Abstract
Purpose
Pharmacodynamic studies are frequently incorporated into phase I trials, but it is uncommon that they guide dose selection. We conducted a dose selection study with daily rapamycin (sirolimus) in patients with solid tumors employing a modified continuous reassessment method (mCRM) using real-time pharmacodynamic data as primary dose-estimation parameter.
Patients and Methods
We adapted the mCRM logit function from its classic toxicity-based input data to a pharmacodynamic-based input. The pharmacodynamic end point was skin phospho-P70 change after 28 days. Pharmacodynamic effect was defined as at least 80% inhibition from baseline. The first two dose levels (2 and 3 mg) were evaluated before implementing the mCRM, and the data used to estimate the next dose level based on statistical modeling. Toxicity-based boundaries limited the escalation steps. Other correlates analyzed were positron emission tomography (PET) and computed tomography, pharmacokinetics, phospho-P70 in peripheral-blood mononuclear cells, and tumor biopsies in patients at the maximum-tolerated dose (MTD).
Results
Twenty-one patients were enrolled at doses between 2 and 9 mg. Pharmacodynamic effect occurred across dose levels, and toxicity boundaries ultimately drove dose selection. The MTD of daily oral rapamycin was 6 mg. Toxicities in at least 20% were hyperglycemia, hyperlipidemia, elevated transaminases, anemia, leucopenia, neutropenia, and mucositis. Pharmacokinetics were consistent with prior data, and exposure increased with dose. No objective responses occurred, but five previously progressing patients received at least 12 cycles. PET showed generalized stable or decreased glucose uptake unrelated to antitumor effect.
Conclusion
mCRM-based dose escalation using real-time pharmacodynamic assessment was feasible. However, the selected pharmacodynamic end point did not correlate with dose. Toxicity ultimately drove dose selection. Rapamycin is a well-tolerated and active oral anticancer agent.
INTRODUCTION
Mammalian target of rapamycin (mTOR) is a serine-threonine kinase downstream of the phosphoinositide-3 kinase (PI3K)/Akt signaling pathway that mediates multiple biologic functions such as transcriptional and translational control by modulating transducers such as P70 and 4EBP1,1,2 and is a target for anticancer drug development. Rapamycin (sirolimus) is a macrocyclic lactone produced by Streptomyces hygroscopicus with immunosuppressive, antimicrobial, and antitumor properties.3 mTOR signaling also plays a key role in hypoxia-triggered angiogenesis, which is abrogated by rapamycin.4 Rapamycin is used for the prevention of organ rejection after solid organ transplantation.5,6 Phase I studies exploring doses from 0.5 to 6.5 mg/m2 every 12 hours have been conducted in transplant patients.7 Despite a robust rationale, and the benefits of an oral agent, rapamycin has not been evaluated clinically as an anticancer agent.
The concept of targeted therapy relies on the assumption that drugs elicit a pharmacodynamic (PD) effect that is necessary for the expected antitumor activity to occur. However this concept has not been uniformly incorporated in phase I dose selection trials. The modified continuous reassessment method (mCRM) is a toxicity-based method that has shown to be feasible in dose-escalating trials, and that has been hypothesized to allow a reduction in patients needed to reach the recommended dose compared with algorithmic dose escalation designs.8 We hypothesized that rapamycin would exert a PD effect in normal tissues, that this effect would correlate with drug exposure, and that real-time PD monitoring would rationally guide dose selection. Thus, we used an mCRM coupled to a PD end point rather than the more classical toxicity-based approach. We selected phospho-P70 as PD end point because prior work in renal cell cancer showed an association between phospho-70 dynamics in normal tissue on mTOR inhibition and outcome.9
This phase I study was conducted to determine the PD optimal and/or maximum-tolerated doses (MTD), acute and chronic toxicity profile, and pharmacokinetics (PK) of rapamycin in adult patients with solid cancers. To this end, we coupled the mCRM to an end point in normal skin tissue, but additionally explored PD end points in peripheral-blood mononuclear cells (PBMCs) and tumor tissue, as well as functional imaging with positron emission tomography (PET).
PATIENTS AND METHODS
Patient Eligibility
Histologically confirmed malignancy beyond standard curative or palliative measures, age at least 18 years, measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status of no greater than 1, life expectancy of at least 12 weeks, willingness to undergo skin and tumor biopsies where appropriate, and adequate bone marrow, hepatic, and renal function (absolute neutrophil count ≥ 1,500/μL; platelets ≥ 100,000/μL; hemoglobin ≥ 9 g/dL; bilirubin < 2 mg/dL; AST, ALT, and alkaline phosphatase < 5× the upper limit of normal [ULN]; triglycerides and total cholesterol < 2.5× the ULN; and creatinine < 2 mg/dL) were required. Patients could have received any prior therapy ending at least 28 days prior without residual toxicity. Patients with brain metastases or clinically significant medical conditions were excluded. Our institutional review board granted approval, and written informed consent was mandatory.
