Abstract
Purpose
To investigate the effect of socioeconomic status, as measured by education, on the survival of 1,577 lung cancer patients treated on 11 studies conducted by the Cancer and Leukemia Group B.
Patients and Methods
Sociodemographic data, including education, was reported by the patient at the time of clinical trial accrual. Cox proportional hazards model stratified by treatment arm/study was used to examine the effect of education on survival after adjustment for known prognostic factors.
Results
The patient population included 1,177 patients diagnosed with non–small-cell lung cancer (NSCLC; stage III or IV) and 400 patients diagnosed with small-cell lung cancer (SCLC; extensive or limited). Patients with less than an eighth grade education (13% of patients) were significantly more likely to be male, nonwhite, and older; have a performance status (PS) of 1 or 2; and have chest pain. Significant predictors of poor survival in the final model included male sex, PS of 1 or 2, dyspnea, weight loss, liver or bone metastases, unmarried, presence of adrenal metastases and high alkaline phosphatase levels among patients with NSCLC, and high WBC levels among patients with advanced disease. Education was not predictive of survival.
Conclusion
The physical condition of patients with low education who enroll onto clinical trials is worse than patients with higher education. Once enrolled onto a clinical trial, education does not affect the survival of patients with SCLC or stage III or IV NSCLC. The standardization of treatment and follow-up within a clinical trial, regardless of education, is one possible explanation for this lack of effect.
INTRODUCTION
Evidence for the link between socioeconomic status (SES) and health/disease has accumulated over the years, mainly through the theoretical framework from the social determinants of health.1,2 Several investigators have examined the statistically significant effect of income and education on survival in the general population.1-5 The first of two explanations for this relationship is poverty in the form of material deprivation (eg, clean water and adequate nutrition). Alternatively, for rich countries such as the United States, the explanation given by Marmot6 is that the relationship between income and survival is not the effect of poverty but the effect of relative differences in “opportunities for social participation, for leading a fulfilling and satisfying life, and for control over one's life.” Social conditions including health practices, psychosocial characteristics of work (control, variety, and satisfaction), social support, and sense of control over the future are important in the determination of mortality. Much of the literature referenced earlier focuses on income; however, Marmot6 notes that education may be an even better indicator of factors linked to social position that are important to health and survival.
In an often quoted article about the relationship between SES and the survival of cancer patients, Cella et al7 reported that income and education were significant predictors of survival among patients treated for cancer on clinical trials conducted by the Cancer and Leukemia Group B (CALGB), a national cooperative group funded by the National Cancer Institute. That study showed that, after adjustment for known prognostic factors including cancer type, performance status, age, and protocol-specific prognostic factors, patients with annual incomes less than $5,000 and patients with only a grade school education had poorer survival than patients with higher SES treated on CALGB trials. The data used in these analyses were drawn from eight CALGB studies conducted between 1977 and 1983 that involved 2,089 patients, including patients with lung cancer (n = 961), multiple myeloma (n = 577), gastric cancer (n = 231), pancreatic cancer (n = 174), breast cancer (n = 87), and Hodgkin's disease (n = 58).
Questions about the relationship between SES and cancer survival persist. Numerous articles have addressed the effect of various measures of SES on cancer survival.8-29 However, inferences have not always been consistent because of differing research methodology, such as differences in the research question being asked (eg, impact of education on survival in the general population or impact in a clinical trial population), the patient population (eg, homogeneous or heterogeneous histology), the source of the data (eg, census, regulatory, Surveillance, Epidemiology, and End Results, or clinical treatment trial), and the measure of SES and its source (eg, patient reported or administrative databases).
For lung cancer, the reviewed literature is inconsistent relative to a relationship between SES and survival of patients. Eight publications that included or completely focused on patients with lung cancer were reviewed. Four articles focused on patient-reported measures of SES, including education,17 education and income,14,21 and occupation.22 Two of these articles focused on a mixed cancer patient population, with Vigano et al's14 advanced cancer patient population including 77 lung cancer patients and Stavraky et al's17 population including 202 lung cancer deaths. Both articles reported that the relationship of SES-related variables with survival was not statistically significant. The remaining articles that focused on patient-reported measures of SES included patients with early-stage cancer.21,22 Greenwald et al21 reported that income, not education, was a significant predictor of survival among 125 lung cancer patients; however, Bouchardy et al22 reported that SES based on occupation was not a significant predictor of long-term survival. Coleman et al13 examined a population base of 3 million cancer patients, including approximately 150,000 lung cancer patients. Without adjustment for stage and other prognostic factors, this article showed that the survival patterns of rich and poor cancer patients were significantly different. After adjustment for known prognostic factors and comorbidity, Tammemagi et al8 found no significant relationship between income estimated from census-tract data and survival among a heterogeneous population of 1,155 lung cancer patients from a cancer registry. Sowah et al20 reported that income estimated from census tract data was not predictive of survival among 367 patients with non–small-cell lung cancer (NSCLC) overall; however, among patients with stage I disease, the relationship was statistically significant. Recently, Ou et al19 presented similar results among 19,702 patients from the California Cancer Registry with stage I disease based on a composite measure stemming from census block data.
This report is part of a larger project the purpose of which is to examine a large database of Background Information Forms that CALGB has routinely collected between 1990 and 1998 and to validate the findings of Cella et al7 concerning the relationship of education and survival within larger, homogeneous cancer patient populations that participated in CALGB clinical trials. This report focuses specifically on the relationship between education and survival among patients with NSCLC or small-cell lung cancer (SCLC) who participated in 11 CALGB clinical trials (CALGB 8831, 8931, 9532, 9033, 9130, 9235, 9430, 9431, 9534, 9730, and 9731).31-43 The strength of this research is that it uses a measure of SES provided by the patient, explores the relationship between education and survival within relatively homogeneous patient groups, and has power (ie, sufficient patient numbers) to detect clinically important effect sizes within the unique context of clinical trials.
