Abstract
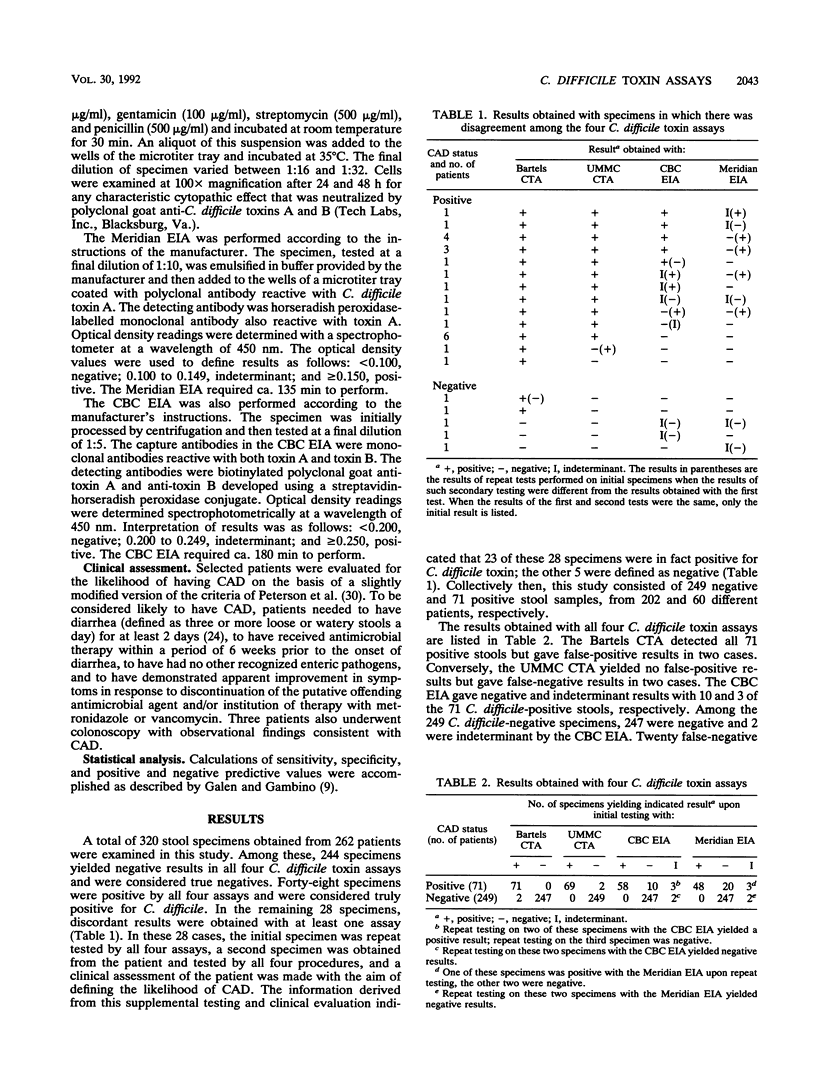
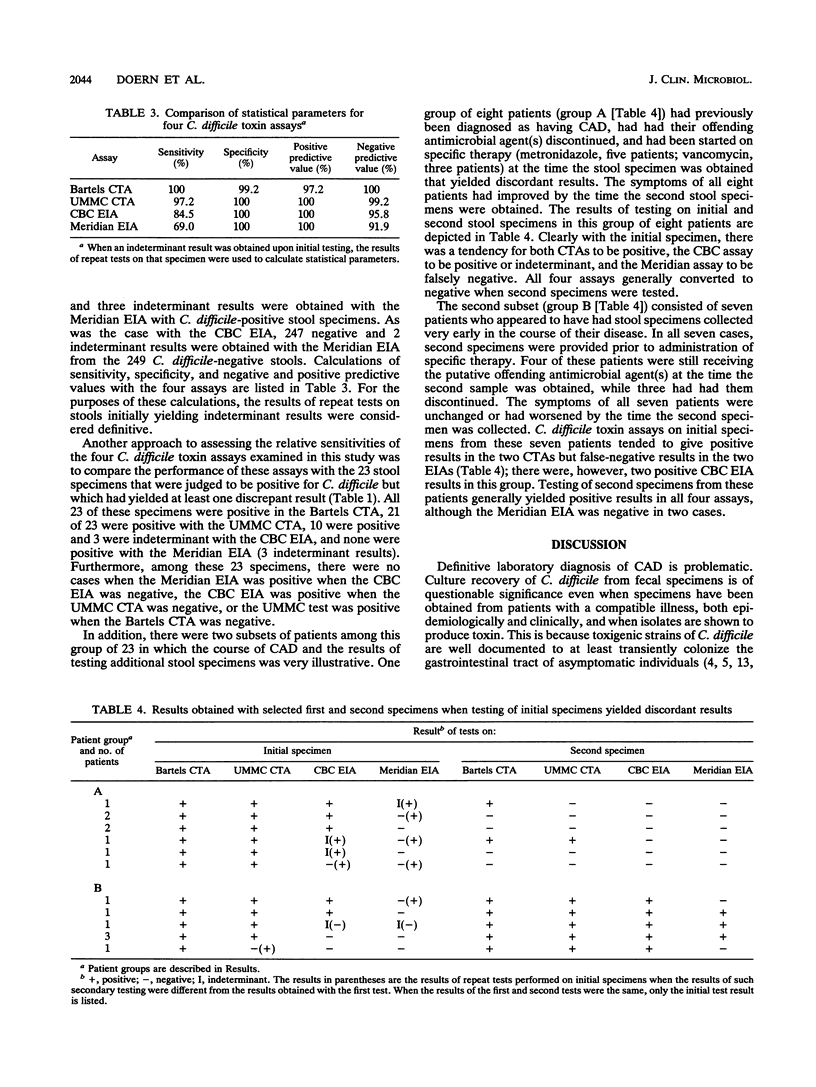
A total of 320 stool specimens obtained from 262 patients suspected of having Clostridium difficile-associated gastrointestinal disease were examined with two cytotoxicity assays (CTAs) and two commercially available enzyme immunoassays (EIAs). The CTAs were an in-house-developed procedure (University of Massachusetts Medical Center [UMMC], Worcester, Mass.) and a commercial test (Bartels CTA; Baxter Healthcare Corp., West Sacramento, Calif.). One EIA was a monoclonal antibody-based assay for C. difficile toxins A and B (Cambridge Biotech Corp. [CBC], Worcester, Mass.). The other EIA employed monoclonal antibodies directed against only toxin A (Meridian Diagnostics, Cincinnati, Ohio). True-positive and true-negative results were defined on the basis of the results of the four assays, clinical assessments of patients, and the results of other laboratory tests. The sensitivities of the four assays were as follows: Bartels CTA, 100%; UMMC CTA, 97.2%; CBC EIA, 84.5%; and Meridian EIA, 69.0%. The Bartels CTA demonstrated a specificity of 99.2%. The other three assays had a specificity of 100%.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jumaili I. J., Shibley M., Lishman A. H., Record C. O. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984 Jan;19(1):77–78. doi: 10.1128/jcm.19.1.77-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson B., Granström M., Möllby R., Nord C. E. Enzyme immunoassay for detection of Clostridium difficile toxins A and B in patients with antibiotic-associated diarrhoea and colitis. Eur J Clin Microbiol. 1985 Apr;4(2):102–107. doi: 10.1007/BF02013572. [DOI] [PubMed] [Google Scholar]
- Aronsson B., Möllby R., Nord C. E. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980-1982. J Infect Dis. 1985 Mar;151(3):476–481. doi: 10.1093/infdis/151.3.476. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Taylor N. S., Chang T., Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2521–2526. doi: 10.1093/ajcn/33.11.2521. [DOI] [PubMed] [Google Scholar]
