Abstract
Purpose
Obesity is a risk factor for the development of colon cancer. However, the influence of body mass index (BMI) on the outcome of patients with established colon cancer remains uncertain. Moreover, the impact of change in body habitus after diagnosis has not been studied.
Patients and Methods
We conducted a prospective, observational study of 1,053 patients who had stage III colon cancer and who were enrolled on a randomized trial of adjuvant chemotherapy. Patients reported on height and weight during and 6 months after adjuvant chemotherapy. Patients were observed for cancer recurrence or death.
Results
In this cohort of patients with stage III cancer, 35% of patients were overweight (BMI, 25 to 29.9 kg/m2), and 34% were obese (BMI ≥ 30 kg/m2). Increased BMI was not significantly associated with a higher risk of colon cancer recurrence or death (P trend = .54). Compared with normal-weight patients (BMI, 21 to 24.9 kg/m2), the multivariate hazard ratio for disease-free survival was 1.00 (95% CI, 0.72 to 1.40) for patients with class I obesity (BMI, 30 to 34.9 kg/m2) and 1.24 (95% CI, 0.84 to 1.83) for those with class II to III obesity (BMI ≥ 35 kg/m2) after analysis was adjusted for tumor-related prognostic factors, physical activity, tobacco history, performance status, age, and sex. Similarly, after analysis was controlled for BMI, weight change (either loss or gain) during the time period between ongoing adjuvant therapy and 6 months after completion of therapy did not significantly impact on cancer recurrence and/or mortality.
Conclusion
Neither BMI nor weight change was significantly associated with an increased risk of cancer recurrence and death in patients with colon cancer.
INTRODUCTION
Prospective cohort studies support the relationship between adiposity and the risk of developing colon cancer.1-12 The mechanism by which obesity influences the incidence of colorectal neoplasia remains uncertain. Elevated glucose and insulin levels appear to promote the growth of adenomatous and malignant lesions in the bowel.13-15 Both body mass index (BMI) and visceral adiposity are critical determinants of insulin resistance and of hyperinsulinemia.16-18
Recent studies have examined the association between obesity and the risk of cancer recurrence in patients who were diagnosed with colon cancer. In a large, randomized trial of adjuvant chemotherapy for stages II to III colon cancer (INT 0089), obesity (BMI ≥ 30 kg/m2) was associated with a 24%, nonstatistically significant, poorer disease-free survival (DFS) compared with normal-weight women (BMI, 21 to 24.9 kg/m2).19 Among patients enrolled on two National Surgical Adjuvant Breast and Bowel Project (NSABP) adjuvant chemotherapy trials for colon cancer, very obese patients (BMI ≥ 35 kg/m2) experienced a 27% statistically significant increase in cancer recurrence or death compared with normal-weight participants.20 However, because neither study updated anthropometric data after completion of adjuvant therapy, the impact of change in weight after recovery from all treatments could not be assessed. Among women who received adjuvant therapy for breast cancer, weight gain after completion of therapy has been associated with an increased risk of recurrence in some,21,22 though not all, studies.23
We therefore examined the influence of body habitus on outcome among patients who participated in a large National Cancer Institute (NCI)–sponsored clinical trial of adjuvant chemotherapy for stage III colon cancer. As part of this trial, we prospectively assessed weight and other covariates at study baseline and approximately 6 months after completion of postoperative chemotherapy, and we monitored patients thereafter for cancer recurrence. Because patient eligibility, cancer treatment, and follow-up were rigorously defined by the trial, we were able to examine directly the influence of body habitus and weight gain on patient outcome while we minimized confounding by other disease and treatment characteristics.
PATIENTS AND METHODS
Study Population
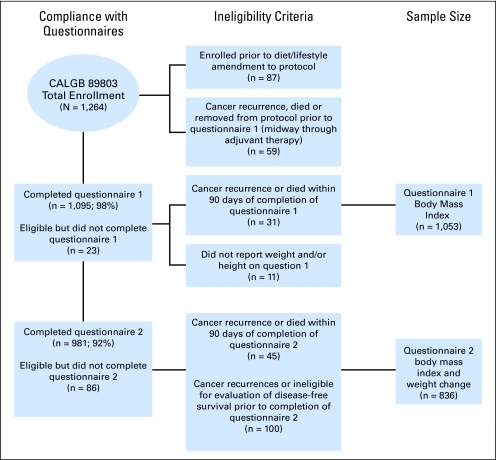
Patients in this prospective cohort study were participants in the NCI-sponsored Cancer and Leukemia Group B (CALGB) adjuvant therapy trial for stage III colon cancer that compared therapy with weekly fluorouracil (FU) and leucovorin to weekly irinotecan, FU, and leucovorin (CALGB 89803).24 Between April 1999 and May 2001, 1,264 patients were enrolled on the treatment trial. A self-administered questionnaire that captured diet and lifestyle habits was given to patients midway through their adjuvant therapy (4 months after surgery) and again 6 months after completion of adjuvant therapy (14 months after surgery). After the first 87 patients were enrolled, the protocol was amended to include a survey of diet and lifestyle; only the subsequent 1,177 patients were eligible for this companion study. Figure 1 illustrates the compliance with completion of the questionnaires and derivation of the final sample sizes for both evaluation of BMI at the time of questionnaire one (Q1), BMI at time of questionnaire two (Q2), and weight change.
