Live cell imaging is a powerful method for studying protein dynamics at the cell surface, but conventional probes, such as antibodies and fluorescent ligands, are bulky, interfere with protein function1,2, or dissociate after internalization3,4. To overcome these limitations, we developed a method to covalently tag any cell surface protein with any chemical probe with remarkable specificity. Through rational design, we re-directed a microbial lipoic acid ligase (LplA)5 to specifically ligate an alkyl azide to an engineered LplA acceptor peptide (LAP) tag. The alkyl azide is then selectively derivatized with a cyclooctyne6 conjugated to any probe of interest. We demonstrate the utility of this method by first labeling LAP fusion proteins expressed on the surface of living mammalian cells with Cy3, Alexa Fluor 568, and biotin. Next, we combined LAP-tagging with our previously reported tagging method7,8 to simultaneously monitor the dynamics of two receptors, co-expressed in the same cell, with different fluorophores. Using a wound-healing assay, we found that while the LDL receptor maintains a uniform distribution on the cell surface, the ephrin receptor EphA3 is polarized to the leading edge. Our methodology should provide general access to biochemical and imaging studies of cell surface proteins, using small fluorophores introduced via a short peptide tag.
Fluorescent labeling of cell surface proteins enables imaging of the trafficking and function of receptors, channels, and transporters. Many protein labeling methods have been developed in recent years9, but none currently allows the covalent attachment of small fluorophores of any structure onto cell surface proteins modified only by a small peptide tag, with short labeling times and with extremely high specificity over a wide range of expression levels and labeling conditions. To address this shortcoming, we developed a new protein labeling method based on the E. coli enzyme lipoic acid ligase (LplA)5. In E. coli, LplA catalyzes the ATP-dependent covalent ligation of lipoic acid to one of three proteins involved in oxidative metabolism (E2p, E2o, and H-protein5) (Fig. 1a, top). LplA naturally exhibits extremely high sequence specificity, but previous work showing that the enzyme accepts octanoic acid, 6-thio-octanoic acid, and selenolipoic acid in place of lipoic acid5 suggest that the small-molecule binding site has considerable plasticity. To harness LplA for fluorescent labeling, we re-engineered the system in three stages. First, through synthesis and testing of ten different substrate analogs, we discovered an alkyl azide substrate that can be efficiently used by LplA in place of lipoic acid. Once ligated to the target protein, the azide functional group can be selectively derivatized with any fluorescent probe conjugated to a cyclooctyne reaction partner6 (Figure 1a). Second, to create a minimally invasive tag to direct the ligation of the alkyl azide, we engineered, through iterative cycles of rational design, a 22-amino acid replacement for LplA's natural protein substrates, which can be fused to the N- or C-terminus of any protein of interest. Third, we tested the specificity of LplA in the mammalian cell context and found no background labeling of endogenous proteins.
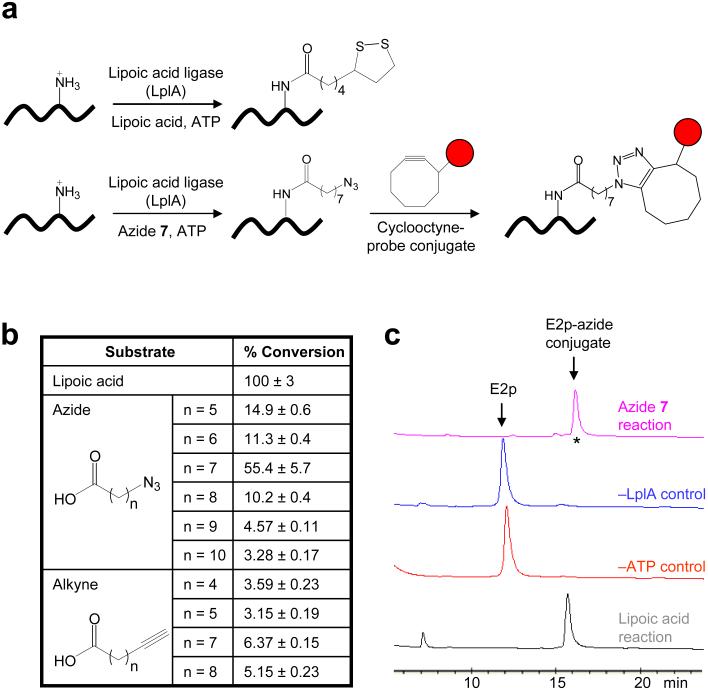
Figure 1. Re-directing LplA for site-specific protein labeling with fluorescent probes.
