Abstract
The liver is a major metastasis-susceptible site and majority of patients with hepatic metastasis die from the disease in the absence of efficient treatments. The intrahepatic circulation and microvascular arrest of cancer cells trigger a local inflammatory reaction leading to cancer cell apoptosis and cytotoxicity via oxidative stress mediators (mainly nitric oxide and hydrogen peroxide) and hepatic natural killer cells. However, certain cancer cells that resist or even deactivate these anti-tumoral defense mechanisms still can adhere to endothelial cells of the hepatic microvasculature through proinflammatory cytokine-mediated mechanisms. During their temporary residence, some of these cancer cells ignore growth-inhibitory factors while respond to proliferation-stimulating factors released from tumor-activated hepatocytes and sinusoidal cells. This leads to avascular micrometastasis generation in periportal areas of hepatic lobules. Hepatocytes and myofibroblasts derived from portal tracts and activated hepatic stellate cells are next recruited into some of these avascular micrometastases. These create a private microenvironment that supports their development through the specific release of both proangiogenic factors and cancer cell invasion- and proliferation-stimulating factors. Moreover, both soluble factors from tumor-activated hepatocytes and myofibroblasts also contribute to the regulation of metastatic cancer cell genes. Therefore, the liver offers a prometastatic microenvironment to circulating cancer cells that supports metastasis development. The ability to resist anti-tumor hepatic defense and to take advantage of hepatic cell-derived factors are key phenotypic properties of liver-metastasizing cancer cells. Knowledge on hepatic metastasis regulation by microenvironment opens multiple opportunities for metastasis inhibition at both subclinical and advanced stages. In addition, together with metastasis-related gene profiles revealing the existence of liver metastasis potential in primary tumors, new biomarkers on the prometastatic microenvironment of the liver may be helpful for the individual assessment of hepatic metastasis risk in cancer patients.
Keywords: Liver metastasis, Tumor microenvironment, Liver sinusoidal cells, Portal fibroblasts, Hepatocytes, Hepatic zonation, Inflammatory cytokines, Angiogenesis, Stromal cells, Tumor myofibroblasts, Immune tolerance, Colorectal carcinoma, Metastasis-related genes
Introduction
The liver is the second most commonly involved organ by cancer metastasis, after the lymph nodes. The true prevalence of metastatic liver disease is unknown but, depending on the site of the primary tumor, 30–70% of patients dying of cancer have hepatic metastases. The liver may be the site of metastasis from virtually any primary malignant tumor, and the most common primary sites are uveal melanoma, gastrointestinal cancers, breast and lung carcinomas, neuroendocrine tumors and sarcomas. In children, the most common liver metastases are from neuroblastoma, Wilms tumor, or leukemia [1].
In Europe and North America, a focal liver lesion is more likely to represent a metastatic tumor than a primary malignancy. However, most liver metastases are multiple, involving various lobes in 77% patients, and only 10% are solitary. In addition, multiple tumors often vary in diameter suggesting that cancer cell seeding occurs in episodes. Most patients with hepatic metastases still will die of their disease and surgical resection offers around a 50% 5-year survival rate to selected patients with hepatic metastases [2]. Numerous studies have focused on factors determining metastasis recurrence to the liver, but at the moment, the genetic and phenotypic properties of specific cancer cells able to implant and grow in the liver have not yet been established for any tumor type. Neither is it known the contribution of the heterogeneous biologic backgrounds from individual patients to hepatic metastasis diathesis and regulation.
In this review we summarize our current knowledge on the pathogenic mechanisms of hepatic metastasis in order to define the contribution of architectural and functional aspects of the hepatic microenvironment to the regulation of infiltrating cancer cells. Four consecutive phases and their specific mechanisms were considered in this review during hepatic metastasis development (Table 1). Then, the role of both the hepatic microvascular structure and the functional heterogeneity of hepatic parenchymal and non-parenchymal cell types in the regulation of metastatic cancer cells were analyzed. Particular emphasis was done on the contribution of tumor-activated hepatic cells and tumor-induced hepatic inflammation to cancer–hepatic cell interactions leading to immune escape, intratumor stromal and angiogenic cell recruitment and gene expression and growth of metastatic cells. However, it has to be mentioned that most of available information derives from experimental hepatic metastasis models and, therefore, needs to be clinically validated.
Table 1.
Phases and major mechanisms of the hepatic metastasis process
| 1. The microvascular phase of liver-infiltrating cancer cells | |
| Cancer cell retention and death in the hepatic microvasculature. | |
| Cancer cell survival and adhesion to the hepatic microvasculature. | |
| Cancer cell extravasation. | |
| Tumor-induced inflammation and immune suppression. | |
| Anti-tumor cytotoxicity of liver-associated lymphocytes. | |
| 2. The intralobular micrometastasis phase | |
| Onset of cancer cell growth at periportal areas of hepatic lobules. | |
| Stromal cell recruitment into avascular micrometastases. | |
| Hepatic stellate cell activation by tumor-derived factors. | |
| Role of tumor-activated hepatocytes. | |
| 3. The angiogenic micrometastasis phase | |
| Proangiogenic effects of intrametastatic stroma. | |
| Role of hypoxia. | |
| Role of tumor-derived factors. | |
| Hepatic metastasis angiogenic patterns. | |
| Replacement/Sinusoidal-type metastasis. | |
| Pushing/Portal-type metastasis. | |
| 4. The established hepatic metastasis phase | |
| Development and effects of tumor-infiltrating stromal cells. | |
| Pathophysiology of unaffected hepatic areas. | |
| Hepatic metastasis-related genes. | |
| Genes encoded at the primary tumor | |
| Hepatic microenvironment-dependent genes |
Why Cancer Cells Metastasize to the Liver?
Since the end of the nineteenth century, two non-excluding theories have been considered to explain the phenomenon of organ-specific spreading of cancer cells [3]. First, it is a process directed passively due to mechanical factors that result from the anatomical arrangement of the vascular system, the blood flow rate and the nonspecific trapping of cancer cells by microvascular size constraints—Ewing’s Theory [4]—. Second, the process is due to a fertile environment provided by the organ in which cancer cells can survive and proliferate—Paget’ Seed and Soil Theory [5]—. In the case of the liver, both anatomical/hemodynamical and functional (microenvironmental) factors work together to trap and kill circulating cancer cells, but also to make this territory one of the most common sites for cancer metastasis.
On the one hand, the liver’s important role within the circulatory system makes it a common stopping point for tumor cell emboli carried in the blood from tumors located in other organs. The liver filters the venous drainage from the majority of intra-abdominal viscera and around 30% of the cardiac output. In addition, hepatic microcirculation is slow and tortuous due to the anastomotic arrangement of networking sinusoidal capillaries within hepatic lobules (Fig. 1), and to the blood flow control role of intrasinusoidal macrophages—Kupffer cells—and perisinusoidal stellate cells [6]. These features confer the hepatic territory with a great accessibility for circulating cancer cells, but also with an efficient filtration capability facilitating the mechanical arrest of the majority of circulating cancer cells.
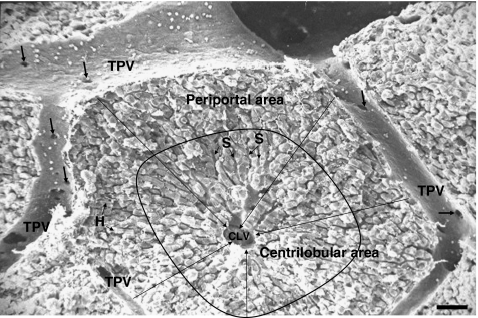
Fig. 1.
Scanning electron microscopic image on the hepatic lobule. The hepatic lobule has the form of a polyhedral prism that in this picture has been sectioned horizontally. At the lobule corners, portal tracts are situated containing terminal portal veins (TPV), perilobular arteries, lymphatic vessels, nerve fibers and bile ducts (not visible). TPV surround the lobule and are also connected to sinusoids through occasional gates serving for the intralobular access of blood (arrows). Hepatic parenchymal cells—hepatocytes (H)—are organized in plates radially arranged around the centrilobular vein (CLV) located in the center of the hepatic lobule. Sinusoids (S) form a microvasculature among hepatocytes within the hepatic lobules. They form an anastomotic network in the periportal area, while are straits in the centrilobular area around the central vein. Blood passes through openings in the TPV into sinusoids and circulates among hepatocytes to be collected by the CLV. There, blood continues to the interlobular veins and, then, into collecting veins draining finally into the hepatic veins leaving the liver through the suprahepatic vein (not shown). Bar: 50 μm
On the other hand, the microenvironment of the liver is biologically unique (Fig. 2a–c). First of all, fenestrated endothelial cells and organ-specific macrophages (Kupffer cells) lining hepatic sinusoids constitutively express a rich profile of surface oligosaccharides [7], cell adhesion proteins [8], and recognition receptors for a variety of pathogen-associated molecular patterns [9], and are endowed with very efficient receptor-mediated endocytotic mechanisms [10]. These properties are cytokine-inducible [11, 12] and account for the efficient hepatic uptake and clearance of circulating waste molecules, death cells, microorganisms, and even cancer cells. Second, the liver also contains a large resident population of activated defense cells—including macrophages [13], dendritic cells [14], mast cells [15], cytotoxic natural killer (NK) cells and T lymphocytes [16]—that provide an enhanced innate immune response, while are maintaining a tolerogenic state to avoid chronic inflammation [17, 18]. In turn, hepatic immune tolerance may be responsible for the increased prevalence of autoimmunity, infectious diseases and malignancies, because the hepatic territory does not significantly object the implantation and growth of microorganisms—as for example malaria sporozoites [19], fungi [20], progenitor hematopoietic cells [21], and even cancer cells. Third, the liver contains a heterogeneous population of parenchyma cells—hepatocytes and cholangiocytes—and non-parenchymal stromal cells—mainly portal fibroblasts and perisinusoidal stellate cells— [22, 23]. These cells can contribute to intratumoral stroma and blood vessel generation in response to tumor-derived factors, providing a favorable milieu for the survival and growth of cancer cells from outside the liver.