- Bennett R. G., Laughon B. E., Mundy L. M., Bobo L. D., Gaydos C. A., Greenough W. B., 3rd, Bartlett J. G. Evaluation of a latex agglutination test for Clostridium difficile in two nursing home outbreaks. J Clin Microbiol. 1989 May;27(5):889–893. doi: 10.1128/jcm.27.5.889-893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenning D. A., Fleisher G. R., Campos J. M., Hulkower C. W., Quinlan R. W. Clostridium difficile in a pediatric outpatient population. Pediatr Infect Dis. 1982 Sep-Oct;1(5):336–338. doi: 10.1097/00006454-198209000-00011. [DOI] [PubMed] [Google Scholar]
- Fluit A. C., Wolfhagen M. J., Verdonk G. P., Jansze M., Torensma R., Verhoef J. Nontoxigenic strains of Clostridium difficile lack the genes for both toxin A and toxin B. J Clin Microbiol. 1991 Nov;29(11):2666–2667. doi: 10.1128/jcm.29.11.2666-2667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Champagne S. G., Sherlock C. H., Noble M. A., Freeman H. J., Smith J. A. Commercial latex agglutination test for detection of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1987 Jul;25(7):1244–1247. doi: 10.1128/jcm.25.7.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Fekety R., Batts D. H., Brown D., Cudmore M., Silva J., Jr, Waters D. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981 Jan;143(1):42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- Kim K., DuPont H. L., Pickering L. K. Outbreaks of diarrhea associated with Clostridium difficile and its toxin in day-care centers: evidence of person-to-person spread. J Pediatr. 1983 Mar;102(3):376–382. doi: 10.1016/s0022-3476(83)80652-0. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Viscidi R. P., Gdovin S. L., Yolken R. H., Bartlett J. G. Enzyme immunoassays for detection of Clostridium difficile toxins A and B in fecal specimens. J Infect Dis. 1984 May;149(5):781–788. doi: 10.1093/infdis/149.5.781. