Abstract
Alterations in hormone concentrations, including adrenocorticotropin, corticotropin releasing hormone, and cortisol have been reported in patients with obsessive compulsive disorder (OCD). Dehydroepiandrosterone (DHEA) and its sulfated metabolite, DHEA-S, have not been assessed in patients with OCD. We report 24-hour serum DHEA, DHEA-S, and cortisol concentrations in a young man with OCD and 15 healthy young men. Circadian patterns of DHEA and cortisol were markedly different in the subject with OCD than in the control subjects. DHEA and DHEA-S concentrations were substantially higher in the OCD subject than in the control subjects. In contrast, cortisol concentrations were similar in the OCD subject and the control subjects. Future clinical studies are needed to evaluate the significance of DHEA and DHEA-S in OCD.
To the Editor
Alterations in hormone concentrations have been reported in patients with obsessive compulsive disorder (OCD). Studies have reported differences in concentrations of cortisol (Catapano et al., 1992; Monteleone et al., 1994), melatonin (Catapano et al., 1992; Monteleone et al., 1994), corticotropin releasing hormone (Altemus et al., 1992), adrenocorticotropin (ACTH) (Bailly et al., 1994), arginine vasopressin (Altemus et al., 1992), and oxytocin (Leckman et al., 1994). Some patients with OCD demonstrate a lack of inhibition of cortisol secretion after a dexamethasone suppression test (Catapano et al., 1990; Cottraux et al., 1984), indicating hyperactivity of the hypothalamic-pituitary-adrenal axis.
Two important steroids, dehydroepiandrosterone (DHEA) and its sulfated metabolite DHEA-S, have not been assessed in patients with OCD. DHEA is secreted synchronously with cortisol from the adrenal cortex in response to ACTH. DHEA and DHEA-S are also neurosteroids, which are both synthesized and active in the brain. DHEA-S, and to a lesser extent DHEA, is a potent noncompetitive antagonist of γ-aminobutyric acid type A (GABAA) receptors (Majewska, 1992; Majewska et al., 1990), and induces anxiogenic activity when injected into rodents (Le Mellédo & Baker, 2004; Majewska et al., 1990; Reddy & Kulkarni, 1997). Neurosteroid-induced decreased GABAergic tone may be relevant to the pathophysiology of mood and anxiety disorders.
METHODS
We measured 24-hour concentrations of DHEA, DHEA-S, and cortisol in a young man with OCD and in 15 healthy control subjects, as part of a larger study conducted at the University of Pittsburgh Medical Center. As a result of the hormonal data reported herein, OCD subsequently was considered a criterion for exclusion; thus, data from only one OCD subject is available. All subjects gave written informed consent prior to any research procedures. Volunteers were evaluated for eligibility in the study using the Structured Clinical Interview for Diagnosis of DSM IV Disorders (First et al., 1995). One subject met DSM IV diagnostic criteria for OCD, which was verified by the study psychologist. OCD severity was considered moderate to severe (i.e. the patient experienced symptoms 70-80% of the time over the previous 5 years). The OCD subject had no current or past history of depression or depressive episodes. The OCD subject had never been treated with any selective serotonin reuptake inhibitor or other antidepressant. The 15 control subjects were psychiatrically healthy. All subjects were screened by medical history, physical examination, and biochemical and hematological laboratory tests within 28 days of the study and were determined physically healthy by those criteria. Subjects were nonsmoking (by self-report), young men, with control subjects ranging in age from 20 to 29 years; the subject with OCD was 29 years old. Subjects with active seizure disorder, unstable chronic disease, or drug or alcohol abuse were excluded, as were subjects who had taken enzyme inducing or inhibiting agents within one month or any chronic medications within one week of the study.
