Abstract
Purpose
The combination of doxorubicin and cyclophosphamide (AC) is a standard adjuvant regimen. Doxorubicin and docetaxel (AT) is one of the most active cytotoxic regimens for metastatic breast cancer. The purpose of this trial was to determine whether adjuvant AT improved disease-free survival compared with AC in operable breast cancer.
Patients and Methods
Women with invasive breast cancer were eligible if there were one to three positive lymph nodes or if the node-negative tumor was greater than 1 cm. Patients were randomly assigned after surgery to receive doxorubicin (60 mg/m2) plus either cyclophosphamide (600 mg/m2; AC) or docetaxel (60 mg/m2; AT) given every 3 weeks for four cycles, followed by hormone therapy for patients with estrogen receptor (ER) and/or progesterone receptor (PR)–positive tumors.
Results
There were 2,882 eligible patients enrolled. After a median follow-up of 79.5 months, there was no significant difference in disease-free survival (DFS; 85% in both arms) or overall survival (91% v 92%) at 5 years. The hazard ratio for AC versus AT was 1.02 (95% CI for DFS, 0.86 to 1.22; P = .78). In an exploratory analysis of prespecified stratification factors by ER and PR expression there were trends toward improved DFS for AT in ER/PR-negative disease. Grade 3 neutropenia associated with fever or infection occurred more often with AT (26% v 10%; P < .05).
Conclusion
AT did not improve DFS or overall survival in this population, and was associated with more toxicity.
INTRODUCTION
The combination of doxorubicin (adriamycin) and cyclophosphamide (AC) has been a standard adjuvant breast cancer regimen.1 Taxanes have become part of the mainstay of the treatment of advanced breast cancer for the past 15 years.2-6 Given their single-agent activity, relative noncrossresistance, partially nonoverlapping toxicities, and different mechanisms of action, there was clear rationale for combining the taxanes with doxorubicin.
The combination of anthracyclines plus either paclitaxel or docetaxel were reported to have high response rates exceeding 50% or more in advanced breast cancer in multiple phase II trials.6-9 The high level of activity was confirmed in two sequentially performed phase II trials performed by the Eastern Cooperative Oncology Group (ECOG) that evaluated the doxorubicin and paclitaxel combination (E4195) and doxorubicin and docetaxel combination (E1196), which were associated with responses rates of 52% and 57%, respectively.7,10 The superior efficacy of the combination was also confirmed in a phase III ECOG trial (E1193) that compared single-agent doxorubicin, single-agent paclitaxel, and the doxorubicin and paclitaxel combination in patients with metastatic disease, although the improvement in objective response for the combination (46%) compared with each single agent (33% to 34%) was not as impressive as in the phase II trials.6 There appeared to be a higher risk of cardiac toxicity associated with doxorubicin and paclitaxel but not docetaxel which was attributed to sequence dependent alteration of doxorubicin pharmacokinetics by paclitaxel that was not observed with docetaxel.11
At the time that this trial was developed, there was no evidence indicating that adjuvant taxane therapy was effective. Soon after the trial was activated, the sequential use of four courses of paclitaxel after four courses of AC was shown to be associated with improved disease-free survival, a finding that was subsequently confirmed in other trials.12-16 The purpose of E2197 was to determine whether a short course of four courses of adjuvant chemotherapy, using concurrent rather than sequential treatment strategy, might also be more effective than standard AC chemotherapy.
PATIENTS AND METHODS
Study Design
E2197 included women with operable, histologically confirmed adenocarcinoma of the breast with histologically involved lymph nodes (one to three) or if lymph node negative, tumor that was greater than 1.0 cm.
Patients were enrolled within 84 days of complete surgical excision of the primary tumor (lumpectomy or mastectomy) and an axillary dissection (with at least six nodes removed), or a sentinel node biopsy alone (if the sentinel node was negative). Patients with T4 or N2-3 were not eligible. Patients were required to have adequate hematologic, hepatic, cardiac, and renal function (neutrophil count ≥ 1,500/mm3, platelet count ≥ 100,000, normal left ventricular ejection fraction (LVEF) ≥ 50%, and total bilirubin ≤ upper limit of normal) ≤ 8 weeks before random assignment. Patients must have been disease free of prior invasive malignancies for ≥ 5 years with the exception of curatively treated basal cell or squamous cell carcinoma of the skin or carcinoma in situ of the cervix. No prior chemotherapy or radiation therapy was allowed. Patients who received radiation to the breast for ductal carcinoma in situ were eligible. Patients may have received tamoxifen for chemoprevention or up to 4 weeks of tamoxifen for this malignancy, but were required to discontinue its use before enrollment. All subjects were required to sign an institutional review board–approved informed consent before being enrolled on this study.