(a) Natural reaction catalyzed by LplA (top), and scheme for LplA-catalyzed fluorescent tagging in cells (bottom). Instead of lipoic acid, LplA ligates an alkyl azide to a lysine sidechain within a peptide recognition sequence. The azide is then selectively functionalized with a cyclooctyne-probe conjugate (red circle), to give a triazole adduct. (b) Comparison of alkyl azide and alkyne substrates of LplA. Conversions are given relative to lipoic acid, which is normalized to 100%. (c) HPLC assay showing the ligation of the azide 7 substrate to E2p protein. The starred peak was analyzed by mass-spectrometry in Supplementary Figure 2 online.
For the first stage of LplA engineering, we considered a range of small molecule structures to replace lipoic acid. Direct ligation of a fluorophore would offer a simpler and shorter labeling procedure, but incorporation of a “functional group handle” is more feasible due to the small size of the lipoate binding pocket, and provides greater versatility for subsequent incorporation of probes of any structure. Many functional group handles have been used in chemical biology, including ketones, organic azides, and alkynes10. Organic azides are the most suitable for live cell applications, because the azide group is both abiotic and non-toxic in animals and can be selectively derivatized under physiological conditions (without any added metals or cofactors) with cyclooctynes, which are also unnatural6. To test if LplA could accept an azide substrate in place of lipoic acid, we synthesized a panel of alkyl azide carboxylic acids of varying lengths (Supplementary Fig. 1), and tested them for ligation onto a 9 kDa lipoyl domain derived from the full-length E2p protein11 (abbreviated “E2p”) using an HPLC assay. As additional probes of the lipoate binding pocket we also synthesized a series of alkyne carboxylic acids (Supplementary Fig. 1). Figure 1b shows that all probes were incorporated by LplA to some degree, but the efficiency of ligation exhibited a clear length-dependence, with azide 7 giving the fastest kinetics. Figure 1c shows the HPLC trace associated with azide 7 ligation to E2p, in addition to negative control reactions with LplA or ATP omitted. We collected the product peak (starred) from the top trace and analyzed it by mass-spectrometry, which confirmed that one molecule of azide 7 had been site-specifically conjugated to E2p (Supplementary Fig. 2 online). We also measured the kinetics of azide 7 ligation to E2p (Supplementary Fig. 2 online), and compared the values to those of lipoic acid ligation. The kcat values were only slightly different (0.111 ± 0.003 s-1 vs 0.253 ± 0.003 s-1) but the Km increased 75- or 30- fold for azide 7 (127 ± 11 μM) compared to lipoic acid (1.7 μM5 or 4.5 μM12). As seen below, however, it is straightforward and non-toxic to provide azide 7 at concentrations higher than 127 μM for live cell labeling, thus maximizing the rate of ligation.
For the second stage of engineering, we wished to design a peptide substrate for LplA to replace the protein substrates. It was necessary for the peptide to be fully transposable (recognized when fused to the N- or C-terminal ends of any protein) and to be recognized by LplA with similar efficiency to the natural protein substrates. As described in Supplementary Figure 3 online, we accomplished this through multiple rounds of rational design. A major challenge was presented by the fact that E2p presents the lysine modification site at the tip of a sharp hairpin turn13, a conformation that is difficult to recapitulate in a peptide. Nevertheless, we designed an initial panel of peptides by analyzing lipoate acceptor proteins from different species, as well as structurally-related biotin acceptor proteins. Peptides that were active in the initial screen were then improved through site-directed mutagenesis and tested for recognition at either terminus of a model protein. The final 22-amino acid sequence, called the LplA acceptor peptide (LAP), had a kcat of 0.048 ± 0.001 s-1, only 2.3-fold lower than the corresponding kcat for full-length E2p.