Blood samples were collected in labeled vacuum tubes at -1 h, 1, 2, 3, 3.5, 4.5, 5.5, 7, 8.5, 10, 11.5, 12.5, 16, 20, and 23 h. Samples were centrifuged and serum was decanted and stored at -80°C. DHEA, DHEA-S, and cortisol concentrations were determined using 125I-radioimmunoassay kits from Diagnostic Systems Lab, Inc. (Webster, TX). The lower limit of quantitation was 0.20 ng/mL for DHEA, 50 ng/mL for DHEA-S, and 0.5 μg/dL for cortisol. Area under the concentration-time curve (AUC) was calculated for each subject using the trapezoid rule. Mean steroid concentrations from 2 to 12.5 h were calculated by dividing the AUC for that interval by 10.5 hours. Statistics and graphing were done using Prism version 5.00 (GraphPad Software; San Diego, California USA; www.graphpad.com).
RESULTS
Twenty-four-hour serum DHEA concentrations are shown in Figure 1. The patterns of DHEA concentrations in the OCD and the control subjects differed visually; peaks were evident several times during the afternoon in the OCD subject. DHEA AUC in the patient with OCD (308.5 ng/mL*h) was 69% higher than in control subjects (183.0 ng/mL*h). Similarly, mean DHEA concentrations from 2 to 12.5 h were 72% higher in the OCD subject than in the control subjects (13.9 ng/mL compared to 8.07 ng/mL).
Figure 1. Twenty-four-hour DHEA concentrations.
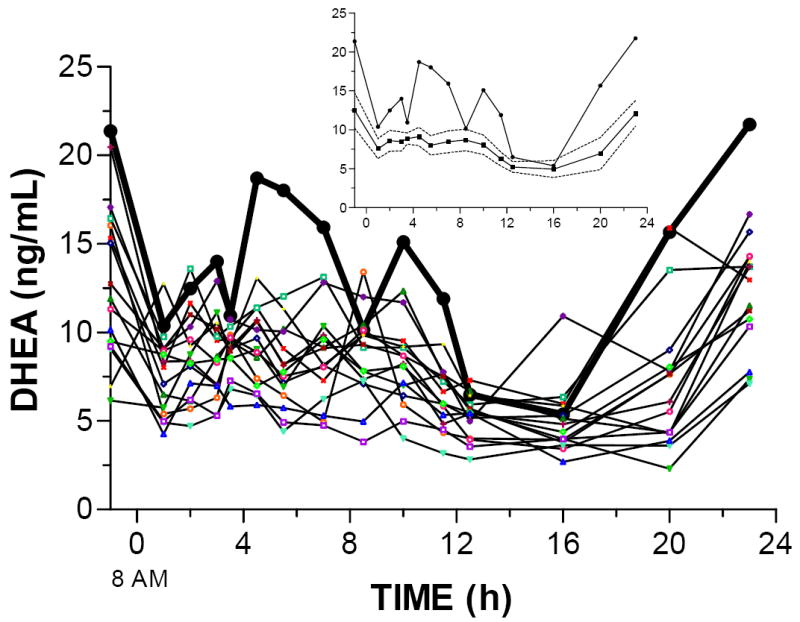
DHEA concentrations in the OCD subject (dark black line) and 15 individual control subjects. The inset shows the OCD subject (circles) and the mean (squares) and 95% confidence interval (dashed lines) for the control subjects.
Twenty-four-hour serum DHEA-S concentrations are shown in Figure 2. DHEA-S AUC in the OCD subject was nearly double the AUC in the control subjects (116245 ng/mL*h compared to 59123 ng/mL*h). Mean DHEA-S concentrations from 2 to 12.5 h were 93% higher in the OCD subject than the control subjects (4921.0 ng/mL compared to 2547.7 ng/mL).
Figure 2. Twenty-four-hour DHEA-S concentrations.