After stratification for nodal status (positive, negative); menopausal status (premenopausal, postmenopausal); and ER/PR status (ER/PR unknown, ER+/PR+, ER+/PR−, ER−/PR+, ER−/PR−) patients were randomly assigned to arm A or B. Arm A consisted of AT (doxorubicin 60 mg/m2 intravenously [IV], docetaxel 60 mg/m2 IV over 1 hour infusion with ciprofloxacin 500 mg twice per day starting days 8 to17 and decadron 8 mg orally twice per day beginning 1 day before treatment with docetaxel and continued for 2 additional days). Arm B consisted of AC (doxorubicin 60 mg/m2 IV, cyclophosphamide 600 mg/m2 IV with ciprofloxacin given at physician's discretion). Treatments were assigned using permuted blocks within strata with dynamic balancing within main institutions and their affiliate networks. Both treatments were given every 3 weeks for four cycles unless tumor recurred, toxicity was excessive, or the patient withdrew consent. Patients with febrile neutropenia were to be placed on granulocyte colony-stimulating factor (G-CSF) according to American Society of Clinical Oncology guidelines of the time, but primary prophylaxis with G-CSF was not used.17 Patients with continuing neutropenia after a subsequent dose of chemotherapy despite G-CSF or who had grade 3 to 4 toxicity, had the chemotherapy dose reduced by 25%. Postoperative irradiation was given at the completion of all chemotherapy for all patients treated with breast conservation and in selected high-risk patients after mastectomy at the discretion of the treating physician. Patients with tumors classified as ER+ and/or PR+ were to receive tamoxifen 20 mg orally daily for 5 years after chemotherapy. In June 2005, the protocol was modified to permit switching from tamoxifen to an aromatase inhibitor (AI) before completing 5 years of tamoxifen or to initiate an AI after completing a course of tamoxifen in postmenopausal women.
Patients were seen before each course of chemotherapy for physical and hematologic evaluations. After chemotherapy ended, mammography and hematologic exams were performed annually and patients were seen for a history and physical every 3 months for the first 2 years from study entry, every 6 months for the next 3 years, and annually thereafter.
Statistical Considerations
The primary end point was disease-free survival (DFS), defined as the time from date of random assignment to date of invasive breast cancer recurrence, invasive contralateral breast cancer, or death from any cause, whichever occurred first. Patients with incomplete follow-up or, without documented DFS event (including those who developed in situ contralateral breast cancer or a nonbreast second primary cancer), were censored at the date last known to be alive. Overall survival (OS) was defined as time from date of random assignment to death from any cause.
The trial was designed to detect a 25% reduction in the failure hazard rate, and assumed a 78% 5-year DFS for the AC arm (based on data from E1180 and E5188).18,19 Assuming 1,000 eligible patients enrolled per year for 2.5 years with an additional 3 years of follow-up, 2,500 eligible patients provided 83% power to detect this difference using a two-sided .05 level log-rank test.
Full information corresponded to 420 DFS failures among the eligible patients who began protocol treatment. O'Brien-Fleming boundaries were used at interim analyses to monitor for early stopping.20 The ECOG Data Monitoring Committee (DMC) reviewed safety and outcome (when prespecified) data twice per year. Two prespecified analyses of outcome data were reviewed by the ECOG DMC in September 2001 and April 2003. Study continuation was recommended after both meetings.
In May and October 1999, the ECOG DMC reviewed pre- and postchemotherapy LVEF data. No significant differences were found between the two arms with respect to percentage of patients with a drop in LVEF. At the time of those analyses, two cases of congestive heart failure (CHF) had been reported.
The primary analysis of outcome was an intent-to-treat analysis among patients classified as eligible. All reported P values were two sided. The Kruskal-Wallis test for ordered data was used to compare maximum toxicity grade between treatment groups.21 The Kaplan-Meier method22 was used to estimate distributions for DFS and OS and the log-rank test23 was used to assess differences between these distributions with respect to treatment. Cox proportional hazards models were used to estimate the effect of treatment alone, effect of treatment after adjustment for baseline covariates, and to test for interactions between prognostic factors and treatment.24 The Wald test was used to test for significant covariates in the proportional hazards models.25 Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0).
RESULTS
Patient Eligibility and Characteristics
Between July 30, 1998, and January 21, 2000, 2,952 patients were enrolled by ECOG (44%), Cancer and Leukemia Group B (15%), Southwest Oncology Group (29%), North Central Cancer Treatment Group (9%), and the Expanded Participation Project (3%). Seventy patients (2.4%) were classified as ineligible, leaving 2,882 patients eligible (Fig 1).
Fig 1.
CONSORT diagram. AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide; DFS, disease-free survival.
Patient characteristics were well balanced between treatment groups (Table 1). Assigned therapy was started in 99.5% of patients and was completed in 94% of the patients in the AT arm and 97% in the AC arm. Among the eligible patients, 11.5% and 4.6% of patients on the AT and AC arms, respectively, received lower than 90% of the planned cumulative dose. Three percent of patients who received a breast sparing procedure did not receive radiation therapy. One thousand eight hundred eighty-six patients began tamoxifen. For patients where both a start and end date for tamoxifen was available (n = 1,521), median time on tamoxifen was 59 months (range, < 1 to 84) for patients receiving AT and 58 months (range, < 1 to 74) for patients receiving AC. Limited data for AI use were available for 374 patients, 168 and 206 on the AT and AC arms, respectively. Of these patients, 34% and 38% on the AT and AC arms respectively completed at least 5 years of tamoxifen.
Table 1.