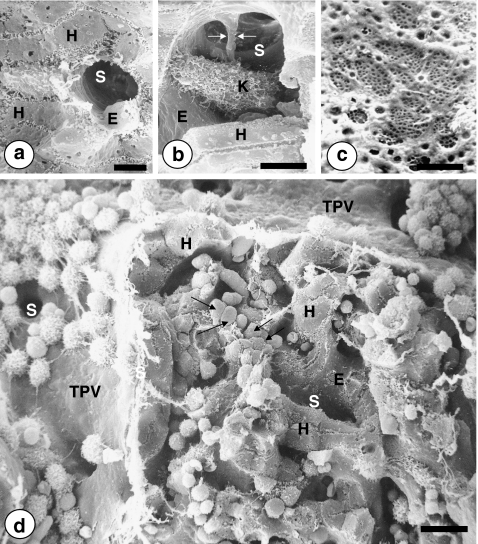
Fig. 2.
Scanning electron microscopic images on the intrahepatic pathway of metastatic cancer cells. a Cross section of a 9-μm in diameter hepatic sinusoid (S), lined by the fenestrated sinusoidal endothelium (E), and surrounded by hepatocytes (H), bar: 5 μm. b An intrasinusoidal Kupffer cell occupying the sinusoidal lumen and connected to the endothelial wall by filopodia and a long citoplasmic prolongation (arrows), bar: 5 μm. c Fenestrated surface of the hepatic sinusoidal endothelium. Under physiological conditions, endothelial fenestrae are transcellular structures of 100–150 nm in diameter that cluster forming highly filtrating microdomains named sieve plates, bar: 1 μm. d Mouse liver tissue on the fifth day post-intrasplenic injection of Lewis lung carcinoma cells. Circulating cancer cells first interact with non-fenestrated endothelial cells and adhered leukocytes and macrophages at perilobular terminal portal veins (TPV). Pre-sinusoidal gates for intralobular access of cancer cells (S). Intrasinusoidal retention of cancer cells (arrows) within the periportal area of the hepatic lobule. Surrounding hepatocytes (H) and endothelial cells (E) lining sinusoids (S). Bar: 10 μm
Therefore, concomitant to anatomical and hemodynamical features facilitating the intrahepatic lodgment of circulating cancer cells, it appears that the functional microenvironment created by hepatic cells may further contribute to retention and destruction of circulating cancer cells, but also to facilitate the ability of some of them to grow in the liver as a metastasis.
The availability of methods for isolating and primary culturing hepatic parenchymal and non-parenchyma cells has greatly facilitated the research on molecular mechanisms of cancer-host cell interactions in the liver using in vitro models. New features of the hepatic metastasis microenvironment have been uncovered and molecular targets of interest for anti-tumor therapeutic innovation have been identified using these experimental models. However, if the same microenvironmental factors operate for every metastasis, irrespective of the tumor type, and if we can prevent or treat hepatic metastasis by blocking hepatic prometastatic factors, or by upregulating anti-metastatic factors from the hepatic microenvironment, needs further preclinical research prior to the clinical translation.
Once circulating cancer cells have reached the liver, metastasis generation depends on the sum of well-defined colonization phenomena that a very small fraction of liver-infiltrating cancer cell clones appear to successfully accomplish. The complexity of involved mechanisms and the control role of microenvironmental factors make hepatic metastasis a highly inefficient process for cancer cells [24] that frequently leads to incomplete metastases, without clinical impact due to their stromal and angiogenic deficiencies. Below is analyzed the sequence of interrelated mechanisms contributing to the development of hepatic metastasis. These have been aggregated into four pathophysiological rate-limiting phases that may demand different treatment strategies.
The Microvascular Phase of Liver-infiltrating Cancer Cells
It includes the mechanisms of occult intravascular arrest, death and survival of liver-infiltrating cancer cells, and their interaction with sinusoidal cells via inflammation-mediated mechanisms.
Cancer Cell Retention and Death in the Hepatic Microvasculature
The hepatic metastasis process begins with the microvascular retention of circulating cancer cells. Cancer cell clumps reaching the liver normally arrest in the terminal portal venules that surround hepatic lobules, while single cancer cells can also arrest in the proximal segments of sinusoids located in the periportal area of hepatic lobules [25, 26]. This happens when single cancer cells pass through pre-sinusoidal sphincters regulating blood access to intralobular sinusoids (Fig. 2d). Intravital videomicroscopy and confocal microscopy on cancer cells experimentally delivered to the portal circulation [27, 28] have demonstrated that this step is in part regulated by sinusoidal structure and hemodynamical mechanisms at same time promoting microvascular arrest and destruction of cancer cells. Not surprisingly, very few of the cancer cells delivered to periportal areas travel along sinusoids to centrilobular areas and reach the lungs in a viable state [29]. Very few cancer cells (around 0.5%) may also traverse the liver through portal-centrilobular venous shunts and implant in the lung [27]. Therefore, deformation-associated trauma and mechanical stress, suffered by cancer cells on entry and residence in the hepatic microvasculature, are important factors contributing to cancer cell death [30, 31].
Infiltrating cancer cells can also induce obstruction of affected sinusoidal segments (Fig. 3a), leading to blood flow blockade and transient micro-infarcts at specific sites of cancer cell inflow and arrest. This mainly occurs in the periportal segment of hepatic sinusoids and can damage sinusoidal cells and even parenchymal cells (Fig. 1d). In turn, re-oxygenation of these ischemic sinusoids induces the proinflammatory activation of sinusoids affected by cancer cell trapping [32], leading to additional killing of cancer cells in the following hours, as a result of the release of nitric oxide [33] and reactive oxygen intermediates [34] by hepatic sinusoidal cells. Moreover, nitric oxide-induced tumor cytotoxicity is increased by sinusoidal cell production of hydrogen peroxide due to the formation of potent oxidants, likely OH and NO3 radicals, via a trace metal-dependent process [35].
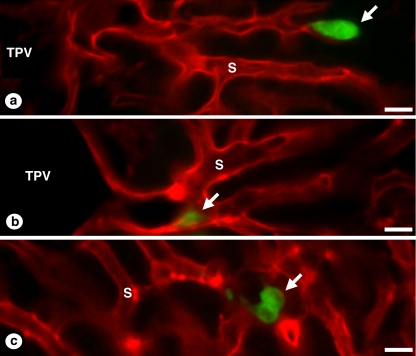
Fig. 3.
Confocal microscopy on experimental cancer cell entry and residence in the hepatic microvasculature. Carboxyfluorescein-labeled CT26 murine colorectal carcinoma cells were intrasplenically injected in syngeneic mice and their livers were perfused on the 24th and 48th hour with fluorescence-labeled wheat germ agglutinin (WGA), as previously described [7], for the specific staining of hepatic microvascular walls (cancer cells had not WGA-binding sites. a Retention of a cancer cell (in green) in the lumen of a hepatic sinusoid (in red). Notice that the body of the cancer cell completely occupied the sinusoidal lumen preventing endothelial cell labeling with the perfused lectin beyond this point. b Cancer cell adhesion to the endothelial wall of a periportal sinusoidal segment. c Onset of cancer cell proliferation on the 48th after injection. Notice that cancer cell clump did not alter microvascular staining in the area, suggesting the extravascular position of cancer cells. Terminal portal vein (TPV); sinusoid (S); cancer cells (arrows). Bar: 20 μm
When cancer cells invade the liver, they also encounter defense mechanisms specific to the organ, operated by the orchestrated actions of Kupffer cells, hepatic NK cells and endothelial cells. Kupffer cells can phagocytose and clear cancer cells [36–38]. Using CC531 colon carcinoma cells, it has been reported that cancer cell clearing may be done by a minority of Kupffer cells mainly located in the periportal area of hepatic lobules [39]. Kupffer cells can also modulate the host immune response to cancer cells by releasing cytotoxic products and immune stimulating factors, such as interferon-gamma, that activate hepatic NK cells [16, 40]. In turn, these cells produce anti-tumor cytotoxicity via perforin/granzyme-containing granule secretion and death receptor-mediated mechanisms, including Fas/FasL pathway [41, 42].
Therefore, the special arrangement of sinusoids in the periportal areas of hepatic lobule, in combination with the preferential location of cytotoxic and phagocytotic cells in this precise territory account for the removal of most circulating cancer cells passing through the liver after leaving their primary tumor. These early microvascular events may serve to severely limit further direct spread of liver-infiltrating cancer cells to other organs, and therefore may contribute to metastatic inefficiency of disseminated tumors. However, some arrested cancer cells can resist to, and even deactivate, the hepatic anti-tumor microenvironment. For example, when cancer cell arrest occurs in clumps inner cells are protected to attacks from anti-tumor immune defenses. In the case of colorectal carcinoma cells, tumor-derived carcinoembryonic antigen can prevent cancer cell death by inducing IL-10 to inhibit inducible NO synthase upregulation in hepatic cells and NO-dependent cancer cell death [43, 44]. Expression of major histocompatibility complex (MHC) class I on colon carcinoma cells is a mechanism of immune escape that can also negatively regulate hepatic NK cell-mediated apoptosis and cytolysis by blocking the perforin/granzyme pathway [45], leading to incomplete killing by NK cells of intrasinusoidal colon carcinoma cells. In the case of metastatic melanoma, the high intracellular level of glutathione protects B16 melanoma cells from oxidative stress induced by sinusoidal cell-derived factors, contributing to cancer cell survival within the hepatic microvasculature [46]. This mechanism appears highly dependent on the glutathione peroxidase/glutathione reductase system required to eliminate hydrogen peroxide [47].