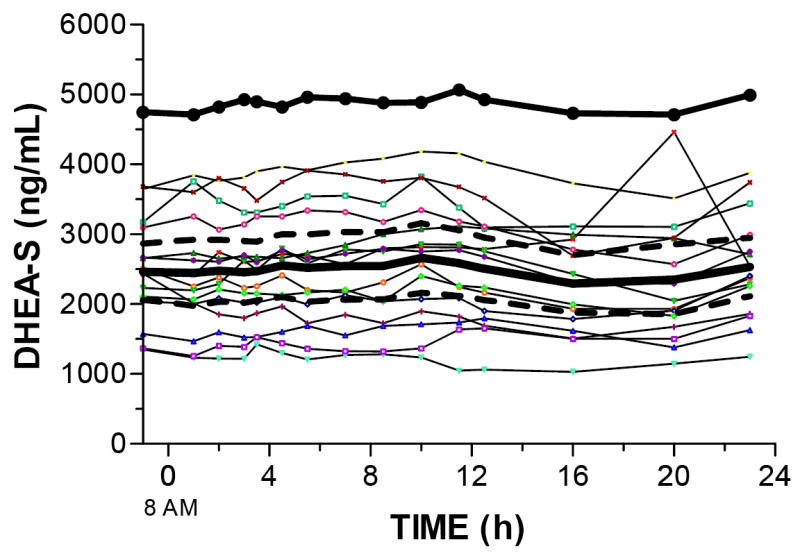
DHEA-S concentrations in the OCD subject (dark black line) and 15 individual control subjects. The mean and 95% confidence interval (dashed line) for the control subjects overlaps the individual subjects.
Figure 3 shows the twenty-four-hour serum cortisol concentrations in the OCD and control subjects. As with DHEA concentrations, the pattern of cortisol concentrations visually differed in the subject with OCD and the control subjects. However, AUCs were similar in the OCD subject and the control subjects (289.5 and 227.7 ng/dL*h), as were mean cortisol concentrations from 2 to 12.5 h (12.26 μg/dL: OCD; and 10.05 μg/dL: controls).
Figure 3. Twenty-four-hour cortisol concentrations.
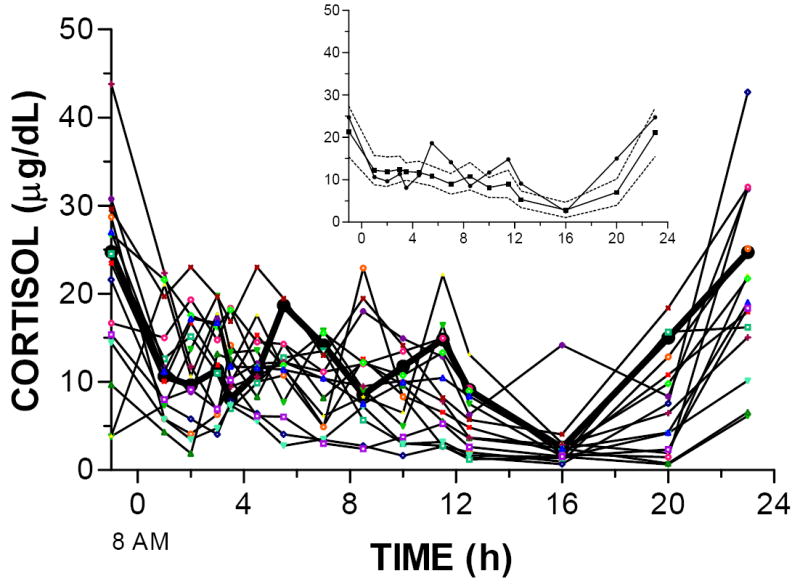
Cortisol concentrations in the OCD subject (dark black line) and 15 individual control subjects. The inset shows the OCD subject (circles) and the mean (squares) and 95% confidence interval (dashed lines) for the control subjects.
DISCUSSION
This is the first report of DHEA and DHEA-S concentrations in a patient with OCD and in control subjects. The data showed that not only were the patterns of DHEA and cortisol different from the control subjects, the mean concentration of DHEA and DHEA-S were higher in the OCD patient by a magnitude of 72% and 93% respectively.