Patient Characteristics Among Patients Classified as Eligible (n = 2,882)
| Characteristic | Arm
|
Total
|
||||
|---|---|---|---|---|---|---|
| A (AT)
|
B (AC)
|
|||||
| No. | % | No. | % | No. | % | |
| No. of patients | 1,441 | 1,441 | 2,882 | |||
| Race* | ||||||
| White | 1,256 | 87 | 1,259 | 88 | 2,515 | 87 |
| Other | 182 | 13 | 179 | 12 | 361 | 13 |
| Bilateral breast cancer† | ||||||
| Yes | 13 | 0.9 | 7 | 0.5 | 20 | 0.7 |
| Age | ||||||
| Minimum | 24 | 25 | 24 | |||
| 25th percentile | 44 | 44 | 44 | |||
| Median | 51 | 51 | 51 | |||
| 75th percentile | 58 | 58 | 58 | |||
| Maximum | 85 | 80 | 85 | |||
| Age group | ||||||
| < 40 | 165 | 11 | 157 | 11 | 322 | 11 |
| ≥ 40 | 1,276 | 89 | 1,284 | 89 | 2,560 | 89 |
| Menopausal status | ||||||
| Pre/peri | 692 | 48 | 683 | 47 | 1,375 | 48 |
| Post | 749 | 52 | 758 | 53 | 1,507 | 52 |
| Surgery | ||||||
| Less than mastectomy | 769 | 53 | 775 | 54 | 1,544 | 54 |
| Total mastectomy | 67 | 5 | 84 | 6 | 151 | 5 |
| Modified radical mastectomy | 605 | 42 | 582 | 40 | 1,187 | 41 |
| SNB/axillary dissection | 376 | 26 | 401 | 28 | 777 | 27 |
| SNB/no axillary dissection | 86 | 6 | 91 | 6 | 177 | 6 |
| No SNB/axillary dissection | 979 | 68 | 949 | 66 | 1,928 | 67 |
| ER/PR status‡ | ||||||
| ER−PR− | 453 | 32 | 465 | 32 | 918 | 32 |
| ER−PR+ | 52 | 4 | 38 | 3 | 90 | 3 |
| ER+PR− | 162 | 11 | 163 | 11 | 325 | 11 |
| ER+PR+ | 765 | 53 | 769 | 54 | 1,534 | 54 |
| Nodal status | ||||||
| Negative | 955 | 66 | 938 | 65 | 1,893 | 66 |
| Positive§ | 486 | 34 | 503 | 35 | 989 | 34 |
| No. of positive nodes | ||||||
| 0 | 955 | 66 | 938 | 65 | 1,893 | 66 |
| 1 | 288 | 20 | 289 | 20 | 577 | 20 |
| 2 | 137 | 9 | 131 | 9 | 268 | 9 |
| 3 | 57 | 4 | 77 | 5 | 134 | 4 |
| At least 1‖ | 4 | 1 | 6 | 1 | 10 | 1 |
| Tumor size¶ | ||||||
| Minimum | 0.1 | 0.2 | 0.1 | |||
| 25th percentile | 1.5 | 1.5 | 1.5 | |||
| Median | 2.0 | 2.0 | 2.0 | |||
| 75th percentile | 3.0 | 2.8 | 2.8 | |||
| Maximum | 8.5 | 12.5 | 12.5 | |||
| Tumor size group, cm | ||||||
| < 2 | 608 | 42 | 637 | 44 | 1,245 | 43 |
| ≥ 2 | 832 | 58 | 800 | 56 | 1,632 | 57 |
| Tumor grade# | ||||||
| Low | 152 | 11 | 145 | 11 | 297 | 11 |
| Intermediate | 552 | 41 | 548 | 40 | 1,100 | 40 |
| High | 659 | 48 | 672 | 49 | 1,331 | 49 |
Abbreviations: AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide; SNB, sentinel node biopsy; ER, estrogen receptor; PR, progesterone receptor.
Data on race not available for three patients within each arm.
For patients with bilateral breast cancer, the worst (ie, highest tumor size and/or highest grade) is reported in this Table.
Based on results from local institution review. ER and/or PR status not available for nine patients on the AT arm and six patients on the AC arm.
Defined as positive if sentinel node positive by standard exam (hematoxylin and eosin) or number of positive nodes from axillary dissection greater than zero.
Result of test available but number of positive nodes from SNB and/or axillary dissection unknown.
Tumor size not available for one patient on the AT arm and four patients on the AC arm.
Tumor grade not available for 78 patients on the AT arm and 76 patients on the AC arm.
DFS and OS
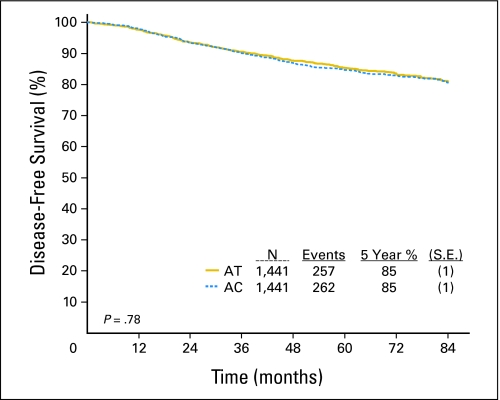
Table 2 summarizes sites of recurrence, deaths, and other clinically significant events that were not included in the DFS end point. In the current analysis (January 2007), there were 257 DFS events in the AT arm, including 216 recurrences and 41 deaths without recurrence. There were 262 DFS events in the AC arm, including 220 recurrences and 42 deaths without recurrence. Figure 2 shows DFS Kaplan-Meier curves for each treatment arm which demonstrates an 85% DFS rate at 5 years with no significant difference in DFS between the two treatments (hazard ratio [HR] for AC v AT 1.02; 95% CI, 0.86 to 1.22; P = .78; Table 3). When adjusting for baseline characteristics including age, menopausal status, primary surgery, ER/PR status, nodal status, tumor size, and tumor grade, the effect of treatment on DFS is similar to the result when accounting for treatment alone (HR for AC v AT 1.03; 95% CI, 0.87 to 1.22; P = .74; Table 3). If all patients (N = 2,952) were analyzed (including ineligible patients), there were 530 DFS events. Results were similar to the results for patients classified as eligible (HR for AC v AT 1.02; 95% CI, 0.86 to 1.21; P = .83; Table 3).
Table 2.