Cancer Cell Survival and Adhesion to the Hepatic Microvasculature
Certain liver-infiltrating cancer cells that survive in the hepatic microvasculature are able to interact with sinusoid-lining endothelium and Kupffer cells [48] (Fig. 3b). This occurs via soluble paracrine and juxtacrine factors and leads to the firm adhesion of certain cancer cells to the sinusoidal wall. The adhesion mechanism is further upregulated by endogenous proinflammatory factors and reactive oxygen metabolites, released or induced by cancer cells in the hepatic sinusoid microenvironment [49–51]. Endotoxins released by gastrointestinal bacteria to the portal circulation, and exogenous pro-inflammatory cytokines and drugs can also stimulate the adhesion of cancer cells to the hepatic microvasculature [52]. However, the proinflammatory response appear to be tumor-specific, and even just some few cancer cell subpopulations within a given tumor may directly release proinflammatory factors or induce their release from hepatic cells.
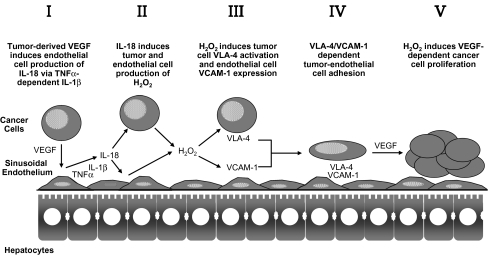
The experimental hepatic colonization of B16 melanoma cells supports the important prometastatic implications of inflammatory cytokine-dependent cancer-sinusoidal cell interactions. Using intrasplenically injected B16F10 melanoma cells, we showed that the expression of VCAM-1 significantly increased in hepatic sinusoidal endothelium cells within the first 24 h of metastatic cancer cell infiltration in the liver. In vivo VCAM-1 blockade with specific antibodies prior to B16 melanoma cell injection decreased microvascular retention of luciferase-transfected B16 melanoma cells by 85%, and metastasis development by 75%, indicating that VCAM-1 expression on tumor-activated hepatic sinusoidal endothelium cells had a prometastatic role [53]. Interestingly, in primary cultured hepatic sinusoidal endothelium cells treated with B16 melanoma-conditioned medium, a proinflammatory cytokine cascade occurred in which TNF-alpha induced IL-1beta; then, IL-1beta, either alone or with TNFalpha, induced IL-18 release [54]. Neither TNF-binding protein nor IL-1 receptor antagonist were able to inhibit the increase in adhesiveness in IL-18-treated hepatic sinusoidal endothelium cells, confirming that neither endogenous TNFalpha nor IL-1 mediated IL-18-induced endothelial cell adhesiveness. Conversely, IL-18 neutralization by using IL-18 binding protein [53] did not reduce tumor-induced TNF-alpha or IL-1beta release from endothelial cells, suggesting that their production was IL-18-independent. As such, TNFalpha and IL-1beta used the production of IL-18 to facilitate the increase in endothelial cell expression of VCAM-1 for arresting cancer cells during their transit through the hepatic microvasculature (Fig. 4).
Fig. 4.
Tumor-induced proinflammatory factors regulate melanoma cell adhesion to hepatic sinusoidal endothelium prior to metastasis formation
Recombinant catalase also abrogated the proadhesive response of hepatic sinusoidal endothelium cells to B16 melanoma cell-conditioned medium, although this did not affect the release of major proinflammatory cytokines by tumor-activated hepatic sinusoidal endothelium cells [51]. In turn, hydrogen peroxide production from B16 melanoma conditioned medium-treated endothelial cells was regulated by IL-18 [53]. Thus, liver-infiltrating B16 melanoma cells activated their adhesion to hepatic sinusoidal endothelium cells through a sequential process involving TNF-alpha-dependent IL-1beta, which induced IL-18 to up-regulate VCAM-1 via hydrogen peroxide. This cytokine cascade can be induced by tumor-derived VEGF [54]. In addition, the pivotal position of IL-18-induced hydrogen peroxide was further supported by the fact that incubation of hepatic sinusoidal endothelium cells with nontoxic concentrations of hydrogen peroxide directly enhanced VCAM-1-dependent B16 melanoma cell adhesion in vitro without proinflammatory cytokine mediation, which emphasizes the key role of oxidative stress in the pathogenesis of IL-18-dependent hepatic metastasis. Consistent with these findings, hepatic metastasis of intrasplenically injected B16 melanoma cells dramatically reduced in IL-1beta KO mice and almost completely inhibited in ICE KO mice [48]. Moreover, a single intraperitoneal dose of naturally occurring IL-18 binding protein given 30 min before intrasplenic injection of B16F10 cells abolished VCAM-1 up-regulation in the hepatic microvasculature and reduced hepatic metastasis by 80% [55]. These data demonstrate a significant role of endogenous IL-18 on hepatic metastasis by up-regulating melanoma cell adhesion to hepatic sinusoidal endothelium cells, implicating a possible antimetastatic benefit of neutralizing IL-18 [56] (Fig. 3).
Khatib et al. [57] also detected that metastatic murine lung carcinoma H-59 or human colorectal carcinoma CX-1 cells triggered TNF-alpha production by Kupffer cells located in sinusoids around the invading cancer cells, during the initial stages of liver metastasis. This was followed by increased expression of the vascular adhesion receptors E-selectin, P-selectin, VCAM-1, and ICAM-1 on sinusoidal endothelium cells. This proinflammatory response was tumor-specific and was not observed with nonmetastatic cancer cells. Again, these results identify sinusoidal cell-mediated TNF-alpha production as an early, tumor-selective host inflammatory response to liver-invading cancer cells that may influence the course of metastasis. Interestingly, over-expression of secretory leukocyte protease inhibitor—a factor attenuating inflammatory response by blocking NF-kappaB-mediated TNF-alpha—in highly metastatic subline (H-59) markedly decreased the ability of these cells to elicit a proinflammatory response in the liver and to form hepatic metastases [58].
Using a combination of immunohistochemistry, confocal microscopy, and three-dimensional reconstruction, Auguste et al. [59] also evidenced E-selectin expression mainly on sinusoids by 6 and 10 h, respectively, following murine H-59 and human CX-1 carcinoma cell inoculation. Cancer cells arrested in E-selectin expressing endothelial cells and appeared to flatten and traverse the sinusoidal lining, away from sites of intense E-selectin staining. This process was evident by 8 (H-59) and 12 h (CX-1) after inoculation, coincided with increased endothelial VCAM-1 expression, and involved tumor cell attachment in areas of intense VCAM-1 and PECAM-1 expression. Non-metastatic (human) MIP-101 and (murine) M-27 cells induced a weaker response and could not be seen to extravasate. The results show that metastatic cancer cells can use newly expressed endothelial cell receptors to arrest and extravasate.
Therefore, cytokine release from tumor-activated sinusoidal cells can be identified as an early, tumor-specific inflammatory response to liver-invading cancer cells, that may influence metastasis occurrence. In addition, factors that either attenuate tumor-induced host proinflammatory response or adhesion receptors for cancer cells may have a therapeutic potential in the prevention of liver metastasis.
Tumor-induced Inflammation Inhibits Anti-tumor Cytotoxicity of Liver-associated Lymphocytes
Tumor-induced hepatic inflammation also involves up-regulation of mannose receptor (ManR)-mediated endocytosis [60], a 175 kDa transmembrane glycoprotein involved in the general processes of endogenous defense [61]. ManR binds and uptakes mannosylated molecules and circulating infectious agents, and contributes to the mechanisms of cancer cell adhesion and antigen processing and presentation to lymphocytes [62, 63]. ManRs are also involved in antigen uptake and presentation to T cells by hepatic sinusoidal endothelium cells and it has been suggested that this process underlies the well-known immune-tolerance occurring in the liver under physiological conditions. Using murine C26 colon carcinoma cells, we demonstrated that ManR expression and endocytotic activity increased in hepatic sinusoidal endothelium cells upon direct interaction with metastatic C26 cells [60]. The mechanism required COX-2-dependent production of IL-1-stimulating factor(s) from tumor cells activated by membrane or soluble ICAM-1. In turn, IL-1-induced ManR led to decreased cytotoxicity in liver lymphocytes interacting with tumor-activated endothelium. This suggests the contribution of mannose receptors to immune suppression during the hepatic colonization of cancer cells, and the secretion of ManR-stimulating factor(s) as a new prometastatic feature of liver-metastasizing cancer cells.
The Intralobular Micrometastasis Phase
It starts with the growth activation of cancer cells evading local immune defense; either intravascularly located and adhered to hepatic endothelial cells, or extravascularly migrated into the Disse’s space and among hepatocytes (Fig. 3c). Irrespective of the location, the proliferation of surviving cancer cells occurs in periportal areas of the liver lobule and results in the formation of subclinical avascular micrometastases [28]. This phase is also supported by the intrametastatic recruitment of perisinusoidal hepatic stellate cells, portal fibroblasts and hepatocytes activated by tumor-derived factors. These cells get integrated among metastatic cancer cells forming a heterogeneous stroma that releases paracrine growth factors for cancer cells, and creates a preangiogenic stromal support [64].
The Prometastatic Microenvironment of Periportal Areas within the Hepatic Lobule
Circulating cancer cell arrest, survival and growth activation is markedly affected by the functional zonation of the liver lobule, leading to the selective generation of micrometastases in periportal areas [65]. Therefore, structural and functional heterogeneity of parenchymal and sinusoidal cells across the liver lobule, or acinus, represents an obligated framework for understanding the contribution of microenvironment to cancer cell regulation and micrometastasis formation during the intralobular phase of the metastatic process.
Liver zonation is characterized by phenotypic variations of hepatocytes and sinusoidal cells along the length of the sinusoid from the portal vein to the central vein (Fig. 5a). Oxygen tension, which decreases by about 50% from the periportal to the perivenous regions, has been considered to be a key regulator for zonated gene expression [66]. Gradients of metabolic substrates, hormones, and extracellular matrix are thought to be other important regulators of these zonal variations. These environmental variations have implications for liver inflammation, fibrosis, aging, regeneration and infection [67].
Fig. 5.