Fig 1.
Derivation of cohort sizes.
Patients in the treatment trial were eligible if they underwent a complete surgical resection of the primary tumor within 56 days of study entry and had regional lymph node metastases (stage III colon cancer) but had no evidence of distant metastases. Moreover, patients were required to have a baseline Eastern Cooperative Oncology Group performance status of 0 to 225 and to have adequate bone marrow, renal, and hepatic function. All patients signed informed consent, which was approved by each site's institutional review board.
Determination of Height, Weight, and BMI
In the initial study questionnaire, Q1 (during adjuvant therapy and approximately 4 months after surgery) and the follow-up questionnaire, Q2 (6 months after adjuvant therapy), patients self-reported their weight. Patients also reported their height on Q1. We compared the weight and height recorded at cancer treatment facilities (at study entry) with the patient self-reports on Q1, and we found correlations of 0.91 and 0.92 (both P < .0001), respectively. For consistency, we utilized self-reported weight and height for all analyses, though analyses with the weight from the treatment center did not change the associations reported. We calculated BMI (kg/m2) by dividing the patient's squared height (meters) into the weight (kilograms). For the primary analysis of BMI, we used cumulative averaging to determine a patient's BMI as follows:
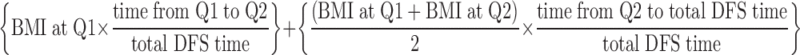 |
BMI categories were created on the basis of WHO classifications and prior analyses.19,26 Change in weight was calculated by subtracting the patient's weight at Q2 from the weight at Q1 for patients that completed both Q1 and Q2 (n = 836).
Study End Points
In analyses of BMI, the primary end point was DFS, which was defined as the time from the completion of Q1 to tumor recurrence, occurrence of a new primary colon tumor, or death from any cause. We defined recurrence-free survival (RFS) as the time from the completion of Q1 to tumor recurrence or occurrence of a new primary colon tumor. For RFS, patients who died without known tumor recurrence were censored at last documented evaluation by the treating provider. Overall survival (OS) was defined as the time from the completion of Q1 to death from any cause. For analyses of weight change, all end points began at the time of completion of Q2. To avoid biases caused by declining health and possible weight changes immediately before recurrence or death, we excluded patients who had either a cancer recurrence or who died within 90 days of Q1 (for BMI analyses) or Q2 (for weight change analyses).
Statistical Analyses
During interim analysis of the treatment trial, it was determined that no difference in either DFS or OS would be observed between the treatment arms.27 Data for patients in both treatment arms were combined and analyzed in BMI and weight-change categories. Cox proportional hazards regression28 was used to determine the simultaneous impact of potential confounders. We conducted our primary analyses by using cumulative averaging of BMI and physical activity. Other potential confounders also were entered into the model as fixed covariates. Covariates with missing variables were coded with indicator variables in adjusted models. Because we observed a quadratic shape to the relationship between BMI and outcomes, we modeled BMI + BMI,2 similar to prior reports.20 A level of significance less than .05 was considered statistically significant. All P values were two-sided and were not adjusted for multiple comparisons.
Patient registration and clinical data collection were managed by the CALGB Statistical Center, and all analyses were based on the study database that was frozen on May 19, 2006.
RESULTS
Baseline Characteristics
Study participants were drawn from a multicenter study of postoperative adjuvant chemotherapy in patients with stage III colon cancer who underwent a curative-intent surgical resection. Within our cohort, 35% of patients were overweight (BMI, 25 to 29.9 kg/m2), and 34% were obese (BMI ≥ 30 kg/m2). Table 1 represents baseline patient characteristics according to updated BMI and weight change between the Q1 and Q2. Underweight (BMI < 21 kg/m2) patients were less likely to be men but were more likely to be current smokers, whereas overweight (BMI, 25 to 29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) patients were more likely to be men, be younger, be less physically active, have well- to moderately differentiated tumors, have less extension through bowel wall, and have greater weight change. There were no significant differences in the number of positive lymph nodes, the number of lymph nodes sampled or node ratio, clinical bowel perforation at time of surgery, race, or treatment arm on the basis of BMI.
Table 1.