Study Design and Dose Escalation
Dose escalation was guided following a computer-based mCRM, a method to estimate the dose-efficacy/toxicity curve.8 Two three-patient dose levels were accrued, and then the PD and toxicity data were used to estimate the parameters that define the dose-efficacy/toxicity curve to determine the next dose. PD efficacy was defined as at least 80% decrease in phospho-P70 in paired skin biopsies. Dose escalation was guided by the mCRM based on PD effect until reaching the pharmacodynamically active dose (PAD) with maximum dose-increments limited by toxicity. The PAD was defined as the dose at which the mCRM recommends a dose for the next cohort that has already been tested in eight patients. Standard mCRM methodology and computer programming systems were followed. A two-parameter logistic model was used to describe the dose-PD association, and the model was estimated using a maximum likelihood approach. The safety boundaries stated that in the absence of grade 2 or worse toxicity, dose was escalated by a maximum of 100%. If grade 2 or worse related toxicity occurred in at least 1 patient of a three-patient cohort, dose was escalated by a maximum of 50%. After one dose-limiting toxicity (DLT) in a three-patient cohort, the dose level was expanded with three more patients. If no further DLT was observed, escalation would be allowed by 33%; if two or more DLTs occurred, the immediate lower dose level was to be expanded. The MTD was defined as the highest dose tested in which fewer than 33% of patients experienced DLT attributable to the study drug. DLT was defined as grade 4 neutropenia with fever or lasting at least 7 days, grade 3 or worse thrombocytopenia for at least 5 days or associated with significant bleeding, any grade 3 or worse nonhematologic toxicity, or any irreversible grade 2 related toxicity during cycle 1.
Treatment
Rapamycin was purchased commercially (Wyeth, Madison, NJ) and administered orally once daily following an uninterrupted schedule in 28-day cycles. The initial dose (2 mg) was chosen because data from transplant series indicated that it was a safe (albeit effective) dose. Treatment was administered until disease progression, intercurrent illness, unacceptable adverse event(s), or withdrawal of consent. Re-treatment at the beginning of each cycle required adequate parameters, and resolution of all nonhematologic toxicities (except alopecia and fatigue) to baseline or grade 1 or better. In case of more than 14-day delay the patient was removed from the study.
Assessments
Before study entry patients had a clinical history and physical examination, performance status, CBC/differential, platelet count, complete chemistry and coagulation, urinalysis, chest x-ray, EKG, and PET and computed tomography (CT) scan. Patients had hematology and chemistry parameters weekly during cycle 1, and then every other week. A pregnancy test was performed for women of childbearing potential. PET-CT scan was repeated every two cycles. Patients fasted for at least 6 hours; 10 to 20 mCi of 18-FDG were injected, and emission images were obtained 50 to 70 minutes postinjection. Analysis was performed by standardized uptake value (SUV) of the maximal 1-cm tumor area, the gross tumor SUV and by visual analysis, and compared per the 1999 European Organisation for Research and Treatment of Cancer (EORTC) recommendations.10 Adverse events were classified and graded per the National Cancer Institute (NCI) Common Toxicity Criteria (CTC) version 3.0. Response was defined using Response Evaluation Criteria in Solid Tumors (RECIST).11
PK Sampling, Analytic Assay, and Data Analysis
PK studies were performed on cycle 1 days 1 and 28. Whole blood was collected in EDTA-containing tubes pretreatment, and 1, 2, 4, 6, and 8 hours after the first dose. Trough samples were collected before drug administration on days 2, 3, 8, 15, and 22 of the first cycle, and on days 1 and 2 of the second cycle. Samples were stored at −20°C or below. Rapamycin concentrations in whole blood were determined by a validated high-performance liquid chromatography with mass spectrometry detection (LC/MS/MS) method. Individual PK parameters were estimated by standard noncompartmental analysis using WINNonlin (Scientific Consultant, Apex, NC) version 5.0 (Pharsight, Mountain View, CA).12
PD Assessment: PBMCs
We collected 8 mL of blood at baseline, and on days 2, 8, and 14 after treatment in a BD Vacutainer CPT cell preparation tube (CPT; Becton Dickinson, Franklin Lakes, NJ), and centrifuged at room temperature 10 minutes at 3,000 rpm. Mononuclear cells were washed with phosphate-buffered saline followed by centrifugation for 10 minutes at 1,000 rpm, and stored at −80°C. Protein 10 μg was loaded onto a Bio-Rad (Hercules, CA) Bio-Plex phosphoprotein bead assay for phospho-P70 expression, following the manufacturer's instructions. Normal skin was collected by 3-mm punch biopsy at baseline and after 28 days. Tumor biopsies were performed in patients at the MTD at baseline and after 28 days after ultrasonographic-guided, fine-needle aspiration–assisted methodology. Formalin-fixed, paraffin-embedded sections (5 μm) were stained. Phospho-Thr389 p70 (9206; Cell Signaling Technology, Beverly, MA), phospho-Ser235/236 S6 (US Biologic, R2031-29, Swampscott, MA) and phospho-Thr 70 4EBP-1 (US Biologic, 0004-20) staining was performed.
