Abstract
Virtually all mammals including humans exhibit neurogenesis throughout life in the hippocampus, a learning and memory center in the brain. Numerous studies in animal models imply that hippocampal neurogenesis is important for functions such as learning, memory and mood. Interestingly, hippocampal neurogenesis is very sensitive to physiological and pathological stimuli. Certain pathological stimuli such as seizures alter both amount and pattern of neurogenesis though the overall effect depends on the type of seizures. Acute seizures are classically associated with augmentation of neurogenesis and migration of newly born neurons into ectopic regions such as the hilus and the molecular layer of the dentate gyrus. Additional studies suggest that abnormally migrated newly born neurons play a role in the occurrence of epileptogenic hippocampal circuitry characteristically seen after acute seizures, status epilepticus or head injury. Recurrent spontaneous seizures such as those typically observed in chronic temporal lobe epilepsy are associated with substantially reduced neurogenesis, which interestingly co-exists with learning and memory impairments and depression. In this review, we discuss both extent and potential implications of abnormal hippocampal neurogenesis induced by acute seizures as well as recurrent spontaneous seizures. We also discuss the consequences of chronic spontaneous seizures on differentiation of neural stem cell progeny in the hippocampus and strategies that are potentially useful for normalizing neurogenesis in chronic temporal lobe epilepsy.
Keywords: adult neurogenesis, dentate neurogenesis, dentate gyrus, epilepsy, seizures, neural stem cells, neural progenitors, stem cell grafts, stem cell proliferation, stem cell differentiation
Introduction
Epilepsy, characterized by periodic and unpredictable occurrence of seizures, affects ∼50 million people worldwide, and temporal lobe epilepsy (TLE) is among the most frequent types of intractable epilepsy [1-4]. While the precise cause of TLE is unknown in most cases, it is typically seen after an initial precipitating injury (IPI) such as status epilepticus (SE), brain injury, tumors, meningitis, encephalitis, and febrile seizures during childhood in other cases [5-9]. There is usually a latent period of several years between this injury and the emergence of chronic TLE characterized by spontaneous recurrent motor seizures (SRMS), learning and memory impairments, depression, substantial neurodegeneration in the dentate hilus (DH) and CA1-CA3 subfields, and aberrant synaptic reorganization [10-16].
Abnormal hippocampal neurogenesis has emerged as another important pathophysiology of TLE over the last decade [17-23]. Neurogenesis is a process of generation of new neurons in the central nervous system (CNS) through division of neural stem cells (NSCs) and neuronal differentiation of newly born cells. Although most of the neurogenesis occurs during the initial development, certain regions of the brain maintain neurogenesis throughout life. These include the dentate gyrus (DG) of the hippocampus and the subventricular zone lining the lateral ventricles [24-28]. Neurogenesis in the adult and aged hippocampus has received great attention [28-37] because of the importance of hippocampus in maintaining normal learning and memory function as well as its dysfunction in diseases such as TLE, Alzheimer's disease and major depressive disorders. Hippocampal NSCs reside in the subgranular zone (SGZ) of the DG, where they proliferate and produce new cells. A great fraction of these new cells differentiate into granule cells of the DG, which migrate up into the granule cell layer (GCL), extend dendrites into the dentate molecular layer, send axons into the dentate hilus and CA3 stratum lucidum. Over time, these newly added granule cells incorporate into the functional hippocampal circuitry through establishment of granule cell specific afferent and efferent synaptic contacts and participate in spatial memory formation [38-44].
However, the extent of hippocampal neurogenesis in the adult brain is not static, as it responds to both physiological and pathological stimuli though the net result of a particular stimulus varies depending on the activation of positive or negative regulators of neurogenesis. For instance, physical exercise or exposure to enriched environment positively enhances the amount of hippocampal neurogenesis through up-regulation of multiple positive regulators of neurogenesis. On the other hand, pathological stimuli such as seizures induce abnormalities in hippocampal neurogenesis though the overall effect depends on the type of seizures. Acute seizures or status epilepticus abnormally increase the amount of hippocampal neurogenesis and induce aberrant migration of a significant fraction of newly born neurons into the dentate hilus and the dentate molecular layer. Spontaneous recurrent motor seizures that occur in chronic temporal lobe epilepsy lead to a radically waned neurogenesis. In the following sections, we review current knowledge on the extent and implications of abnormal hippocampal neurogenesis induced by acute seizures as well as recurrent spontaneous seizures. Additionally, the outcome of recurrent spontaneous seizures on hippocampal NSC activity, neuronal differentiation of the progeny of NSCs, and strategies that are potentially useful for stimulating NSCs and normalizing neurogenesis in chronic TLE are also discussed.
Response of Hippocampal Neurogenesis to Acute Seizures
Pioneering studies on neurogenesis in animal models of TLE by Parent et al [17] and Bengzon et al [45] gave the initial evidence for increased hippocampal neurogenesis following acute seizures. In these studies, a dramatic increase in the production of new cells/neurons was observed in the SGZ-GCL of the DG following pilocarpine induced SE [17] or kindling stimulations [45]. However, by 3-4 weeks after SE, neurogenesis returned to baseline levels [17]. A subsequent study showed that administration of chemoconvulsant kainic acid under anesthesia also increases neurogenesis in the hippocampus [46]. Figure 1 illustrates a schematic of different changes (including increases in DG neurogenesis) that occur following acute seizures. Investigations in a variety of epilepsy models have confirmed the above plasticity of hippocampal neurogenesis to acute seizures [22, 47-54]. This raised a question whether or not a similar phenomenon occurs in humans after acute seizures. While evaluation of hippocampal neurogenesis shortly after acute seizures is yet to be performed in humans, examination of the hippocampus from young TLE patients (<2 years old) suggested increased cell proliferation [55]. Furthermore, epileptic hippocampus from young children (<4 years) also exhibited significant numbers of neural precursor cells [55]. Thus, there is some evidence for increased hippocampal neurogenesis in the early phase of TLE in pediatric patients, which is consistent with studies in animal models of TLE described above.