Baseline Characteristics by Initial Body Mass Index and Weight Change After Completion of Therapy
| Characteristic | Body Mass Index (kg/m2)
|
Weight Change Between Q1 and Q2 (kg)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 21 | 21-24.9 | 25-29.9 | 30-34.9 | ≥ 35 | P* | > 5 Loss | 2.1-5 Loss | ± 2 | 2-4.9 Gain | ≥ 5 Gain | P* | |
| No. of patients | 68 | 258 | 369 | 236 | 122 | 47 | 42 | 105 | 104 | 538 | ||
| % of total cohort | 6 | 25 | 35 | 22 | 12 | 6 | 5 | 13 | 12 | 64 | ||
| Body mass index, kg/m2 | 19.7 | 23.4 | 27.4 | 32.2 | 37.8 | < .0001 | ||||||
| Median weight change, kg | 5 | 6 | 7 | 8 | 10 | .02 | −9 | −4 | 0 | 3 | 12 | < .0001 |
| Age, years | 64 | 63 | 61 | 60 | 55 | < .0001 | 59 | 60 | 59 | 62 | 60 | .82 |
| Sex, % male | 38 | 47 | 66 | 60 | 46 | < .0001 | 64 | 60 | 59 | 49 | 56 | .43 |
| Ethnicity, % | .19 | .89 | ||||||||||
| White | 89 | 90 | 88 | 90 | 82 | 92 | 86 | 92 | 92 | 88 | ||
| Black | 7 | 6 | 6 | 8 | 11 | 6 | 9 | 4 | 4 | 8 | ||
| Other | 4 | 4 | 6 | 2 | 7 | 2 | 5 | 4 | 4 | 4 | ||
| Baseline performance status, % | .04 | .08 | ||||||||||
| 0 | 63 | 75 | 79 | 76 | 71 | 62 | 71 | 82 | 79 | 76 | ||
| 1-2 | 37 | 25 | 21 | 24 | 29 | 38 | 29 | 18 | 21 | 24 | ||
| Smoking status, % | .05 | .006 | ||||||||||
| Current | 15 | 12 | 10 | 4 | 5 | 6 | 17 | 9 | 12 | 6 | ||
| Past | 43 | 40 | 48 | 48 | 48 | 49 | 26 | 42 | 55 | 46 | ||
| Never | 42 | 48 | 42 | 48 | 47 | 45 | 57 | 49 | 33 | 48 | ||
| Number of positive nodes (%) | .18 | .04 | ||||||||||
| 1-3 | 63 | 66 | 62 | 64 | 75 | 66 | 52 | 78 | 66 | 68 | ||
| ≥ 4 | 37 | 34 | 38 | 36 | 25 | 34 | 48 | 22 | 34 | 32 | ||
| Median No. of nodes examined | 11 | 13 | 12 | 12 | 13 | .27 | 12 | 13 | 12 | 14 | 12 | .40 |
| Ratio of No. of positive lymph nodes to No. of examined nodes | 0.25 | 0.25 | 0.25 | 0.22 | 0.17 | .09 | 0.21 | 0.32 | 0.17 | 0.23 | 0.22 | .11 |
| T stage extent through bowel wall, % | .01 | .40 | ||||||||||
| T1-2 | 21 | 10 | 12 | 16 | 21 | 17 | 5 | 16 | 15 | 16 | ||
| T3-4 | 79 | 90 | 88 | 84 | 79 | 83 | 95 | 84 | 85 | 87 | ||
| Bowel perforation, % | 6 | 4 | 4 | 5 | 6 | .84 | 2 | 0 | 4 | 5 | 4 | .65 |
| Clinical bowel obstruction, % | 30 | 26 | 22 | 15 | 29 | .009 | 22 | 21 | 22 | 19 | 23 | .97 |
| Serum CEA ≥ 5 ng/mL | 13 | 7 | 7 | 6 | 7 | .29 | 2 | 5 | 1 | 8 | 5 | .18 |
| Grade of differentiation, % | .003 | .22 | ||||||||||
| Well | 9 | 6 | 5 | 3 | 11 | 9 | 10 | 9 | 5 | 5 | ||
| Moderate | 69 | 65 | 71 | 81 | 69 | 80 | 64 | 72 | 65 | 73 | ||
| Poor | 22 | 29 | 24 | 16 | 20 | 11 | 26 | 19 | 30 | 22 | ||
| Treatment arm, % | .34 | .002 | ||||||||||
| IFL | 46 | 47 | 52 | 52 | 44 | 53 | 71 | 61 | 53 | 46 | ||
| FU/LV | 54 | 43 | 48 | 48 | 56 | 47 | 29 | 39 | 47 | 54 | ||
| Physical activity in MET, h/wk | ||||||||||||
| Q1 | 4.9 | 5.2 | 6.1 | 4.4 | 2.0 | < .0001 | 5.7 | 4.2 | 8.1 | 6.4 | 4.0 | .005 |
| Q2 | 7.8 | 8.9 | 8.2 | 6.3 | 3.2 | .0004 | 8.7 | 7.6 | 11.1 | 12.5 | 6.5 | .02 |
NOTE. Q1 represents questionnaire one, which was administered during adjuvant therapy; Q2 represents questionnaire two, which was administered 6 months after completion of adjuvant therapy.
Abbreviations: CEA, carcinoembryonic antigen; IFL, irinotecan, bolus fluorouracil, and leucovorin; FU, fluorouracil; LV, leucovorin; MET, metabolic equivalent tasks.
P values were obtained by two-sided χ2 testing.
We did not observe any consistent linear trends in baseline characteristics by weight change between Q1 and Q2. Although the number of positive lymph nodes, smoking status, and treatment arm all appeared to differ significantly according to categories of weight change, the relations that emerged were principally U-shaped and had comparable values at the extreme categories of weight change.
