Abstract
Purpose
Raloxifene reduces breast cancer risk in women with osteoporosis, and both tamoxifen and raloxifene prevent breast cancer in high-risk women. However, in vitro, raloxifene does not share the pro-estrogenic effects of tamoxifen on the endometrium. Randomized trials of these agents have provided limited information about endometrial cancer risk in the general population. We sought to compare endometrial cancer risks associated with raloxifene, tamoxifen, and nonusers of a selective estrogen receptor modulator (SERM) in the general population and characterize the endometrial tumors occurring in these groups.
Methods
We performed a case-control study of white and African American women age 50 to 79 years in the Philadelphia area. Patients were diagnosed with endometrial cancer between July 1999 and June 2002. Controls were identified through random-digit dialing.
Results
We analyzed 547 cases and 1,410 controls. Among cases, 3.3% had taken raloxifene; 6.2% had taken tamoxifen. Among controls, 6.6% had taken raloxifene; 2.4% had taken tamoxifen. After adjustment for other risk factors, the odds of endometrial cancer among raloxifene users was 50% that of nonusers (odds ratio [OR] = 0.50; 95% CI, 0.29 to 0.85), whereas tamoxifen users had three times the odds of developing endometrial cancer compared with raloxifene users (OR = 3.0; 95% CI, 1.3 to 6.9). Endometrial tumors in raloxifene users had a more favorable histologic profile and were predominantly International Federation of Gynecology and Obstetrics stage I and low grade.
Conclusion
Raloxifene users had significantly lower odds of endometrial cancer compared with both tamoxifen users and SERM nonusers, suggesting a role for raloxifene in endometrial cancer prevention and individualization of SERM therapy.
INTRODUCTION
Endometrial cancer is the most common gynecologic cancer in the United States, with approximately 40,000 incident cases and 7,500 deaths annually.1 Estrogen exposure seems to play a significant role in endometrial cancer development. Tamoxifen, a selective estrogen receptor modulator (SERM), has been associated with endometrial cancer2 via direct stimulation of endometrial estrogen receptors.3,4 Raloxifene is similar to tamoxifen in imparting antiestrogenic effects in the breast (ie, reducing breast cancer incidence)5,6 and pro-estrogenic effects in bone (ie, reducing or reversing osteoporosis),7 but in vitro studies suggest that raloxifene does not share the pro-estrogenic effects of tamoxifen on the endometrium.8,9
Because raloxifene and tamoxifen seem to confer similar breast cancer prevention benefits, other risks and benefits of these drugs are critically important to physicians and patients in individualizing treatment decisions. Clinical trials of raloxifene for osteoporosis and breast cancer prevention have included endometrial cancer as a secondary end point. Although these studies have suggested either no difference in endometrial cancer incidence with raloxifene compared with placebo6 or a reduced incidence for raloxifene compared with tamoxifen,10 the risk estimates are limited to the specific patient populations studied and may not reflect the true relative incidence of endometrial cancer in women who do or do not use these agents in the general population for a variety of indications.
To address the need for more generalizable risk estimates for endometrial cancer in the general population of SERM users, we used a large, population-based, case-control study of endometrial cancer in Philadelphia, PA, and surrounding areas to examine the odds of endometrial cancer associated with use of raloxifene or tamoxifen compared with nonusers of a SERM and compared with each other, the odds of endometrial cancer associated with duration of SERM use, and the characteristics of endometrial tumors occurring in raloxifene users compared with those occurring in tamoxifen or non-SERM users in the general population.
METHODS
The current report represents a subanalysis of the Women's Insights and Shared Experiences Study, a pair of case-control studies examining associations between exogenous hormone use, parity, estrogen-related genes, and endometrial or breast cancer. Detailed methods have been published previously.10 Briefly, eligible cases were women age 50 to 79 years diagnosed with endometrial cancer between July 1999 and June 2002 while residing in a nine-county area surrounding and including Philadelphia. Cases were identified through all 68 hospitals and multiple physicians’ offices in these counties and through quarterly reviews of the Pennsylvania Cancer Registry. The interval between diagnosis and case identification was limited to 6 months and between ascertainment and contact for the screening interview was limited to 12 months. Only primary, invasive, adenocarcinoma (endometrioid, type I), clear-cell, or papillary serous carcinoma subtypes were included; sarcomas and mixed müllerian histology were excluded as it was anticipated that these would be rare, and evidence supporting hormone-related etiology was less well established. Cancer diagnoses were verified by review of all pathology reports by one of the coauthors (S.C.R.) who had no knowledge of patients’ hormone use and confirmed when possible through state cancer registries. Control patients from within the same geographic region as the cases were identified by a survey research firm through random-digit dialing.
The study sample size was determined based on the primary objectives of the parent study; although the current analyses were specified a priori, the study was not specifically powered to test the hypotheses related to SERM exposure.10 The study was originally designed with a 1:1 control:case ratio and with frequency-matching of controls to cases on age (in 5-year age groups) and race (black or white). Approximately halfway through study accrual, however, the case accrual was lower than anticipated for endometrial cancer overall and for black patients specifically. To compensate, we increased the control:case ratio within specific strata, targeting a 2:1 ratio for white patients and taking all black women within the age group of our study; the details of this change in accrual strategy have been published.10 This resulted in a final control:case ratio of 2.3:1 for white patients and 7.1:1 for black patients.
