Abstract
Purpose
A diagnosis of primary peritoneal serous carcinoma (PPSC) requires exclusion of a source in other reproductive organs. Serous tubal intraepithelial carcinoma (STIC; stage 0) has been described in asymptomatic women with BRCA mutations and linked to a serous cancer precursor in the fimbria. This study examined the frequency of STIC in PPSC and its clinical outcome in BRCA-positive women.
Patients and Methods
Presence or absence of STIC was recorded in consecutive cases meeting the 2001 WHO criteria for PPSC, including 26 patients with nonuniform sampling of the fallopian tubes (group 1) and 19 patients with complete tubal examination (group 2; sectioning and extensively examining the fimbriated end, or SEE-FIM protocol). In selected cases, STIC or its putative precursor and the peritoneal tumor were analyzed for p53 mutations (exons 1 to 11). Outcome of STIC was ascertained by literature review.
Result
Thirteen (50%) of 26 PPSCs in group 1 involved the endosalpinx, with nine STICs (35%). Fifteen (79%) of 19 cases in group 2 contained endosalpingeal involvement, with nine STICs (47%). STIC was typically fimbrial and unifocal, with variable invasion of the tubal wall. In five of five cases, the peritoneal and tubal lesion shared an identical p53 mutation. Of 10 reported STICs in BRCA-positive women, all patients were without disease on follow-up.
Conclusion
The fimbria is the source of nearly one half of PPSCs, suggesting serous malignancy originates in the tubal mucosa but grows preferentially at a remote peritoneal site. The generally low risk of recurrence in stage 0 (STIC) disease further underscores STIC as a possible target for early serous cancer detection and prevention.
INTRODUCTION
Papillary serous carcinomas of the female genital tract are the most lethal of pelvic epithelial malignancies because they are typically discovered after they have spread to the peritoneal surfaces. They are classified as arising in the endometrium, fallopian tube, ovary, and peritoneum.1 A more precise assignment of primary site can be made with tubal and endometrial serous carcinomas, because an early form of the disease (serous tubal or endometrial intraepithelial carcinoma [STIC or EIC]) has been described in both.2,3 Moreover, a putative nonmalignant precursor to STIC, termed the p53 signature, has recently been described in the fallopian tube.4 Because of their mucosal distributions, the STIC (or its precursor) and EIC are considered strong if not irrefutable evidence of a primary tumor in these respective organs. When a serous carcinoma presents as a large (dominant) unilateral ovarian mass, the tumor is usually presumed to be ovarian in origin, particularly when a pre-existing benign condition (cystadenoma, endometriosis) is identified. In the absence of these conditions, the presumed origin is Müllerian epithelium, derived from either the ovarian surface epithelium or fallopian tube (endosalpingiosis). Cortical inclusions derived from these sources are accepted as an origin for ovarian serous epithelial tumors.5
In contrast to the above scenarios, the pathogenesis of serous carcinomas that grow predominately in the omentum or other peritoneal surfaces is unclear. These tumors, designated as primary peritoneal serous carcinomas (PPSCs) have been presumed to arise from Müllerian inclusions or endometriosis involving the peritoneal surfaces. Expression of Müllerian-specific markers such as PAX8 and the lack of coexisting precursors on the ovarian surface have supported an origin in the fallopian tube or Müllerian inclusions.6,7
Recent studies of early epithelial malignancies in women with heritable mutations in the BRCA1 or BRCA2 genes have revealed a greater than expected frequency of these early lesions in the distal fallopian tube.8-10 This discovery has been facilitated by the use of sectioning protocols that extensively sample the tube, including the fimbriated end (sectioning and extensively examining the fimbriated end, or SEE-FIM, protocol).11,12 These studies have raised the possibility that the fimbria is a major site of origin for early serous carcinomas in high-risk women. Moreover, in studies of unselected consecutive women with pelvic serous cancer, we have found STIC in nearly one half, including a small number of women with presumed PPSC.13 Combined with the recent description of a candidate precursor (the p53 signature) in the distal tube, these studies suggest that the distal tube is a sometimes inconspicuous source of pelvic serous cancers.4 The purpose of this study was to examine a larger group of women with PPSC and determine both the frequency of STIC or its precursor and the role of complete tubal sampling in designating a primary source of PPSC.