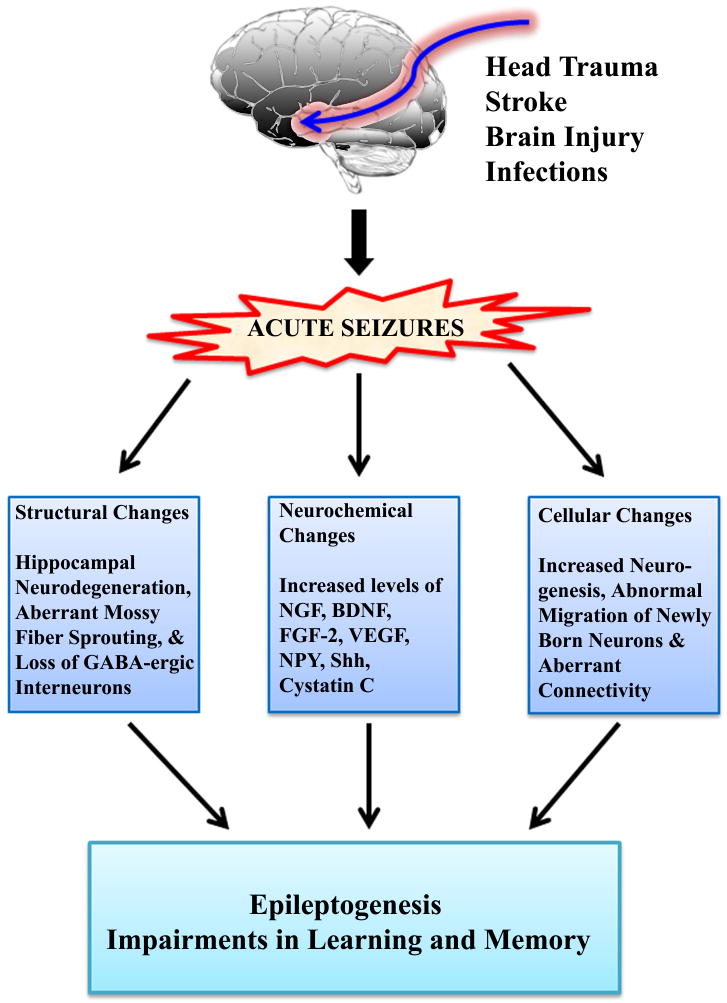
Figure 1.
Schematic of different changes that occur following acute seizures. Acute seizures or status epilepticus typically occur following head trauma, stroke, brain injury or brain infections. Acute seizures induce multiple structural alterations in the brain particularly the hippocampus. The major hippocampal changes include degeneration of fractions of dentate hilar neurons and CA1-CA3 pyramidal neurons, aberrant sprouting of mossy fibers into the dentate supragranular layer and substantial loss of inhibitory interneurons. Acute seizures also transiently up-regulate multiple neurotrophic factors and other proteins in the hippocampus. These include nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), neuropeptide Y (NPY), sonic hedgehog (Shh) and Cystatin C. Additional cellular changes in the hippocampus following acute seizures comprise increased neurogenesis, abnormal migration of newly born neurons into the dentate hilus and dentate molecular layer, and occurrence of hilar basal dendrites in newly added granule cells. All of the above changes are believed to contribute to the formation of aberrant circuitry and epileptogenesis in the hippocampus, and learning and memory impairments.
Potential mechanisms of increased neurogenesis after acute seizures
A proliferative surge occurs in NSCs of the SGZ shortly after SE leading to an increased production of new neurons during the first few weeks after the seizure episode [17, 54, 56, 57]. The precise mechanisms underlying the seizure-induced increase in hippocampal neurogenesis are unclear. However, several potential mechanisms have been proposed. First, it is believed that the release of mitogenic factors from dying neurons, deafferented granule cells and reactive glia likely increase the proliferation of NSCs and the survival of newly formed neurons. This is because, multiple studies demonstrate that several factors that are known to promote NSC proliferation and neuron survival (such as nerve growth factor [NGF], brain-derived neurotrophic factor [BDNF], fibroblast growth factor-2 [FGF-2], vascular endothelial growth factor [VEGF] and sonic hedgehog (Shh) are up-regulated in the hippocampus after acute seizures [58-66]. Second, it is plausible that increased levels of GABA in the DG during the early post-seizure period positively influences neurogenesis, as studies show that GABA has crucial roles in regulating different steps of adult neurogenesis, including proliferation of neural progenitors, migration and differentiation of neuroblasts, and synaptic integration of newborn neurons [67]. Third, it is possible that increased levels of neuropeptide Y (NPY) found typically after acute seizures enhances the proliferation of NSCs in the DG, as studies demonstrate that NSCs increase neurogenesis in the presence of NPY [68-72]. Fourth, modulation of neuron-restrictive silencing factor (NRSF) activity has been suggested to be one of the factors responsible for increased neurogenesis after acute seizures because blocking of NRSF activity through administration of Valproic acid diminishes seizure-induced increases in hippocampal neurogenesis [73]. Fifth, it is likely that augmented neuronal activity during and after seizures promotes an increased proliferation of NSCs, as increased excitatory stimuli can act directly on NSCs and influence the production of new neurons [74]. Thus, it appears that multiple mechanisms underlie increased hippocampal neurogenesis observed after acute seizures.
Adverse effects of increased DG neurogenesis after acute seizures
Numerous studies suggest that increased DG neurogenesis after acute seizures is associated with anomalous migration of a fraction of newly born granule cells into the dentate hilus and/or the dentate molecular layer [11, 17-21, 72, 75-81]. Further investigations demonstrate that aberrantly migrated newly born granule cells exhibit deviant integration with the CA3 network [19], get activated when epileptic rats exhibit spontaneous seizures [73, 82]and respond to perforant path stimulation with a longer latency to onset of evoked responses [21]. Additional studies imply that displaced granule cells in the dentate hilus establish afferent connectivity with mossy fiber terminals [83], exhibit spontaneous bursts of action potentials [19], and contribute to spontaneous seizures in chronically epileptic animals [76, 84]. Similarly displaced granule cells have also been observed in hippocampal tissues obtained from patients with TLE [11, 18, 85, 86]. Thus, it appears that acute seizure induced abnormal DG neurogenesis promotes aberrant circuitry development, which likely contributes to the evolution of initial seizure-induced hippocampal injury into chronic epilepsy.