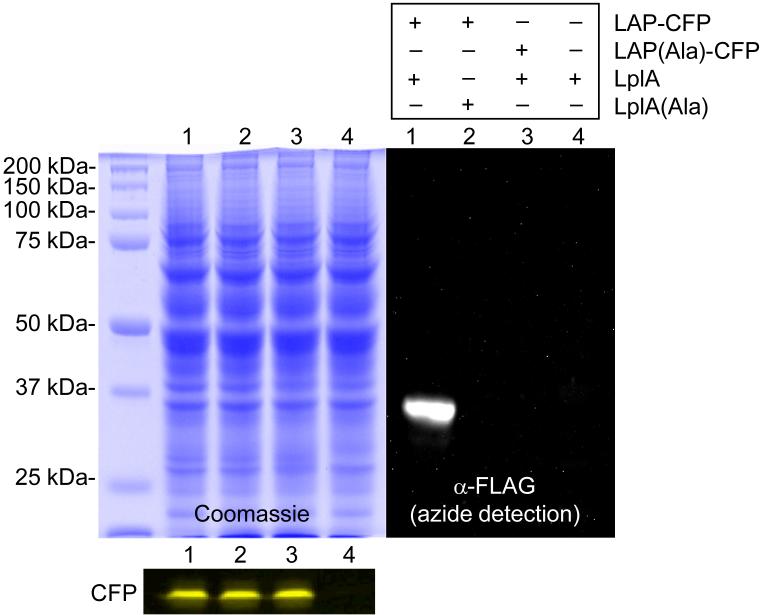
Our third task was to assess the specificity of LplA in the mammalian cell context. To do this, we created a LAP fusion to cyan fluorescent protein (CFP), and expressed it in human embryonic kidney (HEK) cells. HEK lysates were then labeled with LplA, azide 7, and ATP, and the ligated azide was detected by western blot, after functionalization with a FLAG peptide via the Staudinger ligation14. Figure 2 shows that in the presence of thousands of mammalian proteins in lysate, only LAP-CFP is labeled by LplA. The expression level of LAP-CFP is so low that it cannot be seen above endogenous proteins in the Coomassie-stained gel. Negative controls with LplA replaced by a catalytically inactive mutant, or LAP-CFP replaced by an alanine point mutant at the lysine modification site, show that labeling depends on the presence of LplA and an intact LAP sequence. This experiment and the live cell labeling experiments described below demonstrate that LplA is a remarkably specific enzyme at the cell surface, and possibly within the cytosol as well.
Figure 2. LplA labels the LAP peptide without modifying endogenous mammalian proteins.
Lysates from HEK cells expressing a LAP fusion to CFP were labeled in vitro with LplA and azide 7. The azide was derivatized with phosphine-FLAG via the Staudinger ligation14, and the FLAG epitope was detected by blotting with an anti-FLAG antibody. Controls are shown with LAP-CFP replaced by its alanine point mutant (lane 3), or with LplA replaced by its catalytically inactive Lys133Ala mutant (lane 2). Coomassie staining demonstrates equal loading in all lanes. Fluorescence visualization of CFP demonstrates equal expression levels of the LAP fusion in lanes 1-3.
To test our newly engineered small molecule and peptide substrates for LplA in the live cell context, we first created an artificial construct by fusing LAP to CFP, and then fusing this in turn to the extracellular side of the transmembrane (TM) domain of the PDGF receptor (Fig. 3, top). We also synthesized conjugates of our previously reported mono-fluorinated cyclooctyne6 (OCT) to two bright, red-emitting, and membrane-impermeant fluorophores, Alexa Fluor 568 and Cy3 (Supplementary Fig. 4 online).
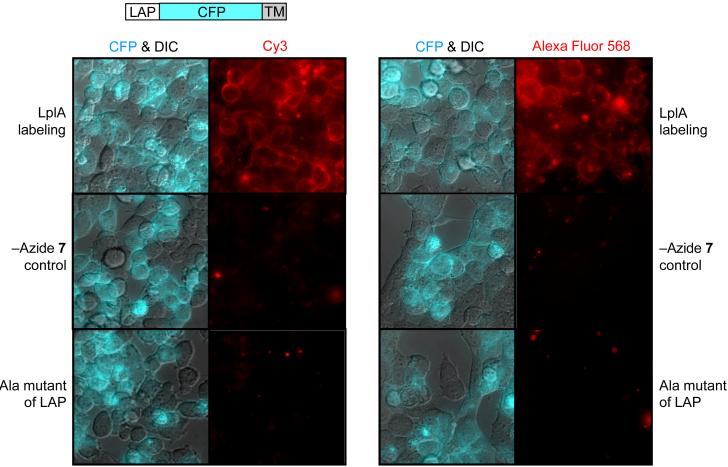
Figure 3. Site-specific labeling of LAP fusion proteins with fluorophores.