PATIENTS AND METHODS
CALGB Collection of Socioeconomic Data
The CALGB Psychiatry Committee (later renamed the Psycho-Oncology Committee) piloted the collection of socioeconomic data from a Background Information Form on two CALGB studies30 and showed the feasibility of collecting such data for all variables except income. In the early 1990s, after completion of the feasibility study, CALGB initiated the collection of the Background Information Form on all active studies. Included among the data provided by the patient were education, race, and marital status. Education was presented as a multiple choice question with the following options: grades 1 to 8, grades 9 to 11, high school graduate, some college, junior college degree, college degree, some postcollege, or advanced degree. Income was not collected because of collection difficulties encountered in the feasibility study. In approximately 1998, CALGB discontinued the collection of the Background Information Form from all studies and limited its collection to studies specifically needing such data to answer the study's primary scientific questions. As of today, the Background Information Form has been collected from more than 18,000 patients on more than 140 studies, including more than 1,500 lung cancer patients. Survival data and baseline clinical data were obtained from the CALGB database and merged with the Background Information Form.
Patient Population
The analyses presented in this article are based on education and clinical data collected in 11 lung cancer studies coordinated by the CALGB. These studies31-43 are listed in Table 1, along with data concerning accrual, patient eligibility, the availability of Background Information Form data, and the patient status (alive or dead) at last follow-up. The submission of the Background Information Form that collected data about the patient's education was required for all studies except for CALGB 9130 and 9033. For these two studies, the requirement to submit such data was instituted during the latter months of patient accrual. The results of all 11 studies, as well as details about treatment regimens administered, have been previously reported.31-43 During the conduct of these clinical studies, the study chair reviewed the eligibility of the patients who were enrolled onto the study by participating institutions. The analyses contained in this article exclude those patients who were not originally eligible for the study and were excluded from the study's primary analysis.
Table 1.
CALGB Lung Cancer Studies (N = 11) From Which Patients Were Drawn
| Disease, Stage, and Study | Treatment Arms | Enrollment Dates | No. of Patients Enrolled | No. of Patients Eligible | Eligible Patients With Education Data
|
No. of Deaths Among Eligible Patients With Education Data | Date of Last Follow-Up* | |
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Total lung | 2,350 | 2,210 | 1,577 | 71 | 1,491 | |||
| NSCLC | 1,616 | 1,505 | 1,177 | 78 | 1,127 | |||
| Advanced | ||||||||
| 8931 | Cisplatin, etoposide, placebo | August 1989-February 1991 | 291 | 266 | 227 | 85 | 225 | June 2000 |
| Cisplatin, etoposide, hydrazine | ||||||||
| 9532 | Vinorelbine, ifosfamide | September 1995-July 1996 | 100 | 93 | 81 | 87 | 74 | October 2002 |
| Paclitaxel, ifosfamide | ||||||||
| 9730 | Paclitaxel | December 1997-January 2001 | 584 | 561 | 505 | 90 | 494 | September 2004 |
| Paclitaxel, carboplatin | ||||||||
| 9731 | Paclitaxel | September 1997-April 1998 | 39 | 38 | 38 | 100 | 35 | February 2002 |
| Stage III | ||||||||
| 8831 | Vinblastine, cisplatin → RT → cisplatin, vinblastine | August 1988-October 1989 | 91 | 85 | 73 | 86 | 67 | October 2000 |
| Vinblastine, cisplatin → RT, carboplatin | ||||||||
| 9130 | Vinblastine, cisplatin → RT | September 1991-November 1996 | 283 | 250 | 69 | 28†‡ | 64 | October 2001 |
| Vinblastine, cisplatin → RT, carboplatin | ||||||||
| 9431 | Cisplatin, gemcitabine → cisplatin, gemcitabine, RT | January 1996-June 1998 | 187 | 172 | 149 | 87 | 136 | February 2004 |
| Cisplatin, paclitaxel → cisplatin, paclitaxel, RT | ||||||||
| Cisplatin, vinorelbine → cisplatin, vinorelbine, RT | ||||||||
| 9534 | Paclitaxel, carboplatin → paclitaxel, carboplatin, RT | August 1996-January 1999 | 41 | 40 | 35 | 88 | 32 | July 2003 |
| SCLC | 734 | 705 | 400 | 57 | 364 | |||
| Limited | ||||||||
| 9235 | Etoposide, cisplatin → RT | August 1993-January 1999 | 319 | 307 | 247 | 80 | 213 | October 2005 |
| Etoposide, cisplatin → RT + carboplatin | ||||||||
| Extensive | ||||||||
| 9033 | Oral etoposide, IV cisplatin | February 1991-October 1993 | 319 | 306 | 74 | 24†‡ | 73 | May 1997 |
| IV etoposide, IV cisplatin | ||||||||
| 9430 | Cisplatin, topotecan, G-CSF | May 1995-June 1999 | 96 | 92 | 79 | 86 | 78 | February 2002 |
| Cisplatin, paclitaxel 230 mg/m2, G-CSF | ||||||||
| Paclitaxel 230 mg/m2, topotecan, G-CSF | ||||||||
| Paclitaxel 175 mg/m2, topotecan, G-CSF | ||||||||
Abbreviations: CALGB, Cancer and Leukemia Group B; NSCLC, non–small-cell lung cancer; RT, radiotherapy; SCLC, small-cell lung cancer; IV, intravenous; G-CSF, granulocyte colony-stimulating factor.
The latest recorded date of death or date of last follow-up used to compute survival time among patients included in analyses.
When CALGB 9033 and 9130 were originally activated, the submission of a Background Information Form to the CALGB Statistical Center was not a requirement.