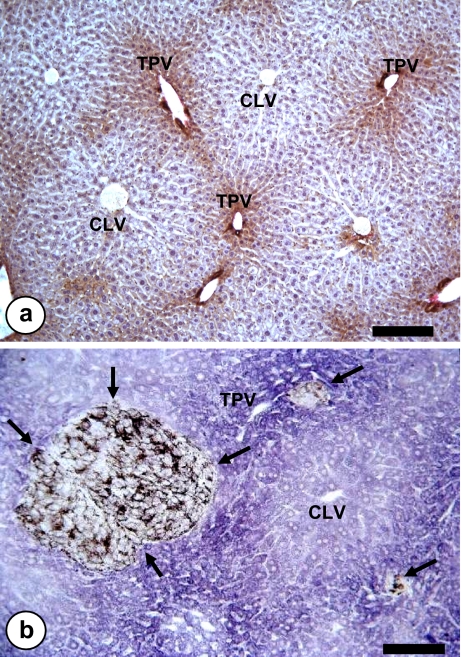
Light microscopic image of hepatic tissue. a Parenchymal cell heterogeneity across the hepatic lobule, as shown by immunohistochemical staining of nerve growth factor-expressing hepatocytes. Bar: 100 μm. b Periportal micrometastases (arrows) from intrasplenically-injected B16F10 melanoma cells. Terminal portal veins (TPV) and centrilobular veins (CLV). Bar: 100 μm
A very accurate method for calibrating the position coordinates of initial micrometastatic foci in the hepatic acini was made possible by using the succinate-dehydrogenase reaction (Fig. 5b), which reveals the functional heterogeneity of parenchymal cells within the hepatic lobule [68]. Based on this approach we reported that experimental micrometastases occur in the periportal zone of liver lobules, without being detected any significant differences resulting from the cancer type (sarcoma, melanoma, carcinoma), its metastatic potential (high and low), or the experimental procedure used for obtaining the metastasis (subcutaneous or intrasplenic injection). In subsequent experiments, metastasis was induced after first altering the zonal distribution of the hepatic extracellular matrix; distribution of the sinusoidal macrophages; and the sinusoidal diameter. However, even under these conditions, metastasis continued to occur exclusively in periportal zones [69]. Thus, metastatic predilection for this sublobular compartment cannot be explained solely in terms of hemodynamic causes, or the influence of extracellular matrix. On the contrary, the functional microenvironment created by cells of the periportal zone account for the metastatic predilection for periportal zones [70]. For example, fenestration pattern, expression of adhesion molecules, phagocytosis, receptor-mediated endocytosis and antigen presentation by hepatic sinusoidal cells have a zonal distribution across the acinus [71, 72], and may influence the metastatic outcome of cancer cells. Hepatocytes and hepatic stellate cells also express a functional heterogeneity across the hepatic lobule [72] that may contribute to the periportal metastasis development.
Regulation of Initial Cancer Cell Growth within the Hepatic Lobule Area
Many liver-infiltrating cancer cells remain dormant in an extravascular position, while growth to form micrometastases is initiated in only a small subpopulation of cancer cells [28]. Therefore, activation of solitary dormant cancer cell proliferation is a key event for hepatic metastasis progression. Several mechanisms may operate at this level. Firstly, when intravascularly located, cancer cell proliferation is activated by paracrine growth factors released by neighboring tumor-activated endothelial cells. The mechanism is significantly stimulated by proinflammatory cytokines [49, 52]. Secondly, when extravascularly located, cancer cell proliferation is activated by paracrine growth factors released by neighboring tumor-activated hepatocytes and hepatic stellate cells. This is facilitated by adhesive mechanisms [73] and junctional specializations, such as desmosomes [74], formed between cancer cells and hepatocytes, soon after invasion of the hepatic cell plate. However, early micrometastatic foci may disappear after a few days, and only a small subset may continue growth. According to Groom et al. [75], critical mechanisms responsible for cancer cell losses and metastatic inefficiency may also occur at this phase of micrometastatic cell growth.
Stromal Cell Recruitment into Avascular Micrometastases
A rich tumor growth-stimulating stroma generated from tumor-activated hepatic cells is recruited into developing intralobular micrometastases. This is driven by cancer cell release of stromal cell migration- and proliferation-stimulating factors. Three main sources of tumor-associated stromal cells have been considered: (1) hepatic stellate cells [68, 76–78], which transdifferentiates into myofibroblasts in response to paracrine factors released by both cancer cells and tumor-activated hepatic sinusoidal endothelium and Kupffer cells. Once intrametastatic located, they express alpha-smooth muscle actin, a marker for hepatic stellate cell activation (Fig. 6a,b). This stromal cell origin mainly operates when micrometastases are developed in the sinusoidal area of liver lobules. (2) Portal tract fibroblasts [79], which contribute to intrametastatic stroma when invading cancer cells are located in the perilobular areas and are unable to activate the hepatic stellate cell-dependent stromagenesis. (3) Hepatocytes, which may suffer an epithelial to mesenchymal transition induced by both tumor-derived factors and tumor-activated hepatic stellate cell-derived factors, are also recruited into metastatic tissue (Fig. 7a,b).
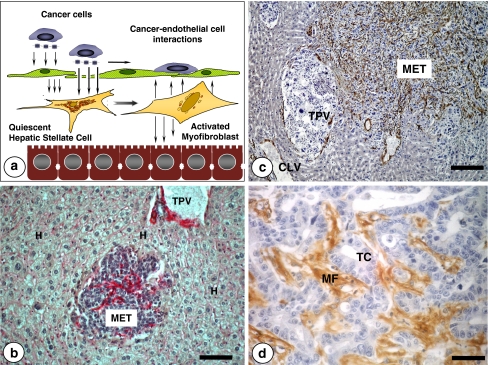
Fig. 6.
a Two mechanisms are proposed for the hepatic stellate cell activation by tumor-derived factors: Indirect, via tumor-activated hepatic sinusoidal endothelial cells; direct, via transendothelial cell diffusion of tumor-derived hepatic stellate cell-stimulating factors. b Intratumoral recruitment of hepatic stellate cells (red stained) at the intralobular micrometastasis phase of the experimental colonization of intrasplenically-injected C26 colon carcinoma cells. Immunohistochemical detection of smooth muscle-alpha actin reveals that intrametastatic, but not extrametastatic, hepatic stellate cells become myofibroblasts by tumor-derived paracrine/juxtacrine factors prior to angiogenesis occurrence. Terminal portal veins (TPV) surrounded by portal tract fibroblasts and smooth muscle cells (in red). Bar: 50 μm. c Panlobular C26 colon carcinoma micrometastasis containing a dense population of tumor-activated myofibroblasts as revealed by immunohistochemical detection of smooth muscle alpha-actin (stained in brown). Bar: 100 μm. d High-magnification picture on smooth muscle alpha-actin-expressing stromal myofibroblasts (MF) in an established hepatic metastasis from a colorectal carcinoma patient. Tumor cells (TC). Bar: 15 μm
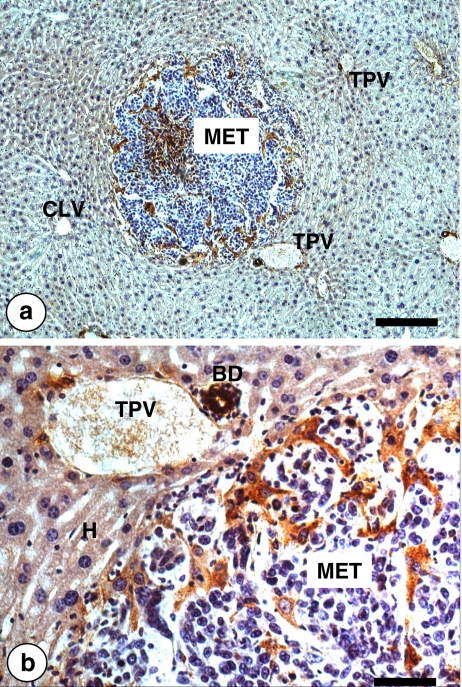
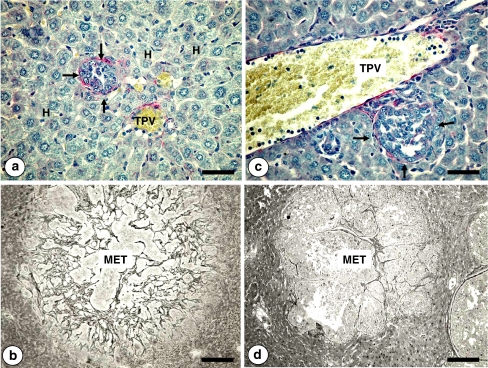
Fig. 7.
Low (a) and high (b) magnification pictures on the intratumoral recruitment of hepatocytes in a C26 colon carcinoma intralobular micrometastasis (MET). Tumor-activated perimetastatic and intrametastatic hepatocytes (in brown) were immunohistochemicaly revealed by anti-mouse nerve growth factor (NGF) antibody. Only cholangiocytes within the perilobular bile ducts and periportal hepatocytes express NGF under normal physiological conditions. However, tumor microenvironmental factors induced hepatocyte expression of NGF. Bar: 100 μm (a) and 10 μm (b)
Whatever its tissue origin, intrametastatic stromal cells exhibit a myofibroblast phenotype, and are already operating in the avascular growth stage of developing hepatic metastasis prior to angiogenic endothelial cell recruitment [68]. There, bidirectional interactions involving paracrine growth factors take place between cancer cells and stromal cells supporting cancer cell invasion and proliferation. Hepatic stellate cell-derived myofibroblasts mainly secrete VEGF, platelet-derived growth factor-AB, hepatocyte growth factor, PGE2 and transforming growth factor-beta. Portal tract-derived myofibroblasts also produce IL-8, a chemokine related to invasion and angiogenesis, in response to TNF-alpha via nuclear factor-kappaB [79]. In the specific case of hepatocytes, they also secrete several cancer cell migration and proliferation-stimulating factors, and even induce VEGF production from cancer cells. However, as shown in a preliminary study using primary cultured hepatocytes from patients with established colon carcinoma metastasis, these prometastatic effects are heterogeneously expressed among patients with established colon carcinoma metastasis (Del Villar et al. submitted for publication).
