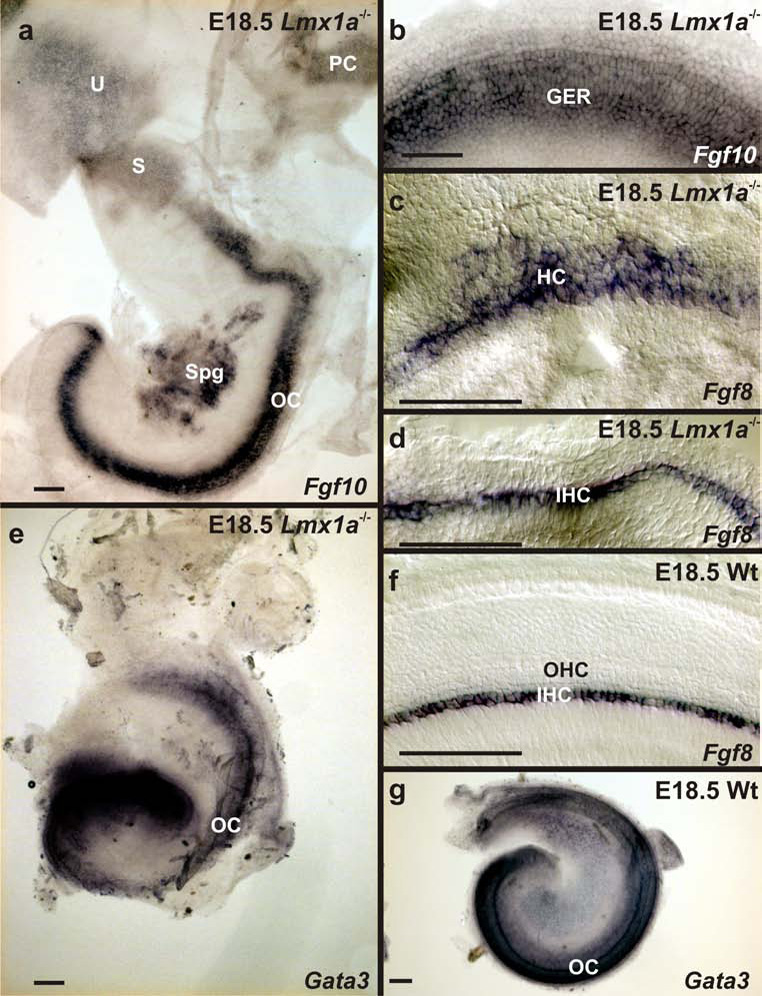
A. In the E18.5 Lmx1a mutant, Fgf10 is strongly expressed in the entire organ of Corti, but more weakly in the vestibular sensory epithelia. B. Mutant Fgf10 expression extends from the greater epithelial ridge (GER) laterally to the inner hair cells in a pattern similar to that of the wildtype. This pattern is the same in the base and the apex. C., D. Fgf8 expression in the base (C) and apex (D) of the mutant. Note expression is confined to the inner hair cells (IHC) in the apex, but scatters among the excess hair cells of the base. F. In the wildtype, Fgf8 expression is neatly confined to the inner hair cells. E. In the E18.5 mutant, Gata3 expression is present in the basal cochlea, but not the saccule. G. Similar Gata3 expression in the wildtype cochlea. Sgl, spiral ganglion; OHC, outer hair cells. Scale bars are 100µm.
The Fgf8 data support the impression derived from the Lmx1a mutant SEM data that all hair cells in the basal portion of the cochlear region develop an inner hair cell phenotype. Furthermore, alterations in Fgf8 and Gata3 expression as well as the near complete absence of Prox1 expressing cells in the base suggest that these too play roles in generating the altered phenotype of basal turn hair cells. Consistent with the Lmx1a expression pattern (Fig. 1N), Lmx1a is apparently necessary to define the lateral boundaries of the organ of Corti (which is more irregular in these mutants) and enhance the interaction of supporting cells and outer hair cells. The absence of functional Lmx1a protein may underlie the disruption of outer hair cells and Deiter’s cells observed in the dr mutant mice (Fig. 3), possibly through indirect effects on the integrity of sensory/ non-sensory boundaries. Proper cellular restriction of early expression of the Lmx1a transcription factor appears to be necessary for coordinated development of the organ of Corti.
The utricle, saccule and organ of Corti never segregate during development
Given that our Lmx1a expression analysis suggests a possible role in ear formation as early as E11 (Fig. 1B,C), the initial upregulation of Atoh1LacZ was compared in Lmx1a mutants and wildtype animals (Chen, et al., 2002, Matei, et al., 2005a). As early as E14.5 the hair cells of the six epithelia of the wildtype ear were distinctly labeled (Fig. 9A). In the mutant, and in contrast to later stages, three cristae could be recognized. However, a single cochlear-gravistatic endorgan was already in place, though distinct hair cell patches were observed within the common endorgan. Specifically, there was an area of constricted hair cell formation indicating the utricular/saccular regional transition Fig. 9B,C) and the cochlea showed little to no upregulation of Atoh1-LacZ in the base. In spite of this absence of staining, a basal cochlear prosensory precursor epithelium could be identified using differential interference microscopy (Fig. 9C). These data suggest a delay in hair cell maturation in the base of the organ of Corti that could contribute to the misexpression of the several of the factors we have already described above and contribute to the histological defects observed in the basal region of the organ of Corti in Lmx1a mutant mice.