Impact of BMI on Cancer Recurrences or Death
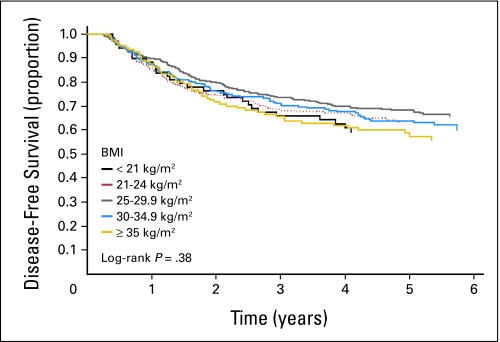
After a median follow-up from completion of Q1 of 5.3 years, 369 of the 1,053 eligible patients experienced cancer recurrence, and 261 patients died. We did not observe any statistically significant differences in DFS, RFS, and OS with increasing BMI (Table 2 and Fig 2). Compared with normal-weight patients (BMI, 21 to 24.9 kg/m2), the multivariate hazard ratio for cancer recurrence or death (DFS) was 1.00 (95% CI, 0.72 to 1.40) for patients with WHO class I obesity (BMI, 30 to 34.9 kg/m2) and was 1.24 (95% CI, 0.84 to 1.83) for those with class II to III obesity (BMI ≥ 35 kg/m2). We conducted our primary analyses with cumulative averaging of BMI, and we utilized data from both Q1 and Q2. However, when we repeated our analysis with only baseline BMI, the results were similar; the hazard ratio for DFS in patients with a BMI of 30 to 34.9 kg/m2 was 1.05 (95% CI, 0.75 to 1.48) and was 1.29 (95% CI, 0.86 to 1.91) for patients with a BMI of ≥ 35 kg/m2 compared with patients who had a BMI of 21 to 25 kg/m2.
Table 2.
Cancer Recurrence and Mortality According to Body Mass Index
| Recurrence and Mortality | Body Mass Index (kg/m2)
|
P* | ||||
|---|---|---|---|---|---|---|
| < 21 | 21-24.9 | 25-29.9 | 30-34.9 | ≥ 35 | ||
| Disease-free survival† | ||||||
| No. of events | 27 | 90 | 117 | 85 | 50 | |
| No. at risk | 68 | 258 | 369 | 236 | 112 | |
| Unadjusted HR | 1.13 | Reference | 0.86 | 1.00 | 1.18 | .86 |
| Unadjusted 95% CI | 0.73 to 1.73 | 0.66 to 1.14 | 0.75 to 1.35 | 0.84 to 1.67 | ||
| Adjusted HR‡ | 1.35 | Reference | 0.81 | 1.00 | 1.24 | |
| Adjusted 95% CI | 0.86 to 2.13 | 0.59 to 1.11 | 0.72 to 1.40 | 0.84 to 1.83 | ||
| Recurrence-free survival§ | ||||||
| No. of events | 23 | 83 | 104 | 79 | 49 | |
| No. at risk | 68 | 258 | 369 | 236 | 112 | |
| Unadjusted HR | 1.04 | Reference | 0.83 | 1.01 | 1.25 | .65 |
| Unadjusted 95% CI | 0.65 to 1.64 | 0.62 to 1.11 | 0.74 to 1.38 | 0.88 to 1.78 | ||
| Adjusted HR‡ | 1.22 | Reference | 0.75 | 0.97 | 1.27 | |
| Adjusted 95% CI | 0.75 to 1.98 | 0.54 to 1.03 | 0.69 to 1.37 | 0.85 to 1.89 | ||
| Overall mortality | ||||||
| No. of events | 17 | 69 | 85 | 60 | 30 | |
| No. at risk | 68 | 258 | 369 | 236 | 112 | |
| Unadjusted HR | 0.91 | Reference | 0.84 | 0.93 | 0.88 | .63 |
| Unadjusted 95% CI | 0.53 to 1.54 | 0.61 to 1.15 | 0.66 to 1.31 | 0.58 to 1.36 | ||
| Adjusted HR‡ | 1.07 | Reference | 0.72 | 0.90 | 0.87 | |
| Adjusted 95% CI | 0.61 to 1.87 | 0.50 to 1.03 | 0.61 to 1.34 | 0.54 to 1.42 | ||
Abbreviation: HR, hazard ratio.
Likelihood-ratio test (two-sided) for the body mass index term for continuous functional form: BMI + BMI2.
Measured by cancer recurrence or death from any cause.
Analyses were adjusted for sex, age, depth of invasion through bowel wall (T1-2 v T3-4), number of positive lymph nodes (1-3 v ≥ 4), presence of clinical perforation at time of surgery, presence of bowel obstruction at time of surgery, baseline performance status (0 v 1-2), treatment arm, weight change between first and second questionnaire, smoking status at time of second questionnaire (current, past, and never), and physical activity level.
Measured by cancer recurrence.
Fig 2.
Disease-free survival by body mass index (BMI).