Percent of eligible patients.
Power Calculations
A priori power calculations were generated to determine whether clinically meaningful effects would be detectable with available data if they existed. Cella et al7 reported that 31% of patients had only a grade school education and that their hazard ratio of death relative to patients with more than a grade school education was approximately 1.2. The current data set includes 1,577 lung cancer patients, of whom 1,491 are dead and 13.1% had only a grade school education (Tables 1 and 2). With a two-tailed log-rank test conducted at the P = .05 level of significance, a hazard ratio of 1.24 is detectable with 80% power in this sample.
Table 2.
Characteristics of Patients With Lung Cancer and Survival Rates
| Predictor | No. of Patients | % | No. of Patients Dead | Survival Time (months)
|
Hazard Ratio | 95% CI | P (χ2) | |
|---|---|---|---|---|---|---|---|---|
| Median | 95% CI | |||||||
| Sex | ||||||||
| Male | 1,047 | 66 | 1,001 | 10.5 | 9.9 to 11.1 | |||
| Female | 530 | 34 | 490 | 12.1 | 11.0 to 13.4 | 0.84 | 0.75 to 0.94 | .0024 |
| Race | ||||||||
| White | 1,331 | 84 | 1,252 | 11.1 | 10.6 to 11.8 | |||
| Black | 168 | 11 | 164 | 10.2 | 8.6 to 12.2 | 1.17 | 0.99 to 1.39 | |
| Hispanic | 40 | 3 | 40 | 9.2 | 6.8 to 13.3 | 1.13 | 0.82 to 1.56 | |
| Other | 38 | 2 | 35 | 9.4 | 7.4 to 14.6 | 1.09 | 0.78 to 1.54 | .2800 |
| Age, years* | ||||||||
| < 50 | 180 | 11 | 171 | 10.0 | 8.5 to 11.0 | |||
| 50-59 | 425 | 27 | 401 | 11.1 | 10.3 to 12.6 | 0.89 | 0.74 to 1.07 | |
| 60-69 | 619 | 39 | 585 | 11.8 | 11.1 to 12.8 | 0.83 | 0.70 to 0.99 | |
| 70+ | 353 | 22 | 334 | 9.5 | 8.4 to 10.9 | 0.93 | 0.77 to 1.12 | .1567 |
| Marital status | ||||||||
| Single | 101 | 6 | 100 | 9.5 | 8.1 to 10.7 | |||
| Married | 1,047 | 66 | 977 | 11.2 | 10.4 to 12.2 | 0.81 | 0.66 to 1.00 | |
| Separated | 39 | 2 | 38 | 12.1 | 8.0 to 14.0 | 0.94 | 0.64 to 1.37 | |
| Divorced | 225 | 14 | 220 | 10.6 | 9.7 to 11.6 | 0.95 | 0.75 to 1.21 | |
| Widowed | 165 | 10 | 156 | 11.7 | 9.5 to 13.5 | 0.81 | 0.62 to 1.04 | .1060 |
| PS | ||||||||
| 0 | 609 | 39 | 556 | 14.7 | 13.6 to 16.1 | |||
| 1 | 820 | 52 | 789 | 9.8 | 9.0 to 10.4 | 1.47 | 1.31 to 1.64 | |
| 2 | 148 | 9 | 146 | 6.1 | 3.9 to 7.5 | 2.52 | 2.06 to 3.08 | < .0001 |
| Chest pain | ||||||||
| No | 1,073 | 68 | 1,006 | 11.8 | 11.0 to 12.7 | |||
| Yes | 504 | 32 | 485 | 9.5 | 8.4 to 10.5 | 1.27 | 1.13 to 1.42 | < .0001 |
| Dyspnea | ||||||||
| No | 802 | 51 | 740 | 12.3 | 11.3 to 13.3 | |||
| Yes | 775 | 49 | 751 | 10.0 | 9.4 to 10.7 | 1.26 | 1.14 to 1.40 | < .0001 |
| Bone pain | ||||||||
| No | 1,325 | 84 | 1,244 | 11.6 | 10.9 to 12.3 | |||
| Yes | 252 | 16 | 247 | 7.6 | 6.7 to 9.2 | 1.28 | 1.11 to 1.48 | .0006 |
| CNS symptoms | ||||||||
| No | 1,513 | 96 | 1,431 | 11.0 | 10.5 to 11.7 | |||
| Yes | 64 | 4 | 60 | 8.5 | 6.0 to 10.9 | 1.24 | 0.95 to 1.62 | .1123 |
| Duration of symptoms, months | ||||||||
| < 3 | 1,050 | 67 | 993 | 10.9 | 10.3 to 11.6 | |||
| 3-6 | 361 | 23 | 342 | 10.7 | 9.0 to 11.8 | 1.00 | 0.88 to 1.13 | |
| > 6 | 166 | 11 | 156 | 11.8 | 10.3 to 13.9 | 0.93 | 0.78 to 1.10 | .6938 |
| Weight loss, % | ||||||||
| None to < 5 | 1,169 | 74 | 1,095 | 12.6 | 11.7 to 13.5 | |||
| ≥ 5 | 408 | 26 | 396 | 7.4 | 6.5 to 7.8 | 1.55 | 1.37 to 1.75 | < .0001 |
| Liver metastases | ||||||||
| No | 1,353 | 86 | 1,271 | 11.8 | 11.1 to 12.7 | |||
| Yes | 224 | 14 | 220 | 6.4 | 5.8 to 7.4 | 1.49 | 1.27 to 1.75 | < .0001 |
| Adrenal metastases | ||||||||
| No | 1,418 | 90 | 1,335 | 11.5 | 11.0 to 12.2 | |||
| Yes | 159 | 10 | 156 | 6.4 | 5.7 to 7.6 | 1.29 | 1.08 to 1.55 | .0055 |
| Bone metastases | ||||||||
| No | 1,285 | 81 | 1,202 | 12.2 | 11.4 to 12.9 | |||
| Yes | 292 | 19 | 289 | 7.4 | 6.2 to 8.0 | 1.34 | 1.16 to 1.54 | < .0001 |
| Brain | ||||||||
| No | 1,555 | 99 | 1,470 | 11.0 | 10.5 to 11.6 | |||
| Yes | 22 | 1 | 21 | 6.5 | 3.2 to 13.6 | 1.24 | 0.78 to 1.98 | .3644 |
| WBC ≥ 8.7 × 103 | ||||||||
| No | 768 | 49 | 718 | 12.2 | 11.4 to 13.6 | |||
| Yes | 809 | 51 | 773 | 9.9 | 9.0 to 10.6 | 1.20 | 1.08 to 1.33 | .0005 |
| Hemoglobin ≥ 14.6 g/dL | ||||||||
| No | 1,271 | 81 | 1,206 | 10.4 | 9.8 to 11.0 | |||
| Yes | 306 | 19 | 285 | 13.4 | 12.2 to 14.6 | 0.80 | 0.70 to 0.92 | .0014 |