Fig. 9. Atoh1-lacZ staining for hair cells and tracer dye studies show initial segregation of sensory epithelia and their innervation.
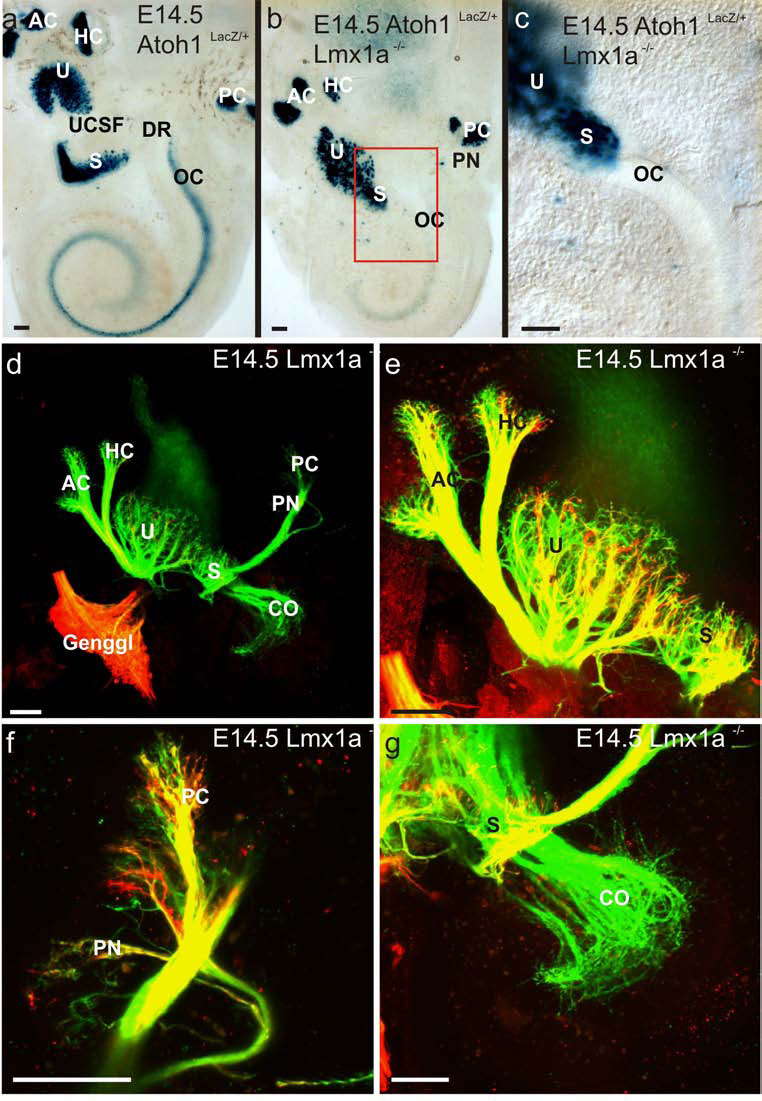
A. Wildtype Atoh1lacZ stained ear. Anterior left; dorsal up. B. An identically stained mutant ear. Note that the cristae are separated at this age and the posterior crista is not grossly enlarged (though a tiny papilla neglecta (PN) is present). The utricle and saccule are, however, joined. C. Higher magnification of the red, boxed field in B. The basal termination of the unstained though translucent organ of Corti (CO) overlaps the stained HC’s of the saccule. D-G. Lipophylic dye tracings in E14.5 mutant ears. The red dye was placed in the solitary nucleus and descending vestibular nucleus. The dye then backfilled collaterals to the vestibular sensory epithelia as well as sensory neurons of the 7th cranial nerve in the geniculate ganglion (Genggl). The green dye was placed in the (otic) efferent nerve fibers near the floor of the fourth ventricle to both the vestibular and cochlear sensory epithelia. D. Overview in which the green channel dominates. All sensory epithelia receive efferents. E. Vestibular afferent (red) and efferent innervation (green) to the anterior crista, horizontal crista, utricle and saccula are almost normal except for the limited segregation of the saccule form the utricle. F. Innervation to the posterior crista is abnormally widespread and includes a large branch to the papilla neglecta. G. The innervation to the basal cochlea lacks a vestibular (yellow) component. Scale bars are 100 µm.