PATIENTS AND METHODS
Overall Study Design and Rationale
The institutional review board at Brigham and Women's Hospital in Boston, MA, approved the study. The study had three goals. The first was to determine the prevalence of STIC and its precursor (p53 signatures) in two consecutively accessioned series of patients diagnosed with PPSC: those with partial and with complete examination of the fallopian tubes. The second was to determine whether p53 signatures and/or STICs shared p53 mutations with the peritoneal tumor. The third was to determine the outcomes of cases where STIC was the only disease present, a circumstance that is largely limited to women undergoing prophylactic salpingo-oophorectomy for BRCA mutations.
Patient Selection
Consecutive cases from January 1, 2000, until February 1, 2007, that met the 2001 WHO criteria for PPSC1 were selected. These criteria specify that (1) both ovaries must be normal in size or enlarged by a benign process, (2) the involvement in the extraovarian sites must be greater than the involvement on the surface of either ovary, (3) the ovarian tumor involvement must be either nonexistent, confined to ovarian surface epithelium without stromal invasion, or involving the cortical stroma with tumor size less than 5 × 5 mm. In each case, the presence or absence of a family history of breast/ovarian cancer was also recorded.
Histologic and Immunohistochemical Analysis of Fallopian Tubes
Fallopian tube sampling was performed in two ways: (1) nonuniform sampling (16 cases), in which only a portion of the tube(s) was submitted for pathologic review; and (2) complete tubal examination, using the SEE-FIM protocol (19 cases).11 All of the sections were available in each case, and hematoxylin and eosin–stained sections were reviewed for the presence of tubal intraepithelial carcinoma, using previously published criteria.
A monoclonal antibody to p53, targeting an epitope spanning amino acids 21 to 25 of the protein, was used to localize the p53 protein (OP43; Oncogene Research Products, San Diego, CA) and was applied to all cases of STIC. The presence of an associated precursor condition—the p53 signature—was recorded when benign-appearing epithelium stained intensely for p53. A monoclonal antibody to Ki-67 (MIB1 corresponding to Ki-67; M7240; DAKO, Carpinteria, CA) was used to further distinguish the highly proliferative STIC from its precursor, as previously described.14
Analysis for p53 Mutations
Some p53 signatures and STICs were analyzed for p53 mutations. In all cases, normal mucosa from the same tissue served as controls. When present, a remote tumor site, typically peritoneal, was also selected for analysis. Laser-capture microdissection was performed to isolate p53 STICs and signatures and control somatic DNA from selected cases using the PALM microbeam instrument (Carl Zeiss Microimaging, Munich, Germany). Genomic DNA was amplified by polymerase chain reaction (PCR) using tailed primers designed to amplify exons 2, 3, 5 to 9, and 11 of p53. A secondary amplification was performed using T3 and T7 primers specific to the tail sequence used in the primary amplification. PCR products were then sequenced from both strands using T3 and T7 primers. Data were analyzed using the Mutation Surveyor program (Soft Genetics, State College, PA). Candidate mutations found by the software were compared with a reference database for cancer-associated p53 mutations (Universal Mutation Database, http://www.umd.be:2072/IFAMTP53A.shtml).4
Because formalin fixation can introduce spurious mutations into somatic DNA, all p53 mutation-positive exons were resequenced from a replicate PCR-amplified product.15 Samples were scored as p53 mutation–positive only if an identical mutation was identified in products of both amplifications.
Literature Review of Outcomes of Stage 0 STIC in BRCA-Positive Women
To ascertain the potential recurrence risk imposed by STIC, the available literature on stage 0 serous carcinoma of the fallopian tube was reviewed.
RESULTS
Case Material and Frequency of Endosalpingeal Involvement and STIC
A total of 45 cases fulfilling the criteria for PPSC were identified from the files, including 26 with nonuniform sampling of the fallopian tube and 19 processed by the SEE-FIM protocol. Thirteen (50%) of 26 cases with incomplete tubal sampling had carcinoma involving the endosalpinx, and nine (35%) had STIC. Additionally, three p53 signatures were identified; two in cases with STICs, and one in a case with endosalpingeal involvement by invasive carcinoma.