Summary of Outcome Information (n = 2,882)
| Parameter | Arm
|
No. | |
|---|---|---|---|
| A (AT) | B (AC) | ||
| Breast recurrence | |||
| Ipsilateral | 22 | 24 | 46 |
| Locoregional | 39 | 38 | 77 |
| Distant | 125 | 128 | 253 |
| Contralateral invasive breast cancer | |||
| Isolated | 26 | 27 | 53 |
| And other site | 4 | 3 | 7 |
| Total* | 216 | 220 | 436 |
| Deaths | 116 | 123 | 239 |
| Without recurrence | 41 | 42 | 83 |
| Total | 157 | 165 | 322 |
| Nonbreast second primaries | |||
| Isolated | 57 | 39 | 96 |
| And recurrence | 14 | 13 | 27 |
| Total | 71 | 52 | 123† |
| Contralateral in situ breast cancer | |||
| Isolated | 6 | 6 | 12‡ |
| And other site (melanoma) | 1 | 0 | 1‡ |
Abbreviations: AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide; DFS, disease-free survival.
Only first recurrence events were collected.
Among 114 patients.
One of these 13 died and was counted as an event in the DFS analysis. The other 12 were censored in the DFS analysis at date last known to be alive.
Fig 2.
Kaplan-Meier curve for disease-free survival. Solid yellow curve indicates doxorubicin and docetaxel (AT); dotted blue curve indicates doxorubicin and cyclophosphamide (AC).
Table 3.
Univariate and Adjusted Hazard Ratios
| Patients | AC v AT
|
||
|---|---|---|---|
| HR* | 95% CI | P | |
| Eligible (n = 2,882) | |||
| DFS | 1.02 | 0.86 to 1.22 | .78 |
| DFS adjusted† | 1.03 | 0.87 to 1.22 | .74 |
| OS | 1.06 | 0.85 to 1.31 | .62 |
| OS adjusted† | 1.06 | 0.85 to 1.31 | .63 |
| All (N = 2,952) | |||
| DFS | 1.02 | 0.86 to 1.21 | .83 |
| DFS adjusted† | 1.03 | 0.87 to 1.22 | .76 |
| OS | 1.03 | 0.83 to 1.28 | .76 |
| OS adjusted† | 1.04 | 0.84 to 1.30 | .73 |
Abbreviations: AC, doxorubicin and cyclophosphamide; AT, doxorubicin and docetaxel; HR, hazard ratio; DFS, disease-free survival; OS, overall survival.
HR > 1 indicates improved outcome for AT.
Adjusted for age, menopausal status, primary surgery, estrogen receptor/progesterone receptor status, nodal status, tumor size, and tumor grade.
Figure 3 shows the effect of treatment on DFS within subgroups of baseline characteristics. There was a statistically significant interaction between ER/PR status and treatment where patients with tumors classified as ER- and PR-negative and ER positive PR negative experienced more favorable outcome with use of AT (P values for interactions terms .02 and < .01, respectively). Figure 4 shows the DFS curves within the ER/PR subgroups. No other interactions between the baseline characteristics and treatment were statistically significant.
Fig 3.
Forest plot: disease-free survival by subgroups. ER, estrogen receptor; PR, progesterone receptor; AC, doxorubicin and cyclophosphamide; AT, doxorubicin and docetaxel.
Fig 4.
Kaplan-Meier disease-free survival curves by estrogen receptor (ER)/progesterone receptor (PR) subgroups: solid yellow curves indicates doxorubicin and docetaxel (AT); dotted blue curves indicates doxorubicin and cyclophosphamide (AC). (A) ER negative, PR negative. (B): ER negative, PR positive (C) ER positive, PR negative. (D) ER positive, PR positive.
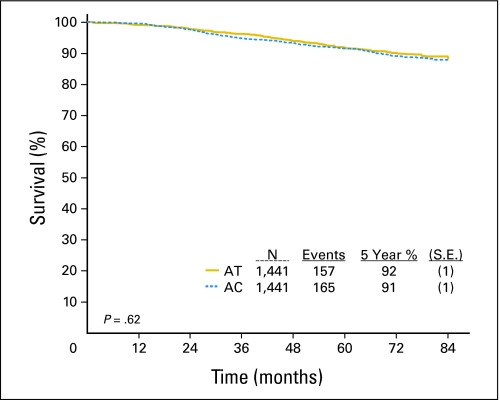
With 79.5 months median follow-up, 322 patients among the eligible population had died. Appendix Figure A1, online only, shows OS Kaplan and Meier curves for each treatment arm which demonstrates 92% survival rate at 5 years for the AT arm and 91% for the AC arm. There was no significant difference in survival between the two treatments (HR for AC v AT 1.06; 95% CI, 0.85 to 1.31; P = .62; Table 3). When adjusting for baseline characteristics, the effect of treatment on OS is similar to the result when accounting for the effect of treatment alone (HR for AC v AT 1.06; 95% CI. 0.85 to 1.31; P = .63; Table 3). No interactions between the baseline characteristics and treatment with respect to survival were statistically significant.
When all patients, eligible and ineligible were analyzed (n = 2952), there were 331 deaths. Results for this analysis were similar to the results for patients classified as eligible and again there was no difference in OS between AT and AC (HR AC v AT 1.03; 95% CI, 0.83 to 1.28; P = .76; Table 3).