In order to verify the presence of distinct subcompartments within the cochlear-gravistatic epithelium vestibular afferents were labeled from the brainstem and vestibule-cochlear efferents were labeled from rhombomere 4, where the olivocochlear bundle crosses (Bruce, et al., 1997, Fritzsch and Nichols, 1993). We had previously shown that with such double labeling, vestibular and cochlear fibers could be distinguished throughout development (Tessarollo, et al., 2004). As expected, brainstem vestibular projections labeled all afferents to vestibular organs and showed discrete innervation of a large utricular area and a smaller saccular portion (Fig. 9D, E). In contrast, the cochlear region received only efferent fibers and was thus identifiable based on this specific innervation. While anterior and horizontal cristae were normal in their innervation pattern, the posterior crista innervation was expanded by fibers targeting the papilla neglecta In summary, hair cell formation in the cristae and papilla neglecta of Lmx1a mutant mice starts as discrete patches, which only later fuse into composite structures. In contrast utricle, saccule and organ of Corti form as a common sensory epithelium. This common epithelium nevertheless shows differences in hair cell organization, maturation and innervation that warrant labeling them as run-on precursors of utricle, saccule and organ of Corti.
The unique phenotype of the Lmx1a mutant is foreshadowed by early alterations in Sox2 expression
In the chick (Giraldez, 1998), as in mice (Faille et al., 2002), Lmx1a has been shown to be excluded from the neurogenic region of the otic placode. In addition, it was found that inhibiting the Notch signaling pathway allowed the spread of Lmx1a expression into the neurogenic region (Abello, et al., 2007). This was reminiscent of the ‘non-neural’ Tbx1 gene of mice, that when mutated allowed neural genes, including Neurog1, to expand their expression domains, and when overexpressed caused the retreat of neural gene expression (Raft, et al., 2004). To obtain further insights into the molecular organization of supporting cells, the distribution of Sox2 was next studied. Sox2 is necessary for prosensory specification (Fritzsch, et al., 2006a, Holmberg, et al., 2008) and is later restricted to expression in supporting cells (Kiernan, et al., 2005). We therefore sought to determine whether prosensory gene expression would expand into non-neural territory in the Lmx1a null mice.
In E10.5 wildtype animals Sox2 expression was in future sensory areas of the antero-ventral quadrant and in the anlage of the posterior crista (Fig. 10A). This general pattern was retained in the mutant, but the areas of expression were greatly expanded, with more diffuse margins. This was most apparent in the region of the posterior crista (Fig. 10B). By E11.5, however, while expression in the wildtype remained strong and neatly confined to future sensory areas, that in the mutant remained broader in the region of the posterior crista (Fig. 10C,D). It was noted that segregation of the anterior cristae from the utricle was completed in both wildtype and mutant mice. In contrast no horizontal crista prosensory patch appeared as a discrete entity in the Lmx1a mutants (Fig. 10C,D). Both wildtype and mutant showed a continuous expression band with focal increases in intensity of the utricle, saccule and organ of Corti (Fig. 10C,D). By E14.5, all six epithelia were distinct in the wildtype, but the mutant showed a combined anterior and horizontal crista, a combined posterior crista and papilla neglecta (including the extension along the cochlear lateral wall) and the fused cochlear-gravistatic endorgan. Most interesting were the pattern and intensity of Sox2 expression in the cochlear region compared to the contiguous saccular portion. In both wildtype and mutant the cochlear expression was much reduced in intensity compared to the saccule. In addition, the cochlear expression was extremely broad in the base of the mutant and much less focused than in wildtype (Fig. 10E, F). This broader and more diffuse pattern of expression persisted in the mutant base through E18.5. The mutant apex developed a focused expression reminiscent of the entire wildtype cochlea (Fig. 10H,I,J,K).
Fig. 10. Sox2 mRNA expression shows Lmx1a defines early prosensory patch development.
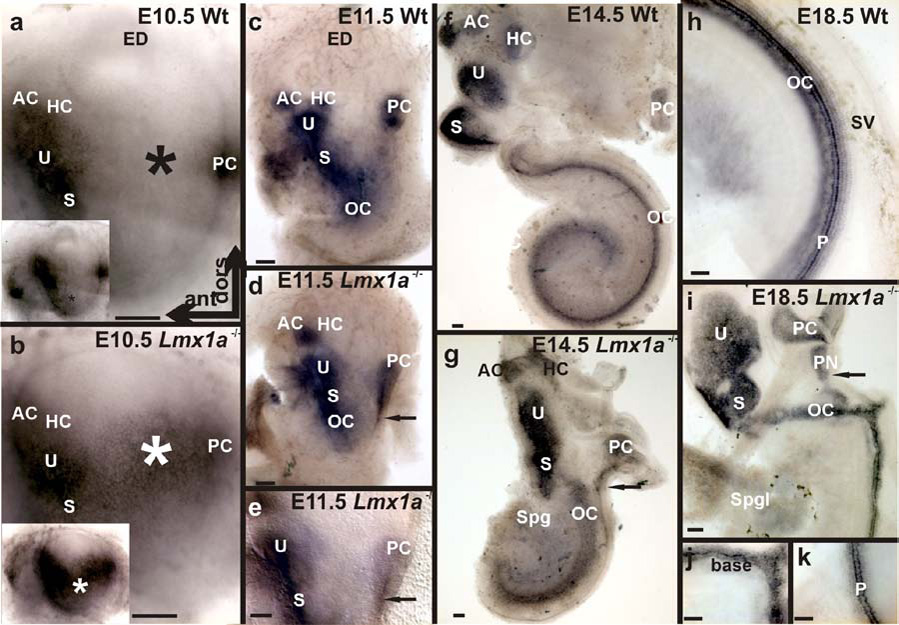
A., B. Sox2 expression is similar, but broader in the mutant without a distinct gap between posterior and anterior prosensory patch expression (asterisk in A,B and lower mag. insets). C.-E. At E11.5 wildtype expression is localizing to individual sensory epithelia (Note separate AC and HC), while in the mutant the AC and HC are joined. Note also the PC/PN extending ventrally toward the cochlea (arrows in D, E, G, I). F,G. By E14.5 the wildtype sensory epithelia are clearly segregated (by Lmx1a expressing epithelia – compare Fig. 8H.), while continuities and near continuities are apparent in the mutant. H. In the E18.5 wildtype cochlea, Sox2 expression is most intense in the inner pillar cells. I. Mutant Sox2 expression identifies the fused U-S-C and enlarged PC-PN. J., K. At E18.5, Sox2 expression in the mutant cochlear base is broad and diffuse, while that in the apex (K.) is, like the wildtype, focused on the inner pillar cells (P). All whole ears oriented as in A. Scale bars are 100 µm in A–G and 50 µm in H–K.