A summary of the 19 cases that underwent complete examination of the fallopian tube is included in Table 1. From one to six sections of each fallopian tube were evaluated. Seven patients had recorded a family history of breast or ovarian cancer; one additional patient had a documented BRCA mutation. Differences in the frequency of endosalpingeal involvement between those with a positive and negative family history were not significant. Fifteen (79%) of 19 cases with complete tubal sampling had endosalpingeal involvement, and nine cases (47%) had STIC. No p53 signatures were identified in this group after immunostaining for p53.
Table 1.
Tubal Sampling, Frequency of Endosalpingeal Involvement and Tubal Intraepithelial Carcinoma, and Clinical History in Patients With Complete Tubal Examination (group 2)
| Case | Evaluation of Fimbria | Sections Evaluated (R, L) | Endosalpinx Involvement | STIC Identified | Gynecologic Cancer History |
|---|---|---|---|---|---|
| 1 | Complete | 3, 3 | Yes | Yes | BRCA2+ |
| 2 | Complete | 4, 3 | Yes | No | Negative family history |
| 3 | Complete | 3, 3 | Yes | No | Negative family history |
| 4 | Complete | 2, 2 | No | No | Breast cancer (aunt) |
| 5 | Complete | 4, 3 | No | No | Negative family history |
| 6 | Complete | 5, 3 | Yes | Yes | Breast cancer (sister) |
| 7 | Complete | 3, 2 | No | No | Negative family history |
| Concurrent endometrioid carcinoma (uterus) | |||||
| 8 | Complete | 5, 2 | Yes | No | Negative family history |
| 9 | Complete | 2, 2 | Yes | No | Negative family history |
| 10 | Complete | 5, NA | No | No | Breast cancer (mother); prior left ectopic pregnancy |
| 11 | Complete | 3, 3 | Yes | Yes | Negative family history |
| 12 | Complete | 3, 2 | Yes | No | Breast cancer (sister) |
| 13 | Complete | 3, 3 | Yes | Yes | Negative family history |
| 14 | Complete | 3, 4 | Yes | Yes | Negative family history |
| 15 | Complete | 2, 2 | Yes | No | Breast cancer (sister) |
| 16 | Complete | 2, 2 | Yes | Yes | Negative family history |
| 17 | Complete | 2, 2 | Yes | Yes | Multiple ovarian/breast cancer (family) |
| 18 | Complete | 6, 4 | Yes | Yes | Negative family history |
| 19 | Complete | 1, 3 | Yes | Yes | Breast cancer (cousin) |
Abbreviations: R, right; L, left; STIC, serous tubal intraepithelial carcinoma; NA, no left tube was present for evaluation.
p53 Mutations in STICs and p53 Signatures
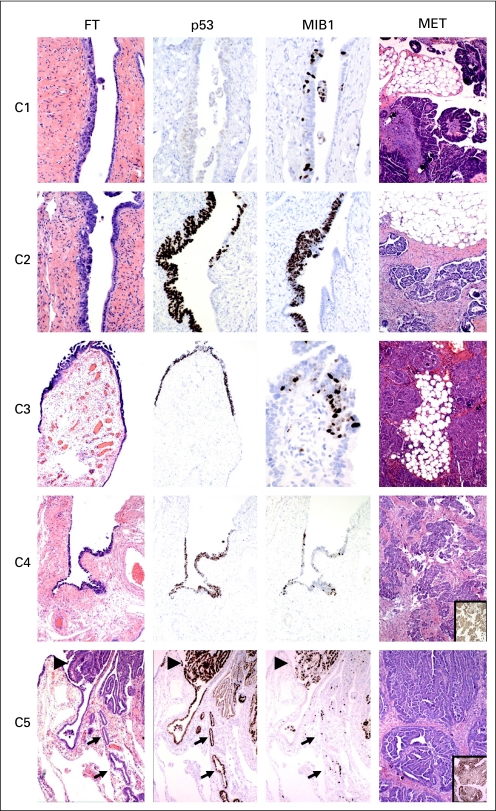
Table 2 summarizes the p53 sequencing data. In five of 10 cases, at least one possible primary site (either STIC or a p53 signature) and one remote (omental or peritoneal surface) tumor site was successfully analyzed for mutations in exons 1 to 11. In five, one or more assays did not reveal sufficient information to make a comparison because of either a limited amount of extracted DNA or poor DNA quality. In four cases, DNA from both a STIC and peritoneal tumor site was completely analyzed and revealed an identical p53 mutation (cases 1 through 4; Fig 1). In a fifth, obtained from the first group (case 5), DNA was analyzed from a contiguous precursor lesion with a low proliferative index (p53 signature) and compared with that of the peritoneal tumor (Fig 1). An identical p53 mutation was identified in both on replicate analysis (Table 2).