Statistical Analysis
Biologic parameters were summarized using descriptive statistics. The association between categoric variables was assessed using Fisher's exact test. Differences between PD parameters between sampling periods and between baseline and post-treatment respectively were compared by t test. Differences between pharmacokinetic parameters between sampling periods were compared by a Wilcoxon matched pairs signed-rank test. A Kruskal-Wallis analysis of variance by ranks was used to compare the differences in apparent systemic clearance and dose-normalized maximal plasma concentration (Cmax) and area under the concentration-time curve (AUC) as a function of dose level. These tests were performed using JMP Statistical Discovery software (version 4.0.4; SAS Institute, Cary, NC) or SPPS version 15 (SPSS, Chicago, IL).
RESULTS
Patient Characteristics
Between February 2005 and January 2007, 21 patients with advanced solid tumors were accrued. All patients were assessable for toxicity and efficacy. In the first cohort one patient developed rapid progression before completing her first cycle and was replaced. Demographic characteristics of the subjects are summarized in Table 1. A total of 129 cycles of the study drug were delivered (median, two cycles; range, one to ≥ 26 cycles).
Table 1.
Patient Characteristics (n = 21)
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Male | 9 |
| Female | 12 |
| Age, years | |
| Median | 59 |
| Range | 30-80 |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 16 |
| 1 | 5 |
| Primary tumor site | |
| Sarcoma | 4 |
| Pancreas (adenocarcinoma) | 4 |
| Colorectal | 3 |
| Hepatocellular | 3 |
| Neuroendocrine | 3 |
| Other* | 4 |
| Prior regimens for advanced disease | |
| 0-2 ≥ 3 | 12:9 |
Other tumor sites (one patient each) include non–small-cell lung, cholangiocarcinoma, desmoplastic round-cell, and unknown primary carcinoma.
Dose-Escalation Process
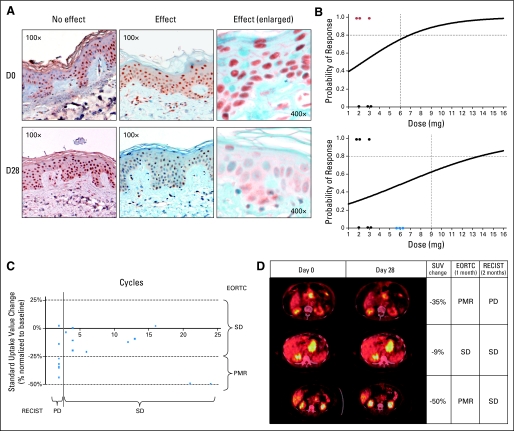
The starting dose of rapamycin was 2 mg. The first two dose levels of 2 and 3 mg were evaluated before implementing the mCRM. Of the first six patients, only three reached the defined level of PD efficacy (Fig 1A). These data was used in the mCRM to define the next dose level, which was between 6 and 7 mg (Fig 1B). Because no related grade 2 or greater toxicities were encountered, a 100% dose escalation was allowed; 6 mg was evaluated in three patients who did not meet PD efficacy criteria. The mCRM indicated a next dose level of 13 mg. However, several grade 2 toxicities had occurred, dose escalation was limited to a 50% increase, and 9 mg was selected. At 9 mg the second patient experienced a DLT consisting in grade 3 mucositis, the cohort was expanded, and the fifth patient suffered a second DLT consisting of grade 3 thrombocytopenia, diarrhea, and hyperglycemia. Dose for these two patients was reduced to 6 mg, and therapy continued without recurrence of the toxicities. The previous cohort was expanded to nine total patients, without documenting any DLT. Therefore, the MTD of rapamycin administered orally once daily on an uninterrupted schedule in solid cancer patients is 6 mg.
Fig 1.