A LAP-CFP fusion was targeted to the cell surface using a transmembrane (TM) domain. Cell-surface LAP was first labeled with azide 7 by LplA, and the introduced azide was then labeled with a cyclooctyne probe conjugated to Cy3 (left) or Alexa Fluor 568 (right). Live cell images of the introduced fluorophores are shown to the right of the merged CFP and DIC images, which highlight the transfected cells. Negative controls with azide 7 omitted from the labeling reaction, or with the LAP-CFP-TM replaced by its alanine point mutant are shown.
To perform labeling, LAP-CFP-TM was expressed in HEK cells, and 350 μM azide 7 was added in the presence of LplA for 1 hour, followed by one of the fluorophore-OCT conjugates for 20 minutes. The live cell images in Figure 3 show that transfected cells (indicated by CFP fluorescence) are labeled with Alexa Fluor 568 or Cy3, while neighboring untransfected cells in the same field of view are not labeled. Interestingly, labeling with Alexa Fluor 568 generated higher background than Cy3 labeling, due to faster non-specific internalization of the probe. We performed additional negative controls with omission of azide 7 or replacement of LAP-CFP-TM by its alanine mutant, and observed no labeling in either case. Unlike sodium azide, organic azides such as the clinically approved drug AZT15 are not known to be toxic to cells, but we nevertheless examined the effect of 24-hour exposure to azide 7 on mitochondrial respiration, and found no effect at concentrations less than 750 μM (data not shown).
We also compared the speed, sensitivity, and specificity of LplA labeling to two other peptide-based labeling methods previously described by our lab (Supplementary Fig. 5 online). Biotin ligase (BirA)/ketone tagging makes use of a ketone isostere of biotin, which can be functionalized with hydrazide conjugates to label proteins fused to a 15-amino acid “acceptor peptide” (AP)16. Transglutaminase labeling attaches cadaverine-functionalized fluorophores to a glutamine-containing peptide recognition sequence17. For the comparison experiments, we used LplA to label LAP-CFP-TM with azide 7, followed by OCT-biotin, and followed by streptavidin-Alexa Fluor 568 to detect the biotin (Supplementary Fig. 5 online). A total labeling time of only 20 minutes was required for all three steps, in order to achieve a signal to background ratio ≥3:1. In contrast, BirA/ketone labeling of an analogous AP-CFP-TM construct with a biotin-hydrazide compound followed by streptavidin detection required 2 hours and 15 minutes to achieve a similar signal to background ratio. We also quantified the sensitivity of LplA labeling using the wedge method18 and determined that cells expressing as little as 5 μM LAP-CFP-TM could be specifically labeled with OCT-biotin, with a signal to background ratio ≥ 3:1. Similar experiments, described in Supplementary Figure 5 online, demonstrate that LplA is also superior to transglutaminase, particularly in terms of labeling specificity under a wide range of conditions.
To illustrate the use of LplA labeling for imaging actual receptors, we created a LAP fusion to the low-density lipoprotein receptor (LDLR), which functions in the uptake of cholesterol in peripheral tissues of the body19, and we established that LAP-LDLR could be labeled with OCT-Cy3 or OCT-biotin in HEK cells, even when expressed at levels matching endogenous LDLR (data not shown). We then wished to image LAP-LDLR in the context of a biological assay. For many imaging studies, it is desirable to visualize two different receptors at once in the same cell, in order to compare their distribution or trafficking patterns. To develop this capability, we investigated the compatibility of LplA labeling with BirA/streptavidin targeting. Unlike BirA/ketone labeling, BirA/streptavidin targeting7,8 makes use of site-specific biotin ligation onto AP-tagged proteins, followed by recognition with streptavidin-fluorophore conjugates. While the use of streptavidin increases the total size of the label, the femtomolar affinity of the biotin-streptavidin interaction makes this labeling approach much faster and much more sensitive than BirA/ketone labeling7.