Table 2.
p53 Mutation Analysis of Noninvasive Tubal Lesions and Remote Tumors
| Case | Classification | Epithelium | Base Change | Designation | Codon | Effect | OVCA | Total Citations |
|---|---|---|---|---|---|---|---|---|
| 1 | PPSC | Normal | None | None | 275 | Missense | 3 | 47 |
| STIC | c.824G > T | C275F | ||||||
| Omentum | c.824G > T | C275F | ||||||
| 2 | PPSC | Normal | None | None | 241 | Missense | 3 | 37 |
| STIC | c.722C > G | S241C | ||||||
| Omentum | c.722C > G | S241C | ||||||
| 3 | PPSC | Normal | None | None | 306 | Missense | 11 | 162 |
| STIC | c.916C > T | R306X | ||||||
| Omentum | c.916C > T | R306X | ||||||
| 4 | PPSC | Normal | None | None | 195 | Missense | 20 | 92 |
| STIC | c.584T > C | I195T | ||||||
| Peritoneum | c.584T > C | I195T | ||||||
| 5 | PPSC | Normal | None | None | — | Missense | 2 | 19 |
| P53 Sig | c.434T > C | L145P | 145 | |||||
| Peritoneum | c.434T > C | L145P |
NOTE. Data adapted (http://www.umd.be:2072/IFAMTP53A.shtml).
Abbreviations: PPSC, primary peritoneal serous carcinoma; STIC, serous tubal intraepithelial carcinoma; OVCA, reported number of ovarian cancers with this mutation.
Fig 1.
Sections of fallopian tube (FT) and omental or peritoneal tumors corresponding to areas from which DNA was obtained by laser capture microdissection and subsequently analyzed in duplicate for mutations in exons 1 to 11 of p53 (Table 2). Numbers and locations correspond to cases summarized in Table 2. Cases (C) 1 through C4 contain serous tubal intraepithelial carcinoma based on morphologic criteria, with variable proliferative (MIB-1) index, and are strongly p53-positive in C2 through C4. In C5, benign-appearing p53-positive epithelium (p53 signature, arrows) is adjacent to cancer (large arrowheads), both containing identical p53 mutations (Table 2). Insets in images of the peritoneal tumors in C4 and C5 illustrate strong p53 immunostaining.
Literature Review
The outcome of STIC, with or without positive peritoneal cytology, is listed in Table 3. Of 10 cases from the literature discovered in women with BRCA mutations, four cases were associated with positive washings. Of nine patients with available information, six patients received chemotherapy. To date, none have experienced recurrence over follow-up periods ranging from 4 to 87 months.8,9,16-18
Table 3.