(A) Pharmacodynamic effect on skin samples. On the left column, the pre- and post-treatment phospho-P70 skin staining of a patient where no effect was observed; on the right, the paired samples in a subject with a significant decrease in staining (with enlarged details at far right). (B) Modified continuous reassessment method plots. The dots highlighted in red indicate the three cases with pharmacodynamic effect, the dots in blue patients where no effect was seen. (C) Relationship between standardized uptake value (SUV) change and clinical efficacy measured in terms of cycles received. Patients receiving only two cycles were those presenting disease progression (PD). (D) Positron emission tomography (PET)-computed tomography (CT) scanning of selected subjects, showing PET-CT results as SUV change from baseline, and indicating the observed clinical response by Response Evaluation Criteria in Solid Tumors (RECIST). PET-CT was not predictive of clinical benefit. Above is a pancreatic cancer patient who met PET response criteria but had PD by RECIST both at the site of PET assessment and in new sites. The patient in the middle row had an unknown primary tumor with para-aortic nodes and had stable disease (SD) both by PET and RECIST. The pancreatic neuroendocrine cancer patient in the lower row had also a partial metabolic response (PMR) that has been followed by SD for more than 2 years. EORTC, European Organisation for Research and Treatment of Cancer.
Toxicity
Treatment was generally well tolerated and in concordance with the expected adverse effect profile of rapamycin (Table 2). No grade 4 or 5 toxicities were documented. The most common nonhematologic adverse events observed in greater than 20% of patients were elevated triglycerides, glucose, AST/ALT and cholesterol, rash and mucositis. Hyperlipidemias responded well to statin treatment. A single urinary tract infection was documented. Hematologic toxicity was mild and characterized by anemia, leucopenia, and thrombocytopenia.
Table 2.
Cycle 1 Toxicity
| Toxicity | Patients | Grade
|
Toxicity Events by Dose Level (No.)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mg (n = 4)
|
3 mg (n = 3)
|
6 mg (n = 9)
|
9 mg (n = 5)
|
||||||||||
| 1 | 2 | 3 | 4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | ||
| Nonhematologic | |||||||||||||
| Mucositis | 4 | 1 | 2 | 1* | 2 | 1 | 1 | ||||||
| Fatigue | 4 | 4 | 2 | 2 | |||||||||
| Pain | 3 | 2 | 1 | 2 | 1 | ||||||||
| Rash | 3 | 3 | 3 | ||||||||||
| Nausea | 2 | 2 | 1 | 1 | |||||||||
| Diarrhea | 2 | 1 | 1* | 1 | 1 | ||||||||
| Sinus congestion | 2 | 2 | 1 | 1 | |||||||||
| Anorexia | 2 | 2 | 2 | ||||||||||
| Fever | 2 | 2 | 1 | 1 | |||||||||
| Urinary infection | 1 | 1 | 1 | ||||||||||
| Sore throat | 1 | 1 | 1 | ||||||||||
| Cough | 1 | 1 | 1 | ||||||||||
| Edema | 1 | 1 | 1 | ||||||||||
| Dyspepsia | 1 | 1 | 1 | ||||||||||
| Hypertriglyceridemia | 10 | 9 | 1 | 1 | 1 | 5 | 3 | ||||||
| Hyperglycemia | 8 | 5 | 2 | 1* | 4 | 1 | 3 | ||||||
| Elevated AST | 7 | 4 | 3 | 1 | 4 | 2 | |||||||
| Elevated ALT | 5 | 4 | 1 | 3 | 2 | ||||||||
| Hypercholesterolemia | 5 | 4 | 1 | 1 | 3 | 1 | |||||||
| Elevated AP | 2 | 1 | 1 | 1 | 1 | ||||||||
| Hypercalcemia | 2 | 1 | 1 | 2 | |||||||||
| Hematologic | |||||||||||||
| Leukopenia | 9 | 6 | 3 | 1 | 2 | 5 | 1 | ||||||
| Thrombocytopenia | 7 | 6 | 1* | 5 | 1 | 1 | |||||||
| Lymphopenia | 6 | 2 | 4 | 4 | 2 | ||||||||
| Anemia | 6 | 5 | 1 | 2 | 3 | 1 | |||||||
| Neutropenia | 3 | 2 | 1 | 2 | 1 | ||||||||
Abbreviation: AP, alkaline phosphatase.
Dose-limiting toxicity.
Clinical Efficacy
In 10 patients, the best response was progressive disease, 10 had stable disease (SD), and one hepatocellular carcinoma patient had a minor response by RECIST that was sufficient to allow a surgical resection by autologous liver transplant that rendered the patient disease free for a year. All seven patients receiving 2 and 3 mg presented with progressive disease at first evaluation, whereas 11 of 14 patients at 6 and 9 mg had SD (P = .001). The ECOG performance status and number of prior lines of treatment were similar between groups (P = .53 and .36, respectively). Five patients with unknown-origin adenocarcinoma, cholangiocarcinoma, and neuroendocrine cancer (n = 3) have been on study for 12, 13, 14+, 22+, and 26+ months, respectively. All five had shown progression to other therapies immediately before study entry, with prior time-to-treatment-failure intervals of 6, 6, 7, 5, and 4 months, respectively.