In summary, the wildtype and Lmx1adr mutant exhibited major differences in initial hair cell formation as revealed by Atoh1LacZ staining (Fig. 9B) and in prosensory patch formation as revealed by Sox2 expression (Fig. 10G). Whereas prosensory patch formation maintained from its onset the fusion/combination of sensory epithelia, hair cell formation initially showed all six sensory epithelia as discrete entities embedded in the unsegregated prosensory patches specified by Sox2. These differences suggest that initial Atoh1 upregulation as well as cell cycle exit of hair cells (Matei, et al., 2005b) and expression of Sox2 might be under different regulatory control. However, Atoh1 expression and initial cell cycle exit take place in the Sox2 expression domains. These data also suggest that in the mutant, it is the initial expression changes in Sox2 that foreshadow the later expansion and disorganization of hair cells in the mutant cochlea and the overgrowth of the papilla neglecta. It should also be noted that in the wildtype animal an Lmx1a expressing caudal constriction separates the posterior crista from the cochlea and thus contributes to the formation of the Lmx1a expressing ductus reuniens. This constriction remains rudimentary, and the ductus nonexistentin the Lmx1a mutant, while the papilla neglecta overgrows the site preserved by the failure to form the constriction. Thus, in the wildtype, an enlarged papilla neglecta does not form and fuse with the posterior crista both because Lmx1a expression confines Sox2 expression and because it creates a non-sensory constriction at the site where the enlarged papilla would form in the mutant.
DISCUSSION
Lmx1a mutants show that non-sensory otic epithelium facilitates ear morphogenesis
Our Lmx1a expression data show a unique association with specific areas of non-sensory otic epithelium in older ears (>E10.5). These Lmx1a expressing non-sensory epithelia are involved in:
Separating and constricting the endolymphatic sac from the saccule by formation of the endolymphatic duct,
Separating and constricting the utricle from the saccule by forming the utriculo-saccular foramen, and
Separating and constricting the cochlea from the saccule and posterior crista by forming the ductus reuniens,
None of these non-sensory constrictions form in Lmx1a null mutants, implying that Lmx1a plays a direct or indirect role in all of these morphogenetic events and that these events are driven in part by the Lmx1a expression in the non-sensory epithelia. In addition Lmx1a is expressed in the outer spiral sulcus separating the organ of Corti from the stria vascularis (Fig. 1K; 2A–D). It is possible that a lack of Lmx1a expression adjacent to developing sensory epithelia is interfering with signaling from the sensory epithelia to govern coordinated morphogenesis of the non-sensory otic epithelia. Alternatively, Lmx1a expression might initiate the secretion of diffusible factors(s) from the non-sensory otic epithelium required for proper sensory epithelia maturation.
The hypothesis that Lmx1a interferes with a sensory epithelium signal is in line with the emerging concept that crista epithelia express diffusible factors such as Fgf10 and BMP4 that regulate growth of the non-sensory part of the vertical canals (Chang, et al., 2004b, Chang, et al., 2008, Fritzsch, et al., 2006b, Pauley, et al., 2003). In contrast, the cochlea grows by intercalation of sensory and non-sensory epithelia (Wang, et al., 2006) and does so even when differentiating hair cells never form (Fritzsch, et al., 2005a) or when the prosensory anlage of the organ of Corti is disrupted (Kiernan, et al., 2005). The data support models that propose more sophisticated molecular interactions and possible feedback loops of sensory and non-sensory epithelia to complete morphogenesis (Chang et al., 2008). These hypothesis are in line with the known inner ear otic mesenchyme interactions that require bilateral signals, only some of which are presently understood (Pirvola, et al., 2004).