Reported Outcomes of Serous Tubal Intraepithelial Carcinoma (stage 0) in BRCA-Positive Women
| First Author | Age (years) | BRCA | Washings | Chemotherapy | Follow-Up (months) |
|---|---|---|---|---|---|
| Agoff16 | 65 | 2558inA BRCA2 | Positive | Yes | NED, 36 |
| Finch8 | 64 | BRCA1 | Negative | UK | NED, 4 |
| Carcangiu17 | 49 | BRCA1 | Negative | None | NED, 87 |
| 61 | BRCA1 | Negative | None | NED, 38 | |
| 48 | BRCA1 | Negative | None | NED, 7 | |
| Paley18 | 65 | BRCA2.2558insA | Positive | No | NED, 36 |
| 47 | BRCA1.2800delAA | Positive | Yes | NED, 48 | |
| Callahan9 | 44 | BRCA2.W2598X | Positive | Yes | NED, 36 |
| 66 | BRCA1.5301insA | Negative | Yes | NED, 36 | |
| 44 | BRCA1.1294del40 | Negative | Yes | NED, 36 |
Abbreviations: BRCA, heterozygous BRCA mutation (1 or 2); NED, no evidence of disease; UK, unknown.
DISCUSSION
Epithelial tumors that arise as a dominant mass or masses on the peritoneal surfaces represent a rare group of tumors, specifically when associated with normal ovaries. The so-called normal-sized ovarian carcinoma syndrome has traditionally included both tumors arising from the ovaries and those presumed to be PPSCs. Criteria for the latter that are stipulated by WHO and the Gynecologic Oncology Group include the following: (1) ovaries must be normal in size or enlarged by a benign process only, (2) extraovarian tumors must exceed the ovarian tumors in size, and (3) microscopic carcinoma in the ovary must be superficial and less than 5 × 5 mm.1,19-21 However, studies have not found these criteria to reliably distinguish tumors on either side of this threshold into distinct subsets. Differences between such tumors assigned to the ovary versus the peritoneum can be largely explained by the inherently smaller tumor burden with primary ovarian tumors (younger age, lower CA125, less omental involvement, and more favorable response to chemotherapy).20 Others have found little difference between advanced ovarian serous and primary peritoneal carcinomas.22,23
Studies in women with heritable mutations in the BRCA1 or BRCA2 genes have highlighted the distal fallopian tube as a site of noninvasive serous carcinomas in high-risk women.8-11 These noninvasive carcinomas, termed tubal intraepithelial carcinomas, are presumed to be an early phase of malignancy, signifying the primary site; they are often the only manifestation of carcinoma in prophylactic salpingo-oophorectomies and, when found in isolation, have a low risk of recurrence.24 Recently, detailed analysis of the distal fallopian tubes in consecutively accessioned women with ovarian serous carcinomas, most of whom had no family history or documented BRCA mutation, revealed STIC in nearly one half of patients.13 To address the existing hypothesis that the earliest events leading to serous carcinoma might occur in the salpingeal mucosa, a study was conducted to characterize nonmalignant mucosal changes that shared properties with serous carcinomas.4,25 In this and subsequent studies, small stretches of linear p53 positivity were identified in the fimbria in a relatively high proportion of women, whether or not they were BRCA mutation carriers or not. These p53-positive regions (p53 signatures) possess several properties attributed to early serous carcinomas, including fimbrial location, secretory cell type, p53 mutations, immunostaining for γ-H2AX (signifying DNA damage), and occasional coexistence with STIC. Thus there is now evidence of a pathway to serous carcinogenesis in the fallopian tube with both benign and malignant components, and it seems to be dominant in women with BRCA mutations.4,14,26
In a prior study that included seven women classified on pathology reports as having PPSC, we found STIC in four women.13 The current study has confirmed this initial observation, demonstrating STIC in the distal fallopian tubes of a significant percentage (47%) of women with PPSC. In four cases, reproducible sequence mutations in the p53 tumor suppressor gene were identified in both the STIC and the tumor in the omental or peritoneal surfaces. In a fifth case (Fig 1 and Table 2, case 5), identical p53 mutations were confirmed in benign-appearing (precursor) epithelium and a serous malignancy. We acknowledge that the direction of tumor spread cannot be guaranteed with absolute certainty and that the possibility exists that peritoneal carcinoma could seed the mucosa of the distal fallopian tube. Nevertheless, the findings of this study coupled with prior work are a strong endorsement of the fallopian tube as the source of a significant proportion of PPSCs.