We examined whether the influence of BMI on patient outcome was significantly modified by any baseline patient or disease characteristics. Among men, the hazard ratio for DFS in those with a BMI of 30 to 34.9 kg/m2 was 1.04 (95% CI, 0.66 to 1.63) and was 1.41 (95% CI, 0.83 to 2.41) in those who had a BMI ≥ 35 kg/m2 compared with normal-weight men. Among women, the hazard ratio forDFS in those with a BMI of 30 to 34.9 kg/m2 was 1.07 (95% CI, 0.64 to 1.80) and was 1.11 (95% CI, 0.61 to 2.02) in those with a BMI of ≥ 35 kg/m2 compared with normal-weight women. The P value for the interaction between sex and BMI was not significant (P = .31). Beyond sex, the effect of BMI on DFS was not significantly different for patients who exercised regularly compared with those who were relatively sedentary (Pinteraction = .98). Similarly, the relation between BMI and DFS was not modified by age (Pinteraction = .82), baseline performance status (Pinteraction = .86), number of positive lymph nodes (Pinteraction = .92), or treatment arm (Pinteraction = .29).
Impact of Weight Change on Cancer Recurrence or Death
In addition, we examined patient outcome according to change in weight as measured from Q1 to Q2. To minimize any potential bias by an undetected cancer recurrence or other life-threatening condition, we excluded recurrence or death that occurred within 90 days of completion of Q2. As listed in Table 3, neither weight loss or weight gain was significantly associated with DFS, RFS, or OS. If we did not exclude recurrence or death that occurred within the first 90 days, greater weight loss between Q1 and Q2 was associated with a poorer outcome (hazard ratio for DFS, 1.87; 95% CI, 1.02 to 3.41 for patients who experienced a weight loss of ≥ 5 kg compared with patients who had a weight change < 2 kg; P trend = .007). In addition, weight change was not associated with outcome even among baseline BMI subgroups (data not shown).
Table 3.
Impact of Weight Change on Recurrences and Survival
| Recurrence and Survival | Weight Change (kg)
|
P
|
|||||
|---|---|---|---|---|---|---|---|
| > 5 Loss | 2.1-5 Loss | ± 2 | 2-4.9 Gain | ≥ 5 Gain | Weight Loss Trend | Weight Gain Trend | |
| Disease-free survival* | |||||||
| No. of events | 14 | 11 | 20 | 22 | 126 | ||
| No. at risk | 47 | 42 | 105 | 104 | 538 | ||
| Unadjusted HR | 1.65 | 1.43 | Reference | 1.15 | 1.31 | .02 | .41 |
| Unadjusted 95% CI | 0.84 to 3.28 | 0.69 to 2.99 | 0.63 to 2.11 | 0.82 to 2.10 | |||
| Adjusted HR† | 1.39 | 1.15 | Reference | 1.11 | 1.19 | .13 | .90 |
| Adjusted 95% CI | 0.69 to 2.79 | 0.54 to 2.44 | 0.60 to 2.06 | 0.73 to 1.94 | |||
| Recurrence-free survival‡ | |||||||
| No. of events | 12 | 9 | 18 | 18 | 116 | ||
| No. at risk | 47 | 42 | 105 | 104 | 538 | ||
| Unadjusted HR | 1.55 | 1.29 | Reference | 1.04 | 1.34 | .02 | .34 |
| Unadjusted 95% CI | 0.75 to 3.22 | 0.58 to 2.87 | 0.54 to 2.00 | 0.81 to 2.19 | |||
| Adjusted HR† | 1.35 | 1.04 | Reference | 1.00 | 1.17 | .07 | .98 |
| Adjusted 95% CI | 0.64 to 2.81 | 0.46 to 2.35 | 0.52 to 1.95 | 0.70 to 1.96 | |||
| Overall mortality | |||||||
| No. of events | 7 | 5 | 12 | 12 | 75 | ||
| No. at risk | 47 | 42 | 105 | 104 | 538 | ||
| Unadjusted HR | 1.29 | 1.11 | Reference | 1.07 | 1.30 | .43 | .60 |
| Unadjusted 95% CI | 0.51 to 3.29 | 0.39 to 3.14 | 0.48 to 2.38 | 0.71 to 2.39 | |||
| Adjusted† | 1.13 | 0.89 | Reference | 0.97 | 1.23 | .53 | .85 |
| Adjusted 95% CI | 0.44 to 2.93 | 0.31 to 2.57 | 0.43 to 2.18 | 0.65 to 2.31 | |||
Abbreviation: HR, hazard ratio.
Measured by cancer recurrence or death from any cause.
Analyses were adjusted for sex, age, depth of invasion through bowel wall (T1-2 v T3-4), number of positive lymph nodes (1-3 v ≥ 4), presence of clinical perforation at time of surgery, presence of bowel obstruction at time of surgery, baseline performance status (0 v 1-2), treatment arm, time between questionnaire one and questionnaire two, time-varying body mass index, smoking status at time of questionnaire two (current, past, and never), and physical activity level.
Measured by cancer recurrence.