Overall, the Lmx1a mutant dysmorphogenesis is a more exaggerated form of the Otx1 null dysmorphogenesis where utricle and saccule stay in communication via an open utriculo-saccular foramen and no ductus reuniens forms (Fritzsch, et al., 2001, Morsli, et al., 1999). The two phenotypes differ, however, in that the Otx1 mutant organ of Corti remains distinct from the saccule in most cases and develops a normal histology. In contrast to Lmx1a expression, Otx1 expression is found in both non-sensory and sensory compartments during ear morphogenesis (Morsli, et al., 1999) and thus does not permit the contention that its non-sensory expression that is uniquely involved in ear morphogenesis. Several other genes also affect ear morphogenesis (Chang, et al., 2004b, Fritzsch, et al., 2007). However, the limited characterization of their expression patterns does not allow making the distinctions we can make here for Lmx1a gene expression and function. In addition, the primary action of some of these genes is in the brain with the ear being secondarily affected. Others are overlappingly expressed with both sensory and non-sensory parts of the ear, as in the case with Otx1 and Foxg1 (Pauley, et al., 2006, Raft, et al., 2004). Based on the Lmx1a expression pattern and defects in null mutants, we show here for the first time that a gene expressing a non-diffusible factor exclusively in non-sensory areas of the differentiating ear is essential for aspects of ear morphogenesis and sensory organ histogenesis.
Lmx proteins are known to regulate Fgfs, Wnts and Bmps in parts of the CNS (Adams, et al., 2000, Alexandre, et al., 2006, Chizhikov and Millen, 2004, Guo, et al., 2007, Matsunaga, et al., 2002, O'Hara, et al., 2005). These secreted factors are major players for ear morphogenesis (Chang, et al., 2008, Pauley, et al., 2003, Riccomagno, et al., 2005, Wright and Mansour, 2003). Clearly, there is an altered expression of Fgf8 in the basal organ of Corti of Lmx1a mutants, showing that indeed disregulation of at least one Fgf factor occurs in the ear and there is severe dysmorphogenesis that could be related to disregulation of both Fgfs and Bmps. Wnts have also been shown to be crucial for ear placode formation (Ohyama, et al., 2006) and to play a major role in ear morphogenesis (Riccomagno, et al., 2005). Moreover, both Wnt’s and some Fgfs are secreted by the non-sensory part of the developing ear. It is therefore possible that a Wnt or Fgf factor such as Fgf9 (Pirvola, et al., 2004) or Wnt4 (Daudet, et al., 2002) is directly regulated by Lmx1a and that these and other factor(s) released from the Lmx1a expressing non-sensory areas of the developing ear regulate those crucial aspects of ear morphogenesis and provide a feed-back loop for sensory epithelium development. Absence of the endolymphatic duct in Lmx1a mutant could certainly relate to the similar absence of the endolymphatic duct in Ffg3 null mice (Hatch, et al., 2007) and could explain the ultimate loss of all hair cells in Lmx1a null mice as a consequence of disturbed endolymphatic homeostasis, such as that reported in Foxi1 and Pendrin mutant mice (Blomqvist, et al., 2006, Hulander, et al., 2003). The absence of an endolymphatic duct does in fact result in the absence of Foxi1 expression (data not shown) and a likely lack of pendrin expression in the missing endolymphatic duct. Further studies are needed to test for ionic disregulation in the Lmx1a mutant mice that could also play a role in the adult hair cell loss.
In this context it is also important to note that no pigment cells reach the lateral wall of the cochlea but rather accumulate near the radial fibers in Lmx1a null mutant. WNTs, in interaction with Wnt signaling modulators, such as the Dickkopf (DKK) family of secreted factors, set up gradients along which cells migrate. For example, in the skin, DKK interacts with WNTs in a reactiondiffusion mechanism that sets up the spacing of hair follicles (Sick, et al., 2006). It also interacts with WNTs in head morphogensis (Lewis, et al., 2008). Similar issues of spacing between sensory epithelia are in part the cause of dysmorphogenesis in the Lmx1a mutant ear. The similarity of several aspects of the Otx1 and Lmx1a phenotypes combined with the fact that Dkk1 can rescue the Otx2 phenotype (Kimura-Yoshida, et al., 2005, Lewis, et al., 2008) implies that Lmx1a may play an unspecified part in the Wnt-Otx mediated morphogenesis. It is similarly possible that the close proximity of pigment cells to Lmx1a expressing areas is suggests a modulated Wnt signaling. Such signaling may guide pigment epithelial cells to known areas of endolymph production (stria vascularis, dark cells of the utricle, and canal cristae) and resorption (endolymphatic duct). This possible involvement of Lmx1a protein in formation and resorption of endolymph, once verified in the Lmx1a mutant ear, could reflect a conserved function of this gene in the ear and hindbrain where Lmx1a is involved in the formation of CSF secreting choroid plexus (Chizhikov, et al., 2006, Elsen, et al., 2008). Wnts and their intracellular effector, β-catenin play important roles in ear formation (Ohyama, et al., 2006, Riccomagno, et al., 2005). However, more work is needed to elucidate the role of Lmx1a in local otic Wnt secretion (Daudet et al., 2002) and its modulation.