| Platelets ≥ 400,000 | ||||||||
| No | 1,138 | 72 | 1,072 | 12.2 | 11.5 to 13.3 | |||
| Yes | 437 | 28 | 417 | 8.5 | 7.8 to 9.3 | 1.34 | 1.19 to 1.51 | < .0001 |
| Alkaline phosphatase ≥ 100 U/mL | ||||||||
| No | 853 | 54 | 798 | 12.6 | 11.6 to 13.6 | |||
| Yes | 721 | 46 | 690 | 9.1 | 8.5 to 10.2 | 1.24 | 1.11 to 1.38 | < .0001 |
| Education | ||||||||
| Grades 1-8 | 206 | 13 | 196 | 11.3 | 9.7 to 13.9 | |||
| Grades 9-11 | 284 | 18 | 273 | 11.0 | 9.8 to 12.9 | 1.04 | 0.86 to 1.26 | |
| High school graduate | 524 | 33 | 496 | 10.5 | 9.5 to 11.4 | 1.06 | 0.90 to 1.26 | |
| Some college | 355 | 23 | 333 | 11.3 | 10.3 to 12.6 | 0.94 | 0.78 to 1.12 | |
| College degree | 208 | 13 | 193 | 11.1 | 10.0 to 13.1 | 1.00 | 0.81 to 1.23 | .5141 |
Abbreviation: PS, performance status.
Age at enrollment onto clinical trial.
Analytic Methods
Most patients on the studies considered in this project had a Background Information Form submitted. To guard against possible bias, the characteristics of patients (age, sex, race, performance status, and weight loss) who had a Background Information Form with education data were compared with the characteristics of all other patients accrued to these studies, including patients without a Background Information Form and patients with a Background Information Form lacking education data, using Cochran-Mantel-Haenszel and Fisher's exact χ2 tests and t tests.44,45
Survival time was defined as the period between the date of study enrollment and the date of death. For those patients who were censored in analyses, survival time was defined as the time between study entry and the date of last contact. The Kaplan-Meier product-limit estimator was used to describe the survival experience within patient subgroups defined by various prognostic factors.46 Median survival estimates were generated using the estimator of Brookmeyer and Crowley.47 The Cox proportional hazards model stratified by treatment arm/study was used to assess the relationship between survival and known prognostic factors.48 Known clinical and sociodemographic predictors that were considered in analyses are listed in Table 2. If the relationship between an individual factor and survival was statistically significant in univariate analyses at the P = .25 level of significance, that factor was included in subsequent multivariable analysis using backwards elimination.49 Interactions were also considered in analyses, including factor × histology (NSCLC or SCLC), factor × stage (advanced/extensive v other), and factor × histology × stage. Martingale and Schoenfeld residuals were used to assess the adequacy of the proportional hazards assumption.50 Once a final multivariable clinical model was determined, factors describing the effect of education and interactions between education, histology, stage, and the model's strata were added to the Cox model. The Cox analysis described earlier was stratified by treatment arm/study to allow different nonproportional hazard rates for the various combinations of studies and treatment arms.
RESULTS
The analyses described in this report are based on the experiences of 1,577 lung cancer patients (Table 1). This patient cohort constitutes 67% of the patients originally accrued to the 11 lung cancer studies considered. A comparison of the characteristics of the 1,577 patients included in these analyses and the 633 other eligible patients accrued to these 11 studies showed no significant difference relative to age, sex, race, performance status, and weight loss.
Among the 1,577 patients are those with advanced NSCLC (851), inoperable stage III NSCLC (326), extensive SCLC (153), and limited SCLC (247). The majority of patients were male (66%), white (84%), over 59 years of age (61%), and had performance status (PS) = 1 or PS = 2 (62%). Additional characteristics of this patient population are provided in Table 2.
Table 3 summarizes the relationship between grade school education and other patient characteristics. Specifically, the table shows that patients with less education are more likely to be male, nonwhite, older, had a PS of 1 to 2, or presented with chest pain.
Table 3.