We tested whether more extremes of weight gain (10 or 20 kg) would impact DFS. Patients who gained 10 or more kilograms (n = 391) had an adjusted hazard ratio for DFS of 1.11 (95% CI, 0.60 to 2.32) compared with those who maintained weight within 2 kilograms. Similarly, the adjusted hazard ratio for patients who gained 20 or more kilograms (n = 135) was 1.11 (95% CI, 0.63 to 1.95). We did not detect any interactions between weight gain and sex (Pinteraction = .22), level of physical activity (Pinteraction = .09), or initial BMI (Pinteraction = .84).
We also considered change in BMI, as utilized in other analyses in breast cancer.22 The hazard ratio for DFS was 1.32 (95% CI, 0.73 to 2.38) for patients whose BMI decreased by 0.5 to 2.0 kg/m2 and was 1.44 (95% CI, 0.59 to 2.54) for those whose BMI decreased by greater than 2.0 kg/m2 compared with patients who maintained their BMI within 0.5 kg/m2. Similarly, there were no significant differences in DFS for patients with BMI increases of 0.5 to 2.0 kg/m2 (hazard ratio, 1.26; 95% CI, 0.81 to 1.94) nor for those with BMI increases of greater than 2.0 kg/m2 (hazard ratio, 1.05; 95% CI, 0.65 to 1.69).
DISCUSSION
Among patients enrolled on an NCI-sponsored clinical trial of postoperative adjuvant chemotherapy for stage III colon cancer, we did not observe any significant associations between BMI and cancer recurrence and mortality. Similarly, weight gain after completion of adjuvant therapy did not negatively impact survival for patients with stage III colon cancer.
In CALGB 89803, data on weight, smoking habits, physical activity, diet, and other factors were collected prospectively shortly after study initiation and approximately 6 months after the completion of therapy. Because such patient behavior and lifestyle habits may change over time, particularly in the period after adjuvant therapy, repeated measurements of diet, lifestyle, and anthropometric factors may reduce misclassification with time and may better reflect the influence of these factors on cancer recurrence and survival. By using updated assessments of body habitus, we did not find a statistically significant association between BMI and cancer recurrence or mortality. However, our results are similar to those of Dignam et al,20 who reported adjusted hazard ratios of 1.06 (95% CI, 0.93 to 1.21) for patients with a BMI of 30 to 34.9 kg/m2 and of 1.27 (95% CI, 1.05 to 1.53) for those with a BMI of 35 kg/m2 or greater. In comparison, we found hazard ratios of 1.00 (95% CI, 0.72 to 1.40) and 1.24 (95% CI, 0.84 to 1.83), respectively. The difference in statistical significance between these two studies could be related to the power of each study (2,074 events in 4,288 patients in the study by Dignam et al compared with 369 events in 1,053 patients in this study). Additional, treatment in the two trials evaluated by Dignam et al required dose capping with a maximum body surface area of 2.0 m2;20 thus, the very obese patients in their analyses may have received suboptimal adjuvant therapy.
Obesity and sedentary lifestyle have been shown fairly consistently to increase the risk of developing colorectal cancer.29,30 Until recently, few studies had addressed whether these factors influence the outcomes of survivors of colorectal cancer. Because increased circulating levels of insulin and free insulin-like growth factor 1 have been associated with obesity and physical inactivity,14,31,32 and because both insulin and insulin-like growth factor 1 promote cell proliferation and inhibit apoptosis in colon cancer cells,33-36 one could hypothesize that such lifestyle factors may influence the risk of colon cancer recurrence by promoting growth of micrometastases through insulin-related growth factors.
We recently reported a beneficial association between physical activity and outcomes in colon cancer survivors with two prospective cohorts, including CALGB 89803.37,38 In both reports, BMI did not influence the benefits of exercise. Conversely, in this analysis, physical activity did not significantly modify the lack of association between BMI and DFS.
Three other recent studies have demonstrated that obesity may be associated with colon cancer recurrence and mortality.19,20,39 Haydon et al39 found that waist circumference was the most predictive measure of adiposity, as a 20% increase in risk of disease recurrence or death was noted for every 10-cm increase in circumference.39 Two other studies19,20 utilized height and weight data collected from NCI-sponsored cooperative group studies of adjuvant therapy in patients with stages II to III disease, though neither study could account for other factors that could confound the results (eg, smoking and physical activity).
In contrast with studies of patients with early-stage breast cancer, weight gain after adjuvant therapy in this study did not significantly influence survival. One hypothesis of the negative impact of weight gain in women with breast cancer is that such gain will increase circulating estrogens. In the survivors of colon cancer, estrogen may be protective, as seen in a recent analysis of hormone replacement therapy in survivors of colorectal cancer.40 Nonetheless, there was no interaction with sex and weight change in this study. In contrast, although weight loss did not affect outcomes in our primary analyses, weight loss was significantly associated with poorer outcomes when we included patients whose disease recurred or who died within 90 days of Q1. Such a result confirms the utility of restrictions in the analyses of factors that may be a result of, rather than the cause of, an outcome.
Patients who enroll on randomized trials may differ from the population at-large. However, the distribution of BMI in our cohort is similar to that of the general population.41 Compared with the National Health and Nutrition Examination Survey (NHANES), our cohort had similar prevalence of overweight status (BMI, 25 to 29.9 kg/m2; 34% in NHANES and 36% in CALGB 89803) and obesity (BMI ≥ 30 kg/m2; 30.5% in NHANES and 30.5% in CALGB 89803).