Odds ratios (OR) and 95% CIs11 were calculated for three targeted comparisons: (1) tamoxifen use versus never use of a SERM, (2) raloxifene use versus never use of a SERM, and (3) raloxifene versus tamoxifen use, as well as for duration of SERM use (defined as < 3 years v ≥ 3 years). Potential confounders were accepted for inclusion in subsequent conditional logistic regression models if they changed one or more of the target SERM ORs by ≥ 10% when added to the unadjusted model of case-control status and SERM.12,13 All analyses were performed in STATA (version 8.0; STATA Corp, College Station, TX).
This study was approved by the University of Pennsylvania institutional review board as well as the institutional review boards of all participating hospitals. The study was conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
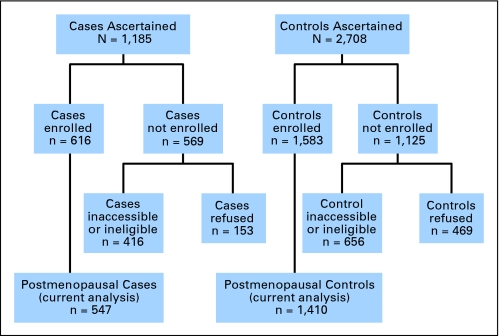
Figure 1 shows the ascertainment and enrollment for cases and controls. Overall, 547 cases and 1,410 controls were analyzed. Among the 1,185 cases with incident endometrial cancer meeting eligibility criteria, 386 (32.6%) were inaccessible for enrollment (seven patients were nursing home residents, 29 patients were non-English speakers, 17 patients were mentally or physically unable to participate, 194 patients were without physician consent, 70 patients were without correct address and/or phone number, and 69 patients died before contact). Of the 799 remaining, 153 patients refused participation, and 30 patients could not be reached for interview before the study ended. Thus 616 cases (52% ascertained, 77% eligible and accessible) were interviewed and available for analysis. Finally, we excluded cases self-identified as premenopausal at the time of the main interview (n = 69) because raloxifene is indicated only in postmenopausal women.
Fig 1.
Patient ascertainment, enrollment, and eligibility for the current analysis.
The survey research firm provided contact information for 2,708 potential random-digit dialing controls. Of these, 405 were ineligible because of age, sex, county, race, or history of hysterectomy or endometrial cancer. Additionally, 25 controls could not participate because of physical or mental impairments, 12 controls did not speak English, seven controls were deceased, 207 controls could not be recontacted because they moved or changed their phone number, and 469 controls refused. The remaining 1,583 controls completed the interview (58% referred, 77% eligible and could be contacted) and were available for analysis. Again, we excluded controls self-identified as premenopausal at the time of the main interview (n = 161). We also excluded two controls who had used both tamoxifen and raloxifene.
Demographic and clinical characteristics of both the overall study population and the population of SERM users are listed in Table 1. Overall, cases differed significantly from controls with regard to age, race, body mass index (BMI), smoking history, breast cancer history, family history of endometrial cancer, duration of active menses, and parity. Cases were also more likely to have used oral contraceptives and less likely to have taken combination estrogen/progestin replacement, though there was no difference in estrogen-only use. Among the SERM users in the study, only race, BMI, and breast cancer history differed significantly between cases and controls.
Table 1.
Potential Risk Factors for Endometrial Cancer by Case-Control Status Among Postmenopausal Women in This Study* and for SERM Users Only
| Risk Factor | Overall Study
|
SERM Users Only
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 547)
|
Controls(n = 1,410)
|
P† | Cases(n = 52)
|
Controls(n = 127)
|
P† | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| Age at index date, years | < .001 | .823 | ||||||||
| 50-59 | 169 | 30.9 | 605 | 42.9 | 20 | 38.5 | 43 | 42.9 | ||
| 60-79 | 233 | 42.6 | 502 | 35.6 | 19 | 36.5 | 48 | 37.8 | ||
| 70-79 | 145 | 26.5 | 303 | 21.5 | 13 | 25.0 | 36 | 28.4 | ||
| Race | < .001 | .027 | ||||||||