Likely reasons for anomalous migration of newly born granule cells after acute seizures
The precise reasons for anomalous migration of newly born granule cells are still being examined. However, indirect evidences propose the following. A study by Jessberger et al [54]demonstrates that acute seizures do not significantly influence the proliferation of nestin-expressing NSCs (type 1 cells) but rather stimulate the division of doublecortin (DCX) expressing cells (transit amplifying cells [type 2 cells] and immature neurons [type 3 cells]). Based on this, it is presumed that delayed proliferation during the process of neurogenesis interferes with migration, leading to a significant dispersion of DCX-positive early post-mitotic neurons away from the GCL into the dentate hilus and the molecular layer. However, a recent study suggests that loss of reelin (a secreted migration guidance cue) expression after acute seizures largely contributes to the chain migration and aberrant integration of newly born dentate granule cells into ectopic locations [77]. This study demonstrates that subclasses of interneurons that are typically lost in TLE express reelin and dentate granule cell progenitors express the downstream reelin signaling molecule Disabled 1 (Dab1). This arrangement in normal conditions is believed to promote appropriate migration of newly born neurons into the GCL. However, prolonged seizures decrease reelin (likely due to the loss of reelin expressing interneurons) but increase Dab1 expression in hilar-ectopic neuroblasts. Because exogenous reelin increases detachment of chain-migrating neuroblasts and blockade of reelin signaling increases chain migration in DG explants, it has been suggested that reelin deficiency after acute seizures contributes to ectopic chain migration and aberrant integration of newborn dentate granule cells into the dentate hilus.
Dendritic abnormalities in granule cells that are born after acute seizures
A study by Shapiro et al [53] showed that a significant fraction of new dentate granule cells that are born after acute seizures exhibit abnormal morphological features in the form of basal dendrites. As these basal dendrites seem to run along the GFAP-labeled astrocytic processes in the hilus, involvement of an ectopic glial scaffold in the hilus has been proposed for the formation of hilar basal dendrites after acute seizures. Further studies demonstrate that these basal dendrites persist for prolonged periods and exhibit immature synapses [87], suggesting their involvement in the formation of epileptogenic circuitry. A recent study by Walter and colleagues [88] has elegantly demonstrated that ∼50% of immature granule cells exposed to pilocarpine-induced seizures (i.e. new neurons that were born shortly before the induction of seizures) and ∼40% of new granule cells that are born after the induction of seizures exhibit aberrant hilar basal dendrites. These results suggest the existence of a critical period after the birth of adult-generated neurons during which they are vulnerable to being recruited into epileptogenic neuronal circuits. Furthermore, seizures seem to accelerate the morphological development of newly born granule cells, causing their dendrites to extend swiftly through the molecular layer leading to a rapid functional integration of adult-generated granule cells [89]. Interestingly, neurons born one month after acute seizures also exhibit alterations in dendrite morphology, suggesting persistent effects of seizures on granule cell maturation [89]. A recent study by Arisi and Garcia-Cairasco [90] reports that apical dendrites of newly born neurons born shortly after the SE exhibit more bifurcations inside the granular cell layer and more terminations in the inner molecular layer, implying that a concentration of apical dendrites occurs in the inner molecular layer where mossy fibers typically sprout after SE. This dendritic arrangement likely facilitates formation of greater number of synapses between aberrantly sprouted mossy fibers and the dendrites of newly born granule cells and contributes to increased epileptogenesis. Thus, the overall dendritic properties of newly born neurons that are born shortly after SE are predisposed for epileptogenesis.
Will suppression of abnormal neurogenesis after acute seizures reduce epileptogenesis and prevent cognitive impairment?
As acute seizure induced abnormal DG neurogenesis promotes aberrant circuitry development and likely contributes to the evolution of initial seizure-induced hippocampal injury into chronic epilepsy, an important question emerges. Will prevention or suppression of acute seizure-induced abnormal neurogenesis reduce the frequency and intensity of spontaneous seizures in the chronic phase of epilepsy? Jung and associates [84] reduced SE-induced hippocampal neurogenesis through administration of an antimitotic agent cytosine-b-D-arabinofuranoside (Ara-C) into the lateral ventricle in an animal model and evaluated the frequency of spontaneous seizures. They reported reduction in both frequency and duration of spontaneous seizures in animals that received Ara-C following SE, in comparison to animals that received vehicle after SE. These results are supportive of the notion that seizure-induced abnormal neurogenesis contributes to the development of chronic epilepsy. However, there are some caveats that need to be addressed in future studies. First, it remains to be addressed whether the beneficial effects of Ara-C would persist for prolonged periods after SE, as spontaneous seizures were quantified only during the early phase (28-34 days) after SE. Second, it will be necessary to resolve whether the positive effects of Ara-C treatment after SE is a result of decreased number of ectopic granule cells or decreased proliferation of glia. Third, it is critical to determine whether Ara-C treatment blocks other epileptogenic changes that promote chronic epilepsy. It is interesting to note that, studies by Raedt et al. [91] and Pekcec et al. [92, 93] report contrasting results. Exposure of rats to whole brain low-dose (8 Gy) γ -radiation one day before the initiation of rapid hippocampal kindling significantly suppressed the generation of new granule cells but had no effect on the final establishment of the permanent fully kindled state [91]. Based on these results authors argue that seizure-induced neurogenesis does not play a prominent role in epileptogenesis. Pekcec et al [92, 93] examined the effects of reducing the proliferation rate of hippocampal NSCs and the fate of newborn neurons via transient enzymatic depolysialylation of neural cell adhesion molecule (NCAM) in two different models of epilepsy. They found no changes in the generation of a hyperexcitable kindled network in the kindling model of epilepsy or development of spontaneous seizures in the SE model of epilepsy following the above suppression of neurogenesis. Because both radiation exposure and enzymatic depolysialylation of NCAM might lead to multiple side effects [94-99], additional studies that selectively ablate neurogenesis are however required to confirm these findings. Thus, it remains to be seen whether complete elimination of aberrant neurogenesis after the SE would prevent the evolution of SE into chronic epilepsy.