Relationship Between Education and Selected Prognostic Factors
| Predictor | Patients With Grade 1-8 Education
|
Patients With Grade 9-11 Education
|
Patients Who Graduated High School
|
Patients With Some College Education
|
Patients Who Graduated College
|
Total Patients
|
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Sex | .0002 | ||||||||||||
| Male | 156 | 15 | 191 | 18 | 326 | 31 | 219 | 21 | 155 | 15 | 1,047 | 66 | |
| Female | 50 | 9 | 93 | 18 | 198 | 37 | 136 | 26 | 53 | 10 | 530 | 34 | |
| Race | < .0001 | ||||||||||||
| White | 151 | 11 | 226 | 17 | 469 | 35 | 304 | 23 | 181 | 14 | 1,331 | 84 | |
| Black | 29 | 17 | 47 | 28 | 40 | 24 | 38 | 23 | 14 | 8 | 168 | 11 | |
| Hispanic | 17 | 43 | 5 | 13 | 9 | 23 | 4 | 10 | 5 | 13 | 40 | 3 | |
| Other | 9 | 24 | 6 | 16 | 6 | 16 | 9 | 24 | 8 | 21 | 38 | 2 | |
| Age group, years | .0048 | ||||||||||||
| < 50 | 7 | 4 | 35 | 19 | 66 | 37 | 54 | 30 | 18 | 10 | 180 | 11 | |
| 50-59 | 54 | 13 | 80 | 19 | 144 | 34 | 95 | 22 | 52 | 12 | 425 | 27 | |
| 60-69 | 83 | 13 | 110 | 18 | 207 | 33 | 136 | 22 | 83 | 13 | 619 | 39 | |
| > 69 | 62 | 18 | 59 | 17 | 107 | 30 | 70 | 20 | 55 | 16 | 353 | 22 | |
| Performance status | .0080 | ||||||||||||
| 0 | 62 | 10 | 106 | 17 | 196 | 32 | 144 | 24 | 101 | 17 | 609 | 39 | |
| 1 | 128 | 16 | 144 | 18 | 277 | 34 | 178 | 22 | 93 | 11 | 820 | 52 | |
| 2 | 16 | 11 | 34 | 23 | 51 | 34 | 33 | 22 | 14 | 9 | 148 | 9 | |
| Chest pain | .0097 | ||||||||||||
| No | 123 | 11 | 190 | 18 | 354 | 33 | 248 | 23 | 158 | 15 | 1,073 | 68 | |
| Yes | 83 | 16 | 94 | 19 | 170 | 34 | 107 | 21 | 50 | 10 | 504 | 32 | |
Table 2 also provides information about the relationship of each individual baseline patient characteristic to survival. These analyses show that the following individual factors to be significant predictors of better survival: Female sex, PS = 0, lack of chest pain or dyspnea at diagnosis, low weight loss, lack of liver, adrenal, or bone metastases at diagnosis, WBC less than 8.7 × 103, HGB less than 14.6 g/dL, platelets less than 400,000, and alkaline phosphatase level less than 100 U/mL.
All variables statistically significant at the 0.25 level of significance in univariate analyses presented in Table 2 were considered as candidate variables in a multivariable analysis. Table 4 summarizes the resulting multivariable model that includes the following significant predictors: sex, PS, married, presence of dyspnea, weight loss, presence of liver metastases, presence of bone metastases, hemoglobin levels, and platelet levels. Statistically significant interactions were also included in the final model; they showed that the presence of adrenal metastases and levels of alkaline phosphatase levels were important prognostically among patients with NSCLC, and that WBC level was an important predictor among patients with advanced or extensive disease. A residual analysis confirmed the adequacy of the proportional hazards assumption.
Table 4.
Multivariable Cox Model Predictive of Survival As a Function of Clinical Predictors
| Variable | df | Parameter Estimate | SE | χ2 | P (χ2) | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|---|
| Female | 1 | −0.17131 | 0.06073 | 7.9579 | .0048 | 0.843 | 0.748 to 0.949 |
| PS: 1 v 0 | 1 | 0.28039 | 0.05973 | 22.0387 | < .0001 | 1.324 | 1.177 to 1.488 |
| PS: 2 v 0 | 1 | 0.66433 | 0.10804 | 37.8117 | < .0001 | 1.943 | 1.572 to 2.401 |
| Married | 1 | −0.12290 | 0.05821 | 4.4578 | .0347 | 0.884 | 0.789 to 0.991 |
| Dyspnea | 1 | 0.16148 | 0.05522 | 8.5526 | .0035 | 1.175 | 1.055 to 1.310 |
| Weight loss | 1 | 0.18400 | 0.06575 | 7.8308 | .0051 | 1.202 | 1.057 to 1.367 |
| Liver metastases | 1 | 0.27325 | 0.08402 | 10.5765 | .0011 | 1.314 | 1.115 to 1.549 |
| Bone metastases | 1 | 0.30869 | 0.07287 | 17.9454 | < .0001 | 1.362 | 1.180 to 1.571 |
| Hemoglobin ≥ 14.6 g/dL | 1 | −0.15781 | 0.07118 | 4.9157 | .0266 | 0.854 | 0.743 to 0.982 |
| Platelets ≥ 400,000 | 1 | 0.13783 | 0.06214 | 4.9196 | .0266 | 1.148 | 1.016 to 1.296 |
| Adrenal metastases among NSCLC | 1 | 0.36004 | 0.09861 | 13.3302 | .0003 | 1.433 | 1.181 to 1.739 |
| Alkaline phosphatase > 100 U/mL among NSCLC | 1 | 0.17243 | 0.06385 | 7.2932 | .0069 | 1.188 | 1.048 to 1.347 |
| WBC ≥ 8.7 × 103 among extensive/advanced patients | 1 | 0.19829 | 0.06865 | 8.3430 | .0039 | 1.219 | 1.066 to 1.395 |
Abbreviations: PS, performance status; NSCLC, non–small-cell lung cancer.