The Angiogenic Micrometastasis Phase
It takes place in prevascular stromagenic micrometastases having an average diameter larger than 300 μm, and therefore, that are growing beyond the limits of liver lobule. Main activities at this phase are intrametastatic endothelial cell recruitment and blood vessel formation. Proangiogenic factors from tumor-activated stromal cells and hypoxic cancer cells initiate this phase [80]. Therefore, it cannot occur in hypoxic hepatic micrometastases that did not previously develop a myofibroblastic stromal support. This angiogenic phase progresses stepwise as the hepatic metastasis enlarges, and is a prerequisite for its continued growth until it becomes a clinically visible established metastasis.
Hypoxia Induces Proangiogenic Activation of Intrametastatic Myofibroblasts
As above reported, infiltration of tumor-activated myofibroblasts precedes endothelial cell recruitment into avascular micrometastases. Endothelial cell migration only occurred towards avascular micrometastases containing a high density of myofibroblasts and not towards metastases not containing myofibroblasts (Fig. 8). Both myofibroblasts and endothelial cells co-localized, and their densities consistently correlated with the development of well-vascularized metastases [80]. Because hypoxic tissue has been identified as a potential source of angiogenic factors within the tumor, we analyzed the effect of hypoxia on myofibroblast production of angiogenic-stimulating factors. As confirmed by pimonidazole staining, hypoxia occurred in hepatic metastases of greater than 300 μm in diameter. However, onset of myofibroblast recruitment occurred in normoxic avascular micrometastases, whereas new intratumoral capillaries are constituted once micrometastases become hypoxic. In vitro, we [76] reported that hypoxia contributes to hepatic stellate cell production of VEGF, which in turn increases endothelial cell migration, reduction of apoptosis, and proliferation. Using an experimental model of liver cirrhosis, Corpechot et al. [81] also reported VEGF production by hypoxic hepatic stellate cell. Thus, their recruitment under normoxic conditions, followed by tumor growth-associated hypoxia, may constitute two synergistic stimuli for intratumoral migration and survival of endothelial cells during tumor blood vessel formation. Interestingly, endothelial cell recruitment also follows the penetration of hepatic stellate cells into hepatocyte clusters of regenerating liver [82]. This physiologic mechanism of tissue reconstitution may also account for the recruitment of endothelial cells into micrometastases containing activated hepatic stellate cells. Therefore, tumor-activated hepatic stellate cells may promote blood delivery to liver metastasis by triggering the onset of angiogenesis in avascular micrometastases and, then, by supporting their progressive vascularization. Not surprisingly, hepatic stellate cells exhibit pericyte-like functions [83] and their activation into a myofibroblast-like phenotype is involved in several hepatic disease processes associated to chronic and acute liver injury [84].
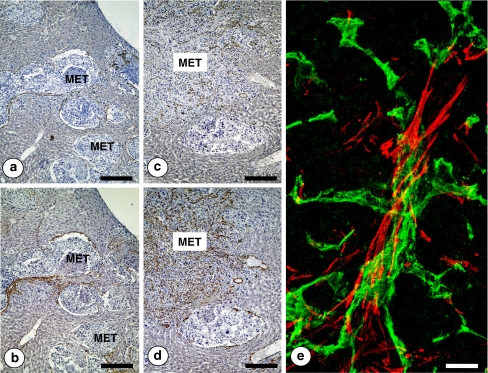
Fig. 8.
Immunohistochemical detection of CD31-expressing angiogenic endothelial cells in portal-type (pushing growth pattern) (a) and sinusoidal-type (replacement growth pattern) (c) hepatic metastases from intrasplenically-injected head and neck squamous cell murine PAN-LY2 carcinoma cells. Immunohistochemical staining for smooth muscle alpha actin expression of serial tissue sections from the same livers (b and d). Notice the co-localization of CD31 and smooth muscle alpha actin expressing cells in both kinds of hepatic metastases. Bar: 150 μm. e High-magnification confocal microscopic image on intrametastatic neo-angiogenic vessels. CD31-expressing endothelial cells (green-stained) were surrounded by smooth muscle alpha actin-expressing vascular coverage cells (red stained). Bar: 20 μm
Angiogenic Patterns in Developing Hepatic Metastasis
In an experimental model of hepatic metastasis by Lewis lung carcinoma, Paku and Lapis [85] identified two types of metastases with different angiogenic patterns: a sinusoidal type, devoid of immunohistochemically detectable basement membrane, and a portal type, located in the vicinity of portal tracts, characterized by staining positively for basement membrane components. Their association to specific cancer cell implantation sites in the hepatic microvasculature was further supported by the fact that intrasplenically injected cancer cells (portal route) produced only 18.2% of the portal type-metastases, whereas a significantly higher percentage of the metastases (33.2%) proved to be portal-type when cancer cells were injected into the left ventricle (arterial route).
Consistent with these observations, Vermeulen et al. [86] demonstrated that liver metastases from patients with colorectal adenocarcinoma were also heterogeneous with respect to angiogenesis and growth patterns. Three different growth patterns were found in these liver metastases. In the desmoplastic and in the pushing growth patterns, the architecture of the liver parenchyma was not preserved. In the replacement growth pattern, the reticulin pattern of the liver parenchyma was conserved within the metastases at the tumor-liver parenchyma interface. The replacement growth pattern expanded with minimal angiogenesis by co-opting the stroma with the sinusoidal blood vessels of the liver. Indeed, the ratio of the proliferating cancer cell fraction and the proliferating endothelial cell fraction, roughly representing the degree of angiogenesis-dependent growth, was three- to four-fold higher in the replacement-type metastases compared with the other metastases. Cancer cell apoptosis was highest in the pushing-type metastases and was inversely correlated with microvessel density in liver metastases.
Using several liver-metastasizing murine tumors (51b and C26 colon carcinoma, PAM squamous cell carcinomas and B16 melanoma), we also recognized two predominant stromal patterns (Fig. 9), according to expression of alpha-smooth muscle actin by intrametastatic myofibroblast-like cells [87]: sinusoid-associated metastases, which contained infiltrating, but not encapsulating, reticularly-arranged myofibroblasts; and portal tract-associated metastases, which were incompletely encapsulated, but not infiltrated, by fibrous tract-arranged myofibroblasts. Based on reticulin staining, the liver architecture was preserved in myofibroblast-infiltrated metastases because invasive cancer cells co-opted the supportive fibrillar network of sinusoids, and, thus, the limit between tumor and normal tissue was ill-defined (equivalent to replacement-type). In contrast, the reticulin network was not conserved within myofibroblast-encapsulated metastases, because the enlarging mass of cancer cells compressed surrounding parenchyma (pushing-type) and generated the formation of tumor lobules delineated by desmoplastic stroma (desmoplastic-type).
Fig. 9.
a Avascular micrometastasis (arrows) developed in the sinusoidal domain of an hepatic lobule, surrounded by tumor-activated hepatic stellate cells expressing smooth muscle alpha actin (red stained cells). Terminal portal venule (TPV). Hepatocytes (H). Bar: 25 μm. b Sinusoidal-type hepatic micrometastasis (MET) at the angiogenic phase, containing a dense network of sinusoidal neovessels, as revealed by reticulin stain according to Gordon–Sweets silver impregnation technique. Recruited microvessels form concentric interconnections. Liver architecture is not disturbed, and cancer cells co-opt the supportive fibrilar network of the sinusoids. Bar: 100 μm. c Avascular micrometastasis (arrows) developed in close proximity to a terminal portal vein (TPV) and surrounded by portal tract-derived cells expressing smooth muscle alpha actin (red stained cells). Bar: 25 μm. d Portal-type micrometastasis (MET) at the angiogenic phase. Here, the reticulin network supporting intratumoral angiogenesis is not conserved. Desmoplastic stroma surrounds and traverses metastasis, facilitating invasion of vascular-type angiogenic vessels. Necrotic areas frequently develop in this metastasis type. Bar: 100 μm
The precise mechanisms underlying different stromal and angiogenic arrangements for replacement-/sinusoidal-type and pushing-desmoplastic-/portal-type metastases remain unclear. One possibility is that the myofibroblast-inducing activities of cancer cells is dependent on the site of cancer cell implantation, and that hepatic stellate cells represent the main source of myofibroblasts for sinusoidal-type metastases, whereas portal tract-derived fibroblasts constitute the stromal support of portal-type metastases. This possibility is also supported by the predominant expression of desmin and glial fibrillary acidic protein—two hepatic stellate cell markers—by myofibroblasts located in sinusoidal-type metastases [80]; while myofibroblasts located in portal-type metastases display a vimentin and Thy-1 phenotype similar to resident portal tract hepatic fibroblasts [79].
These stromagenic and angiogenic patterns are also associated to aggressiveness and treatment resistance of hepatic metastases. In this regard, hepatic metastases from low metastatic squamous cell carcinoma PAM-212 cancer cells replaced hepatocytes at the cancer cell-liver parenchymal cell interface, preserving the liver architecture, co-opting the sinusoidal blood vessels and having a low fraction of proliferating cancer cells; in contrast, hepatic metastases from (highly-metastatic) PAM-LY2, the architecture of the liver parenchyma was not preserved, stromagenic and angiogenic activity derived from portal tracts and the fraction of proliferating cancer cells was high. Interestingly, PAM-212 produced low concentrations of pro-angiogenic cytokines while they released thrombospondin and insulin-like growth factor binding protein-1. In contrast, PAM-LY2 cells produced a high amount of VEGF and GM-colony stimulating factor. Therefore, specific cytokine patterns of these two squamous carcinoma cell variants were associated to different hepatic metastasis growth and angiogenesis patterns [88]. More importantly, anti-angiogenic agents did not similarly affect sinusoidal-type and portal-type hepatic metastases. Treatment of 51b colon carcinoma hepatic metastases with recombinant human endostatin did not affect portal-type metastases while it almost eradicated sinusoidal-type metastases [87]. Similar results were recently obtained (Salado et al., submitted) in hepatic metastases from B16 melanoma treated with resveratrol, a natural product with anti-angiogenic properties.