Suppression of abnormal hippocampal neurogenesis after acute seizures might also be important for reducing behavioral abnormalities such as learning and memory impairments. Indeed, a study demonstrated that blockage of seizure-induced neurogenesis through an antiepileptic drug Valproic acid (VPA) protected animals from seizure-induced cognitive impairment in a hippocampus-dependent learning task [73]. These results imply that seizure-generated granule cells have the potential to interfere with hippocampal function and contribute to cognitive impairment caused by epileptic activity within the hippocampal circuitry. Similarly, Pekcec et al [93] demonstrated that blockage of seizure-induced neurogenesis through enzymatic depolysialylation of NCAM during SE and in the early phase of epileptogenesis resulted in a cognition sparing effect as revealed by the water maze test. Thus, blocking of seizure-induced abnormal neurogenesis appears useful for minimizing cognitive impairments associated with acute seizures. Hence, rigorous studies on early interventions that thwart seizure-induced abnormal neurogenesis are needed in the future to develop this strategy for diminishing both chronic epilepsy development and cognitive impairments.
Response of Hippocampal Neurogenesis to Chronic Spontaneous Seizures
Changes in the extent of neurogenesis in animal models of chronic epilepsy
Increased neurogenesis observed shortly after acute seizures returns to baseline by about 2 months after the initial seizure episode in rats [73]. However, the extent of neurogenesis declines radically in the chronic phase of epilepsy when significant numbers of spontaneous seizures manifest [22]. By employing DCX as a marker of newly born neurons in two distinct rat models of TLE, Hattiangady et al. [22] reported 64-81% decrease in neurogenesis. Furthermore, an inverse relationship was evident between the frequency of spontaneous seizures and the extent of neurogenesis, as the overall decrease in neurogenesis was considerably greater in rats exhibiting increased number of spontaneous seizures. Additional evaluation using pulsed injections of 5′-bromodeoxyuridine (BrdU) at 5 months post-SE demonstrated that the overall addition of new cells to the SGZ-GCL and the extent of long-term survival in chronic epilepsy are analogous to that observed in the age-matched intact hippocampus [100]. However, phenotypical characterization revealed that only ∼4% of newly generated cells differentiated into mature neurons in chronic epileptic conditions, in contrast to 80% of newly born cells exhibiting neuronal differentiation in the age-matched intact hippocampus. A subsequent study using a mouse model of TLE also reported similar changes in neuronal differentiation of newly born cells in chronic epilepsy where reduced neurogenesis was associated with increased production of new astrocytes [52]. Another study by Heinrich et al [101] reported a gradual fall in neurogenesis at one week and virtual loss of all neurogenesis by 4-6 weeks after the initial seizure episode, which interestingly paralleled granule cell dispersion and widening of GCL. In contrast to the above findings, Bonde et al [102], using an electrically evoked SE model, reported no changes in neurogenesis within the dorsal hippocampus at 6 months post-SE. Similarly, in a lithium-pilocarpine model of epilepsy using postnatal day 20 rats, a modest increase in neurogenesis was observed even at 2 months post-SE [103]. From the above findings, it emerges that decreased levels of hippocampal neurogenesis in chronic epilepsy depends on the model and age of the animal at the time of the initial seizure episode. Adult animals seem to be vulnerable for an almost complete loss of neurogenesis in the chronic phase after the initial seizure episode.
Characteristics of hippocampal neurogenesis in human TLE
Only a few studies have so far examined neurogenesis in hippocampal tissues of TLE patients. Accurate interpretation of results from these specimens has been difficult because of several constraints, which include non-availability of hippocampal tissues at different stages of epilepsy and apt age-matched control samples, and lack of apt markers that detect neurogenesis. Mathern et al [104]demonstrated decreased density of cells positive for PSA-NCAM (a putative marker of newly born neurons) in the DG from children exhibiting frequent spontaneous seizures than the DG from age-matched autopsy samples, implying that severe seizures during early childhood are associated with decreased hippocampal neurogenesis. Examination of hippocampal tissues from adult patients with chronic TLE in subsequent studies also revealed reduced density of PSA-NCAM+ cells [23, 105]. Another study by Crespel et al [106] also found minimal numbers of DCX+ cells in the SGZ of hippocampal tissues from patients with mesial TLE, despite an evidence for increased proliferation of cells immunopositive for Musashi-1 (a putative marker of NSCs) in these samples. Furthermore, a study by Fahrner et al [107] demonstrated both decreased synthesis of mRNA for DCX and absence of cells positive for Ki-67 (an endogenous marker of proliferative cells) in hippocampal tissues from chronic TLE patients. Thus, available reports are supportive of the finding in animal models that chronic epilepsy is associated with declined hippocampal neurogenesis. Considering the potential functions of hippocampal neurogenesis, it is likely that hippocampal-dependent learning and memory deficits and depressive behavior observed in TLE are at least partially linked to decreased hippocampal neurogenesis.
Potential mechanisms underlying decreased neurogenesis in chronic TLE
While the precise mechanisms underlying decreased neurogenesis in chronic TLE are unknown, several potential reasons have been proposed. Although a role for chronic inflammation in this decline is an attractive hypothesis, a study on activated microglial cells has almost ruled out this possibility as only minimal density of such cells was found in the hippocampus during chronic epilepsy [22]. Another potential reason underlying decreased neurogenesis includes the presence of adverse hippocampal milieu for neurogenesis from NSCs and decreased numbers of NSCs. While unfavorable NSC milieu can be gleaned from decreased levels of NSC mitogenic factors such as FGF-2, IGF-1 and BDNF in chronic epilepsy [22, 63], numbers of NSCs do not appear to change drastically in chronic epilepsy. In fact, a human study suggests an increased number of putative NSCs positive for Musashi-1 in chronic TLE [106]. Furthermore, a recent study reports only moderate decreases in numbers of putative NSCs positive for Sox-2 or vimentin in the SGZ of chronically epileptic animals [108]. Thus, significant numbers of NSCs persist during chronic epilepsy. However, neuronal differentiation of the progeny of NSCs is impaired in chronic epilepsy [100, 52], likely due to an unfavorable hippocampal milieu discussed above. Increased concentration of Wnt protein inhibitor Dickkopf-1 in chronic epilepsy [109] further supports the unfavorable milieu hypothesis. This is because blockade of Wnt signaling abolishes DG neurogenesis [110].