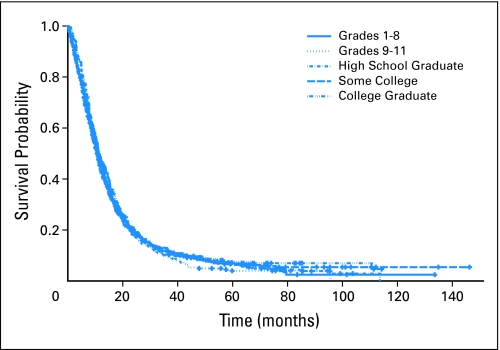
The relationship between education and survival without adjustment for known prognostic factors was not statistically significant (Fig 1; Table 2). Two descriptors of low education were added to the multivariable clinical model presented in Table 4 to determine whether low education provided any prognostic information beyond that provided by known prognostic factors. The resulting likelihood ratio test showed that neither an education less than 8 years nor an education less than high school, were significant predictors of poor survival.
Fig 1.
Survival stratified by educational level attained by patient.
DISCUSSION
Statistical analyses reported in this article showed that among lung cancer patients enrolled onto CALGB trials education level was not predictive of survival. Clinical trials are characterized by patient populations that are relatively homogeneous clinically at study entry due to strict eligibility criteria, and by standardized treatment plans. These results substantiated the a priori hypothesis that during “active” protocol treatment and during the lifetime of protocol follow-up among patients with poor prognosis, social conditions as measured by education level do not have an impact on survival. Patients with low education, defined as less than an eighth grade education, enroll onto CALGB clinical studies with poorer prognostic factors, as measured by low ECOG performance status, old age, and presence of chest pain (Table 3). However, after adjustment for the patient's baseline clinical status, education or social condition did not have an impact on survival after clinical trial enrollment, given that all study participants, regardless of education, were carefully and intensively followed until treatment failure and death. Two recent CALGB publications authored by Blackstock51,52 studied one aspect of the patient's social condition—race—in a population of patients with advanced NSCLC and extensive SCLC, respectively, and found that social conditions which lead to African Americans presenting with poorer prognostic factors, did not have an impact on survival after enrollment onto the clinical trial.
Dale18 has developed the minimal requirements for a well-designed study examining race-cancer mortality issues that is easily adapted to studies examining the relationship between SES and cancer survival: (1) measures of SES should be on the individual level and not be estimated from census data, (2) SES should include at least (individual level) measures of income and education, (3) sample sizes should be adequate for the relevant population to make scientifically and statistically sound inferences, and (4) specific cancer sites should be studied separately.
The criteria adapted from Dale18 suggest that the study described in this article is well-designed to investigate the relationship between SES and cancer survival in that SES, as measured by education, is available on the individual patient level, the sample size is large enough to assure statistically sound inferences, and a relatively homogeneous population (ie, one cancer site) has been studied. The inclusion of patient-reported income would have strengthened the study; however, such data was purposely not collected as previous pilot work had indicated that a large percentage of patients would not provide such data.30
The results reported in this article are not consistent with that reported by Cella et al.7 In contrast to the relatively homogeneous population considered in this study, Cella considered a heterogeneous cancer patient population that had varying prognoses or expected survival times. Some of Cella's patient population had relatively poor prognoses such as the patients included in the current article; however, others had relatively better prognoses. It is hypothesized that patients who have a prognosis that is much longer than the treatment regimen and the initial period of intensive clinical trial follow-up will ultimately be followed by a period of less intensive monitoring during which a patient's poorer social condition (or education) will negatively influence patient health and survival. The inclusion of these patients with better prognoses in Cella's analyses could have influenced the overall results of his analyses.
The current study did not include patients with early stage NSCLC as such patients were not represented in the database used. A natural question to ask is whether results found in this study would be applicable to such patients if they had participated in CALGB clinical trials. The prognosis of patients with early stage NSCLC is much longer than the treatment regimen and the initial period of intensive clinical trial follow-up. As discussed above, it is hypothesized that patients with such a prognosis will ultimately be in a position where they are not monitored or followed as intensively, and a patient's poorer social condition (or education) will negatively influence patient health and survival. The research of Ou et al19 and Sowah et al20 are supportive of this hypothesis in that they found patients with early stage NSCLC who were of low SES to have higher mortality. However, these inferences were based on analyses that involved composite estimates of income derived from census data, and not patient-reported data. In addition, these analyses were also not generated within the context of a clinical trial. Additional research needs to be conducted to assess the relationship between SES/education and survival among patients with early stage NSCLC enrolled onto clinical trials.