Histological defects of Lmx1a mutants relate to gene misexpression
Our data show that the enlarged anterior and posterior canal cristae of Lmx1a mutants result from the fusion of the horizontal crista and negelected papilla with the anterior and posterior crista, respectively. Interestingly, the initial upregulation of Atoh1 to differentiate hair cells is discrete but embedded in an enlarged or fused Sox2 expression domains (Fig. 10). It is only in late embryonic stages that the hair cell patches fuse into these enlarged epithelia (Fig. 3B, 9B). These data imply that two independent, but topographically related processes focus Sox2 expression to the prosensory patches and Atoh1 expression to the first differentiating hair cells inside the Sox2 expression domains. Obviously, the effect of the absence of Lmx1a expression is more profound on prosensory patch formation which foreshadows the later phenotype that simply fills in the prosensory domain with differentiated hair cells. Neither the focal prosensory patch formation nor the focal Atoh1 upregulation is understood at a molecular level (Kiernan, et al., 2005, Matei, et al., 2005b). Like the Lim domain factor tailup in insect sensory development (Biryukova and Heitzler, 2005), Lmx1a may counteract the bHLH gene upregulation mediated by Gata3 (Karis, et al., 2001) and other factors. However, a complete inventory of Lim-homeodomain factors in ear development is necessary before such multimeric interactions (Bhati, et al., 2008a, Matthews and Visvader, 2003) can be understood.
We suggest that the common function of the Lim genes Isl/tailup (flies) and Lmx1a (mice) is to define non-sensory cells. This suggestion is in agreement with existing models comparing insect and vertebrate mechanosensory development (Caldwell and Eberl, 2002, Fritzsch, et al., 2000). However, whereas the sorting of insect sensory and non-sensory cells is reinforced by delta-notch signaling, we argue here that diffusible factor gradients may be altered in the multicellular non-sensory spacers that form between sensory epithelia of mice, complementing the delta-notch function in sensory epithelium segregation (Daudet and Lewis, 2005).
In contrast to canal cristae, the wildtype utricle, saccule and cochlea form as a single elongated epithelium that only segregates later in development (Farinas, et al., 2001, Fritzsch, et al., 2002, Morsli, et al., 1998). Obviously, neither prosensory epithelium formation nor hair cell differentiation demonstrate any such segregation in Lmx1a mutants. Segregation appears only during senescence of the adult ear when hair cells die. The blending of vestibular with cochlear hair cell types near the saccular-cochlear transition might be due to cell-type mingling across an undetected border. However, it might also suggest that proximity prevents specific factors associated with vestibular and cochlear hair cell differentiation or segregation from functioning normally. In addition, the absence of Lmx1a might change radial gradients of Wnts or other diffusible factors and cause the altered morphology. Certainly, the broad expression of Fgf8 in Lmx1a mutant basal turn hair cells combined with the wider expression of Sox2 and the absence of Prox1 could result in sophisticated changes in signaling that would lead to the observed dismorphogenesis. Mutating Fgfr3 can clearly alter pillar and Deiter’s cell differentiation (Puligilla et al., 2007). Obviously, changing the expression of Fgf8 from a single to multiple rows can affect differentiation of pillar and Deiter’s cells as shown for mutations of Fgfr3 receptor (Puligilla, et al., 2007). Clearly, the next step is to define such factors that are disregulated by Lmx1a and cause dysmorphogenesis and/or altered histogenesis.
Evolutionary implications
The morphological and histological defects reported here suggest that in the absence of Lmx1a protein, the mouse ear reverts to a hagfish-like ear consisting of a simple torus with two canal cristae and a single common macula for the gravistatic organs (Fritzsch, et al., 2006b). Once DNA sequencing efforts in hagfish have been completed it will be possible to begin determining how Lmx1a expression differs across vertebrate phyla. It is possible that Lmx1a plays the same role for the segregation of the common macula into multiple end organs as Otx1 (Fritzsch, et al., 2001) and Foxg1 (Pauley, et al., 2006) play for the formation of the horizontal canal and horizontal canal crista , respectively, in development and evolution. Further work is needed to show whether the fly Lmx1a ortholog, CG32105 is associated with chordotonal organ development. Alternatively, Lmx1a may play a conserved role in ionic homeostasis, which is critical for proper function of ear and mechanosensors alike (Fritzsch, et al., 2000, Fritzsch, et al., 2007, Todi, et al., 2004, Walker, et al., 2000). Irrespetive of the details, our results suggest local Lmx1a expression is a major player in ear morpho-and histogenesis and this function is likely conserved at the level of interacting modules of transcription factors.