Functional Imaging Evaluation
Rapamycin induced a generalized decrease in SUV that was not correlated with dose or best response (average SUV −15%, −35%, −17% and −26% at 2-, 3-, 6-, and 9-mg dose levels, respectively; Fig 1C to 1D). PET and CT assessment correlated in six patients that showed SD by both methods, but PET suggested a better-than-observed outcome in the rest (showing partial metabolic response in five patients with SD, SD in three subjects with PD, and partial metabolic response in five patients with PD).
PK Evaluation
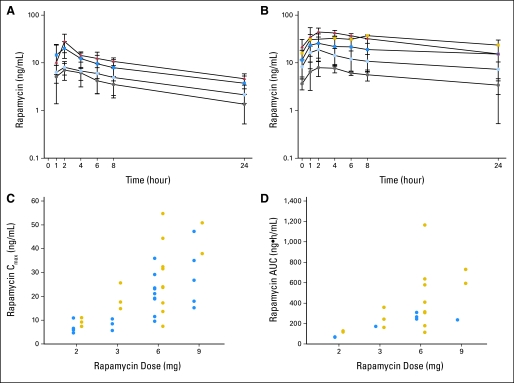
PK data were assessable for 21 patients (Fig 2; Table 3). Day-28 PK was not obtained in three patients. The rapamycin PK profile was characterized by rapid absorption and a slow elimination phase after oral administration. By assessment of pretreatment trough concentrations, steady state was reached by day 8. Accumulation was noted since there was an increase in rapamycin exposure (Cmax; day 1 AUCinf v day 28 AUC0-24 hours) between days 1 and 28 (P < .05; Fig 2A to 2B). Fourteen patients were not assessable for AUCinf on day 1 because of a large percentage extrapolation of the AUC, suggesting that an additional washout period was necessary to accurately assess single-dose PK. There was a statistically significant increase in the day 28 half-life (12.95 [n = 19] v 23.79 [n = 9] hours on days 1 and 28, respectively; P < .05) and decrease in the apparent systemic clearance (26.0 [n = 7] v 17.7 [n = 17] hours on days 1 and 28, respectively; P < .05). Rapamycin showed extensive distribution in excess of blood volume (apparent volume of distribution mean ± SD, 314.3 ± 80.6 [n = 7] and 277.5 [n = 1] L on days 1 and 28, respectively). System exposure (Cmax and AUC) increased proportionally with rapamycin dose from 2 to 9 mg (P > .05; Figs 2C to 2D).
Fig 2.
Average plasma concentration time curve for rapamycin administered orally during day (A) 1 and (B) 28. (A and B) The gray circles, light blue circles, dark blue squares, and red triangles represent 2-, 3-, 6-, and 9-mg dose levels, respectively. Yellow squares represent two patients who had their doses reduced from 9 to 6 mg. The error bars are the standard deviation. Rapamycin maximum plasma concentrations (Cmax; C) and area under the concentration-time curve (AUCinf v day 28 AUC0-24; D) as a function of dose level. (C and D) The blue and yellow circles represent days 1 and 28, respectively.
Table 3.
Pharmacokinetic Parameters of Rapamycin
| Dose (mg) | Day | Pharmacokinetic Parameters
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmax (hours)
|
Cmax (ng/mL)
|
Vz/F (L)
|
Cls/F (L/h)
|
T1/2,z (hours)
|
AUC (ng · h/mL)
|
Css,min (ng/mL)
|
||||||||||||||||
| Median | Range | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | ||
| 2 | 1 | 2.02 | 1.98-3.00 | 4 | 7.0 | 2.7* | 4 | 354.7 | 371.5 | 2 | 29.0 | 30.6 | 2 | 11.13 | 3.15 | 4 | 65.3 | 69.1* | 2 | 6.6 | 6.6 | 4 |
| 2 | 28 | 2.00 | 1.07-4.05 | 3 | 9.2 | 1.8* | 3 | 277.5 | 473.2 | 2 | 16.3 | 0.8 | 3 | 11.23 | 1 | 122.7 | 5.6* | 3 | ||||
| 3 | 1 | 2.00 | 1.00-3.92 | 3 | 8.2 | 2.4* | 3 | 231.9 | 1 | 17.5 | 1 | 9.20 | 16.57 | 2 | 171.6* | 1 | 7.3 | 2.8 | 3 | |||
| 3 | 28 | 1.93 | 1.00-2.00 | 3 | 19.3 | 5.6* | 3 | 541.5 | 228.4 | 3 | 13.1 | 5.1 | 3 | 29.04 | 9.92 | 3 | 254.1 | 98.7* | 3 | |||
| 6 | 1 | 2.00 | 1.00-6.00 | 9 | 21.3 | 8.1* | 9 | 356.1 | 33.8 | 3 | 22.3 | 2.6 | 3 | 14.46 | 3.11 | 9 | 272.1 | 33.2* | 3 | 12.7 | 7.1 | 9 |
| 6 | 28 | 1.98 | 1.00-5.12 | 9 | 27.6 | 15.0* | 9 | 562.5 | 367.6 | 3 | 20.5 | 14.7 | 9 | 28.52 | 8.19 | 3 | 445.2 | 318.1* | 9 | |||
| 9 | 1 | 2.00 | 1.00-2.08 | 5 | 28.4 | 13.1* | 5 | 173.8 | 1 | 38.2 | 1 | 11.44 | 6.05 | 4 | 235.7* | 1 | 23.6 | 7.4 | 5 | |||
| 9 | 28 | 2.00 | 4.00 | 2 | 37.9 | 50.8* | 2 | 217.3 | 395.4 | 2 | 12.3 | 15.2 | 2 | 12.21 | 18.05 | 2 | 592.7 | 729.5* | 2 | |||
| All | 1 | 2.00 | 1.00-6.00 | 21 | 314.3 | 80.6 | 7 | 26.0 | 7.1 | 7 | 12.95* | 3.99 | 19 | |||||||||
| 28 | 2.00 | 1.00-8.08 | 18 | 467.6 | 240.8 | 10 | 17.7 | 11.1 | 17 | 23.79* | 10.03 | 9 | ||||||||||
NOTE. When n ≤ 2, individual values are reported. AUCinf is reported for all dose levels for day 1; AUC0-24 hours is reported for all dose levels for day 28.