Dysregulation of DHEA and DHEA-S has been reported in other mood and anxiety disorders (Eser et al., 2006; Le Mellédo & Baker, 2002; Le Mellédo & Baker, 2004). For example, plasma DHEA is higher in drug-free panic disorder patients than in control subjects (Brambilla et al., 2005). Patients with major depression have increased diurnal plasma DHEA (Heuser et al., 1998); notably, DHEA and DHEA-S have been reported to decrease with remission of depression in older adults (Fabian et al., 2001). One study identified a positive correlation between morning serum DHEA-S concentration and anxiety ratings in drug-free major depressive patients, but no relationship to depression ratings (Hsiao, 2006). Patients with anorexia or bulimia nervosa have significantly higher DHEA, DHEA-S, and cortisol concentrations than controls (Monteleone et al., 2001). Since patients with OCD experience high levels of anxiety similar to panic disorder and anorexia, the elevated DHEA and DHEA-S concentrations observed in this patient is not surprising.
Several studies have reported the relative amounts of DHEA and/or DHEA-S are maintained between brain and blood (Bernardi et al., 2005; Guazzo et al., 1996). In rats, oral DHEA administration increased DHEA-S content in a dose-dependent manner in hippocampus, hypothalamus, and serum (Bernardi et al., 2005). In humans, the proportional levels of DHEA and DHEA-S in cerebrospinal fluid (CSF) compared to blood are 5.4% and 0.15% respectively, and these blood/CSF ratios are similar in subjects taking steroids and steroid-free subjects (Guazzo et al., 1996). There are significant correlations between blood and CSF levels for DHEA (r = 0.65) and DHEAS (r = 0.88) (Guazzo et al., 1996), therefore it is reasonable to believe that elevated DHEA and/or DHEA-S concentrations in serum would reflect elevated DHEA and/or DHEA-S in the brain.
While the DHEA and DHEA-S data described herein are exciting and previously unreported, the data are limited by the fact that we have data from only a single subject with OCD. The data raise questions as to whether the DHEA pattern and magnitude of DHEA or DHEA-S concentrations could be used as a biomarker for or provide insight into the pathophysiology of OCD. DHEA and DHEA-S are modulators of GABAA (Le Mellédo & Baker, 2004; Majewska, 1992; Majewska et al., 1990; Reddy & Kulkarni, 1997) and N-methyl-D-aspartate (NMDA) receptors (Compagnone & Mellon, 2000; Rupprecht, 1997); they also play a role in NMDA-evoked norepinephrine release (Monnet et al., 1995). Alterations in norepinephrine have been implicated in other anxiety disorders, and therefore may also be a link between DHEA dysregulation and anxiogenesis resulting in OCD (Sullivan et al., 1999). Due to these neuropsychopharmacological properties, future clinical studies are needed to evaluate the role of DHEA and DHEA-S in OCD, which may lead to a greater understanding of the neurocircuitry behind this illness.
Acknowledgments
This study was funded by the Pharmacodynamic Research Center at the University of Pittsburgh School of Pharmacy.
Role of the Funding Source
The funding source had no role in the study.
Footnotes
Conflict of Interest
Kristin Bigos has no conflicts of interest. Other authors will send their conflicts separately.
Contributors
Kristin Bigos was involved with data acquisition, data analysis, and manuscript writing.
Mary Folan was involved with data acquisition and data analysis, and manuscript revisions.
Mark Jones was the social worker and therapist for the patients in this study and was involved with recruitment.
Gretchen Haas completed all the psychiatric evaluations (SCID).
Frank Kroboth completed all the medical histories and physicals.