| White | 489 | 89.4 | 1,065 | 75.5 | 51 | 98.1 | 111 | 87.5 | ||
| Black | 58 | 10.6 | 345 | 24.5 | 1 | 1.9 | 16 | 12.6 | ||
| BMI | < .001 | .001 | ||||||||
| Mean | 26.2 | 24.1 | 24.5 | 22.8 | ||||||
| SD | 5.81 | 4.28 | 5.77 | 3.10 | ||||||
| BMI in NIH categories | ||||||||||
| < 18.5, underweight | 8 | 1.5 | 53 | 3.8 | < .001 | 2 | 3.9 | 5 | 4.7 | .034 |
| 18.5-24.9, average | 273 | 49.9 | 922 | 65.4 | 34 | 65.4 | 94 | 74.0 | ||
| 25.0-29.9, overweight | 154 | 28.2 | 301 | 21.4 | 10 | 19.2 | 25 | 19.7 | ||
| ≥ 30, obese | 106 | 19.4 | 133 | 9.4 | 6 | 11.5 | 2 | 1.6 | ||
| Unknown | 6 | 1.1 | 1 | 0.1 | — | — | ||||
| Cancer history | ||||||||||
| Ovarian tumors, benign | 24 | 4.4 | 52 | 3.7 | .472 | 2 | 3.9 | 4 | 3.2 | .814 |
| Breast cancer | 71 | 13.0 | 84 | 6.0 | < .001 | 37 | 71.2 | 36 | 28.4 | < .001 |
| Any other cancer‡ | 60 | 11.0 | 100 | 7.1 | .005 | 8 | 15.4 | 10 | 7.9 | .129 |
| Age at menarche, years | < .001 | .491 | ||||||||
| Mean | 12.4 | 12.7 | 12.6 | 12.7 | ||||||
| SD | 1.59 | 1.66 | 1.42 | 1.33 | ||||||
| Age at menopause, years | .017 | .517 | ||||||||
| Mean | 50.1 | 49.5 | 50.0 | 49.4 | ||||||
| SD | 4.62 | 4.86 | 4.82 | 5.08 | ||||||
| Duration of menses, years | < .001 | .397 | ||||||||
| Mean | 37.6 | 36.8 | 37.4 | 36.7 | ||||||
| SD | 4.80 | 5.10 | 5.01 | 5.26 | ||||||
| No. of full-term pregnancies | < .001 | .495 | ||||||||
| Mean | 2.4 | 2.8 | 2.5 | 2.7 | ||||||
| SD | 1.67 | 1.86 | 1.64 | 1.86 | ||||||
| Age at first full-term pregnancy, years | .331 | .248 | ||||||||
| Mean | 24.1 | 23.9 | 24.6 | 25.4 | ||||||
| SD | 4.56 | 4.97 | 4.42 | 4.42 | ||||||
| Duration or oral contraceptive use, years | < .001 | .517 | ||||||||
| Never | 342 | 62.5 | 669 | 47.4 | 24 | 46.2 | 68 | 53.5 | ||
| < 3 | 113 | 20.7 | 333 | 23.6 | 12 | 23.1 | 30 | 23.6 | ||
| ≥ 3 | 92 | 16.8 | 404 | 28.6 | 16 | 30.8 | 29 | 22.8 | ||
| Smoker | < .001 | .445 | ||||||||
| Never | 285 | 52.1 | 575 | 40.8 | 23 | 44.2 | 56 | 44.1 | ||
| Former | 220 | 40.2 | 562 | 39.9 | 26 | 50.0 | 56 | 44.1 | ||
| Current | 42 | 7.7 | 271 | 19.2 | 3 | 5.8 | 15 | 11.8 | ||
| Unknown | 0 | 0.0 | 2 | 0.1 | — | — | ||||
| Unopposed ERT | .130 | .381 | ||||||||
| Never any HRT | 322 | 58.9 | 771 | 54.7 | 32 | 61.5 | 64 | 50.4 | ||
| Ever any ERT | 74 | 13.5 | 184 | 13.0 | 5 | 9.6 | 18 | 14.2 | ||
| Other HRT exclusively | 151 | 27.6 | 455 | 32.3 | 15 | 28.9 | 45 | 35.4 | ||
| Duration of ERT, years | .008 | .483 | ||||||||
| Never any HRT | 322 | 58.9 | 771 | 54.7 | 32 | 61.5 | 64 | 50.4 | ||
| < 3 | 43 | 7.9 | 139 | 9.9 | 3 | 5.8 | 14 | 11.0 | ||
| ≥ 3 | 31 | 5.7 | 45 | 3.2 | 2 | 3.9 | 4 | 3.2 | ||
| Other HRT exclusively | 151 | 27.6 | 455 | 32.3 | 15 | 28.9 | 45 | 35.4 | ||
| CHRT | .003 | .374 | ||||||||
| Never any HRT | 322 | 58.9 | 771 | 54.7 | 32 | 61.5 | 64 | 50.4 | ||
| Ever any CHRT | 132 | 24.1 | 446 | 31.6 | 13 | 25.0 | 38 | 29.9 | ||
| Other HRT exclusively | 93 | 17.0 | 193 | 13.7 | 7 | 13.5 | 25 | 19.7 | ||
| Duration of CHRT, years | .008 | .162 | ||||||||
| Never any HRT | 322 | 58.9 | 771 | 54.7 | 32 | 61.5 | 64 | 50.4 | ||
| < 3 | 51 | 9.3 | 183 | 13.0 | 4 | 7.7 | 23 | 18.1 | ||
| ≥ 3 | 81 | 14.8 | 263 | 18.6 | 9 | 17.3 | 15 | 11.8 | ||
| Other HRT exclusively | 93 | 17.0 | 193 | 13.7 | 7 | 13.5 | 25 | 19.7 | ||
| Used other hormones§ | 45 | 8.2 | 109 | 7.7 | .714 | 10 | 19.2 | 13 | 10.2 | .103 |
| Known first-degree family history of cancer | ||||||||||
| Endometrial | 42 | 7.7 | 72 | 5.1 | .029 | 4 | 7.7 | 4 | 3.2 | .182 |
| Breast or ovarian | 93 | 17.0 | 261 | 18.5 | .436 | 11 | 21.2 | 34 | 26.8 | .432 |
| SERM | < .001 | |||||||||
| Never any SERM | 495 | 90.5 | 1,283 | 91.0 | — | — | ||||
| Tamoxifen only | 34 | 6.2 | 34 | 2.4 | — | — | ||||
| Raloxifene only | 18 | 3.3 | 93 | 6.6 | — | — | ||||
Abbreviations: SERM, selective estrogen receptor modulator; SD, standard deviation; BMI, body mass index; NIH, National Institutes of Health; ERT, estrogen replacement therapy; HRT, hormone replacement therapy; CHRT, combination hormone replacement therapy (estrogen plus progestin).