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximal plasma concentration; Cls/F, apparent systemic clearance; Css,min, steady state plasma concentration at trough; NR, not reportable; Tmax, time of the maximal plasma concentration; T1/2,z, terminal half-life; Vz/F, apparent volume of distribution; SD, standard deviation.
P < .05 using a Wilcoxon matched pairs signed-rank test.
PD Evaluation
No correlation was found between phospho-P70 inhibition in the skin and dose received or observed clinical benefit. At the completion of the trial, we assessed phospho-S6 and phospho-4EBP1. There was no correlation between the latter and dose or outcome parameters, but phospho-S6 dynamics roughly correlated with dose despite a lower inhibition at 9 mg compared with 6 mg (normalized activation 175%, 88%, 34%, and 81% at 2-, 3-, 6-, and 9-mg levels, respectively; analysis of variance P = .003). Had phospho-S6 evaluation been used in lieu of phospho-P70, the escalation result would have been similar, because at higher doses, toxicity-driven decisions would have also prevailed. We assessed phospho-P70 inhibition in the PBMCs evidencing no correlation with skin phospho-P70, dose, or outcome.
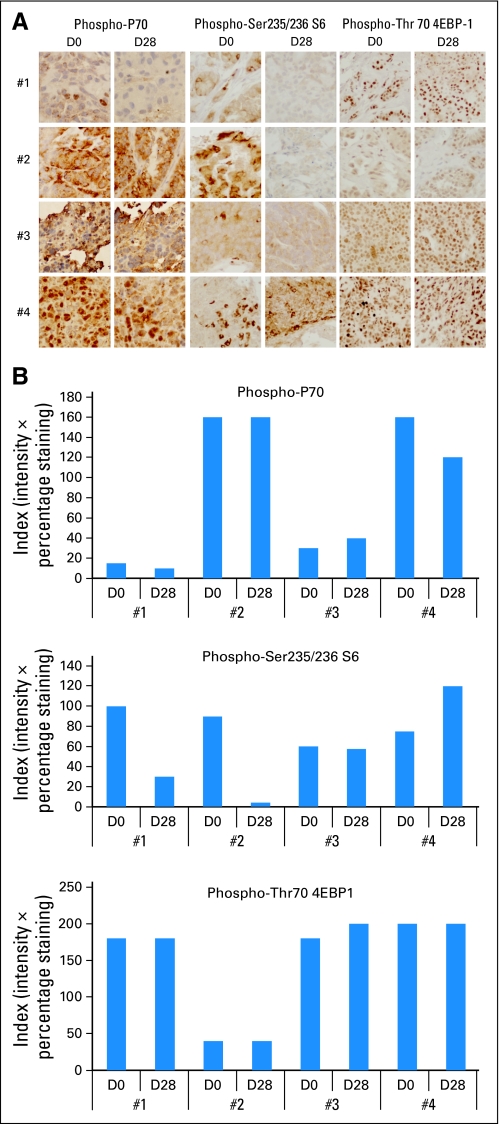
Four paired tumor biopsies were obtained at the MTD, from patients with unknown primary and neuroendocrine cancers and two sarcomas. The first two received treatment for at least 12 months; the latter two received two and four cycles only. Phospho-S6 only decreased in both patients showing benefit (Fig 3).
Fig 3.