Patricia Kroboth was involved with funding, data analysis, and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Pigott T, Kalogeras KT, Demitrack M, Dubbert B, Murphy DL, Gold PW. Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49:9–20. doi: 10.1001/archpsyc.1992.01820010009002. [DOI] [PubMed] [Google Scholar]
- Bailly D, Servant D, Dewailly D, Beuscart R, Racadot A, Fossati P. Corticotropin releasing factor stimulation test in obsessive compulsive disorder. Biological Psychiatry. 1994;35:143–146. doi: 10.1016/0006-3223(94)91206-8. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Casarosa E, Pluchino N, Palumbo M, Genazzani AD, Luisi S, Genazzani AR. Effect of dehydroepiandrosterone on central and peripheral levels of allopregnanolone and beta-endorphin. Fertility and Sterility. 2005;83:1161–1168. doi: 10.1016/j.fertnstert.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Mellado C, Alciati A, Pisu MG, Purdy RH, Zanone S, Perini G, Serra M, Biggio G. Plasma concentrations of anxiolytic neuroactive steroids in men with panic disorder. Psychiatry Research. 2005;135:185–190. doi: 10.1016/j.psychres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Catapano F, Monteleone P, Fuschino A, Maj M, Kemali D. Melatonin and cortisol secretion in patients with primary obsessive-compulsive disorder. Psychiatry Research. 1992;44:217–225. doi: 10.1016/0165-1781(92)90025-x. [DOI] [PubMed] [Google Scholar]
- Catapano F, Monteleone P, Maj M, Kemali D. Dexamethasone suppression test in patients with primary obsessive-compulsive disorder and in healthy controls. Neuropsychobiology. 1990;23:53–56. doi: 10.1159/000119427. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Cottraux JA, Bouvard M, Claustrat B, Juenet C. Abnormal dexamethasone suppression test in primary obsessive-compulsive patients: a confirmatory report. Psychiatry Research. 1984;13:157–165. doi: 10.1016/0165-1781(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Eser D, Schüle C, Romeo E, Baghai TC, di Michele F, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuropsychopharmacological properties of neuroactive steroids in depression and anxiety disorders. Psychopharmacology. 2006;186:373–387. doi: 10.1007/s00213-005-0188-z. [DOI] [PubMed] [Google Scholar]
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF, III, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trotini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry. 2001;50:767–774. doi: 10.1016/s0006-3223(01)01198-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department; 1995. [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. Journal of Clinical Endocrinology & Metabolism. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. Journal of Clinical Endocrinology and Metabolism. 1998;83:3130–3133. doi: 10.1210/jcem.83.9.5081. [DOI] [PubMed] [Google Scholar]
- Hsiao C-C. Positive correlation between anxiety severity and plasma levels of dehydroepiandrosterone sulfate in medication-free patients experiencing a major episode of depression. Psychiatry and Clinical Neurosciences. 2006;60:746–750. doi: 10.1111/j.1440-1819.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- Le Mellédo J-M, Baker GB. Neuroactive steroids and anxiety disorders. Journal of Psychiatry and Neuroscience. 2002;27:161–165. [PMC free article] [PubMed] [Google Scholar]
- Le Mellédo J-M, Baker GB. Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Review of Neurotherapeutics. 2004;4:851–860. doi: 10.1586/14737175.4.5.851. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McSwiggan-Hardin M, McDougle CJ, Barr LC, Cohen DJ. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Archives of General Psychiatry. 1994;51:782–792. doi: 10.1001/archpsyc.1994.03950100030003. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgören S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Mahe V, Robel P, Baulieu E-E. Neurosteroids, via σ receptors, modulate the [3H]norepianephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Catapano F, Del Buono G, Maj M. Circadian rhythms of melatonin, cortisol, and prolactin in patients with obsessive-compulsive disorder. Acta Psychiatr Scand. 1994;89:411–415. doi: 10.1111/j.1600-0447.1994.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi M, Colurgio B, Casarosa E, Ioime R, Genazzani AR, Maj M. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosomatic Medicine. 2001;63:62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Research. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. The neuropsychopharmacological potential of neuroactive steroids. J psychiat Res. 1997;31:297–314. doi: 10.1016/s0022-3956(96)00060-x. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biological Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]