E. coli LplA and biotin ligase are mechanistically related, and their natural acceptor proteins share some structural and sequence overlap20. However, the engineered LAP and AP sequences are dissimilar, as are the azide 7 and biotin structures. To test the orthogonality of these two labeling methods, we prepared separate dishes of HEK cells expressing LAP-LDLR (with a GFP tag to serve as a transfection marker), or AP-EGFR (AP fused to the extracellular N-terminus of the EGF receptor16) together with a CFP transfection marker. After 16-24 hours of expression, the cells were re-plated together in a single dish. We performed labeling by first adding a mixture of LplA, BirA, azide 7, biotin, and ATP to the cells. Thereafter, OCT-Cy3 was added to derivatize the azide, and streptavidin was added to detect the biotin. Supplementary Figure 6 online shows that cells expressing LAP-LDLR were selectively labeled with Cy3, while cells expressing AP-EGFR were selectively labeled with streptavidin. The same results were obtained using LAP-LDLR in combination with an AP-tagged receptor for ephrinA3 (AP-EphA3). Thus, simultaneous labeling of cells with LplA and BirA is possible, without sacrificing the extremely high specificity of each system.
We then used this two-color labeling protocol to image LAP- and AP-fused receptors co-expressed within the same cell. EGF receptor and EphA3 are both known to function in cell migration21,22, and thus we performed imaging on cells migrating toward an artificial wound. HEK cells were co-transfected with either LAP-LDLR and AP-EGFR, or LAP-LDLR and AP-EphA3. After 16-24 hours of expression, the confluent cells were wounded with a pipet tip. We allowed the wound to partially close over 12-18 hours, and then performed simultaneous labeling with Cy3, and Alexa Fluor 488 conjugated to monovalent streptavidin8. Figure 4 shows that Cy3-labeled LDLR was evenly distributed on the surface of the HEK cells, whereas Alexa Fluor 488-labeled EGFR and EphA3 were both asymmetrically concentrated at the leading edge of the polarized cells. The same patterns were also observed when the LAP and AP tags were swapped (AP-LDLR and LAP-EGFR), suggesting that the localization patterns do not reflect artifacts of AP and LAP labeling (data not shown).
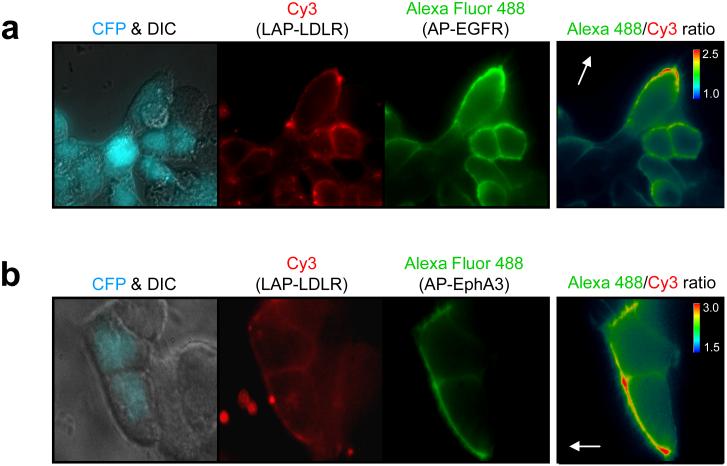
Figure 4. Simultaneous labeling and imaging of two receptors in polarized cells in a wound healing assay.
HEK cells co-expressing a LAP-LDLR fusion and either AP-EGFR (a) or AP-EphA3 (b) were labeled during wound healing by first treating with LplA, BirA, azide 7, and biotin, followed by OCT-Cy3 to derivatize the azide, followed by monovalent streptavidin-Alexa Fluor 4888 to detect the biotin. The Cy3 images show the non-polarized distribution of surface LAP-LDLR. The Alexa Fluor 488 images show the polarized distribution of AP-EGFR (a) and AP-EphA3 (b) at the wound edge. CFP is a transfection marker. The images on the far right depict the intensity ratios of Alexa Fluor 488 and Cy3. The white arrows point toward the wound.