(A) Pharmacodynamics of rapamycin in tumors. All images were taken at 40× magnification. (B) The intensity (0, 1, 2, 3) and percentage (0% to 100%) of cells positive were considered, and an index multiplying both was calculated. Graphs of the numeric assessment of the three end points in the four tumor biopsy pairs. Only phospho-S6 showed significant decrease in the patients with clinical benefit (#1 and #2) compared with the two patients showing rapidly progressing disease (#3 and #4).
PK/PD and Outcome-PD Correlations
We correlated PK/PD parameters from our study (Appendix Table A1, online only). No correlation was found between PK and functional imaging or PBMC phospho-P70. No trend was observed between PK and skin phospho-P70 or phospho-4EBP1 dynamics, but there was a strong correlation between PK and phospho-S6 dynamics, indicating increasing inhibition with exposure. No PD parameter showed differences when comparing patients receiving at least12 cycles with the rest.
DISCUSSION
This study explored whether a PD-based modified continuous reassessment method was a feasible tool to rationally determine the PAD or MTD of rapamycin in solid cancer patients. A comprehensive PK/PD plan was built in parallel to further elucidate the relationship between pharmacologic and biologic end points, and identify biomarkers in normal and finally in tumor tissue. The mCRM-based escalation was feasible, but we encountered several obstacles: The selected PD end point did not correlate with dose, target inhibition in the normal tissue was uninformative regarding efficacy, and toxicity ultimately drove dose-selection decisions. A classic dose-escalation approach would have likely led us to the same dose with approximately the same number of patients. Dose level 3, consisting of 6 mg per day of oral rapamycin continuously was safe with no DLTs in nine patients, as was the MTD. Our PK data challenge the widely accepted notion that rapamycin has poor and erratic absorption; we documented systemic drug exposure increasing proportionally with dose and comparable PK results to those reported in transplant studies.
mTOR is now a validated anticancer target. Intravenous weekly temsirolimus (Torisel; Wyeth), a synthetic rapamycin ester, demonstrated superior survival in advanced renal cell carcinoma patients compared with interferon.13 During preclinical testing, temsirolimus demonstrated cytostatic activity rather than tumor shrinkage, prompting the use of end points such as time to progression during clinical development.14,15 We observed minimal efficacy in terms of tumor regression, but SD periods that tripled prior time-to-treatment-failure intervals were documented, especially at the higher dose levels. This, put in context with deeper PD effect in normal skin and higher toxicity than at the lower dose levels, may indicate a dose effect. Five patients remained on study for at least 12 months, which is unusual in a phase I setting. The efficacy in all three neuroendocrine patients is noteworthy considering the negative results of temsirolimus in this disease.16 Higher dose density and deeper pathway shutdown with daily administration may explain these differences. Given mTOR's primary function as a nutrient regulator, the higher incidence of elevations in glucose and lipids compared with temsirolimus also suggest more sustained effect. These alterations may account for the PET efficacy overestimation; alternative tracers should be considered for mTOR inhibitor studies.
PD studies are increasingly being incorporated into phase I trials, but basing dose selection in PD effects is uncommon. In this study, we evaluated a novel statistical tool adapted for a PD end point in a normal tissue. Phospho-70 per a radioactivity-based assay in PBMCs from renal cancer patients treated with temsirolimus correlated with outcome.9 This, together with phospho-P70 suggesting predictive value in glioblastoma patients receiving temsirolimus,17 led us to apply this end point to the mCRM using a nonradioactive and seemingly more applicable assay. The chosen threshold was set arbitrarily and is supported by the temsirolimus data in normal tissues, where a 80% decrease in phospho-P70 was documented at steady state.9 Additionally we explored phospho-S6 ribosomal protein and phospho-4EBP1, which has shown prognostic value in ovarian and breast cancer.18,19 Skin phospho-P70 inhibition did not correlate with pharmacologic exposure and toxicity ultimately dictated dosing decisions. In retrospect, skin phospho-S6 dynamics correlated with rapamycin exposure; however, had we used this end point for the mCRM, decisions would have been similar. In tumor biopsies from patients at the MTD, phospho-S6 decreased in patients deriving a clinical benefit, but it needs to be emphasized that this was a subset analysis and its interpretation requires caution.