Sixty-nine premenopausal cases and 171 premenopausal controls are excluded. Two menopausal women who took both tamoxifen and raloxifene are also excluded.
χ2 test used for categorical variables and F test used for continuous variables. Those with unknown value are not included in the test.
Except ovarian, cervical, and breast cancers. History of ovarian and history of cervical cancer are collinear with yes/no endometrial cancer.
To get pregnant, to prevent pregnancy, or to prevent miscarriage.
Table 2 lists endometrial cancer risk factors among tamoxifen and raloxifene users in the analysis cohort. Use of either tamoxifen or raloxifene was not associated with age, race, or BMI. Tamoxifen users were significantly more likely to have had a history of breast cancer. Notably, raloxifene users were more likely to have received either unopposed estrogen replacement or combined hormone replacement and had longer duration of use.
Table 2.
Potential Risk Factors for Endometrial Cancer Among Postmenopausal Users of Tamoxifen or Raloxifene in This Study*
| Risk Factor | Tamoxifen Only (n = 68)
|
Raloxifene Only (n = 111)
|
P† | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at index date, years | .190 | ||||
| 50-59 | 19 | 27.9 | 44 | 39.6 | |
| 60-79 | 26 | 38.2 | 41 | 36.9 | |
| 70-79 | 23 | 33.2 | 26 | 23.4 | |
| Race | .063 | ||||
| White | 58 | 85.3 | 104 | 93.7 | |
| Black | 10 | 14.7 | 7 | 6.3 | |
| BMI | .258 | ||||
| Mean | 23.7 | 3.0 | |||
| SD | 3.75 | 4.32 | |||
| BMI in NIH categories | .266 | ||||
| < 18.5, underweight | 1 | 1.5 | 7 | 6.3 | |
| 18.5-24.9, average | 47 | 69.1 | 81 | 73.0 | |
| 25.0-29.9, overweight | 17 | 25.0 | 18 | 16.2 | |
| ≥ 30, obese | 3 | 4.4 | 5 | 4.5 | |
| History of cancer | |||||
| Ovarian tumors, benign | 3 | 4.4 | 3 | 2.7 | .538 |
| Breast cancer | 67 | 98.5 | 6 | 5.4 | < .001 |
| Any other cancer‡ | 10 | 14.7 | 8 | 7.2 | .105 |
| Age at menarche, years | .269 | ||||
| Mean | 12.5 | 12.8 | |||
| SD | 1.33 | 1.36 | |||
| Age at menopause, years | .298 | ||||
| Mean | 50.1 | 49.3 | |||
| SD | 4.25 | 5.40 | |||
| Duration of menses, years | .179 | ||||
| Mean | 37.6 | 36.5 | |||
| SD | 4.31 | 5.63 | |||
| No. of full-term pregnancies | .060 | ||||
| Mean | 2.9 | 2.5 | |||
| SD | 1.83 | 1.49 | |||
| Age at first full-term pregnancy, years | .027 | ||||
| Mean | 24.2 | 25.8 | |||
| SD | 4.50 | 4.29 | |||
| Duration of oral contraceptive use, years | .176 | ||||
| Never | 41 | 60.3 | 51 | 46.0 | |
| < 3 | 13 | 19.1 | 29 | 26.1 | |
| ≥ 3 | 14 | 20.6 | 31 | 27.9 | |
| Smoker | .314 | ||||
| Never | 30 | 44.1 | 49 | 44.1 | |
| Former | 34 | 50.0 | 48 | 43.2 | |
| Current | 4 | 5.9 | 14 | 12.6 | |
| Unopposed ERT | < .001 | ||||
| Never any HRT | 49 | 72.1 | 47 | 42.3 | |
| Ever any ERT | 3 | 4.4 | 20 | 18.0 | |
| Other HRT exclusively | 16 | 23.5 | 44 | 39.6 | |
| Duration of ERT, years | .001 | ||||
| Never any HRT | 49 | 72.1 | 47 | 42.3 | |
| < 3 | 1 | 1.5 | 16 | 14.4 | |
| ≥ 3 | 2 | 2.9 | 4 | 3.6 | |
| Other HRT exclusively | 16 | 23.5 | 44 | 39.6 | |
| CHRT | < .001 | ||||
| Never any HRT | 49 | 72.1 | 47 | 42.3 | |
| Ever any CHRT | 13 | 19.1 | 38 | 34.2 | |
| Other HRT exclusively | 6 | 8.8 | 26 | 23.4 | |
| Duration of CHRT, years | .001 | ||||
| Never any HRT | 49 | 72.1 | 47 | 42.3 | |
| < 3 | 5 | 7.4 | 22 | 19.8 | |
| ≥ 3 | 8 | 11.8 | 16 | 14.4 | |
| Other HRT exclusively | 6 | 8.8 | 26 | 23.4 | |
| Used other hormones§ | 10 | 14.7 | 13 | 11.7 | .561 |
| Known first-degree family history of cancer | |||||
| Endometrial | 5 | 7.4 | 3 | 2.7 | .144 |
| Breast or ovarian | 15 | 22.1 | 30 | 27.0 | .457 |
Abbreviations: SERM, selective estrogen receptor modulator; SD, standard deviation; BMI, body mass index; NIH, National Institutes of Health; ERT, estrogen replacement therapy; HRT, hormone replacement therapy; CHRT, combination hormone replacement therapy (estrogen plus progestin).