While the polarization of AP-EGFR to the leading edge of migrating cells was expected, and has previously been observed using antibody detection23, the pattern of AP-EphA3 staining is surprising. Previous work has shown that trans interactions between EphA3 and ephrin ligand expressed on the surface of contacting cells play a role in developmental cell migration24 and tumor invasion25. However, it is unclear that unliganded EphA3 should function in migratory processes. Our observation of EphA3 accumulation at the free, leading edge of polarized cells suggests that unactivated EphA3 may play a role in cell signaling, or that EphA3 may be constitutively linked to the actin cytoskeleton.
In summary, we have developed new methodology for labeling cell surface proteins fused to a 22-amino acid recognition sequence for E. coli LplA. Small, noncrosslinking probes such as Cy3, Alexa Fluor, and biotin can be site-specifically and covalently conjugated to the LAP peptide in as little as 20 minutes. An important feature of our methodology is its generality; any cell surface protein in any cell type can be labeled with any chemical moiety that can be functionalized with a cyclooctyne.
Many new protein labeling methods have been developed in recent years9, and a survey of these techniques reveals that a general trade-off exists between labeling specificity and tag size. Protein-based tags, such as SNAP/AGT26 generally give higher labeling specificity than peptide tags, such as FlAsH27. However, protein tags have greater potential to interfere with protein folding, trafficking, and activity, as GFP often does28,29. We and others (for example, ACP/PCP labeling methodology30) have tried to bridge the requirements of small tag size and high labeling specificity, by making use of enzyme ligases. By capitalizing on the intrinsic sequence specificity of enzymes such as biotin ligase and LplA, highly specific probe conjugation can be achieved, without sacrificing the small size of the directing tag.
In previous work with BirA, we found that a ketone isostere of biotin could be accepted16, but not compounds with more dissimilar structures, such as alkyne and azide derivatives of biotin, due to the structural requirements of the biotin binding pocket. In contrast, LplA exhibits much more relaxed specificity for its small molecule substrate, while maintaining extremely high specificity for its protein or peptide substrate5. This property allowed us to harness LplA for unnatural ligation reactions in this study. Important next challenges will be to extend this methodology to labeling of intracellular protein targets and to re-engineer LplA for one-step-ligation of fluorophore or photoaffinity probes.
We also used LplA in combination with biotin ligase to image two different receptors in the same cell. Many problems in receptor biology would benefit from simultaneous imaging of two or more different proteins in the same living cell, instead of separate experiments involving one-color labeling of each receptor. The combination of LplA and BirA tagging, which can be performed simultaneously due to the orthogonality of the labeling reaction components, will provide access to such experiments.
Methods
In vitro LplA activity assays
LplA reactions contained 2 μM LplA, 200 μM E2p, 350 μM probe, 1 mM ATP, 2 mM magnesium acetate, and 25 mM sodium phosphate pH 7.0. Reactions were incubated at 30 °C for 30 minutes, and then quenched with EDTA (final concentration 50 mM). Conversion to product was determined by HPLC on a C18 reverse-phase column with a 40-57% gradient of acetonitrile in water with 0.1% trifluoroacetic acid over 20 minutes (flow rate 1.0 mL/minute). Unmodified E2p had a retention time of ~12 minutes while E2p-probe conjugates eluted at 15-18 minutes. Percent conversion to product was calculated from the ratio of the E2p-probe peak area to the sum of (E2p + E2p-probe) peak areas. All measurements were performed in triplicate.
LplA specificity test on mammalian lysate
Human embryonic kidney (HEK) 293T cells were transfected with LAP-CFP-pcDNA3 plasmid using Lipofectamine 2000 (1 μg DNA/well of a 6-well plate). Lysates were generated 48 hours later by hypotonic lysis to minimize protease release, as follows. Cells were lifted from the plates, concentrated by centrifugation, and resuspended in 1 mM HEPES pH 7.5, 5 mM magnesium chloride, 1 mM phenylmethylsulphonyl fluoride, and protease inhibitor cocktail (Calbiochem). After incubation at 4 °C for 10 minutes, the cells were lysed by vigorous vortexing for 2 minutes at 21 °C. Crude lysate was clarified by centrifugation, and stored at -80 °C. Lysate was labeled by incubating at 30 °C for 10 hours with 25 mM sodium phosphate pH 7.0, 1 μM LplA, 250 μM azide 7, 1 mM ATP, and 4 mM magnesium acetate. Thereafter, Staudinger ligation was performed by adding FLAG-phosphine14 to a final concentration of 500 μM, and incubating at 30 °C for 16 hours. Each reaction sample was then divided into thirds. The first third was analyzed by 12% SDS-PAGE followed by Western blotting with anti-FLAG(M2)-peroxidase antibody conjugate (Sigma, 1:1000 dilution). The second sample was analyzed by 12% SDS-PAGE followed by Coomassie staining. The last third was analyzed by 12% SDS-PAGE without boiling the samples, in order to prevent unfolding of CFP, and in-gel fluorescence was visualized on a Storm 860 instrument (Amersham).