Overall, the data support the use of phospho-S6 as the marker of choice in mTOR PD studies, as reported by other groups.20 Recent data indicate that its baseline expression level could have predictive value.21 We can conclude that normal tissue assessment is a valid strategy to elucidate drug effect, but our results suggest that tumor tissue exploration is more promising because of a wider dynamic range. Rapamycin was well tolerated, had predictable toxicity and PK profiles, and showed evidence of antitumor activity.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Antonio Jimeno, Elizabeth Garrett-Mayer, Sharyn D. Baker, Manuel Hidalgo
Financial support: Manuel Hidalgo
Administrative support: Yasmin Khan, Manuel Hidalgo
Provision of study materials or patients: Antonio Jimeno, Michelle A. Rudek, Peter Kulesza, Wen Wee Ma, Anna Howard, Yasmin Khan, Heather Jacene, Wells A. Messersmith, Daniel Laheru, Ross C. Donehower, Manuel Hidalgo
Collection and assembly of data: Antonio Jimeno, Michelle A. Rudek, Peter Kulesza, Wen Wee Ma, Jenna Wheelhouse, Ming Zhao, Heather Jacene, Elizabeth Garrett-Mayer, Sharyn D. Baker, Manuel Hidalgo
Data analysis and interpretation: Antonio Jimeno, Michelle A. Rudek, Elizabeth Garrett-Mayer, Sharyn D. Baker, Manuel Hidalgo
Manuscript writing: Antonio Jimeno, Michelle A. Rudek, Elizabeth Garrett-Mayer, Sharyn D. Baker, Manuel Hidalgo
Final approval of manuscript: Antonio Jimeno, Michelle A. Rudek, Sharyn D. Baker, Manuel Hidalgo
Appendix
Table A1.
Pharmacokinetic/Pharmacodynamic Correlations
| End Point | Day 28 Pearson's Correlation Coefficient
|
||
|---|---|---|---|
| Cmax | AUC0-8 hours | AUC0-24 hours | |
| SUV | −0.071 | −0.088 | 0.036 |
| P | .779 | .729 | .888 |
| Day 2 PBMC phospho-P70 variation | −0.095 | −0.204 | −0.226 |
| P | .795 | .572 | .529 |
| Average PBMC phospho-P70 variation | −0.056 | −0.091 | −0.147 |
| P | .871 | .790 | .666 |
| Day 28 skin phospho-P70 variation | 0.388 | 0.368 | 0.350 |
| P | .171 | .196 | .220 |
| Day 28 skin phospho-S6 variation | –.633 | –.590 | –.553 |
| P | .02 | .034 | .05 |
| Day 28 skin phospho-4EBP1 variation | −0.271 | −0.296 | −0.362 |
| P | .371 | .326 | .224 |
NOTE. Of the explored PD end points, the only one that showed a solid correlation with the PK profile was the variation (defined as % normalized to baseline) in skin phospho-S6 activation (indicated by boldfacing), where exposure was inversely correlated to phosphorylation levels.
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximal plasma concentration; PBMC, peripheral-blood mononuclear cell; SUV, standard uptake value.
Supported by Grant No. R21CA112919.
Presented at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Schmelzle T, Hall MN: TOR, a central controller of cell growth. Cell 103:253-262, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Thomas G, Hall MN: TOR signalling and control of cell growth. Curr Opin Cell Biol 9:782-787, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Garber K: Rapamycin's resurrection: A new way to target the cancer cell cycle. J Natl Cancer Inst 93:1517-1519, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Humar R, Kiefer FN, Berns H, et al: Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. Faseb J 16:771-780, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Kahan BD: Two-year results of multicenter phase III trials on the effect of the addition of sirolimus to cyclosporine-based immunosuppressive regimens in renal transplantation. Transplant Proc 35:S37-S51, 2003 [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AS: A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 71:271-280, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman JJ, Kahan BD: Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol 37:405-415, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Piantadosi S, Fisher JD, Grossman S: Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol 41:429-436, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Peralba JM, DeGraffenried L, Friedrichs W, et al: Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin Cancer Res 9:2887-2892, 2003 [PubMed] [Google Scholar]
- 10.Young H, Baum R, Cremerius U, et al: Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations: European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773-1782, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gibaldi M, Perrier D: Pharmacokinetics (ed 2nd). New York, NY, Dekker, 1982
- 13.Hudes GR, Carducci M, Tomczak P, et al: A phase III, randomized 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). J Clin Oncol 24:,2s 2006. (suppl; abstr LBA4) [Google Scholar]
- 14.Gibbons JJ, Discafani C, Peterson R, et al: The effect of CCI-779, a novel macrolide anti-tumor agent, on the growth of human tumor cells in vitro and in nude mouse xenograft in vivo. Proc Am Assoc Cancer Res 40, 1999. (abstr 301)
- 15.Dudkin L, Dilling MB, Cheshire PJ, et al: Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res 7:1758-1764, 2001 [PubMed] [Google Scholar]
- 16.Duran I, Kortmansky J, Singh D, et al: A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 95:1148-1154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanis E, Buckner JC, Maurer MJ, et al: Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol 23:5294-5304, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Castellvi J, Garcia A, Rojo F, et al: Phosphorylated 4E binding protein 1: A hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer 107:1801-1811, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Rojo F, Najera L, Lirola J, et al: 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res 13:81-89, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al: Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5:e8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwenofu OH, Lackman RD, Staddon AP, et al: Phospho-S6 ribosomal protein: A potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol 21:231-237, 2008 [DOI] [PubMed] [Google Scholar]