Sixty-nine premenopausal cases and 171 premenopausal controls are excluded. Two menopausal women who took both tamoxifen and raloxifene are also excluded.
χ2 test used for categorical variables and F test used for continuous variables. Those with unknown value are not included in the test.
Except ovarian, cervical, and breast cancers. History of ovarian and history of cervical cancer are collinear with yes/no endometrial cancer.
To get pregnant, to prevent pregnancy, or to prevent miscarriage.
Table 3 lists the adjusted ORs for endometrial cancer among tamoxifen users and raloxifene users relative to non-SERM users and the relationships with duration of use. Overall, raloxifene users had a 50% reduction in the odds of developing endometrial cancer compared with those who had not used a SERM in an adjusted model (OR = 0.50; 95% CI, 0.29 to 0.85). In contrast, tamoxifen users had an increased adjusted odds of endometrial cancer compared with nonusers of a SERM, though this did not reach statistical significance (OR = 1.5; 95% CI, 0.77 to 2.92). Compared with raloxifene users, the OR for endometrial cancer for tamoxifen users was 3.0 (95% CI, 1.3 to 6.9).
Table 3.
Adjusted* Odds of Endometrial Cancer by Type of SERM Use and Duration of Use
| Factor | Cases (n = 547†)
|
Controls (n = 1,410†)
|
Adjusted OR | 95% CI | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Nonusers compared with both raloxifene and tamoxifen users | ||||||
| Nonusers | 495 | 90 | 1,283 | 91 | 1.0 | Reference |
| Raloxifene | 18 | 3.3 | 93 | 6.6 | 0.50 | 0.29 to 0.85 |
| Tamoxifen | 34 | 6.3 | 34 | 2.4 | 1.5 | 0.77 to 2.92 |
| Tamoxifen compared with raloxifene users | ||||||
| Raloxifene | 18 | 3.3 | 93 | 6.6 | 1.0 | Reference |
| Tamoxifen | 34 | 6.3 | 34 | 2.4 | 3.0 | 1.3 to 6.9 |
| Raloxifene compared with nonusers by duration of use, years | ||||||
| < 3 | 11 | 2.2 | 74 | 5.3 | 0.41 | 0.21 to 0.80 |
| ≥ 3 | 7 | 1.4 | 19 | 1.3 | 0.78 | 0.31 to 1.95 |
| Tamoxifen compared with nonusers by duration of use, years | ||||||
| < 3 | 12 | 2.2 | 16 | 1.1 | 1.32 | 0.54 to 3.23 |
| ≥ 3 | 22 | 4 | 18 | 1.3 | 1.69 | 0.77 to 3.69 |
| Tamoxifen use compared with raloxifene use, < 3 years of use | ||||||
| Raloxifene | 11 | 2.2 | 74 | 5.3 | 1.0 | Reference |
| Tamoxifen | 12 | 2.2 | 16 | 1.1 | 3.19 | 1.06 to 9.65 |
| Tamoxifen use compared with raloxifene use, ≥ 3 years of use | ||||||
| Raloxifene | 7 | 1.4 | 19 | 1.3 | 1.0 | Reference |
| Tamoxifen | 22 | 4 | 18 | 1.3 | 2.15 | 0.67 to 6.95 |
Abbreviation: SERM, selective estrogen receptor modulator.
Adjusted for age, race, body mass index (BMI), and history of breast cancer.
Six cases missing data on BMI, one control missing data on BMI, and one control missing data on history of breast cancer were excluded from the multivariate model.
The only other confounding factors independently associated with endometrial cancer were a history of breast cancer and increasing BMI. Increasing BMI was significantly associated with odds of having endometrial cancer (OR = 1.12; 95% CI, 1.10 to 1.15) and changed the OR for the SERM-endometrial cancer relationship by more than 10% when included in the models for both raloxifene (unadjusted OR = 0.43 v 0.50 adjusted) and tamoxifen (unadjusted OR = 2.35 v 2.73 adjusted). A history of breast cancer was also significantly associated with endometrial cancer (OR = 1.87; 95% CI, 1.16 to 3.0), and modified the SERM-endometrial cancer relationship, but just for tamoxifen users (unadjusted OR = 2.35 v 1.29 adjusted), with no effect on raloxifene users (unadjusted OR = 0.43 v 0.43 adjusted). Factors that did not change the estimate of the SERM-endometrial cancer relationship for either drug included marital status; Jewish ethnicity; education; household income; health insurance; height; birth weight; diabetes; hypercholesterolemia; hypertension; myocardial infarction; stroke; venous thromboembolic disease; gallbladder disease; migraine headaches; liver disease; Stein-Leventhal Syndrome; ovarian cancer history; history of nonovarian, nonbreast cancer; oophorectomy; age at menarche; age at menopause; duration of menses; menopause type; age at first full-term pregnancy; number of full-term pregnancies; duration of breast feeding; use/duration of oral contraceptives; bilateral tubal ligation; previous dilation and curettage; tobacco use; alcohol use; hormone replacement therapies; use of natural or herbal remedies; and family history of endometrial cancer.