Several potential limitations for our study should be considered. In this study, we could only assess obesity by BMI and not other measures of body habitus, such as waist-hip ratio or waist circumference. In some studies, these measures were more predictive of the risk of developing colon cancer than BMI.3,4 Such measures of body habitus may better distinguish abdominal adiposity, which has been correlated with serum insulin level. Another limitation is that we assessed weight change up to 6 to 8 months after the completion of adjuvant therapy. Whether longer follow-up of weight change would influence outcomes cannot be addressed in this study.
Although our study did not reach statistical significance, this is the second NCI-sponsored intergroup study of adjuvant chemotherapy for colon cancer to demonstrate an approximately 25% increased risk of cancer recurrence and survival in patients with resected colon cancer who had a BMI ≥ 35 kg/m2 (class II to III obesity). Of greatest concern is the rising incidence of obesity in the United States in the last two decades.41 Patients enrolled on the study of Dignam et al20 were diagnosed with colon cancer between 1989 and 1994, whereas those in this study were diagnosed between 1999 and 2001. In the Dignam study, 5.5% of patients were classified as class II to III obesity. One decade later, in this study, 11.6% of patients had class II to III obesity. Thus, if the 25% increase is the true relationship between class II to III obesity and cancer recurrence and mortality, an increasing trend in obesity will be an important prognostic factor. Future research is necessary to better understand the impact of obesity on survivors of colon cancer and whether interventions among obese survivors of colon cancer will improve overall outcomes.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey A. Meyerhardt, Leonard B. Saltz, Charles S. Fuchs
Financial support: Jeffrey A. Meyerhardt, Robert J. Mayer, Charles S. Fuchs
Administrative support: Jeffrey A. Meyerhardt, Donna Hollis
Provision of study materials or patients: Donna Niedzwiecki, Donna Hollis, Leonard B. Saltz, Robert J. Mayer, Heidi Nelson, Renaud Whittom, Alexander Hantel, James Thomas
Collection and assembly of data: Jeffrey A. Meyerhardt, Donna Niedzwiecki, Donna Hollis, Leonard B. Saltz, Robert J. Mayer, Heidi Nelson, Renaud Whittom, Alexander Hantel, James Thomas, Charles S. Fuchs
Data analysis and interpretation: Jeffrey A. Meyerhardt, Donna Niedzwiecki, Donna Hollis, Charles S. Fuchs
Manuscript writing: Jeffrey A. Meyerhardt, Charles S. Fuchs
Final approval of manuscript: Jeffrey A. Meyerhardt, Donna Niedzwiecki, Donna Hollis, Leonard B. Saltz, Robert J. Mayer, Heidi Nelson, Renaud Whittom, Alexander Hantel, James Thomas, Charles S. Fuchs
Appendix
The following institutions participated in this study: Baptist Cancer Institute CCOP, Memphis, TN–Lee S. Schwartzberg, MD, supported by CA71323; Christiana Care Health Services Inc, CCOP, Wilmington, DE–Stephen Grubbs, MD, supported by CA45418; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, MD, supported by CA47559; University of Chicago, Chicago, IL–Gini Fleming, MD, supported by CA41287; Dartmouth Medical School –Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, MD, supported by CA32291; Georgetown University Medical Center, Washington, DC–Edward Gelmann, MD, supported by CA77597; Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, MD, supported by CA29165; University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, MD, supported by CA74811; University of Iowa, Iowa City, IA–Gerald Clamon, MD, supported by CA47642; North Shore –Long Island Jewish Medical Center, Manhasset, NY–Daniel R. Budman, MD, supported by CA35279; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical School, Worcester, MA–William V. Walsh, MD, supported by CA37135; Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, MD, supported by CA12449; Mount Sinai Medical Center, Miami, FL–Rogerio Lilenbaum, MD, supported by CA45564; University of Minnesota, Minneapolis, MN–Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C. Perry, MD, supported by CA12046; Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, MD, supported by CA04457; Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford Hudis, MD, supported by CA77651; University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, MD, supported by CA77298; Long Island Jewish Medical Center, Lake Success, NY–Marc Citron, MD, supported by CA11028; The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, MD, supported by CA77658; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA02599; Southeast Cancer Control Consortium Inc, CCOP, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John Ellerton, MD, supported by CA35421; Syracuse Hematology-Oncology Associates, CCOP, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, MD, supported by CA47555; University of California at San Diego, San Diego, CA–Joanne Mortimer, MD, supported by CA11789; University of California at San Francisco, San Francisco, CA–Alan P. Venook, MD, supported by CA60138; Vermont Cancer Center, Burlington, VT–Hyman B. Muss, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC–Thomas Reid, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO–Nancy Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY–Scott Wadler, MD, supported by CA07968.