Is diminished neurogenesis linked to persistence of spontaneous seizures, cognitive impairments and depressive behavior in chronic TLE?
Diminished hippocampal neurogenesis might contribute to the persistence of spontaneous seizures, learning and memory impairments and depression prevailing in chronic TLE. First, it is plausible that decreased addition of new neurons to the GCL interferes with the possible spontaneous repair of hyperexcitability in the DG. This is because, a study using an electrical stimulation model of SE implies that granule cells that are born and integrated into the GCL at extended time-points after the SE receive reduced excitatory synaptic input and display an enhanced inhibitory synaptic drive [111]. Second, in view of findings that newly formed neurons get incorporated into the hippocampal circuitry and actively participate in learning and memory function [40, 42, 112-114], decreased addition of new functional granule cells into the GCL in chronic epilepsy likely contributes to impairments in hippocampal-dependent learning and memory functions observed in TLE. This notion is also supported by the finding that the overall granule cell density is the most significant predictor accounting for the total memory capacity in an individual TLE patient [115, 116]. Third, from the perspective of findings that increased production of new neurons in the hippocampus is essential for effective action of antidepressants [117-121], diminished addition of new granule cells into the GCL perhaps plays a role in depressive like behavior prevailing in chronic epilepsy. Thus, available evidence is supportive of the perception that diminished hippocampal neurogenesis plays a role in maintaining spontaneous seizures, learning and memory impairments and depression in chronic TLE. It remains to be determined however whether strategies that have the potential to increase neurogenesis in chronic epileptic conditions would be capable of easing any of these impairments.
Potential strategies for augmenting dentate neurogenesis in chronic TLE
Based on studies in animal models of brain disease and injury, the following strategies appear promising for increasing neurogenesis in chronic epilepsy: administration of distinct neurotrophic factors, physical exercise, exposure to an enriched environment, antidepressant therapy and grafting of NSCs. Figure 2 illustrates various time-points after acute seizures at which interventional strategies may be applied to alleviate the acute seizure induced chronic epilepsy, learning and memory impairments, and depression. Administration of neurotrophic factors is relevant because many factors that promote neurogenesis (such as FGF-2, IGF-1 and BDNF) exhibit decreased levels in chronic epilepsy [22, 63]. While no studies are currently available on the effects of administration of these factors into the chronically epileptic hippocampus, this approach has promise based on their ability to enhance neurogenesis in both intact and injured aged hippocampus [122-126]. Performing physical exercise and exposure to enriched environment for increasing neurogenesis in chronic epilepsy are very appealing because these approaches are non-invasive and have many beneficial effects [127-129] other than increasing neurogenesis [130-132]. Pertaining to physical exercise, studies imply that physical exercise decreases the incidence and severity of seizures in epileptic patients as well as in animal models of epilepsy [133-137]. Furthermore, physical exercise enhances the concentration of several factors (such as BDNF, NGF, VEGF) and phosphorylated cAMP response element binding protein (pCREB) that promote neurogenesis and cognitive function [102, 138-141]. Regarding exposure to enrichment, studies demonstrate that environmental enrichment of rats prior to KA administration increases the seizure threshold, decreases hippocampal neurodegeneration, improves learning and memory abilities and alleviates depressive-like behavior [142-146].
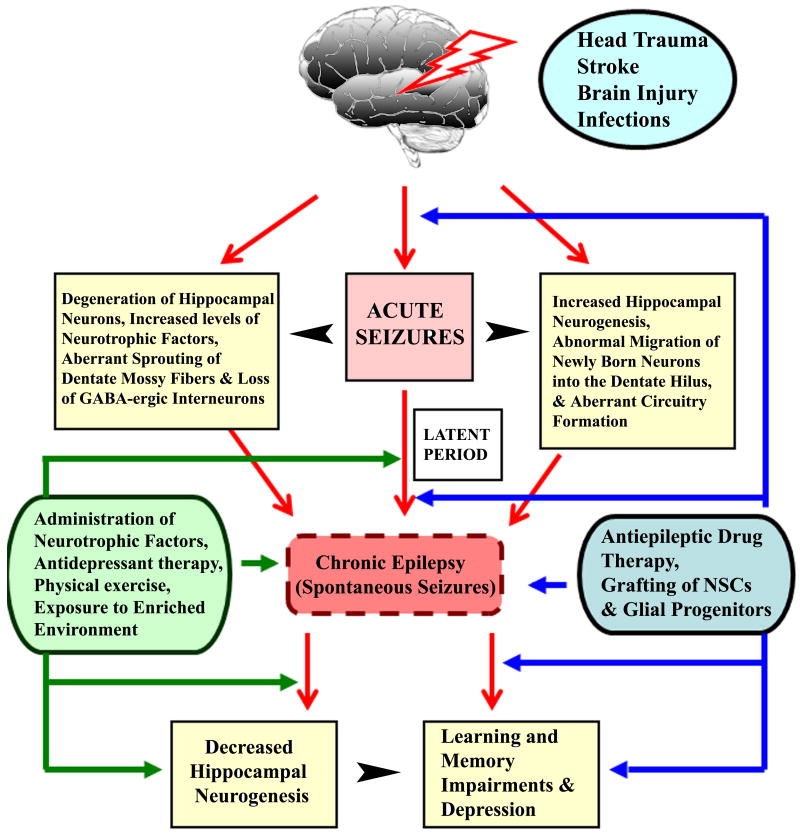
Figure 2.