The Established Hepatic Metastasis Phase
Once liver-infiltrating cancer cells have accomplished the three phases of the hepatic metastasis process, generated tumors get the status of “established metastasis” whose clinical detection will depend on the size, intrahepatic location, blood flow alterations and aggressiveness. Even for a given tumor type, established metastases are markedly heterogeneous among patients and within the same patient. Differences mainly concern the structure and density of tumor-associated stroma and blood vessels [80], the hemodynamic alterations in the portal vein and hepatic artery [89], the phenotypic composition of tumor-infiltrating lymphocytes [90], and the cancer cell profile with regard to proliferation, invasion, gene expression and protein secretion. However, at the moment the clinical and prognostic significances of these biological aspects of hepatic metastases are unclear. Moreover, none of the identified gene signatures [91, 92] or molecular markers has been successfully validated as a diagnostic or prognostic tool applicable to routine clinical practice.
Hepatic Metastasis-related Genes
The development of established hepatic metastasis is associated with overexpression or downregulation of specific genes and cell regulatory pathways. Some of the gene alterations may be originated in the primary tumor and, thereafter, may support hepatic metastasis development. In a recent study, Yamasaki et al. [92] investigated the existence of liver metastatic potential in primary colorectal tumors using metastasis-related genes detected by chronological gene expression profiling of colorectal samples corresponding to consecutive oncogenic stages. Interestingly, the profile of metastasized primary tumors resembled one of a metastatic lesion apart from a primary lesion rather than one of a non-metastasized primary tumor. Moreover, the expression profile of these genes allowed the classification of tumors diagnosed as localized cancer into two classes, the localized and the metastasized class, according to their final metastatic status. The disease-free survival and overall survival were significantly longer in the localized class than the metastasized class suggesting that the metastatic potential is already encoded in the primary tumor and detectable, which allows the prediction of liver metastasis in patients diagnosed with localized tumors.
Other studies suggest that different microenvironments can differentially affect the expression of metastasis-related genes [93], and overexpression or underexpression of these genes need not be present when cancer cells are in primary tumors or initially disseminated. However, if the hepatic microenvironment can regulate at a gene expression level the ability of a cancer cell to metastasize is unclear. Using RNA from hepatic metastases, tumor-unaffected hepatic tissues and peripheral blood mononuclear cells from the same patients we determined by DNA microarray and RT-PCR the specific gene cluster representing the transcriptome of cancer cells at the established hepatic metastasis phase in patients with advanced colorectal carcinoma (Del Villar et al. submitted for publication). This gene cluster did neither include genes expressed by tumor-unaffected hepatic tissues nor those from peripheral blood mononuclear cells from the same patients. To determine if hepatic cells can still contribute to the regulation of hepatic metastasis genes at established hepatic metastases, we evaluated the transcriptome of HT-29 colon carcinoma cells given the conditioned media from highly-prometastatic hepatocytes and hepatic stellate cell-derived myofibroblasts. HT-29 cancer cells contained 235 genes upregulated by hepatocyte-conditioned medium and 67 genes upregulated by hepatic myofibroblast-conditioned medium. Majority of genes upregulated by both hepatic cell types were different, but a significant number of genes induced by each cell type overlapped with those included in the specific gene cluster representing the hepatic metastasis transcriptome from patients with colon carcinoma. These results demonstrate the differential contribution of hepatocytes and hepatic myofibroblasts to the microenvironmental regulation of hepatic metastasis-related genes from colon carcinoma patients. Consistent with tumor growth-stimulating properties of hepatic stromal cells, around 50% of hepatic metastasis-related genes regulated by soluble factors from hepatic cells belonged to the cell cycle-regulation class further supporting the role of these hepatic cells in the control of metastatic cell proliferation. Hence, despite the possible expression of certain hepatic metastasis-related genes in the primary colorectal tumors, that even may allow detecting metastasis risk in cancer patients, tumor-activated hepatic cells may also contribute to the expression of genes operating at established hepatic metastases. This microenvironmental control occurs at an advanced phase of the hepatic metastasis process and, therefore may have implications for therapy. Moreover, these results demand research efforts to identify biomarkers on the contribution of the hepatic biology backgrounds from individual patients to hepatic metastasis development.
Conclusions
Certain circulating cancer cells are resistant to the specialized anti-tumoral mechanisms of the liver microvasculature, while take advantage of the hepatic microenvironment for developing metastases. The influence of the hepatic microenvironment on the cancer metastasis process operates at various levels, allowing the division of the hepatic metastasis process in three microenvironment-associated stages that offer different therapeutic opportunities (Table 2).
Table 2.
Microenvironment-associated stages of the hepatic metastasis process
| Features | Stage I | Stage II | Stage III |
|---|---|---|---|
| Metastasis size | <300 μm; not detectable | 0.3 mm–5 mm; sometimes detectable | >5 mm; detectable |
| Clinical impact | Subclinical | Organ-specific effects | Systemic effects and risk of extrahepatic dissemination |
| Metastasis phase | Intralobular: Microvascular and Avascular micrometastasis phases | Panlobular status: angiogenic phase | Lobar status: established metastasis phase |
| Interaction with hepatic cells | Avascular and stromal-free | In cooperation with hepatic cells for intrametastatic stroma and angiogenesis development | Affected by both hepatic cells from tumor-unaffected areas, and tumor-infiltrating hepatic cells |
| Intrahepatic impact | Cancer cells interact with hepatic cells located in its original tissue organization | Cancer cells interact with tumor-infiltrating migratory hepatic cells | Cancer cells alter hepatic tissue structure, blood supply and parenchymal cell metabolism |
| Therapeutic targets | Proinflammatory cytokines | Angiogenic factors | Tumor-growth factors |
| Immune suppressant factors | Stromagenic factors | Immune suppressant factors | |
| Myofibroblast-stimulating factors | |||
| Oxidative stress-inducing factors |
Hepatic Metastasis Stage I When the behavior of liver-infiltrating cancer cells is mainly affected by the microvascular anatomy and the functional zonation occurring within the hepatic lobules or acini. This operates since the initial arrest of circulating cancer cells in the hepatic microvasculature until the formation of an avascular micrometastasis that has not yet progressed beyond the limits of the hepatic lobule because its diameter is smaller than 300 μm. At this stage, cancer cells interact with hepatic cells located in its original tissue organization. This stage includes both the microvascular and the avascular micrometastasis phases of the hepatic metastasis process. Micrometastases are completely subclinical, avascular, sometimes even stromal-free, and have not yet a significant influence on the functionality of the whole organ. Therapeutic targets at this stage are those factors inducing tumor-dependent hepatic inflammation and immune response inhibition, intrametastatic myofibroblast recruitment and endogenous antioxidant machinery upregulation in cancer cells.
Hepatic Metastasis Stage II When liver-infiltrating cancer cells have formed a micrometastasis whose mechanism of development is no longer affected by hepatic zonation. However, the process is still strongly hepatic cell-dependent and requires the cooperation of hepatic cells for successfully developing intrametastatic stroma and angiogenesis. This is the panlobular stage of the hepatic metastasis process because it involves several hepatic lobules. Here, cancer cells mainly interact with migratory hepatic parenchymal and non-parenchymal cells that have lost their original position coordinates in the hepatic tissue organization, and are recruited into the metastatic tissue to form the tumor stroma. This stage corresponds to the angiogenic phase of the hepatic metastasis process, and it implies the transition from avascular to vascularized metastasis. Lesions can be detectable by non-invasive methods [94], but their existence is still subclinical because metastasis size ranges from 0.3 up to 5 mm. These metastases have the potential of becoming clinically relevant and, if multifocal, they may affect liver functions and hemodynamics. Therapeutic targets at this stage are cancer cell invasion and proliferation-stimulating factors, and mediators of tumor-induced angiogenesis and stromagenesis.
Hepatic Metastasis Stage III When liver-infiltrating cancer cells have formed a clinically detectable established metastasis, bigger than 5 mm in diameter, whose development is no longer affected by hepatic tissue organization. This is the most advanced stage of the hepatic metastasis process. It involves the whole hepatic organ, to which it affects through the secretion of tumor-derived factors, and it progressively alters in terms of tissue structure, blood supply and metabolic substrate availability, jeopardizing parenchymal cell function. However, at this stage, hepatic metastases are still microenvironmentally modulated. On the one hand, metastases contain tumor-infiltrating lymphocytes [95]—including immunosuppressant CD4/CD25 regulatory T cells [90]—and stromal cells mainly derived from hepatic tissue [96], and whose specific phenotypes have prognostic implications. On the other hand, both normal and cancer cells within metastatic tissue are also transcriptionally affected by hepatic soluble factors, including proinflammatory and angiogenic cytokines [56, 97], type I-insulin-like growth factor [98], and immunosuppressant factors (TGFbeta, IL-10, soluble ICAM-1, etc), whose elevated concentration also have regulatory effects. Therefore, at this stage established hepatic metastases are affected again by two interrelated microenvironments whose control may require different therapeutic approaches: parenchymal and non-parenchymal cells from tumor-unaffected hepatic areas, and tumor-activated hepatic cells recruited into the metastatic tissue.
In summary, besides its natural antitumor defense mechanisms, the liver has prometastatic effects accounting for the high incidence and aggressiveness of hepatic metastasis. Our growing knowledge on the molecular basis of hepatic metastasis regulation by microenvironment opens new avenues for hepatic metastasis prevention at a subclinical stage, and for treatment at more advanced stages. In addition, together with possible metastasis-related gene profiles revealing the existence of liver metastatic potential in primary tumors, some genes from metastatic cells are hepatic cell-dependent and, therefore, new biomarkers of the specific biologic background of the liver supporting its prometastatic microenvironment are needed for the individual assessment of hepatic metastasis risk in cancer patients.