Regarding duration of SERM use (Table 3), raloxifene users had a 59% reduction in the odds of endometrial cancer with less than 3 years of use (OR = 0.41; 95% CI, 0.21 to 0.80), which decreased to a 22% reduction among users of raloxifene for 3 or more years (OR = 0.78; 95% CI, 0.31 to 1.95). Duration of tamoxifen use ≥ 3 years was associated with a higher odds of endometrial cancer than a shorter duration of use, but these estimates were not statistically significantly different (OR = 1.32; 95% CI, 0.54 to 3.23 for < 3 years v OR = 1.69, 95%, 0.77 to 3.69 for ≥ 3 years).
Table 4 shows the histopathologic characteristics of endometrial cancers identified among cases by SERM use. Twenty-four percent of tamoxifen users and 6% of non-SERM users had papillary-serous and clear-cell histologic subtypes not seen in raloxifene users. The stage and grade at presentation of any histologic type in tamoxifen or raloxifene users were not significantly different from those occurring in non-SERM users, with a predominance of early-stage cancers primarily of low histologic grade.
Table 4.
Histopathologic Characteristics of Endometrial Cancer Cases by SERM Use
| Category | Nonusers
|
Raloxifene
|
Tamoxifen
|
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Histologic type | |||||||
| Adenocarcinoma | 467 | 94 | 18 | 100 | 26 | 76 | < .001 |
| Papillary-serous or clear cell | 28 | 6 | 0 | 0 | 8 | 24 | |
| Total | 495 | 18 | 34 | ||||
| FIGO stage*† | |||||||
| I | 363 | 83 | 16 | 94 | 22 | 76 | .53‡ |
| II | 35 | 8 | 0 | 0 | 4 | 14 | |
| III | 27 | 6 | 1 | 6 | 3 | 10 | |
| IV | 13 | 3 | 0 | 0 | 0 | 0 | |
| Total | 438 | 17 | 29 | ||||
| Histologic grade*§ | |||||||
| 1 | 214 | 46 | 12 | 67 | 16 | 52 | .21‖ |
| 2 | 155 | 33 | 4 | 22 | 6 | 19 | |
| 3 | 97 | 21 | 2 | 11 | 9 | 29 | |
| Total | 466 | 18 | 31 | ||||
Abbreviations: SERM, selective estrogen receptor modulator; FIGO, International Federation of Gynecology and Obstetrics.
Among all histologic types.
Sixty-three cases are missing FIGO stage.
P = .28 for association of FIGO stages I/II+ with SERM use.
Thirty-two cases are missing histologic grade.
P = .19 for association of grades 1/2+ with SERM use.
DISCUSSION
This population-based study demonstrates a 50% reduction in odds of endometrial cancer associated with use of raloxifene compared with those women not taking a SERM, and shows that tamoxifen users were three times more likely to develop endometrial cancer compared with raloxifene users. These data confirm and extend the findings of two randomized controlled trials reporting endometrial cancer with raloxifene use as a secondary end point. The Multiple Outcomes of Raloxifene Evaluation Trial5 was a study of 7,705 postmenopausal women randomly assigned to either raloxifene or placebo for prevention of osteoporosis.14 At 8 years of follow-up, there was a nonsignificant decrease in the rate of endometrial cancer among raloxifene users (0.32% v 0.39% in raloxifene and placebo groups, respectively; P = .75). The National Surgical Adjuvant Breast and Bowel Project P2 Study of Tamoxifen and Raloxifene randomly assigned 19,747 postmenopausal women at increased risk of breast cancer to either tamoxifen or raloxifene.15 In this study, there were 36 cases of endometrial cancer in tamoxifen users compared with only 23 in raloxifene users. At 7 year cumulative follow-up, this translated to 14.7 cases/1,000 women treated with tamoxifen compared with 8.1 cases/1,000 women treated with raloxifene (P = .07). However, more than 50% of women in the Study of Tamoxifen and Raloxifene had had hysterectomies before enrollment, and an additional 355 patients underwent hysterectomy for noncancer indications while participating in the study, with the majority of these occurring in the tamoxifen group. Although endometrial cancer relative risk estimates were only considered among those at risk by virtue of having an intact uterus, the high proportion of patients who underwent hysterectomy may have underestimated the true magnitude of endometrial cancer attributable to these agents and the true difference between the groups. Numerous randomized, controlled trials and case-control studies in breast cancer have demonstrated elevations in the relative risk of endometrial cancer from tamoxifen, varying from 1.3 to 15.2,16-20 Our finding that tamoxifen use was associated with an odds of endometrial cancer that was 50% higher than that of nonusers, although not statistically significant (OR = 1.5; 95% CI, 0.77 to 2.92) is in line with those reported by earlier studies.