Live cell labeling with fluorescent probes
HEK 293T cells were transfected with the LAP-CFP-TM expression plasmid using Lipofectamine 2000. After 36-48 hours at 37 °C, the cells were washed twice with fresh growth media (Dulbecco's Modified Eagle's Medium with 10% fetal bovine serum and 1% penicillin/streptomycin). Enzymatic ligation of azide 7 was performed in complete growth media with 10 μM LplA, 350 μM azide 7, 1 mM ATP, and 5 mM magnesium acetate for 60 minutes at 32 °C. Cells were rinsed three times with growth media, and incubated for 20 minutes at 21 °C with 200-400 μM OCT-Cy3 or 100-200 μM OCT-Alexa Fluor 568. Thereafter, the cells were washed once with growth media, twice with a 1% bovine serum albumin (BSA) solution in Dulbecco's Phosphate-Buffered Saline (DPBS) pH 7.4, and twice more with DPBS alone. Labeled cells were imaged in the same buffer on a Zeiss Axiovert 200M inverted epifluorescence microscope using a 40x oil-immersion lens. CFP (420/20 excitation, 450 dichroic, 475/40 emission), Cy3 and Alexa Fluor 568 (560/20 excitation, 585 dichroic, 605/30) and differential interference contrast (DIC) images (630/10 emission) were collected and analyzed using Slidebook software (Intelligent Imaging Innovations). Fluorescence images were normalized to the same intensity range. Acquisition times ranged from 10-250 milliseconds.
Two-color live cell labeling with LplA and biotin ligase
HEK 239T cells were co-transfected with the LAP-LDLR and AP-EGFR16 plasmids in a 1:2 ratio, or with the LAP-LDLR and AP-EphA3 (a gift from M. Lackmann, Monash University) plasmids in a 2:1 ratio. 24 hours after transfection, the cells were wounded with a pipet tip and allowed to heal over 16-24 hours. For labeling, cells were washed twice with complete growth media, and then incubated with 5 μM BirA, 10 μM LplA, 50 μM biotin, 350 μM azide 7, 1 mM ATP, and 5 mM magnesium acetate for 60 minutes at 32 °C. Cells were then rinsed three times with growth media, and incubated for 20 minutes at 21 °C with 200-400 μM OCT-Cy3. Biotin was detected by staining with 50 μg/mL monovalent streptavidin-Alexa Fluor 4888 for 10 minutes at 4 °C. The cells were washed once with ice-cold 1% BSA in DPBS pH 7.4, then twice with ice-cold DPBS, before imaging in the same buffer using the configuration described above. The Alexa Fluor 488 filter set was 495/20 excitation, 515 dichroic, 530/30 emission.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mark Howarth, John Cronan, Irwin Chen, Chi-Wang Lin, and Martin Lackmann for their assistance and advice. This work was supported by the National Institutes of Health (R01 GM072670-01), the Sloan Foundation, the Dreyfus Foundation, a La Caixa Foundation pre-doctoral fellowship (to M.F.S.), and a National Science Foundation pre-doctoral fellowship (to J.M.B.).
Footnotes
Competing interests statement: The authors declare competing financial interests; details accompany the full-text HTML version of the paper at http://www.nature.com/naturebiotechnology/.
Note: Supplementary information is available on the Nature Biotechnology website.