Despite the above, it should be noted that half of PPSCs did not have a STIC present. Some could have still arisen in the endosalpinx, with the STIC obscured by the malignancy. However, one fourth did not involve the endosalpinx, and a source elsewhere must be considered. In our experience and that of others, the cell type responsible for PPSC, tubal carcinomas and classic ovarian serous carcinomas seems the same—Müllerian—based on the immunohistochemical data.6 Going forward, a clear distinction of these two mechanisms would permit a more precise pathogenetic subclassification of this unique subset of serous carcinomas. In view of studies that suggest an increasing incidence of PPSC in recent years, the potential role of precursor lesions in this changing incidence merits closer attention.27
Ultimately, the significance of the fallopian tube as a source of pelvic serous cancer resides in the prospect that this disease can be interrupted before it has spread to the pelvic surfaces. The data supporting STIC as an established step in the serous carcinogenic sequence are outlined above, and the occasional discovery of STIC as the only lesion in tubes from women with established pelvic serous cancer emphasizes the potential of this entity to exfoliate cells with metastatic potential. Nevertheless, of the small number of BRCA-positive women with stage 0 carcinoma (STIC) and prospectively observed, no recurrences were identified (Table 3). Currently, there is controversy regarding the best way to manage a diagnosis of STIC in a BRCA-positive woman. It is possible that postoperative adjuvant chemotherapy may not be necessary if the peritoneal cytology is normal and there is no evidence of spread. Nonetheless, the natural history of these lesions in the absence of further treatment remains unclear.17 Given the low rate of adverse outcomes in large series of prophylactic salpingo-oophorectomies, many without thorough sampling of the distal fallopian tube, it is reasonable to assume that many STICs are missed, yet most (if not all) have not been able to spread.28 The favorable outcome for these individuals is not unlike that of women with serous endometrial intraepithelial carcinoma. Women with the latter experience a low (but not nonexistent) rate of recurrence, provided disease is not documented beyond the uterus at the time of surgery. Although the behavior of STICs may vary between women with and without BRCA mutations, they are otherwise identical in appearance and location.10 The likelihood that a proliferating intraepithelial neoplasm could exist in the distal tube for a period of time before metastasizing, coupled with its strong link to pelvic serous cancer, raises the distinct possibility that if measures could be designed to detect this lesion, the death rate from pelvic serous cancer could be reduced.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph W. Carlson, Alexander Miron, Elke A. Jarboe, Mana M. Parast, Yonghee Lee, Michael G. Muto, David Kindelberger, Christopher P. Crum
Financial support: Alexander Miron, Christopher P. Crum
Administrative support: Alexander Miron, Michael G. Muto, Christopher P. Crum
Provision of study materials or patients: Michelle S. Hirsch, Michael G. Muto
Collection and assembly of data: Joseph W. Carlson, Alexander Miron, Elke A. Jarboe, Mana M. Parast, Yonghee Lee, Michael G. Muto, David Kindelberger, Christopher P. Crum
Data analysis and interpretation: Joseph W. Carlson, Alexander Miron, Christopher P. Crum
Manuscript writing: Joseph W. Carlson, Alexander Miron, Elke A. Jarboe, David Kindelberger, Christopher P. Crum
Final approval of manuscript: Joseph W. Carlson, Alexander Miron, Elke A. Jarboe, Mana M. Parast, Michelle S. Hirsch, Yonghee Lee, Michael G. Muto, David Kindelberger, Christopher P. Crum
Supported by grants from the National Cancer Institute (Grants No. P50 CA10500 [SPORE]: D. Cramer, principal investigator; NCI KO8 CA108748, R. Drapkin, principal investigator; NCI 1R21CA124688-01A1, C.P.C., principal investigator), the Charlotte Geyer Foundation (C.P.C., principal investigator), the Columbia Hospital for Women Research Foundation (C.P.C., principal investigator), the Dana-Farber Cancer Institute, and the Francis Ward Paine and TSA Pemberton Funds from the Division of Women's and Perinatal Pathology, Brigham and Women's Hospital.