Toxicity
There was a higher incidence of grade 3 neutropenia associated with fever or infection in the AT arm compared with the AC arm (26% v 10%; P < .05); primary G-CSF prophylaxis was not used, which was the standard of care at the time by ASCO guidelines.17 Other most frequently clinically important grade 3/4 adverse events included neutropenia (54% with AT v 38% with AC; P < .05) and leucopenia (22% with AT v 8% with AC; P < .05; Appendix Tables A1 and A2, online only). There were six deaths classified as related to treatment including four in the AT arm (visceral arterial ischemia, infection with grade 3/4 neutropenia, cardiac arrest, acute respiratory distress syndrome) and two deaths in the AC arm (myocardial infarction and acute myeloid leukemia). There were seven cases of myelodysplastic syndromes/acute myeloid leukemia on each arm. Twelve patients developed CHF during chemotherapy—eight on AT (six grade 3, one grade 4, one grade 5), four on AC (all grade 3), and 19 patients developed CHF more than 30 days postchemotherapy—12 on AT (11 grade 3, one grade 4), seven on AC (all grade 3). There was no significant difference in changes in LVEF from baseline between the two arms (Appendix Table A3, online only).
DISCUSSION
The purpose of this trial was to determine if concurrent administration of adjuvant AT every 3 weeks for four cycles was more effective than the standard concurrent doxorubicin and cyclophosphamide regimen in patients with operable breast cancer and 0 to three positive axillary lymph nodes. We believed that there was a reasonable likelihood that this would be the case based on data from phase II and phase III trials available at the time, and subsequently by a phase III trial confirming a significantly higher objective response rate for the doxorubicin and docetaxel combination compared with the doxorubicin and cyclophosphamide combination in patients with metastatic breast cancer.26-28 However, the DFS and OS rates were essentially identical between the two arms in our trial, although the 5-year DFS survival rates observed in both treatment arms (85%), was substantially better than had been predicted based on historical data (78%). In an exploratory analysis, the AT arm was associated with a strong trend toward improved DFS in patients with ER- and PR-negative disease. Although the AT arm was not associated with more cardiac toxicity, it was associated with significantly higher rates of severe neutropenia, febrile neutropenia, and other severe nonhematologic toxicities.
Several other trials have demonstrated a clear benefit for adjuvant docetaxel or paclitaxel, whether used concurrently with doxorubicin-containing therapy, or sequentially after anthracycline-based therapy. Martin et al reported that concurrent administration of docetaxel with doxorubicin and cyclophosphamide (TAC) significantly improved DFS and OS when compared with fluorouracil, doxorubicin, and cyclophsophamide.15 Several studies also demonstrated that sequential administration of paclitaxel or docetaxel after anthracycline-based therapy was more effective than the same regimen without taxane therapy.12,13,29 Moreover, meta-analyses that included studies incorporating adjuvant taxanes also demonstrated a benefit for taxane therapy.30,31 It appears that the concurrent administration of a short course of doxorubicin and docetaxel, as used in our trial, is not an effective way of intergrating taxanes into adjuvant therapy.
Most recently, Jones at al reported the results of a US Oncology (USO) trial comparing AC to docetaxel and cyclophosphamide (TC).32 TC resulted in a superior DFS compared with AC in this trial (5-year DFS 86% for TC v 80% AC; P = .015; HR, 0.67) without a significant difference in OS (TC 90% v AC 87%; P = .13; HR, 0.76) although 7-year follow-up has now demonstrated an improvement in OS for TC (TC 87% v AC 82%; P = .032; HR, 0.69).32,33 All of these data would have led the unbiased investigator to expect that AT would be superior to AC in E2197. While one cannot do cross trial comparisons, the TC arm of this trial is similar to the overall outcome on E2197. There are some specific differences between the trials that may account for the divergence. First, the dose of docetaxel in E2197 was 60 mg/m2 to maintain the dose of adriamycin constant in both arms where as the docetaxel dose in the USO study was 75 mg/m2. This may be important since it has been shown that there is a docetaxel dose response effect.34 In addition, the patient population was of slightly higher risk in the USO study with 53% of patients having positive lymph nodes compared with 34% in E2197. The National Surgical Adjuvant Breast and Bowel Project B30 is designed to compare AC for four cycles followed by docetaxel for four cycles versus AT for four cycles versus TAC for four cycles. This study has completed accrual but the results have not yet been reported.