Supported by Grants No. CA32291; CA33601; CA77651; CA25224; CO15; CA38926, CA32101, CA46282; CA21076; P50CA127003; R01 CA 118553-01A2; and K07CA097992 from the National Cancer Institute. Also supported by Pharmacia & Upjohn Co, now Pfizer Oncology.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Larsson SC, Rutegard J, Bergkvist L, et al: Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer 42:2590-2597, 2006 [DOI] [PubMed] [Google Scholar]
- 2.MacInnis RJ, English DR, Hopper JL, et al: Body size and composition and colon cancer risk in women. Int J Cancer 118:1496-1500, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Moore LL, Bradlee ML, Singer MR, et al: BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham study adults. Int J Obes Relat Metab Disord 28:559-567, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pischon T, Lahmann PH, Boeing H, et al: Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 98:920-931, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Rapp K, Schroeder J, Klenk J, et al: Obesity and incidence of cancer: A large cohort study of over 145,000 adults in Austria. Br J Cancer 93:1062-1067, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IM, Paffenbarger RS Jr: Quetelet's index and risk of colon cancer in college alumni. J Natl Cancer Inst 84:1326-1331, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Ascherio A, Rimm EB, et al: Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122:327-334, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Le Marchand L, Wilkins LR, Mi MP: Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control 3:349-354, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Chyou PH, Nomura AM, Stemmermann GN: A prospective study of colon and rectal cancer among Hawaii Japanese men. Ann Epidemiol 6:276-282, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Bostick RM, Potter JD, Kushi LH, et al: Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 5:38-52, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Martinez ME, Giovannucci E, Spiegelman D, et al: Leisure-time physical activity, body size, and colon cancer in women: Nurses’ Health Study Research Group. J Natl Cancer Inst 89:948-955, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Ford ES: Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol 150:390-398, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Bruce WR, Wolever TM, Giacca A: Mechanisms linking diet and colorectal cancer: The possible role of insulin resistance. Nutr Cancer 37:19-26, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kaaks R, Lukanova A: Energy balance and cancer: The role of insulin and insulinlike growth factor-I. Proc Nutr Soc 60:91-106, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E: Insulin and colon cancer. Cancer Causes Control 6:164-179, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Kissebah AH, Vydelingum N, Murray R, et al: Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 54:254-260, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Krotkiewski M, Bjorntorp P, Sjostrom L, et al: Impact of obesity on metabolism in men and women: Importance of regional adipose tissue distribution. J Clin Invest 72:1150-1162, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao SV, Donahue M, Pi-Sunyer FX, et al: Results of Expert Meetings: Obesity and Cardiovascular Disease—Obesity as a risk factor in coronary artery disease. Am Heart J 142:1102-1107, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Catalano PJ, Haller DG, et al: Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 98:484-495, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Dignam JJ, Polite BN, Yothers G, et al: Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 98:1647-1654, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Camoriano JK, Loprinzi CL, Ingle JN, et al: Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol 8:1327-1334, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke CH, Chen WY, Rosner B, et al: Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol 23:1370-1378, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Caan BJ, Emond JA, Natarajan L, et al: Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat 99:47-57, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Saltz LB, Niedzwiecki D, Hollis D, et al: Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cancer (intergroup trial CALGB C89803). J Clin Oncol 22:246s, 2004. (suppl; abstr 3500) [Google Scholar]
- 25.Zubrod C, Scheiderman M, Frei E, et al: Appraisal of methods for the study of chemotherapy in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chron Dis 11:7-33, 1960 [Google Scholar]
- 26.Physical status: The use of interpretation of anthropometry—Report of a WHO expert committee. World Health Organ Tech Rep Ser 854:1-452, 1995 [PubMed] [Google Scholar]
- 27.Saltz LB, Niedzwiecki D, Hollis D, et al: Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 25:3456-3461, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Cox D: Regression models and life tables. J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 29.Martinez ME: Primary prevention of colorectal cancer: Lifestyle, nutrition, exercise. Recent Results Cancer Res 166:177-211, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E: Diet, body weight, and colorectal cancer: A summary of the epidemiologic evidence. J Womens Health (Larchmt) 12:173-182, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Sandhu MS, Dunger DB, Giovannucci EL: Insulin, insulinlike growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 94:972-980, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E: Insulin, insulinlike growth factors and colon cancer: A review of the evidence. J Nutr 131:3109S-20S, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Pollak MN, Perdue JF, Margolese RG, et al: Presence of somatomedin receptors on primary human breast and colon carcinomas. Cancer Lett 38:223-230, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Guo YS, Narayan S, Yallampalli C, et al: Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology 102:1101-1108, 1992 [PubMed] [Google Scholar]
- 35.Koenuma M, Yamori T, Tsuruo T: Insulin and insulinlike growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res 80:51-58, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjork J, Nilsson J, Hultcrantz R, et al: Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the non-transformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand J Gastroenterol 28:879-884, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Meyerhardt JA, Giovannucci EL, Holmes MD, et al: Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24:3527-3534, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24:3535-3541, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Haydon AM, Macinnis RJ, English DR, et al: Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55:62-67, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JA, Meyerhardt JA, Chan AT, et al: Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol 24:5680-5686, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Hedley AA, Ogden CL, Johnson CL, et al: Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA 291:2847-2850, 2004 [DOI] [PubMed] [Google Scholar]