Schematic showing various time-points after acute seizures at which interventional strategies may be applied to alleviate the acute seizure induced chronic epilepsy, learning and memory impairments, and depression. Acute seizures induce multiple structural, neurochemical and cellular changes in the hippocampus, which are believed to contribute to the development of chronic epilepsy characterized by spontaneous seizures. Chronic epilepsy leads to a substantial decline in hippocampal neurogenesis which is believed to contribute towards learning and memory impairments and depression. Interventional strategies such as administration of neurotrophic factors, antidepressant therapy, physical exercise, exposure to enriched environment, and grafting of neural stem cells (NSCs) and glial progenitors, may be useful when applied during the latent period (or silent phase after acute seizures), at the commencement of spontaneous seizures or after the establishment of chronic epilepsy. Interventional strategies such as antiepileptic drug therapy are likely useful at all of the above time-time points as well as immediately after the onset of acute seizures.
Antidepressant therapy in chronic epilepsy is another interesting approach for increasing neurogenesis and cognitive impairments, as antidepressant treatments enhance DG neurogenesis [117-121] likely via increases in the concentrations of serotonin, norepinephrine, CREB and multiple neurotrophic factors [147-150]. Because decreased neurogenesis, cognitive impairments and depression co-exist in chronic epilepsy, prolonged antidepressant therapy appears to be a useful approach for easing these problems.
Grafting of NSCs or glial progenitors into the chronically epileptic hippocampus might also increase neurogenesis, as a study showed that dramatically diminished neurogenesis in aging hippocampus could be reversed considerably with this approach [151]. Grafting of NSCs into the chronically epileptic hippocampus might also induce other beneficial effects such as seizure control through generation of new GABA-ergic interneurons [79, 152] and improved cognitive function through the release of useful neurotrophic factors by the grafted NSCs. Thus, several approaches appear promising for improving neurogenesis, cognitive function, and mood in chronic epilepsy. However, rigorous long-term studies in chronic epilepsy models are clearly needed in the future to validate these approaches.
Potential consequences of increasing neurogenesis in chronic TLE
Although increasing hippocampal neurogenesis in chronic epilepsy using a variety of strategies proposed above is attractive considering the possible involvement of neurogenesis in hippocampal-dependent learning and memory function and mood, it is difficult to predict the overall impact of increased neurogenesis in chronic epilepsy on the frequency and intensity of spontaneous seizures. This uncertainty stems from the suggested role of aberrant DG neurogenesis occurring at early time-points after SE towards the evolution of SE into chronic epilepsy [18, 19, 21, 72, 78]. Beneficial effects such as restrained spontaneous seizures, reduced learning and memory impairments and better mood are expected, if the majority of newly generated granule cells migrates into the GCL and integrate into the hippocampal circuitry with apt connectivity or with a pattern of connectivity described by Jakubs et al [111]. However, exacerbation of the epileptogenic circuitry in the hippocampus might occur if a greater fraction of newly born neurons exhibit aberrant migration and incorporate inappropriately into the dentate hilus or the molecular layer. Therefore, detailed studies examining these issues are critical in future.
Overall Conclusions
Studies in animal models clearly reveal that, at early time-points after an initial precipitating injury such as acute seizures or SE, increased hippocampal neurogenesis and abnormal recruitment of newly born neurons into the hippocampal circuitry occurs. Studies on hippocampal tissues from pediatric patients with early phase of TLE support these findings. However, the relative contribution of this aberrant circuitry for the evolution of initial seizure-induced hippocampal injury into chronic epilepsy remains to be determined. Approaches that selectively ablate aberrant neurogenesis need to be developed for determining the full impact of blocking the initial seizure-induced abnormal neurogenesis on spontaneous seizures, cognitive function and mood. Studies in both animal models and hippocampal tissues from patients with TLE reveal that the chronic phase of epilepsy is associated with substantially decreased hippocampal neurogenesis. Available analyses suggest that decreased neurogenesis is a consequence of dramatic decreases in the neuronal differentiation of newly born cells rather than decreased production of new cells or substantial decreases in numbers of NSCs. Impaired neuronal differentiation of newly generated cells in chronic epilepsy appears to be related to the presence of an unfavorable hippocampal microenvironment, typified by depletion in the concentration of several factors that promote neurogenesis. Based on the suggested functions of DG neurogenesis, it is possible that dramatically waned DG neurogenesis contributes to persistence of seizures, learning and memory dysfunction and depression observed in chronic epilepsy. Considering these, development of strategies that enhance hippocampal neurogenesis such as administration of distinct neurotrophic factors, physical exercise, exposure to enriched environment, antidepressant therapy, grafting of NSCs are proposed for easing various impairments associated with chronic epilepsy. However, the outcome of increased DG neurogenesis during chronic epilepsy will largely depend upon the behavior and connectivity of newly born neurons. Therefore, studies that examine the effects of increased DG neurogenesis in chronic epilepsy on spontaneous seizures, learning and memory function, and mood are critically needed to make further progress in this field.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS054780 & NS 043507 to A.K.S.), National Institute for Aging (AG20924 to A.K.S.) and the Department of Veterans Affairs (VA Merit Review Award to A.K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engel J., Jr Intractable epilepsy: definition and neurobiology. Epilepsia. 2001;42 6:3. doi: 10.1046/j.1528-1157.2001.0420s6003.x. [DOI] [PubMed] [Google Scholar]
- 2.Litt B, et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron. 2001;30(1):51–64. doi: 10.1016/s0896-6273(01)00262-8. [DOI] [PubMed] [Google Scholar]
- 3.McKeown MJ, McNamara JO. When do epileptic seizures really begin? Neuron. 2001;30(1):1–3. doi: 10.1016/s0896-6273(01)00253-7. [DOI] [PubMed] [Google Scholar]
- 4.Strine TW, et al. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46(7):1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 5.French JA, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34(6):774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 6.Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg. 1995;82(2):220–227. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- 7.Mathern GW, et al. The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res. 1996;26(1):151–61. doi: 10.1016/s0920-1211(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 8.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1(3):173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DV. Losing neurons: selective vulnerability and mesial temporal sclerosis. Epilepsia. 2005;46 7:39–44. doi: 10.1111/j.1528-1167.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 10.Sutula T, et al. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26(3):321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- 11.Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535(2):195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- 12.Devinsky O. Therapy for neurobehavioral disorders in epilepsy. Epilepsia. 2004;45 2:34–40. doi: 10.1111/j.0013-9580.2004.452003.x. [DOI] [PubMed] [Google Scholar]
- 13.Helmstaedter C, et al. Depressed mood and memory impairment in temporal lobe epilepsy as a function of focus lateralization and localization. Epilepsy Behav. 2004;5(5):696–701. doi: 10.1016/j.yebeh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Mazza M, et al. Epilepsy and depression: risk factors for suicide? Clin Ter. 2004;155(10):425–427. [PubMed] [Google Scholar]
- 15.Detour J, et al. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46(4):499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]