The ER/PR subgroups were prespecified stratification groups at randomization designed to balance the treatment arms and are not powered to detect differences between arms within the subgroups. In an exploratory subset analysis, we found that AT tended to be more effective in patients ER- and PR-negative disease. Although we did not perform central ER, PR, and HER2/neu testing for all patients enrolled in our trial, a subset of 776 patients who had central testing performed demonstrated 90% concordance with local ER/PR testing and 79% concordance with local HER2/neu testing; concordance was higher for HER2/neu testing when cases were determined to be HER2/neu negative locally.35 These data support the hypothesis presented by Berry et al, that in the ER positive tumors the large benefit provided by tamoxifen, overwhelms the potential benefit of chemotherapy, or that the prognosis of these tumors is so good it is difficult to detect a difference between the two chemotherapy arms.36 Others have also demonstrated greater treatment benefit for adjuvant taxanes in ER/PR-negative disease, although this has not been consistently demonstrated in other studies.15,36
Of great interest is the biologic hypothesis generated by E2197 from the ER/PR prespecified subset analysis and supports the importance of collecting clinical specimens for future prospective laboratory analysis on archival samples from controlled randomized clinical trials. These data also support the premise of the Trial Assigning IndividuaLized Options for Treatment, which uses Oncotype DX (Genomic Health Inc, Redwood City, CA), using molecular characteristics, to stratify tumors based on genomic profiling to determine prognosis and potential benefit to specific therapy. Studies aimed at the biologic tumor characteristics as determinants of outcome from E2197 will include central review of ER/PR and HER2, Oncotype DX, and genomic profiling to determine if individual genes may predict outcome of specific sensitivity or resistance to a specific therapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Lori J. Goldstein, Sanofi-aventis (C); Joseph A. Sparano, Sanofi-aventis (C); Edith A. Perez, Genentech (U), GlaxoSmithKlein (U), Bristol-Myers Squibb (U) Stock Ownership: None Honoraria: None Research Funding: Lori J. Goldstein, Sanofi-aventis; Joseph A. Sparano, Sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lori J. Goldstein, Anne O'Neill, Joseph A. Sparano, Silvana Martino, Nancy E. Davidson
Administrative support: Joseph A. Sparano, Lawrence N. Shulman
Provision of study materials or patients: Lori J. Goldstein, Joseph A. Sparano, Edith A. Perez, Silvana Martino, Nancy E. Davidson
Collection and assembly of data: Lori J. Goldstein, Edith A. Perez, Lawrence N. Shulman, Silvana Martino
Data analysis and interpretation: Lori J. Goldstein, Anne O'Neill, Joseph A. Sparano, Edith A. Perez, Lawrence N. Shulman, Nancy E. Davidson
Manuscript writing: Lori J. Goldstein, Anne O'Neill, Joseph A. Sparano, Edith A. Perez, Lawrence N. Shulman, Nancy E. Davidson
Final approval of manuscript: Lori J. Goldstein, Anne O'Neill, Joseph A. Sparano, Lawrence N. Shulman, Silvana Martino, Nancy E. Davidson
Acknowledgments
We thank Deborah Nemande and Nicole Williams, Eastern Cooperative Oncology Group Operations and Data Management; and Pat Dessin, Administrative Assistant, Fox Chase Cancer Center.
Appendix
Fig A1.
Kaplan-Meier curve overall survival. Solid yellow curve indicates doxorubicin and docetaxel (AT); dotted blue curve indicates doxorubicin and cyclophosphamide (AC).
Table A1.
Grade 4 and 5 Treatment-Related Hematologic Toxicities
| Toxicity Type | Treatment Arm (%)
|
|||
|---|---|---|---|---|
| A (AT)
|
B (AC)
|
|||
| Grade 4 | Grade 5 | Grade 4 | Grade 5 | |
| No. of patients | 1,469 | 1,469 | ||
| Hemoglobin | < 1 | — | < 1 | — |
| Hemolysis | < 1 | — | — | — |
| Leukocytes | 22 | — | 7 | — |
| Lymphopenia | < 1 | — | < 1 | — |
| Neutrophils | 54 | — | 37 | — |
| Platelets | < 1 | — | < 1 | — |
| Worst degree | 57 | — | 38 | — |
Abbreviations: AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide.
Table A2.
Grade 3 to 5 Treatment-Related Nonhematologic Toxicities
| Toxicity Type | Treatment Arm (%)
|
|||||
|---|---|---|---|---|---|---|
| A (AT)
|
B (AC)
|
|||||
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| No. of patients | 1,469 | 1,469 | ||||
| Allergic reaction | 2 | < 1 | — | < 1 | — | — |
| Middle ear/hearing | — | — | — | < 1 | — | — |
| Sinus tachycardia | < 1 | — | — | — | — | — |
| Arrhythmia | ||||||
| Supraventricular | < 1 | < 1 | — | — | — | — |
| Ventricular | — | < 1 | — | — | — | — |
| Other | < 1 | — | — | — | — | — |
| Cardiac ischemia | — | < 1 | — | — | < 1 | — |
| Cardiac left ventricular function | < 1 | < 1 | < 1 | < 1 | — | — |
| Hypotension | < 1 | < 1 | — | — | — | — |
| Thrombosis/embolism | < 1 | < 1 | — | < 1 | — | — |
| Visceral arterial ischemia | — | — | < 1 | — | — | — |