The patients studied in this article have all participated in a clinical trial, and may not be completely representative of the greater lung cancer patient population. Hence the study conclusion that education, as a proxy for SES, does not affect the survival of patients with SCLC or stage III/IV NSCLC is limited to participants of clinical trials. Standardization of the treatment in clinical trials insures that all patients are given the same treatment, irrespective of their educational status, as well as probably other SES factors. Additional research is needed to determine if it is appropriate to extrapolate study inferences to patients who do not participate in clinical trials.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: James E. Herndon II, Alice B. Kornblith, Jimmie C. Holland, Electra D. Paskett
Collection and assembly of data: James E. Herndon II
Data analysis and interpretation: James E. Herndon II, Alice B. Kornblith, Electra D. Paskett
Manuscript writing: James E. Herndon II, Alice B. Kornblith, Electra D. Paskett
Final approval of manuscript: James E. Herndon II, Alice B. Kornblith, Jimmie C. Holland, Electra D. Paskett
Appendix
The research for studies 8931 (Dr Michael Kosty), 9532 (Dr Michael C. Perry), 9730 (Dr Rogerio C. Lilenbaum), 9731 (Dr Wallace L Akerley), 8831 (Gerald H. Clamon), 9130 (Dr Gerald H. Clamon), 9431 (Dr Everett E. Vokes), 9534 (Dr Wallace L. Akerley), 9235 (Dr Edward F. Mcclay), 9033 (Dr Antonius A. Miller), and 9430 (Dr Thomas J. Lynch) was supported, in part, by grants from the National Cancer Institute to the Cancer and Leukemia Group B (Grant No. CA31946; Richard L. Schilsky, MD, Chairman) and to the Cancer and Leukemia Group B Statistical Center (Grant No. CA33601; Stephen George, PhD). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions and investigators participated in this study: Medical University of South Carolina, Charleston, SC–Mark Green, MD, supported by CA03927; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA02599; University of Massachusetts Medical School, Worcester, MA–William V. Walsh, MD, supported by CA37135; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C. Perry, MD, supported by CA12046; Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, MD, supported by CA32291; Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Georgetown University Medical Center, Washington, DC–Minetta C. Liu, MD, supported by CA77597; Green Mountain Oncology Group Community Clinical Oncology Program (CCOP), Bennington, VT–L. Herbert Maurer, MD, supported by CA35091; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; Illinois Oncology Research Association, Peoria, IL–John W. Kugler, MD, supported by CA35113; Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, MD, supported by CA12449; Missouri Baptist Medical Center, St Louis, MO–Alan P. Lyss, MD, supported by CA114558-02; Mount Sinai Medical Center, Miami, FL–Rogerio Lilenbaum, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, MD, supported by CA04457; Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John A. Ellerton, MD, supported by CA35421; Southeast Cancer Control Consortium Inc CCOP, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by CA21060; University of California at San Diego, San Diego, CA–Joanne Mortimer, MD, supported by CA11789; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of California at San Francisco, San Francisco, CA–Alan P. Venook, MD, supported by CA60138; University of Chicago, Chicago, IL–Gini Fleming, MD, supported by CA41287; University of Illinois Minority-Based CCOP (MBCCOP), Chicago, IL–Lawrence E. Feldman, MD, supported by CA74811; University of Iowa, Iowa City, IA–Gerald Clamon, MD, supported by CA47642; University of Minnesota, Minneapolis, MN–Bruce A. Peterson, MD, supported by CA16450; University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, MD, supported by CA47559; University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, MD, supported by CA47555; Vermont Cancer Center, Burlington, VT–Hyman B. Muss, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC–Thomas Reid, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO–Nancy Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY–Scott Wadler, MD, supported by CA07968; Long Island Jewish Medical Center, Lake Success, NY–Marc Citron, MD, supported by CA11028; North Shore-Long Island Jewish Medical Center, Manhasset, NY–Daniel R. Budman, MD, supported by CA35279; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, MD, supported by CA31983; University of Alabama Birmingham, Birmingham, AL–Robert Diasio, MD, supported by CA47545; McGill University, Montreal, Quebec, Canada–Gerald Batist, MD; Eastern Cooperative Oncology Group, Philadelphia, PA–Robert L. Comis, MD, Chairman; supported by CA23318.
Supported by Grants No. CA32291, CA77651, and CA77658 from the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.House JS, Landis KR, Umberson D: Social relationships and health. Science 241:540-545, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Marmot M: Social determinants of health equalities. Lancet 365:1099-1104, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lynch JW, Smith GD, Kaplan GA, et al: Income inequality and mortality: Importance to health of individual income, psychosocial environment, or material conditions. BMJ 320:1200-1204, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmot M, Ryff CD, Bumpass LL, et al: Social inequalities in health: Next questions and converging evidence. Soc Sci Med 44:901-910, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Marmot M, Wilkinson RG: Psychosocial and material pathways in the relations between income and health: A response to Lynch et al. BMJ 322:1233-1236, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marmot M: The influence of income on health: Views of an epidemiologist. Health Aff 21:31-46, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Cella DF, Orav EJ, Kornblith AB, et al: Socioeconomic status and cancer survival. J Clin Oncol 9:1500-1509, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Tammemagi CM, Neslund-Dudas C, Simoff M, et al: In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol 57:597-609, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Yabroff KR, Gordis L: Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case fatality, and mortality? Soc Sci Med 57:2265-2279, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cross CK, Harris J, Recht A: Race, socioeconomic status, and breast carcinoma in the U.S.: What have we learned from clinical studies? Cancer 95:1988-1999, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gorey KM, Kliewer E, Holowaty EJ, et al: An international comparison of breast cancer survival: Winnipeg, Manitoba and Des Moines, Iowa metropolitan areas. Ann Epidemiol 13:32-41, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariotto A, Capocaccia R, Verdecchia A, et al: Projecting SEER cancer survival rates to the US: An ecological regression approach. Cancer Causes Control 13:101-111, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Coleman MP, Babb P, Sloggett A, et al: Socioeconomic inequalities in cancer survival in England and Wales. Cancer 91:208-216, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Vigano A, Bruera E, Jhangri GS, et al: Clinical survival predictors in patients with advanced cancer. Arch Intern Med 160:861-868, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Mackillop