- 16.Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328(2):143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parent JM, et al. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 19.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20(16):6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharfman HE, et al. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol. 2002;454(4):424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharfman HE, et al. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121(4):1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 22.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17(3):473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Pirttila TJ, et al. Cystatin C modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiol Dis. 2005;20(2):241–253. doi: 10.1016/j.nbd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 25.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90(5):2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4(6):1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron HA, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56(2):337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17(7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould E, et al. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3(5):186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 32.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao MS, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 34.Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- 35.Rao MS, Hattiangady B, Shetty AK. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 2008 doi: 10.1002/hipo.20449. PMID: 18493929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hattiangady B, Rao MS, Shetty AK. Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age. Aging Cell. 2008;7(2):207–224. doi: 10.1111/j.1474-9726.2007.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29(1):129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan MS, Bell DH. Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp Brain Res. 1983;52(1):1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- 39.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406(4):449–460. [PubMed] [Google Scholar]
- 40.Kee N, et al. Imaging activation of adult-generated granule cells in spatial memory. Nat Protoc. 2007;2(12):3033–3044. doi: 10.1038/nprot.2007.415. [DOI] [PubMed] [Google Scholar]
- 41.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 42.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31(4):163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Zhao CS, Overstreet-Wadiche L. Integration of adult generated neurons during epileptogenesis. Epilepsia. 2008;49 5:3–12. doi: 10.1111/j.1528-1167.2008.01632.x. [DOI] [PubMed] [Google Scholar]
- 45.Bengzon J, et al. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94(19):10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790(12):52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa E, et al. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41(1):10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 48.Covolan L, et al. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10(2):169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 49.Sankar R, et al. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41 6:S53–56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 50.Ekdahl CT, et al. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14(6):937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 51.Radley JJ, Jacobs BL. Pilocarpine-induced status epilepticus increases cell proliferation in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent mechanism. Brain Res. 2003;966(1):1–12. doi: 10.1016/s0006-8993(02)03989-6. [DOI] [PubMed] [Google Scholar]
- 52.Kralic JE, Ledergerber DA, Fritschy JM. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur J Neurosci. 2005;22(8):1916–1927. doi: 10.1111/j.1460-9568.2005.04386.x. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro LA, Korn MJ, Ribak CE. Newly generated dentate granule cells from epileptic rats exhibit elongated hilar basal dendrites that align along GFAP-immunolabeled processes. Neuroscience. 2005;136(3):823–831. doi: 10.1016/j.neuroscience.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 54.Jessberger S, et al. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196(2):342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Blumcke I, et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11(3):311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- 56.Parent JM, et al. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19(11):4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huttmann K, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18(10):2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 58.Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56(3):597–604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- 59.Bugra K, et al. aFGF, bFGF and flg mRNAs show distinct patterns of induction in the hippocampus following kainate-induced seizures. Eur J Neurosci. 1994;6(1):58–66. doi: 10.1111/j.1460-9568.1994.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 60.Gall CM, Berschauer R, Isackson PJ. Seizures increase basic fibroblast growth factor mRNA in adult rat forebrain neurons and glia. Brain Res Mol Brain Res. 1994;21(34):190–205. doi: 10.1016/0169-328x(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 61.Riva MA, et al. Short- and long-term induction of basic fibroblast growth factor gene expression in rat central nervous system following kainate injection. Neuroscience. 1994;59(1):55–65. doi: 10.1016/0306-4522(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Pinilla F, van der Wal EA, Cotman CW. Possible coordinated gene expressions for FGF receptor, FGF-5, and FGF-2 following seizures. Exp Neurol. 1995;133(2):164–174. doi: 10.1006/exnr.1995.1019. [DOI] [PubMed] [Google Scholar]
- 63.Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87(1):147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- 64.Shetty AK, et al. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78(4):520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- 65.Croll SD, Goodman JH, Scharfman HE. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv Exp Med Biol. 2004;548:57–68. doi: 10.1007/978-1-4757-6376-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee SB, et al. Recruitment of the Sonic hedgehog signaling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci. 2005;22(7):1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ge S, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Madsen TM, et al. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47(12):1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 69.Howell OW, et al. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86(3):646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 70.Howell OW, et al. Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis. 2007;26(1):174–188. doi: 10.1016/j.nbd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Scharfman HE, Gray WP. Plasticity of neuropeptide Y in the dentate gyrus after seizures, and its relevance to seizure-induced neurogenesis. EXS. 2006;(95):193–211. doi: 10.1007/3-7643-7417-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48 2:33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jessberger S, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27(22):5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 75.Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- 76.McCloskey DP, et al. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24(8):2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong C, et al. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27(8):1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- 79.Shetty AK, Hattiangady B. Prospects of Stem Cell therapy for Temporal Lobe Epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49 5:26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315(5810):336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111(1):71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- 83.Pierce JP, et al. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196(2):316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jung KH, et al. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19(12):3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- 85.Sloviter RS, et al. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308(3):381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- 86.Thom M, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002;61(6):510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69(1):53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Walter C, et al. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci. 2007;27(28):7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Overstreet-Wadiche LS, et al. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26(15):4095–5003. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arisi GM, Garcia-Cairasco N. Doublecortin-positive newly born granule cells of hippocampus have abnormal apical dendritic morphology in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2007;1165:126–134. doi: 10.1016/j.brainres.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 91.Raedt R, et al. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007;48(10):1952–1963. doi: 10.1111/j.1528-1167.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 92.Pekcec A, et al. Impact of the PSA-NCAM system on pathophysiology in a chronic rodent model of temporal lobe epilepsy. Neurobiol Dis. 2007;27(1):54–66. doi: 10.1016/j.nbd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Pekcec A, et al. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem. 2008;105(2):389–400. doi: 10.1111/j.1471-4159.2007.05172.x. [DOI] [PubMed] [Google Scholar]