The reduction in the odds of endometrial cancer with raloxifene use in this study was most pronounced among those with short-term (< 3 years) use. Although benefit was seen beyond 3 years of use, this did not reach statistical significance. The British Tamoxifen Second Cancer Study Group showed that the odds of endometrial cancer associated with tamoxifen use increased significantly with increasing duration of use up to 10 years (Ptrend < .001)21; however, the impact of duration was most pronounced for müllerian and mesodermal mixed tumors and sarcomas, histologies not included in the current study. Although our findings are intriguing, the small sample size per group limit the precision of our estimates, and the clinical significance is unclear. Further study is clearly needed both in vitro and in vivo to better understand the biologic effects of raloxifene on endometrial growth and proliferation over time. Mechanistically, raloxifene could have an initial beneficial effect on endometrial estrogen receptors that is attenuated over time as these receptors lose sensitivity to the beneficial effects of raloxifene, in a similar fashion to changes in estrogen receptor function that have been reported during prolonged exposure to tamoxifen.22
All endometrial cancers identified in the current study among raloxifene users were adenocarcinomas (endometrioid or type I); no clear-cell or papillary-serous cancers were identified (in contrast with tamoxifen users, in whom 24% of tumors were clear-cell or papillary-serous). The majority of endometrial cancers diagnosed in this study were International Federation of Gynecology and Obstetrics stage I, and tumor grade did not differ by group. None of the previous studies of raloxifene that included endometrial outcomes have reported histologic information on endometrial cancers in this group. Thus these data provide new information on the profile of endometrial cancers seen in raloxifene users, suggesting that among raloxifene users who do develop endometrial cancers, the vast majority of tumors are potentially curable. Endometrial cancers among tamoxifen users in the National Surgical Adjuvant Breast and Bowel Project P-1 Trial were observed early in the follow-up period, and all were International Federation of Gynecology and Obstetrics stage I.20 Of the 57 tumors diagnosed, 53 were adenocarcinomas and four were uterine sarcomas, predominantly of the carcinosarcoma type. Our study excluded sarcomas, thus precluding our ability to compare the frequency of this subtype between tamoxifen and raloxifene users in this study or with that reported in other studies. Although sarcomas may be important outcomes in patients taking these drugs, the exclusion of this subtype of endometrial cancer in no way diminishes the findings presented here for endometrial carcinoma.
Several limitations should be noted. First, although the case-control approach we used is particularly suited to the study of this relatively rare event, endometrial cancer, the current analysis was a secondary aim of a study that was not designed to primarily answer the question posed. Thus, despite the relatively large number of patients in this case-control study, the number of tamoxifen or raloxifene users in this population was relatively small, which may have limited the precision of our risk estimates. Nevertheless, our case-control design with a large case sample size allowed us to evaluate this relatively rare outcome of SERM use in a way that existing randomized controlled trials could not. By excluding controls with a history of hysterectomy (thereby precluding their ability to get endometrial cancer), we have potentially underestimated the frequency of SERM use in the general population. However, our focus is on endometrial cancer risk, which is only relevant in women who have not undergone hysterectomy. Many potentially eligible patients were not enrolled, potentially introducing bias. However, reasons for nonparticipation were not significantly different between cases and controls, providing no compelling reason that nonparticipation would be systematically related to use of SERMs. In addition, the changes in accrual strategy that occurred during the study to augment the numbers of patients with endometrial cancer, African Americans, and controls for reasons of sample size resulted in a small imbalance in the age and race distributions between cases and controls, which has the potential to confound our results. We addressed this imbalance by performing conditional logistic regression to examine age and race strata specifically and performed formal tests of interaction to determine whether age or race modified the relationship between SERM use and outcome. Because those interaction tests were nonsignificant, we were able to drop those factors from further consideration in the models. Because use of conditional logistic regression is superior to direct adjustment in the setting of partial matching, and because even partial matching achieves most of the benefits of full matching,23,24 we feel confident that potential confounding and bias have been minimized. Finally, there is the potential for our study to be confounded by indication. That is, if patients in this study were taking raloxifene for osteoporosis, and osteoporosis is associated with endometrial cancer, one must consider the possibility that the risk reduction attributed to raloxifene was a reflection of a lower risk in the population taking raloxifene. Lower estrogen levels might lead to such a connection between osteoporosis and reduced endometrial cancer risk. We did not collect information on estrogen levels or bone density, which would clearly be necessary to explore this hypothesis.