Acknowledgement
We thank Jenifer Kersigo, Anne Lindgren, Yuriko Mishima and Amanda Branch for technical assistance and collection of mouse embryos, Dr. Garret Soukup, Marsha Pierce and Jason Pecka for help in designing and preparing primers, Drs. Engel, Ornitz, Hogan and Chea for providing plasmids with probes and Dr. Huda Zoghbi for mice. This work was supported by grants from the NCRR/COBRE (P20 RR 018788; DHN) and NIH (RO1 DC 005590; BF). Parts of this investigation were conducted in a facility constructed with support from Research Facilities Improvement Program Grant from the National Center for Research Resources, National Institutes of Health. We acknowledge the use of the confocal microscope facility of the NCCB, supported by EPSCoR EPS-0346476 (CFD 47.076), and the University of Nebraska microarray facility supported by NCRR/COBRE.
Abbreviations for all plates
- AC
anterior crista
- CO
cochlea
- D
Deiter’s cell
- DR
ductus reuniens
- GER
greater epithelial ridge
- HP
habenula perforate
- HC
horizontal crista
- IHC
inner hair cell
- OC
organ of Corti
- OHC
outer hair cell
- P
pillar cells
- PC
posterior crista
- PN
papilla neglecta
- RF
radial fibers
- S
saccule
- SL
spiral limbus
- Spg
spiral ganglion
- SV
stria vascularis
- U
utricle
- USF
utriculo-saccular foramen
References
- Abello G, Khatri S, Giraldez F, Alsina B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Adams KA, Maida JM, Golden JA, Riddle RD. The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development. 2000;127:1857–1867. doi: 10.1242/dev.127.9.1857. [DOI] [PubMed] [Google Scholar]
- Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- Alexandre P, Bachy I, Marcou M, Wassef M. Positive and negative regulations by FGF8 contribute to midbrain roof plate developmental plasticity. Development. 2006;133:2905–2913. doi: 10.1242/dev.02460. [DOI] [PubMed] [Google Scholar]
- Asmar J, Biryukova I, Heitzler P. Drosophila dLMO-PA isoform acts as an early activator of achaete/scute proneural expression. Dev Biol. 2008;316:487–497. doi: 10.1016/j.ydbio.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bhati M, Lee C, Nancarrow AL, Lee M, Craig VJ, Bach I, Guss JM, Mackay JP, Matthews JM. Implementing the LIM code: the structural basis for cell type-specific assembly of LIM-homeodomain complexes. Embo J. 2008a;27:2018–2029. doi: 10.1038/emboj.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati M, Lee M, Nancarrow AL, Bach I, Guss JM, Matthews JM. Crystallization of an Lhx3-Isl1 complex. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008b;64:297–299. doi: 10.1107/S174430910800691X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukova I, Heitzler P. The Drosophila LIM-homeo domain protein Islet antagonizes pro-neural cell specification in the peripheral nervous system. Dev Biol. 2005;288:559–570. doi: 10.1016/j.ydbio.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. Embo J. 2006;25:4131–4141. doi: 10.1038/sj.emboj.7601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–692. doi: 10.1016/s0736-5748(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Caldwell JC, Eberl DF. Towards a molecular understanding of Drosophila hearing. J Neurobiol. 2002;53:172–189. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004a;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Chang W, Cole LK, Cantos R, Wu DK. Molecular genetics of vestibular organ development. In: Highstein SM, Fay RR, Popper AN, editors. The Vestibular System, vol 19. New York: Springer Verlag; 2004b. pp. 11–56. [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chizhikov V, Steshina E, Roberts R, Ilkin Y, Washburn L, Millen KJ. Molecular definition of an allelic series of mutations disrupting the mouse Lmx1a (dreher) gene. Mamm Genome. 2006;17:1025–1032. doi: 10.1007/s00335-006-0033-7. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Control of roof plate development and signaling by Lmx1b in the caudal vertebrate CNS. J Neurosci. 2004;24:5694–5703. doi: 10.1523/JNEUROSCI.0758-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ripoll C, Moles JP, Rebillard G. Expression of members of Wnt and Frizzled gene families in the postnatal rat cochlea. Brain Res Mol Brain Res. 2002;105:98–107. doi: 10.1016/s0169-328x(02)00397-2. [DOI] [PubMed] [Google Scholar]
- Deng M, Pan L, Xie X, Gan L. Differential expression of LIM domain-only (LMO) genes in the developing mouse inner ear. Gene Expr Patterns. 2006;6:857–863. doi: 10.1016/j.modgep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Deol MS. The origin of the abnormalities of the inner ear in dreher mice. J Embryol Exp Morph. 1964;12:727–733. [PubMed] [Google Scholar]
- Deol MS. Development of auditory and vestibular systems in mutant mice. In: Romand R, editor. Development of Vestibular and Auditory Systems. New York: Academic Press; 1983. pp. 309–333. [Google Scholar]