Acknowledgement
This work was supported in part by grants from the CICYT (no. SAF2006-09341) and from Basque Country Government to consolidated research groups (no. IT-487-07).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Pickren JW, Tsukada Y, Lane WW (1982) Liver metastasis. In: Weiss L, Gilbert HA (eds) Analysis of autopsy data. GK Hall and Company, Boston, Mass, pp 2–18
- 2.Gilbert HA, Kagan AR, Hintz BL et al (1982) Patterns of metastases. In: Weiss L, Gilbert HA (eds) Liver metastases. GK Hall Medical Publishers, Boston, MA, pp 19–39
- 3.Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458 [DOI] [PubMed]
- 4.Ewing J (1928) Neoplastic diseases, 6th edn. WB Saunders, Philadelphia
- 5.Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 1:571–573 [DOI] [PubMed]
- 6.McCuskey RS (1994) The hepatic microcirculation. In: Arias I, Boyer JL, Fausto N et al (eds) The liver: biology and pathobiology. Raven, New York, pp 1089–1106
- 7.Barberá-Guillem E, Rocha M, Alvarez A et al (1991) Differences in the lectin-binding patterns of the periportal and perivenous endothelial domains in the liver sinusoids. Hepatology 14:131–139 [DOI] [PubMed]
- 8.Scoazec J-Y, Feldman G (1991) Immnunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology 14:789–797 [DOI] [PubMed]
- 9.Szabo G, Mandrekar P, Dolganiuc A (1997) Innate immune response and hepatic inflammation. Semin Liver Dis 27:339–350 [DOI] [PubMed]
- 10.Smedsrød B, Pertoft H, Gustafson S et al (1990) Scavenger functions of the liver endothelial cells. Biochem J 266:313–327 [DOI] [PMC free article] [PubMed]
- 11.Asumendi A, Alvarez A, Martinez I et al (1996) Hepatic sinusoidal endothelium heterogeneity with respect to mannose receptor activity is IL-1-dependent. Hepatology 23:1521–1529 [DOI] [PubMed]
- 12.Martinez I, Sveinbjørnsson B, Vidal-Vanaclocha F et al (1995) Differential cytokine-mediated modulation of endocytosis in rat liver endothelial cells. Biochem Biophys Res Commun 212:235–241 [DOI] [PubMed]
- 13.Smedsrod B, De Bleser PJ, Braet F (1994) Cell biology of liver endothelial and Kupffer cells. Gut 35:1509–1516 [DOI] [PMC free article] [PubMed]
- 14.Sallusto F, Cella M, Danieli C et al (1995) Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182:389–400 [DOI] [PMC free article] [PubMed]
- 15.Farrell DJ, Hines JE, Walls AF et al (1995) Intrahepatic mast cells in chronic liver diseases. Hepatology 22:1175–1181 [DOI] [PubMed]
- 16.Bouwens L, Jacobs R, Remels L (1988) Natural cytotoxicity of rat hepatic natural killer cells and macrophages against a syngeneic colon adenocarcinoma. Cancer Immunol Immunother 27:137–141 [DOI] [PMC free article] [PubMed]
- 17.Knolle PA, Gerken G (2000) Local control of the immune response in the liver. Immunol Rev 174:21–34 [DOI] [PubMed]
- 18.Diehl L, Schurich A, Grochtmann R (2008) Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47:296–305 [DOI] [PubMed]
- 19.Frevert U, Engelmann S, Zougbédé S (2005) Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol 3:e192 [DOI] [PMC free article] [PubMed]
- 20.Lewis JH, Patel HR, Zimmerman HJ (1982) The spectrum of hepatic candidiasis. Hepatology 2:479–487 [DOI] [PubMed]
- 21.Barberá-Guillem E, Ayala R, Vidal-Vanaclocha F (1989) Differential location of hemopoietic colonies within liver acini of postnatal and phenylhydrazine-treated adult mice. Hepatology 9:29–36 [DOI] [PubMed]
- 22.Uchio K, Tuchweber B, Manabe N (2002) Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest 82:619–628 [DOI] [PubMed]
- 23.Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88:125–172 [DOI] [PMC free article] [PubMed]
- 24.Weiss L (1994) Inefficiency of metastasis from colorectal carcinomas. Relationship to local therapy for hepatic metastasis. Cancer Treat Res 69:1–11 [PubMed]
- 25.Vidal-Vanaclocha F, Rocha M, Asumendi A et al (1993) Periportal and perivenous endothelial cell phenotypes in the liver sinusoidal pathway: role in homing of blood and metastatic cancer cells. Sem Liver Dis 13:60–71 [DOI] [PubMed]
- 26.Dingemans KP, Roos E, van den Bergh Weerman MA et al (1978) Invasion of liver tissue by tumor cells and leukocytes: comparative ultrastructure. J Natl Cancer Inst 60:583–598 [DOI] [PubMed]
- 27.Ishii S, Mizoi T, Kawano K (1996) Implantation of human colorectal carcinoma cells in the liver studied by in vivo fluorescence videomicroscopy. Clin Exp Metastasis 14:153–164 [DOI] [PubMed]
- 28.Luzzi KJ, MacDonald IC, Schmidt EE et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873 [DOI] [PMC free article] [PubMed]
- 29.Barbera-Guillem E, Weiss L (1993) Cancer-cell traffic in the liver. III Lethal deformation of B16 melanoma cells in liver sinusoids. Int J Cancer 54:880–884 [DOI] [PubMed]
- 30.Barbera-Guillem E, Smith I, Weiss L (1993) Cancer-cell traffic in the liver. II. Arrest, transit and death of B16F10 and M5076 cells in the sinusoids. Int J Cancer 53:298–301 [DOI] [PubMed]
- 31.Weiss L (1992) Biomechanical interactions of cancer cells with the microvasculature during hematogenous metastasis. Cancer Met Rev 11:227–235 [DOI] [PubMed]
- 32.Jessup J, Battle P, Waller H et al (1999) Reactive nitrogen and oxygen radicals formed during hepatic ischemia-reperfusion kill weakly metastatic colorectal cancer cells. Cancer Res 59:1825–1829 [PubMed]
- 33.Wang H, McIntosh A, Hasinoff B et al (2000) B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 60:5862–5869 [PubMed]
- 34.Anasagasti MJ, Alvarez A, Avivi C et al (1996) Interleukin-1-mediated H2O2 production by hepatic sinusoidal endothelium in response to B16 melanoma cell adhesion. J Cell Physiol 167:314–323 [DOI] [PubMed]
- 35.Carretero J, Obrador E, Anasagasti MJ et al (1999) Growth-associated changes in glutathione content correlate with liver metastatic activity of B16 melanoma cells. Clin Exp Metastasis 17:567–574 [DOI] [PubMed]
- 36.Roos E, Dingemans KP, Van de Pavert IV et al (1978) Mammary-carcinoma cells in mouse liver: infiltration of liver tissue and interaction with Kupffer cells. Br J Cancer 38:88–99 [DOI] [PMC free article] [PubMed]
- 37.Kan Z, Ivancev K, Lunderquist A et al (1995) In vivo microscopy of hepatic metastases: dynamic observation of tumor cell invasion and interaction with Kupffer cells. Hepatology 21:487–494 [PubMed]
- 38.Bayón LG, Izquierdo MA, Sirovich I et al (1996) Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology 23:1224–1231 [DOI] [PubMed]
- 39.Timmers M, Vekemans K, Vermijlen D et al (2004) Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int J Cancer 112:793–802 [DOI] [PubMed]
- 40.Gardner CR, Wasserman AJ, Laskin DL (1991) Liver macrophage mediated cytotoxicity toward mastocytoma cells involves phagocytosis of tumor targets. Hepatology 14:318–324 [PubMed]
- 41.Vermijlen D, Luo D, Robaye B et al (1999) Pit cells (Hepatic natural killer cells) of the rat induce apoptosis in colon carcinoma cells by the perforin/granzyme pathway. Hepatology 29:51–56 [DOI] [PubMed]
- 42.Vekemans K, Timmers M, Vermijlen D et al (2003) CC531 colon carcinoma cells induce apoptosis in rat hepatic endothelial cells by the Fas/FasL-mediated pathway. Liver Int 23:283–293 [DOI] [PubMed]
- 43.Jessup JM, Laguinge L, Lin S et al (2004) Carcinoembryonic antigen induction of IL-10 and IL-6 inhibits hepatic ischemic/reperfusion injury to colorectal carcinoma cells. Int J Cancer 111:332–337 [DOI] [PubMed]
- 44.Jessup JM, Samara R, Battle P et al (2004) Carcinoembryonic antigen promotes tumor cell survival in liver through an IL-10-dependent pathway. Clin Exp Metastasis 21:709–717 [DOI] [PubMed]
- 45.Luo D, Vermijlen D, Kuppen PJ et al (2002) MHC class I expression protects rat colon carcinoma cells from hepatic natural killer cell-mediated apoptosis and cytolysis, by blocking the perforin/granzyme pathway. J Comp Hepatol 1:2 [DOI] [PMC free article] [PubMed]
- 46.Anasagasti MJ, Martin JJ, Mendoza L et al (1998) Glutathione protects metastatic melanoma cells against oxidative stress in the murine hepatic microvasculature. Hepatology 27:1249–1256 [DOI] [PubMed]
- 47.Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43:143–181 [DOI] [PubMed]
- 48.Roos E, Tulp A, Middelkoop OP et al (1982) Interaction between liver-metastasizing lymphoid tumor cells and hepatic sinusoidal endothelial cells. In: Knook DL, Wisse E (eds) Cells of the hepatic sinusoid. Elsevier Biomedical Press, Amsterdam, pp 147–154
- 49.Vidal-Vanaclocha F, Amézaga C, Asumendi A et al (1994) Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res 54:2667–2672 [PubMed]
- 50.Anasagasti MJ, Alvarez A, Martin JJ et al (1997) Sinusoidal endothelium release of hydrogen peroxide enhances very late antigen-4-mediated melanoma cell adherence and tumor cytotoxicity during interleukin-1 promotion of hepatic melanoma metastasis in mice. Hepatology 25:840–846 [DOI] [PubMed]
- 51.Mendoza L, Carrascal T, de Luca M et al (2001) Hydrogen peroxide mediates vascular cell adhesion molecule-1 expression from IL-18-activated hepatic sinusoidal endothelium: Implications for circulating cancer cell arrest in murine liver. Hepatology 34:298–310 [DOI] [PubMed]
- 52.Vidal-Vanaclocha F, Alvarez A, Asumendi A et al (1996) Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst 88:198–205 [DOI] [PubMed]
- 53.Carrascal T, Mendoza L, Vacarcel M et al (2003) Interleukin-18 binding protein reduces B16 Melanoma Hepatic Metastasis by neutralizing the adhesiveness and growth factors of sinusoidal endothelial cell. Cancer Res 63:491–497 [PubMed]
- 54.Mendoza L, Valcarcel M, Carrascal T et al (2004) Inhibition of cytokine-induced microvascular arrest of tumor cells by recombinant endostatin prevents experimental hepatic melanoma metastasis. Cancer Res 64:304–310 [DOI] [PubMed]
- 55.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L et al (2000) IL-18 regulates IL-1 beta-dependent hepatic melanoma metastasis via vascular adhesion molecule-1. Proc Natl Acad Sci U S A 97:734–739 [DOI] [PMC free article] [PubMed]
- 56.Vidal-Vanaclocha F, Mendoza L, Telleria N et al (2006) Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev 25:417–434 [DOI] [PubMed]
- 57.Khatib AM, Auguste P, Fallavollita L et al (2005) Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol 167:749–759 [DOI] [PMC free article] [PubMed]
- 58.Wang N, Thuraisingam T, Fallavollita L et al (2006) The secretory leukocyte protease inhibitor is a type 1 insulin-like growth factor receptor-regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res 66:3062–3070 [DOI] [PubMed]
- 59.Auguste P, Fallavollita L, Wang N et al (2007) The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol 170:1781–1792 [DOI] [PMC free article] [PubMed]
- 60.Arteta B, Lasuen N, Vidal-Vanaclocha F (2007) Tumor-activated endothelial cells inhibit anti-tumor immunity during hepatic colon carcinoma metastasis via mannose receptor upregulation. In: Witz I (ed) Proc 4th Int Conf on Tumor Microenvironment: Progression, Therapy and prevention, Medimond, Italy.