| Fatigue | 5 | 1 | — | 2 | — | — |
| Fever | < 1 | — | — | — | — | — |
| Injection site reaction | < 1 | — | — | — | — | — |
| Radiation dermatitis | < 1 | — | — | < 1 | — | — |
| Rash/desquamation | < 1 | — | — | — | — | — |
| Wound | ||||||
| Infectious | — | — | — | < 1 | — | — |
| Noninfectious | < 1 | — | — | — | — | — |
| Skin, other | < 1 | — | — | — | — | — |
| Hot flashes | < 1 | — | — | — | — | — |
| Anorexia | < 1 | — | — | < 1 | — | — |
| Colitis | — | < 1 | — | < 1 | — | — |
| Constipation | 1 | < 1 | — | < 1 | — | — |
| Dehydration | 1 | < 1 | — | < 1 | — | — |
| Diarrhea | 4 | < 1 | — | 1 | < 1 | — |
| Dyspepsia | 1 | — | — | < 1 | — | — |
| Dysphagia | < 1 | < 1 | — | < 1 | — | — |
| Fistula, rectal/anal | — | — | — | < 1 | — | — |
| Gastric ulcer | < 1 | — | — | — | — | — |
| Gastritis | < 1 | < 1 | — | — | — | — |
| Ileus | < 1 | — | — | — | — | — |
| Mucositis due to radiation | < 1 | < 1 | — | — | — | — |
| Nausea | 5 | — | — | 6 | < 1 | — |
| Proctitis | < 1 | — | — | < 1 | — | — |
| Stomatitis | 4 | < 1 | — | 1 | < 1 | — |
| Taste disturbance | < 1 | — | — | — | — | — |
| Typhlitis | < 1 | < 1 | — | — | — | — |
| Vomiting | 3 | < 1 | — | 5 | < 1 | — |
| Diarrhea without prior colostomy | — | — | — | < 1 | — | — |
| GI, other | < 1 | — | — | — | — | — |
| Hemorrhage without grade 3 or 4 platelet | < 1 | — | — | — | — | — |
| Epistaxis | < 1 | — | — | — | — | — |
| Melena/GI bleeding | — | < 1 | — | — | — | — |
| Vaginal bleeding | < 1 | — | — | — | — | — |
| GGT | < 1 | — | — | — | — | — |
| Liver dysfunction/failure | — | — | — | < 1 | — | — |
| AST | < 1 | — | — | — | — | — |
| ALT | < 1 | — | — | — | — | — |
| Febrile neutropenia | 17 | 2 | — | 5 | 1 | — |
| Infection | ||||||
| With grade 3 or 4 neutropenia | 7 | 2 | < 1 | 4 | < 1 | — |
| With unknown ANC | 1 | — | — | 1 | — | — |
| Without neutropenia | 1 | < 1 | — | < 1 | — | — |
| Hyperglycemia | 2 | 1 | — | 1 | — | — |
| Hypernatremia | < 1 | — | — | — | — | — |
| Hypoglycemia | — | — | — | < 1 | — | — |
| Hypokalemia | < 1 | — | — | — | — | — |
| Hyponatremia | < 1 | — | — | — | < 1 | — |
| Arthritis | — | — | — | < 1 | — | — |
| Muscle weakness | < 1 | — | — | — | — | — |
| Joint, muscle, bone–other | < 1 | — | — | — | — | — |
| Dizziness/lightheadedness | < 1 | — | — | < 1 | — | — |
| Insomnia | < 1 | — | — | < 1 | — | — |
| Anxiety/agitation | < 1 | — | — | — | < 1 | — |
| Depression | < 1 | — | — | < 1 | < 1 | — |
| Neuropathy | ||||||
| Motor | < 1 | — | — | — | — | — |
| Sensory | < 1 | — | — | — | < 1 | — |
| Syncope | 1 | — | — | < 1 | — | — |
| Tremor | < 1 | — | — | — | — | — |
| Neurologic, other | < 1 | — | — | — | — | — |
| Abdominal pain | < 1 | — | — | < 1 | — | — |
| Arthralgia | < 1 | — | — | — | — | — |
| Bone pain | < 1 | — | — | < 1 | — | — |
| Chest pain | < 1 | — | — | < 1 | — | — |
| Headache | < 1 | < 1 | — | < 1 | — | — |
| Myalgia | < 1 | — | — | < 1 | — | — |
| Pleuritic pain | < 1 | — | — | < 1 | — | — |
| Rectal or perirectal pain | — | — | — | < 1 | — | — |
| Pain, other | < 1 | — | — | < 1 | — | — |
| Cough | < 1 | — | — | — | — | — |
| Dyspnea | < 1 | < 1 | — | < 1 | — | — |
| FEV1 | < 1 | — | — | — | — | — |
| Hypoxia | < 1 | — | — | — | — | — |
| Pleural effusion | < 1 | — | — | < 1 | — | — |
| Pneumonitis/pulmonary infiltrates | < 1 | — | — | < 1 | — | — |
| Creatinine | < 1 | — | — | — | — | — |
| Incontinence | < 1 | — | — | — | — | — |
| Renal failure | — | < 1 | — | — | — | — |
| Urinary frequency/urgency | — | — | — | < 1 | — | — |
| Vaginitis | < 1 | — | — | <1 | — | — |
| Renal/GU, other | < 1 | — | — | — | — | — |
| Irregular menses | 1 | — | — | < 1 | — | — |
| Worst degree | 38 | 7 | < 1 | 20 | 2 | — |
Abbreviations: AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide; GGT, gamma glutamate transferase; FEV1, forced expiratory volume in 1 second; GU, genitourinary; ANC, absolute neutrophil count.
Table A3.
Summary for Cases With LVEF Values at Baseline and Postchemotherapy
| Parameter | Arm
|
|||
|---|---|---|---|---|
| A (AT)
|
B (AC)
|
|||
| Median | Range | Median | Range | |
| Total patients who began protocol treatment | 1,469 | 1,469 | ||
| Patients with baseline and postchemotherapy LVEF values* | 1,048 | 1,074 | ||
| Baseline LVEF | 63 | 50-93 | 64 | 49-92 |
| Postchemotherapy LVEF | 60 | 15-92 | 61 | 30-89 |
| Difference baseline minus postchemotherapy | 2 | −21-52 | 2 | −27-30 |
| Postchemotherapy LVEF drop > 10%, No. | 154 | 165 | ||
| % | 15 | 15 | ||
| Postchemotherapy drop > 10% and LVEF < LLN, No.* | 43 | 41 | ||
| Where postchemotherapy drop > 10% and LVEF < LLN | 45 | 15-58 | 46 | 30-54 |
| LLN where postchemotherapy drop > 10% and LVEF < LLN | 50 | 50-60 | 50 | 48-55 |
NOTE. Includes patients classified as ineligible.
Abbreviations: AT, doxorubicin and docetaxel; AC, doxorubicin and cyclophosphamide; LVEF, left ventricular ejection fraction; LLN, lower limits of normal.