- Elsen GE, Choi LY, Millen KJ, Grinblat Y, Prince VE. Zic1 and Zic4 regulate zebrafish roof plate specification and hindbrain ventricle morphogenesis. Dev Biol. 2008;314:376–392. doi: 10.1016/j.ydbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli V, Bachy I, Retaux S. Expression of the LIM-homeodomain gene Lmx1a (dreher) during development of the mouse nervous system. Mech Dev. 2002;118:225–228. doi: 10.1016/s0925-4773(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The ear of Latimeria chalumnae revisited. Zoology (Jena) 2003;106:243–248. doi: 10.1078/0944-2006-00120. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Bermingham NA. Developmental evolutionary biology of the vertebrate ear: conserving mechanoelectric transduction and developmental pathways in diverging morphologies. Neuroreport. 2000;11:R35–R44. doi: 10.1097/00001756-200011270-00013. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006a;28:1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51:663–678. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005a;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVuetrade mark Maroon, NeuroVuetrade mark Red and NeuroVuetrade mark Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005b;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res. 1993;65:51–60. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: the making of the vertebrate ear. Brain Res. 2006b;1091:151–171. doi: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Srinivasan RS, Harvey NL, Nichols DH, Oliver G. Canal cristae growth and fiber extension to the outer hair cells require Prox1 activity. Dev Dyn. 2008 doi: 10.1371/journal.pone.0009377. in revision: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Wake MH. The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system. Zoomorphol. 1988;108:210–217. [Google Scholar]
- Giraldez F. Regionalized organizing activity of the neural tube revealed by the regulation of lmx1 in the otic vesicle. Dev Biol. 1998;203:189–200. doi: 10.1006/dbio.1998.9023. [DOI] [PubMed] [Google Scholar]
- Guo C, Qiu HY, Huang Y, Chen H, Yang RQ, Chen SD, Johnson RL, Chen ZF, Ding YQ. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134:317–325. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- Hatch EP, Noyes CA, Wang X, Wright TJ, Mansour SL. Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development. 2007;134:3615–3625. doi: 10.1242/dev.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzano R, Dror AA, Montcouquiol M, Ahmed ZM, Ellsworth B, Camper S, Friedman TB, Kelley MW, Avraham KB. Lhx3, a LIM domain transcription factor, is regulated by Pou4f3 in the auditory but not in the vestibular system. Eur J Neurosci. 2007;25:999–1005. doi: 10.1111/j.1460-9568.2007.05332.x. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Hansson E, Malewicz M, Sandberg M, Perlmann T, Lendahl U, Muhr J. SoxB1 transcription factors and Notch signaling use distinct mechanisms to regulate proneural gene function and neural progenitor differentiation. Development. 2008;135:1843–1851. doi: 10.1242/dev.020180. [DOI] [PubMed] [Google Scholar]
- Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerback S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development. 2003;130:2013–2025. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Nunes F, Wu DK, Fekete DM. The expression domain of two related homeobox genes defines a compartment in the chicken inner ear that may be involved in semicircular canal formation. Dev Biol. 1997;191:215–229. doi: 10.1006/dbio.1997.8716. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee B, Joshi K, Pfaff SL, Lee JW, Lee SK. A regulatory network to segregate the identity of neuronal subtypes. Dev Cell. 2008;14:877–889. doi: 10.1016/j.devcel.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton: CRC Press; 1985. [Google Scholar]
- Lewis SL, Khoo PL, De Young RA, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson RV, Robb L, Tam PP. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135:1791–1801. doi: 10.1242/dev.018853. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Haugas M, Matilainen T, Pussinen C, Karis A, Salminen M. Gata3 is required for early morphogenesis and Fgf10 expression during otic development. Mech Dev. 2006;123:415–429. doi: 10.1016/j.mod.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Trainor PA, Ariza-McNaughton L, Nonchev S, Krumlauf R. Dorsal patterning defects in the hindbrain, roof plate and skeleton in the dreher (dr(J)) mouse mutant. Mech Dev. 2000;94:147–156. doi: 10.1016/s0925-4773(00)00288-4. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005a;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005b;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei VA, Feng F, Pauley S, Beisel KW, Nichols MG, Fritzsch B. Near-infrared laser illumination transforms the fluorescence absorbing X-Gal reaction product BCI into a transparent, yet brightly fluorescent substance. Brain Res Bull. 2006;70:33–43. doi: 10.1016/j.brainresbull.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Katahira T, Nakamura H. Role of Lmx1b and Wnt1 in mesencephalon and metencephalon development. Development. 2002;129:5269–5277. doi: 10.1242/dev.129.22.5269. [DOI] [PubMed] [Google Scholar]
- Matthews JM, Visvader JE. LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO Rep. 2003;4:1132–1137. doi: 10.1038/sj.embor.7400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- O'Hara FP, Beck E, Barr LK, Wong LL, Kessler DS, Riddle RD. Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development. 2005;132:3163–3173. doi: 10.1242/dev.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev. 2008;10:106–113. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 Is Required for the Development of the Auditory Sensory Epithelium. Neuron. 2002;35:671–685. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol. 2004;273:350–360. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Sienknecht UJ, Fekete DM. Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J Comp Neurol. 2008;510:378–395. doi: 10.1002/cne.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech. 2004;63:388–399. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Devriendt K. Transcription factor GATA3 and the human HDR syndrome. Cell Mol Life Sci. 2001;58:1296–1300. doi: 10.1007/PL00000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]