- 94.Calvo W, et al. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61(731):1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 95.Hodges H, et al. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav Brain Res. 1998;91(12):99–114. doi: 10.1016/s0166-4328(97)00108-3. [DOI] [PubMed] [Google Scholar]
- 96.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 97.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 98.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 99.Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16(3):261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- 100.Hattiangady B, Rao MS, Rai KS, Shetty AK. Enduring Survival of new neurons born at early post-status and during chronic epilepsy in the rat dentate gurus. Epilepsia. 2005;46:s8, 106. [Google Scholar]
- 101.Heinrich C, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006;26(17):4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci. 2006;23(4):965–974. doi: 10.1111/j.1460-9568.2006.04635.x. [DOI] [PubMed] [Google Scholar]
- 103.Cha BH, et al. Spontaneous recurrent seizure following status epilepticus enhances dentate gyrus neurogenesis. Brain Dev. 2004;26(6):394–397. doi: 10.1016/j.braindev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Mathern GW, et al. Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia. 2002;43 5:68–73. doi: 10.1046/j.1528-1157.43.s.5.28.x. [DOI] [PubMed] [Google Scholar]
- 105.Pirttila TJ, et al. Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann Neurol. 2005;58(2):211–223. doi: 10.1002/ana.20545. [DOI] [PubMed] [Google Scholar]
- 106.Crespel A, et al. Increased number of neural progenitors in human temporal lobe epilepsy. Neurobiol Dis. 2005;19(3):436–450. doi: 10.1016/j.nbd.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 107.Fahrner A, et al. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007;203(2):320–332. doi: 10.1016/j.expneurol.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 108.Kuruba R, et al. Hippocampal stem cell activity in a rat model of chronic temporal lobe epilepsy. In Soc Neurosci Abstracts. 2007:237.5. [Google Scholar]
- 109.Busceti CL, et al. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48(4):694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 110.Lie DC, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 111.Jakubs K, et al. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;56(6):1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 112.Shors TJ, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;12(2):513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 114.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 115.Pauli E, et al. Deficient memory acquisition in temporal lobe epilepsy is predicted by hippocampal granule cell loss. Neurology. 2006;67(8):1383–1389. doi: 10.1212/01.wnl.0000239828.36651.73. [DOI] [PubMed] [Google Scholar]
- 116.Siebzehnrubl FA, Blumcke I. Neurogenesis in the human hippocampus and its relevance to temporal lobe epilepsies. Epilepsia. 2008;49 5:55–65. doi: 10.1111/j.1528-1167.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 117.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 118.Drew MR, Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets. 2007;6(3):205–218. doi: 10.2174/187152707780619353. [DOI] [PubMed] [Google Scholar]
- 119.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 120.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perera TD, Park S, Nemirovskaya Y. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14(4):326–338. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- 122.Lichtenwalner RJ, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 123.Jin K, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2(3):175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 124.Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26(7):1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- 125.Hattiangady B, Shetty AK. Intracerebroventricular infusions of FGF-2 or BDNF enhance dentate neurogenesis in the injured senescent hippocampus. Soc Neurosci Abstracts. 2005:24.13. [Google Scholar]
- 126.Hattiangady B, Shetty AK. Subcutaneous Administration of BDNF Dramatically Enhances Dentate neurogenesis in the Injured Aged hippocampus. Soc Neurosci Abstracts. 2007:562.12. [Google Scholar]
- 127.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 128.Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175(1):1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 130.Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- 131.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Denio LS, Drake ME, Jr, Pakalnis A. The effect of exercise on seizure frequency. J Med. 1989;20(2):171–176. [PubMed] [Google Scholar]
- 134.Arida RM, et al. Effect of physical exercise on seizure occurrence in a model of temporal lobe epilepsy in rats. Epilepsy Res. 1999;37(1):45–52. doi: 10.1016/s0920-1211(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 135.Arida RM, et al. Physical training reverts hippocampal electrophysiological changes in rats submitted to the pilocarpine model of epilepsy. Physiol Behav. 2004;83(1):165–171. doi: 10.1016/j.physbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 136.Arida RM, et al. Physical exercise in epilepsy: the case in favor. Epilepsy Behav. 2007;11(3):478–479. doi: 10.1016/j.yebeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Eriksen HR, et al. Physical exercise in women with intractable epilepsy. Epilepsia. 1994;35(6):1256–1264. doi: 10.1111/j.1528-1157.1994.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 138.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 139.Neeper SA, et al. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 140.Neeper SA, et al. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(12):49–56. [PubMed] [Google Scholar]
- 141.Soya H, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961–967. doi: 10.1016/j.bbrc.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 142.Young D, et al. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5(4):448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 143.Auvergne R, et al. Delayed kindling epileptogenesis and increased neurogenesis in adult rats housed in an enriched environment. Brain Res. 2002;954(2):277–85. doi: 10.1016/s0006-8993(02)03355-3. [DOI] [PubMed] [Google Scholar]
- 144.Faverjon S, et al. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology. 2002;59(9):1356–1364. doi: 10.1212/01.wnl.0000033588.59005.55. [DOI] [PubMed] [Google Scholar]
- 145.Rutten A, et al. Memory impairment following status epilepticus in immature rats: time-course and environmental effects. Eur J Neurosci. 2002;16(3):501–513. doi: 10.1046/j.1460-9568.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- 146.Koh S, et al. Depressive behavior and selective down-regulation of serotonin receptor expression after early-life seizures: reversal by environmental enrichment. Epilepsy Behav. 2007;10(1):26–31. doi: 10.1016/j.yebeh.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thome J, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20(11):4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 150.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 151.Hattiangady B, et al. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25(8):2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 152.Chu K, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004;1023(2):213–221. doi: 10.1016/j.brainres.2004.07.045. [DOI] [PubMed] [Google Scholar]