WJ, Zhang-Salomons J, Groome PA, et al: Socioeconomic status and cancer survival in Ontario. J Clin Oncol 15:1680-1689, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Goodwin JS, Samet JM, Hunt WC: Determinants of survival in older cancer patients. J Natl Cancer Inst 88:1031-1038, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Stavraky KM, Skillings JR, Stitt LW, et al: The effect of socioeconomic status on the long-term outcome of cancer. J Clin Epidemiol 49:1155-1160, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Dale W, Vijayakumar S, Lawlor EF, et al: Prostate cancer, race, and socioeconomic status: Inadequate adjustment for social factors in assessing racial differences. Prostate 29:271-281, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Ou SHI, Zell JA, Ziogas A, et al: Prognostic factors for survival of stage I nonsmall cell lung cancer patients: A population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 110:1532-1541, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Sowah LA, Brahmer JR, Alberg AJ, et al: Census tract-level socioeconomic status as a determinant of survival in non-small cell lung cancer. Lung Cancer 46:S61-S62, 2004. (suppl) [Google Scholar]
- 21.Greenwald HP, Borgatta EF, McCorkle R, et al: Explaining reduced cancer survival among the disadvantaged. Milbank Q 72:215-238, 1996 [PubMed] [Google Scholar]
- 22.Bouchardy C, Fioretta G, DePerrot M, et al: Determinants of long term survival. Cancer 86:2229-2237, 1999 [PubMed] [Google Scholar]
- 23.Robbins AS, Yin D, Parikh-Patel A: Differences in prognostic factors and survival among white men and black men with prostate cancer, California, 1995-2004. Am J Epidemiol 166:71-78, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ortiz CA, Goodwin JS, Freeman JL: The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit 11:RA163-RA172, 2005 [PubMed] [Google Scholar]
- 25.Lehto US, Ojanen M, Dyba T, et al: Baseline psychosocial predictors of survival in localized breast cancer. Br J Cancer 94:1245-1252, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dejardin O, Remontet L, Bouvier AM, et al: Socioeconomic and geographic determinants of survival of patients with digestive cancer in France. Br J Cancer 95:944-949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Jawde RM, Baz R, Walker E, et al: The role of race, socioeconomic status, and distance traveled on the outcome of African-American patients with multiple myeloma. Haematologica 91:1410-1413, 2006 [PubMed] [Google Scholar]
- 28.Coker AL, Du XL, Fang S: Socioeconomic status and cervical cancer survival among older women: Findings from the SEER-Medicare linked data cohorts. Gynecol Oncol 102:278-284, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kaffashian F, Godward S, Davies T, et al: Socioeconomic effects on breast cancer survival: Proportion attributable to stage and morphology. Br J Cancer 89:1693-1696, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland JC, Herndon J, Kornblith AB, et al: A sociodemographic data collection model for cooperative clinical trials. Proc Am Soc Clin Oncol 11:157, 1992. (abstr 445) [Google Scholar]
- 31.McClay EF, Bogart J, Herndon JE II, et al: A phase II trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B study (9235). J Clin Oncol 28:81-90, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Clamon G, Herndon J, Eaton W, et al: A feasibility study of extended chemotherapy for locally advanced non-small cell lung cancer: A phase II study of Cancer and Leukemia Group B. Cancer Invest 12:273-282, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Kosty MP, Fleishman S, Herndon JE, et al: Cisplatin, vinblastine, and hydrazine sulfate in advanced, non-small cell lung cancer: A randomized placebo-controlled, double-blind phase III study of the Cancer and Leukemia Group B. J Clin Oncol 12:1113-1120, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Miller A, Herndon JE, Hollis DR, et al: Schedule dependency of 21-day oral versus 3-day intravenous etoposide in combination with intravenous cisplatin in extensive stage small-cell lung cancer: A randomized phase III study of the Cancer and Leukemia Group B (CALGB 9033). J Clin Oncol 13:1871-1879, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Clamon G, Herndon J, Cooper R, et al: Radiosensitization with carboplatin for patients with unresectable stage 3 non-small cell lung cancer: A phase III trial of Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol 17:4-11, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Herndon JE II, Fleishman S, Kornblith AB, et al: Is quality of life predictive of survival among patients with advanced non-small cell lung cancer? Cancer 85:333-340, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Perry MC, Ihde DC, Herndon JE II, et al: Paclitaxel/ifosfamide or navelbine/ifosfamide chemotherapy for advanced non-small cell lung cancer: CALGB 9532. Lung Cancer 28:63-68, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Naughton MF, Shumaker SA, Herndon J, et al: Health-related quality of life in small-cell lung cancer patients: Quality of life companion to CALGB 9033. Qual Life Res 11:235-248, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Lyss AP, Herndon J, Lynch TJ, et al: Novel doublets in extensive small cell lung cancer: A randomized phase II study of topotecan/cisplatin, paclitaxel/cisplatin, and paclitaxel/topotecan. Clin Lung Cancer 3:205-210, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Akerley W, Herndon JE, Lyss AP, et al: Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small cell lung cancer: A limited-access study—CALGB 9534. Clin Lung Cancer 7:47-53, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Vokes EE, Herndon JE II, Crawford J, et al: Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: Cancer and Leukemia Group B Study 9431. J Clin Oncol 20:4191-4198, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Akerley W, Herndon JE, Egorin MJ, et al: Weekly, high-dose paclitaxel in advanced lung carcinoma. Cancer 97:2480-2486, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Lilenbaum RC, Herndon JE II, List MA, et al: Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (study 9730). J Clin Oncol 23:190-196, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Agresti A: Categorical Data Analysis. New York, NY, Wiley, 1990
- 45.Motulsky H: Intuitive Biostatistics. New York, NY, Oxford University Press, 1995
- 46.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 47.Brookmeyer R, Crowley J: A confidence interval for the median survival time. Biometrics 38:29-41, 1982 [Google Scholar]
- 48.Cox DR: Regression models and life tables (with discussion). J R Stat Soc B 34:187-220, 1972 [Google Scholar]
- 49.Hosmer DW, Lemeshow S: Applied Logistic Regression. New York, NY, John Wiley & Sons, 1991
- 50.Hosmer DW, Lemeshow S: Applied Survival Analysis. New York, NY, John Wiley & Sons, 1999
- 51.Blackstock AW, Herndon JE II, Paskett ED, et al: Outcomes among African-American/non-African-American patients with advanced non-small-cell carcinoma: Report from the Cancer and Leukemia Group B. J Natl Cancer Inst 94:284-290, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Blackstock AW, Herndon JE II, Paskett ED, et al: Similar outcomes between African American and non-African American patients with extensive-stage small-cell lung carcinoma: Report from the Cancer and Leukemia Group B. J Clin Oncol 24:407-412, 2006 [DOI] [PubMed] [Google Scholar]