Postchemotherapy assessment performed during the last cycle for 32%, performed within 90 days of the last cycle for 62.5%, and > 90 days for 5.5% of the patients.
published online ahead of print at www.jco.org on August 4, 2008
Supported in part by Grants No. CA027525 and P30-CA006927 from the Department of Health and Human Services and the National Institutes of Health. This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA27525, CA14958, CA25224, CA32291, CA32102, CA16116, and from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 13-17, 2005.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Fisher B, Brown AM, Dimitrov NV, et al: Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8:1483-1496, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Bishop JF, Dewar J, Toner GC, et al: Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol 17:2355-2364, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Chan S, Friedrichs K, Noel D, et al: Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17:2341-2354, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ghersi D, Wilcken N, Simes J, et al: Taxane containing regimens for metastatic breast cancer. Br J Cancer 93:293-301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crown J, Dieras V, Kaufmann M, et al: Chemotherapy for metastatic breast cancer-report of a European expert panel. Lancet Oncol 3:719-727, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Sledge GW, Neuberg D, Bernardo P, et al: Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193). J Clin Oncol 21:588-592, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Hu P, Rao RM, et al: Phase II trial of doxorubicin and paclitaxel plus granulocyte colony-stimulating factor in metastatic breast cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol 17:3828-3834, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Dombernowsky P, Gehl J, Boesgaard M, et al: Treatment of metastatic breast cancer with paclitaxel and doxorubicin. Semin Oncol 22:13-17, 1995 [PubMed] [Google Scholar]
- 9.Gianni L, Munzone E, Capri G, et al: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13:2688-2699, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Sparano JA, O'Neill A, Schaefer PL, et al: Phase II trial of doxorubicin and docetaxel plus granulocyte colony-stimulating factor in metastatic breast cancer: Eastern Cooperative Oncology Group study E1196. J Clin Oncol 18:2369-2377, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Sparano JA, Hu P, Raor RM, et al: Phase II trial of doxorubicin plus paclitaxel plus G-CSF in metastatic breast cancer: An Eastern Cooperative Oncology Group study (E4195). Breast Cancer Res Treat 46, 1997
- 12.Henderson IC, Berry DA, Demetri GD, et al: Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976-983, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Mamounas EP, Bryant J, Lembersky B, et al: Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol 23:3686-3696, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Crown JP, Francis P, Di Leo A: Docetaxel given either concurrently or sequentially to anthracycline-based adjuvant therapy for patients with node-positive breast cancer: comparison with non-taxane combination chemotherapy: First results of the BIG 2-98 trial at 5 years median follow-up. J Clin Oncol 24:7s, 2006. (suppl; abstract LBA519) [Google Scholar]
- 15.Martin M, Pienkowski T, Mackey J, et al: Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302-2313, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Roche H, Fumoleau P, Spielmann M, et al: Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 trial. J Clin Oncol 24:5664-5671, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Update of recommendations for the use of hematopoietic colony-stimulating factors: Evidence-based clinical practice guidelines: American Society of Clinical Oncology. J Clin Oncol 14:1957-1960, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Mansour EG, Gray RJ, Shatila A, et al: Efficacy of adjuvant chemotherapy in high risk node negative breast cancer. N Engl J Med 320:485-490, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Davidson NE, O'Neill AM, Vukov AM, et al: Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: Results from INT 0101 (E5188). J Clin Oncol 23:5973-5982, 2005 [DOI] [PubMed] [Google Scholar]
- 20.O'Brien PC, Fleming TR: A multiple testing procedure for clinical trials. Biometrics 35:5490556, 1979 [PubMed] [Google Scholar]
- 21.Mehta CR, Patel NR, Tsiatis AA: Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics 40:819-825, 1984 [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 23.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 24.Cox DR: Regression models and life tables (with discussion). J R Stat Soc B 187-220, 1972
- 25.Wald A: Tests of statistical hypotheses concerning several parameters when the number of parameters is large. Trans Am Math Soc 54:426-482, 1943 [Google Scholar]
- 26.Misset JL, Dieras V, Gruia G, et al: Dose-finding study of docetaxel and doxorubicin in first-line treatment of patients with metastatic breast cancer. Ann Oncol 10:553-560, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Nabholtz JM, Falkson C, Campos D, et al: Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: Results of a randomized, multicenter, phase III trial. J Clin Oncol 21:968-975, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Cresta S, Grasselli G, Mansutti M, et al: A randomized phase II study of combination, alternating and sequential regimens of doxorubicin and docetaxel as first-line chemotherapy for women with metastatic breast cancer. Ann Oncol 15:433-439, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Lluch A, Segui MA: Toxicity and health-related quality of life in node negative breast cancer patients receiving adjuvant treatment with TAC or FAC: Impact of adding prophylactic growth factors to TAC. GEICAM Study 9805. J Clin Oncol 23:29s, 2005. (suppl; abstr 604). [Google Scholar]
- 30.Ferguson T, Wilcken N, Vagg R, et al: Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev CD004421, 2007 [DOI] [PubMed]
- 31.De Laurentiis M, Cancello G, D'Agostino D, et al: Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials. J Clin Oncol 26:44-53, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Jones SE, Savin MA, Holmes FA, et al: Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24:5381-5387, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Jones SE, Holmes FA, O'Shaughnessy JA, et al: Extended follow-up and analysis by age of the US Oncology Adjuvant trial 9735:Docetaxel/ cyclophosphamide is associated with an overall survival benefit compared to doxorubicin/ cyclophosphamide and is well-tolerated in women 65 or older. Breast Cancer Res Treat 106:S5, 2007. (abstr 12) [Google Scholar]
- 34.Harvey V, Mouridsen H, Semiglazov V, et al: Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24:4963-4970, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Badve SS, Baehhner FM, Gray RP, et al: Estrogen and progesterone receptor status in ECOG 2197: Comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase reaction by central laboratory. J Clin Oncol 26:2473-2481, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Berry DA, Cirrincione C, Henderson IC, et al: Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658-1667, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]