Nonetheless, this population-based, case-control study provides evidence that raloxifene use may be associated with a significantly lower odds of endometrial cancer compared with both SERM nonusers and users of tamoxifen. As of April 2006, more than 500,000 women were taking raloxifene in the United States, the majority of whom were not taking it for breast cancer prevention.9 Because small differences in risk of endometrial cancer between these agents would have a relatively large impact on the absolute numbers of cases of endometrial cancer that develop, these findings warrant further investigation. If confirmed, this information provides important additional information to aid physicians and patients in individualizing SERM therapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a ‘U’ are those for which no compensation was received; those relationships marked with a ‘C’ were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jesse A. Berlin, Johnson & Johnson (U) Consultant or Advisory Role: None Stock Ownership: Jesse A. Berlin, Johnson & Johnson Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Angela DeMichele, Jesse A. Berlin, Greta R. Bunin, Elene Turzo, Rita Schinnar, Michelle Berlin, Timothy R. Rebbeck, Brian L. Strom
Financial support: Timothy R. Rebbeck, Brian L. Strom
Administrative support: Elene Turzo, Rita Schinnar, Desiree Burgh
Provision of study materials or patients: Angela DeMichele, Elene Turzo, Rita Schinnar, Desiree Burgh, Stephen C. Rubin, Timothy R. Rebbeck, Brian L. Strom
Collection and assembly of data: Angela DeMichele, Greta R. Bunin, Elene Turzo, Rita Schinnar, Desiree Burgh, Stephen C. Rubin, Timothy R. Rebbeck, Brian L. Strom
Data analysis and interpretation: Angela DeMichele, Andrea B. Troxel, Jesse A. Berlin, Anita L. Weber, Elene Turzo, Stephen C. Rubin, Brian L. Strom
Manuscript writing: Angela DeMichele, Andrea B. Troxel, Jesse A. Berlin, Greta R. Bunin, Stephen C. Rubin, Brian L. Strom
Final approval of manuscript: Angela DeMichele, Andrea B. Troxel, Jesse A. Berlin, Anita L. Weber, Greta R. Bunin, Elene Turzo, Rita Schinnar, Michelle Berlin, Stephen C. Rubin, Timothy R. Rebbeck, Brian L. Strom
Acknowledgments
We thank Lewis Chodosh, MD, J.A. Grisso, MD, and Mona Baumgarten, PhD, for their contributions to the planning and implementation of this study. We thank Karen Venuto for managing the tracking database and the vast correspondence involved in the study and Shawn Fernandes for performing extensive quality control checks and helping with the development of the questionnaire database. We thank the hospitals in the Greater Delaware Valley for their cooperation and the physicians who sponsored our study in these institutions, as without this help we could not have performed this study.
Supported by the National Cancer Institute, National Institutes of Health, Grant No. P01-CA77596.
A.D. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Ries L, Eisner M, Kosary C, et al: SEER Cancer Statistics Review, 1973-1997. Bethesda, MD, National Cancer Institute, 2000
- 2.Fornander T, Rutqvist LE, Cedermark B, et al: Adjuvant tamoxifen in early breast cancer: Occurrence of new primary cancers. Lancet 1:117-120, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Shang Y, Brown M: Molecular determinants for the tissue specificity of SERMs. Science 295:2465-2468, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Barsalou A, Dayan G, Anghel SI, et al: Growth-stimulatory and transcriptional activation properties of raloxifene in human endometrial Ishikawa cells. Mol Cell Endocrinol 190:65-73, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Eckert S, Krueger KA, et al: The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial—Multiple Outcomes of Raloxifene Evaluation. JAMA 281:2189-2197, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Martino S, Cauley JA, Barrett-Connor E, et al: Continuing outcomes relevant to Evista: Breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96:1751-1761, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ettinger B, Black DM, Mitlak BH, et al: Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial—Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637-645, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, McElrath T, Tong W, et al: The molecular basis of tamoxifen induction of mouse uterine epithelial cell proliferation. J Endocrinol 184:129-140, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Kleinman D, Karas M, Danilenko M, et al: Stimulation of endometrial cancer cell growth by tamoxifen is associated with increased insulin-like growth factor (IGF)-I induced tyrosine phosphorylation and reduction in IGF binding proteins. Endocrinology 137:1089-1095, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Vogel VG, Costantino JP, Wickerham DL, et al: Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727-2741, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bunin GR, Baumgarten M, Norman SA, et al: Practical aspects of sharing controls between case-control studies. Pharmacoepidemiol Drug Saf 14:523-530, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kleinbaum D, Kupper L, Morgenstern H: Epideiologic Research, Principles and Quantitative Methods. Belmont, CA, Lifetime Learning Publications, 1982
- 13.Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 138:923-936, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Mickey RM, Greenland S: The impact of confounder selection criteria on effect estimation. Am J Epidemiol 129:125-137, 1989 [DOI] [PubMed] [Google Scholar]
- 15.National Surgical Adjuvant Breast and Bowel Project: Initial results of the study of tamoxifen and raloxifene (STAR) release: Osteoporosis drug raloxifene shown to be as effective as tamoxifen in preventing invasive breast cancer. Pittsburgh, PA, NSABP Operations Center, 2006
- 16.Fisher B, Costantino JP, Redmond CK, et al: Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 86:527-537, 1994 [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen FE, Benraadt J, Coebergh JW, et al: Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet 343:448-452, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Rydén S, Ferno M, Moller T, et al: Long-term effects of adjuvant tamoxifen and/or radiotherapy: The South Sweden Breast Cancer Trial. Acta Oncol 31:271-274, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Andersson M, Storm HH, Mouridsen HT: Carcinogenic effects of adjuvant tamoxifen treatment and radiotherapy for early breast cancer. Acta Oncol 31:259-263, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, et al: Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371-1388, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow AJ, Jones ME: Tamoxifen treatment for breast cancer and risk of endometrial cancer: A case-control study. J Natl Cancer Inst 97:375-384, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Tonetti DA, Jordan VC: The role of estrogen receptor mutations in tamoxifen-stimulated breast cancer. J Steroid Biochem Mol Biol 62:119-128, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Greenland S: Partial and marginal matching in case-control studies, in Moolgavkar SH, Prentice RL (eds): Modern Statistical Methods in Chronic Disease Epidemiology. New York, NY, Wiley, 1986
- 24.Friedlander Y, Lev Merom D, Kark JD: A comparison of different matching designs in case-control studies: An empirical example using continuous exposures, continuous confounders and incidence of myocardial infarction. Stat Med 12:993-1004, 1993 [DOI] [PubMed] [Google Scholar]