- 61.Stahl PD, Ezekowitz RA (1998) The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol 10:50–55 [DOI] [PubMed]
- 62.Malovic I, Sorensen KK, Elvevold KH et al (2007) The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology 45:1454–1461 [DOI] [PubMed]
- 63.Mendoza L, Olaso E, Anasagasti MJ et al (1998) Mannose receptor-mediated endothelial cell activation contributes to B16 melanoma cell adhesion and metastasis in liver. J Cell Physiol 174:322–330 [DOI] [PubMed]
- 64.Olaso E, Arteta B, Salado C (2006) Proangiogenic implications of hepatic stellate cell transdifferentiation into myofibroblasts induced by tumor microenvironment. In: Chaponnier C (ed) Tissue repair, contraction and the myofibroblast. Landes, Austin, TX
- 65.Vidal-Vanaclocha F, Mendoza L, Anasagasti MJ et al (1997) On the role of sinusoidal endothelium and stellate cells in the progression of primary and secondary hepatic neoplasms. In: Wisse E et al. (ed) Cells of the hepatic sinusoid, vol 6. Kupffer Cell Found, Leiden, pp 393–401
- 66.Jungermann K (1995) Zonation of metabolism and gene expression in liver. Histochem Cell Biol 103:81–91 [DOI] [PubMed]
- 67.Jungermann K, Kietzmann T (2000) Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 31:255–260 [DOI] [PubMed]
- 68.Barbera-Guillem E, Alonso A, Boyano MD et al (1990) Estimating anatomical-functional position coordinates in liver tissue. Anat Rec 228:267–276 [DOI] [PubMed]
- 69.Barberá-Guillem E, Alonso-Varona A, Vidal-Vanaclocha F (1989) Selective implantation and growth in rats and mice of experimental liver metastasis in acinar zone one. Cancer Res 49:4003–4010 [PubMed]
- 70.Vidal-Vanaclocha F, Alonso-Varona A, Ayala R et al (1990) Coincident implantation, growth and interaction sites within the liver of cancer and reactive hematopoietic cells. Int J Cancer 46:267–271 [DOI] [PubMed]
- 71.Wang HH, Nance DM, Orr FW (1999) Murine hepatic microvascular adhesion molecule expression is inducible and has a zonal distribution. Clin Exp Metastasis 17:149–155 [DOI] [PubMed]
- 72.Vidal-Vanaclocha F (ed) (1997) Functional heterogeneity of liver tissue: from cell lineage diversity to sublobular compartment-specific pathogenesis. RG Landes Co, Austin, TX
- 73.Kemperman H, Wijnands Y, Meijne AM et al (1994) TA3/St, but not TA3/Ha, mammary carcinoma cell adhesion to hepatocytes is mediated by alpha 5 beta 1 interacting with surface-associated fibronectin. Cell Adhes Commun 2:45–58 [DOI] [PubMed]
- 74.Shimizu S, Yamada N, Sawada T et al (2000) Ultrastructure of early phase hepatic metastasis of human colon carcinoma cells with special reference to desmosomal junctions with hepatocytes. Pathol Int 50:953–959 [DOI] [PubMed]
- 75.Groom AC, MacDonald IC, Schmidt EE et al (1999) Tumor metastasis to the liver, and the roles of proteinases and adhesion molecules: new concepts from in vivo videomicroscopy. Can J Gastroenterol 13:733–743 [DOI] [PubMed]
- 76.Olaso E, Santisteban A, Bidaurrazaga J et al (1997) Tumor-dependent activation of rodent hepatic stellate cells during experimental melanoma metastasis. Hepatology 26:634–642 [DOI] [PubMed]
- 77.Shimizu S, Yamada N, Sawada T et al (2000) In vivo and in vitro interactions between human colon carcinoma cells and hepatic stellate cells. Jpn J Cancer Res 91:1285–1295 [DOI] [PMC free article] [PubMed]
- 78.Faouzi S, Lepreux S, Bedin C et al (1999) Activation of cultured rat hepatic stellate cells by tumoral hepatocytes. Lab Invest 79:485–493 [PubMed]
- 79.Mueller L, Goumas FA, Affeldt M et al (2007) Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol 171:1608–1618 [DOI] [PMC free article] [PubMed]
- 80.Olaso E, Salado C, Egilegor E et al (2003) Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology 37:674–685 [DOI] [PubMed]
- 81.Corpechot C, Barbu V, Wendun D et al (2002) Hypoxia-induced VEGF and collagen I expression are associated with angiogenesis and fibrogenesis in experimental fibrosis. Heptology 35:1010–1021 [DOI] [PubMed]
- 82.Martinez-Hernandez A, Amenta PS (1995) The extracellular matrix in hepatic regeneration. FASEB J 9:1401–1410 [DOI] [PubMed]
- 83.Wake K (1999) Cell-cell organization and functions of 'sinusoids' in liver microcirculation system. J Electron Microsc 48:89–98 [DOI] [PubMed]
- 84.Rockey DC (2001) Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis 21:337–349 [DOI] [PubMed]
- 85.Paku S, Lapis K (1993) Morphological aspects of angiogenesis in experimental liver metastases. Am J Pathol 143:926–936 [PMC free article] [PubMed]
- 86.Vermeulen PB, Colpaert C, Salgado R et al (2001) Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 195:336–342 [DOI] [PubMed]
- 87.Solaun MS, Mendoza L, de Luca M et al (2002) Endostatin inhibits murine colon carcinoma sinusoidal-type metastases by preferential targeting of hepatic sinusoidal endothelium. Hepatology 35:1104–1116 [DOI] [PubMed]
- 88.Egilegor E, Salado C, Gutierrez V et al (2006) Differential stromagenic and angiogenic cytokine patterns support replacement- and pushing-type hepatic metastasis growth patterns of squamous cell carcinoma. Proc 100th Annual Meeting AACR, Los Angeles, CA
- 89.Oktar SO, Yücel C, Demirogullari T et al (2006) Doppler sonographic evaluation of hemodynamic changes in colorectal liver metastases relative to liver size. J Ultrasound Med 25:575–582 [DOI] [PubMed]
- 90.Kobayashi N, Hiraoka N, Yamagami W et al (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13:902–911 [DOI] [PubMed]
- 91.Friederichs J, Rosenberg R, Mages J (2005) Gene expression profiles of different clinical stages of colorectal carcinoma: toward a molecular genetic understanding of tumor progression. Int J Colorectal Dis 20:391–402 [DOI] [PubMed]
- 92.Yamasaki M, Takemasa I, Komori T et al (2007) The gene expression profile represents the molecular nature of liver metastasis in colorectal cancer. Int J Oncol 30:129–138 [PubMed]
- 93.Ohta S, Lai EW, Morris JC et al (2007) Metastasis-associated gene expression profile of liver and subcutaneous lesions derived from mouse pheochromocytoma cells. Mol Carcinog 47:245–251 [DOI] [PubMed]
- 94.Nomura K, Kadoya M, Ueda K et al (2007) Detection of hepatic metastases from colorectal carcinoma: comparison of histopathologic features of anatomically resected liver with results of preoperative imaging. J Clin Gastroenterol 41:789–795 [DOI] [PubMed]
- 95.Winnock M, Garcia-Barcina M, Huet S et al (1993) Functional characterization of liver-associated lymphocytes in patients with liver metastasis. Gastroenterology 105:1152–1158 [DOI] [PubMed]
- 96.Ooi LP, Crawford DH, Gotley DC et al (1997) Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol 26:798–807 [DOI] [PubMed]
- 97.Stoeltzing O, Liu W, Reinmuth N et al (2003) Angiogenesis and antiangiogenic therapy of colon cancer liver metastasis. Ann Surg Oncol 10:722–733 [DOI] [PubMed]
- 98.Bauer TW, Fan F, Liu W et al (2007) Targeting of insulin-like growth factor-I receptor with a monoclonal antibody inhibits growth of hepatic metastases from human colon carcinoma in mice. Ann Surg Oncol 14:2838–2846 [DOI] [PubMed]