References
- 1.Debant A, Ponzio G, Clauser E, Contreres JO, Rossi B. Receptor cross-linking restores an insulin metabolic effect altered by mutation on tyrosine 1162 and tyrosine 1163. Biochemistry. 1989;28:14–17. doi: 10.1021/bi00427a003. [DOI] [PubMed] [Google Scholar]
- 2.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RG, Brown MS, Beisiegel U, Goldstein JL. Surface distribution and recycling of the low density lipoprotein receptor as visualized with antireceptor antibodies. J. Cell Biol. 1982;93:523–531. doi: 10.1083/jcb.93.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barak LS, Webb WW. Fluorescent low density lipoprotein for observation of dynamics of individual receptor complexes on cultured human fibroblasts. J. Cell Biol. 1981;90:595–604. doi: 10.1083/jcb.90.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DE, Morris TW, Green J, Cronan JE, Jr., Guest JR. Purification and properties of the lipoate protein ligase of Escherichia coli. Biochem. J. 1995;309:853–862. doi: 10.1042/bj3090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. A comparative study of bioorthogonal reactions with azides. ACS Chem. Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 7.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howarth M, et al. A monovalent streptavidin with a single femtomolar biotin binding site. Nature Methods. 2006;3:267–273. doi: 10.1038/NMETHXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks KM, Nolan GP. Chemical labeling strategies for cell biology. Nat. Methods. 2006;3:591–596. doi: 10.1038/nmeth906. [DOI] [PubMed] [Google Scholar]
- 10.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 11.Ali ST, Guest JR. Isolation and characterization of lipoylated and unlipoylated domains of the E2p subunit of the pyruvate dehydrogenase complex of Escherichia coli. Biochem. J. 1990;271:139–145. doi: 10.1042/bj2710139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara K, et al. Crystal structure of lipoate-protein ligase A from Escherichia coli. Determination of the lipoic acid-binding site. J. Biol. Chem. 2005;280:33645–33651. doi: 10.1074/jbc.M505010200. [DOI] [PubMed] [Google Scholar]
- 13.Green JD, Laue ED, Perham RN, Ali ST, Guest JR. Three-dimensional structure of a lipoyl domain from the dihydrolipoyl acetyltransferase component of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J. Mol. Biol. 1995;248:328–343. doi: 10.1016/s0022-2836(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 14.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin RJ. The medicinal chemistry of the azido group. Prog. Med. Chem. 1994;31:121–232. doi: 10.1016/s0079-6468(08)70020-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Methods. 2005;2:99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 17.Lin CW, Ting AY. Transglutaminase-catalyzed site-specific conjugation of small-molecule probes to proteins in vitro and on the surface of living cells. J. Am. Chem. Soc. 2006;128:4542–4543. doi: 10.1021/ja0604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams SR, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 19.Willnow TE. The low-density lipoprotein receptor gene family: multiple roles in lipid metabolism. J. Mol. Med. 1999;77:306–315. doi: 10.1007/s001090050356. [DOI] [PubMed] [Google Scholar]
- 20.Reche P, Perham RN. Structure and selectivity in post-translational modification: attaching the biotinyl-lysine and lipoyl-lysine swinging arms in multifunctional enzymes. EMBO J. 1999;18:2673–2682. doi: 10.1093/emboj/18.10.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 22.Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Tuli SS, et al. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr. Eye Res. 2006;31:709–719. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- 24.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 25.Wimmer-Kleikamp SH, Lackmann M. Eph-modulated cell morphology, adhesion and motility in carcinogenesis. IUBMB. Life. 2005;57:421–431. doi: 10.1080/15216540500138337. [DOI] [PubMed] [Google Scholar]
- 26.George N, Pick H, Vogel H, Johnsson N, Johnsson K. Specific labeling of cell surface proteins with chemically diverse compounds. J. Am. Chem. Soc. 2004;126:8896–8897. doi: 10.1021/ja048396s. [DOI] [PubMed] [Google Scholar]
- 27.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 28.Brock R, Hamelers IH, Jovin TM. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry. 1999;35:353–362. doi: 10.1002/(sici)1097-0320(19990401)35:4<353::aid-cyto8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.McLean AJ, Milligan G. Ligand regulation of green fluorescent protein-tagged forms of the human beta(1)- and beta(2)-adrenoceptors; comparisons with the unmodified receptors. Br. J. Pharmacol. 2000;130:1825–1832. doi: 10.1038/sj.bjp.0703506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z, et al. Genetically encoded short peptide tags for orthogonal protein labeling by Sfp and AcpS phosphopantetheinyl transferases. ACS Chem. Biol. 2007;2:337–346. doi: 10.1021/cb700054k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.