J.W.C. and A.M. contributed equally to this work.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Lee KR, Tavassoli FA, Prat J, Dietel M, et al: Surface epithelial-stromal tumors, in Tavassoli FA, Devilee P (eds): Tumours of the Breast and Female Genital Organs. Lyon, France, IARC Press, 2003, pp 119-120
- 2.Ambros RA, Sherman ME, Zahn CM, et al: Endometrial intraepithelial carcinoma: A distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 26:1260-1267, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Colgan TJ: Challenges in the early diagnosis and staging of Fallopian-tube carcinomas associated with BRCA mutations. Int J Gynecol Pathol 22:109-120, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Miron A, Drapkin R, et al: A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211:26-35, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Drapkin R, Crum CP, Hecht JL: Expression of candidate tumor markers in ovarian carcinoma and benign ovary: Evidence for a link between epithelial phenotype and neoplasia. Hum Pathol 35:1014-1021, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Tong GX, Chiriboga L, Hamele-Bena D, et al: Expression of PAX2 in papillary serous carcinoma of the ovary: Immunohistochemical evidence of fallopian tube or secondary Müllerian system origin? Mod Pathol 20:856-863, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Seidman JD, Wang BG: Evaluation of normal-sized ovaries associated with primary peritoneal serous carcinoma for possible precursors of ovarian serous carcinoma. Gynecol Oncol 106:201-206, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Finch A, Shaw P, Rosen B, et al: Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol 100:58-64, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Callahan MJ, Crum CP, Medeiros F, et al: Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol 25:3985-3990, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cass I, Holschneider C, Datta N, et al: BRCA-mutation-associated fallopian tube carcinoma: A distinct clinical phenotype? Obstet Gynecol 106:1327-1334, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Medeiros F, Muto MG, Lee Y, et al: The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 30:230-236, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Powell CB, Kenley E, Chen LM, et al: Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role of serial sectioning in the detection of occult malignancy. J Clin Oncol 23:127-132, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kindelberger DW, Lee Y, Miron A, et al: Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 31:161-169, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Jarboe E, Folkins A, Nucci MR, et al: Serous carcinogenesis in the fallopian tube: A descriptive classification. Int J Gynecol Pathol 27:1-9, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Williams C, Ponten F, Moberg C, et al: A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol 155:1467-1471, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agoff SN, Garcia RL, Goff B, et al: Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol 28:1112-1114, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Carcangiu ML, Peissel B, Pasini B, et al: Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: Report of 6 cases and review of the literature. Am J Surg Pathol 30:1222-1230, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Paley PJ, Swisher EM, Garcia RL, et al: Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: A case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol 80:176-180, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Feuer GA, Shevchuk M, Calanog A: Normal-sized ovary carcinoma syndrome. Obstet Gynecol 73:786-792, 1989 [PubMed] [Google Scholar]
- 20.Choi CH, Kim TJ, Kim WY, et al: Papillary serous carcinoma in ovaries of normal size: A clinicopathologic study of 20 cases and comparison with extraovarian peritoneal papillary serous carcinoma. Gynecol Oncol 105:762-768, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Jaaback KS, Ludeman L, Clayton NL, et al: Primary peritoneal carcinoma in a UK cancer center: Comparison with advanced ovarian carcinoma over a 5-year period. Int J Gynecol Cancer 16:123-128, 2006. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 23.Barda G, Menczer J, Chetrit A, et al: National Israel Ovarian Cancer Group: Comparison between primary peritoneal and epithelial ovarian carcinoma—A population-based study. Am J Obstet Gynecol 190:1039-1045, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Leeper K, Garcia R, Swisher E, et al: Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol 87:52-56, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Piek JM, van Diest PJ, Zweemer RP, et al: Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195:451-456, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Folkins AK, Jarboe EA, Callahan M, et al: A candidate precursor to pelvic serous cancer (p53 Signature) and its prevalence in ovaries and fallopian tubes from women with heterozygous BRCA mutations. Gynecol Oncol 109:168-173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halperin R, Zehavi S, Langer R, et al: Primary peritoneal serous papillary carcinoma: A new epidemiologic trend? A matched-case comparison with ovarian serous papillary cancer. Int J Gynecol Cancer 11:403-408, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kauff ND, Domchek SM, Friebel TM, et al: Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol 26:1331-1337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]