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Ball D. W., Toth J., Wilkins T. D. Characterization of cross-reactive proteins detected by Culturette Brand Rapid Latex Test for Clostridium difficile. J Clin Microbiol. 1988 Mar;26(3):397–400. doi: 10.1128/jcm.26.3.397-400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Barroso L. A., Wilkins T. D. Identification of the latex test-reactive protein of Clostridium difficile as glutamate dehydrogenase. J Clin Microbiol. 1991 Nov;29(11):2639–2642. doi: 10.1128/jcm.29.11.2639-2642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Wilkins T. D. Commercial latex test for Clostridium difficile toxin A does not detect toxin A. J Clin Microbiol. 1986 Mar;23(3):622–623. doi: 10.1128/jcm.23.3.622-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamou-Ladas H., O'Farrell S., Nash J. Q., Tabaqchali S. Isolation of Clostridium difficile from patients and the environment of hospital wards. J Clin Pathol. 1983 Jan;36(1):88–92. doi: 10.1136/jcp.36.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar A. C., Williams T. W., Hammond G. W. Detection of Clostridium difficile toxin in various tissue culture monolayers. J Clin Microbiol. 1987 Oct;25(10):1999–2000. doi: 10.1128/jcm.25.10.1999-2000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Miles B. L., Siders J. A., Allen S. D. Evaluation of a commercial latex test for Clostridium difficile for reactivity with C. difficile and cross-reactions with other bacteria. J Clin Microbiol. 1988 Nov;26(11):2452–2455. doi: 10.1128/jcm.26.11.2452-2455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E. Epidemiology of Clostridium difficile-induced intestinal disease. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S222–S228. doi: 10.1093/clinids/6.supplement_1.s222. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Lotz-Nolan L., Skalina D. Evaluation of a commercial cytotoxicity assay for detection of Clostridium difficile toxin. J Clin Microbiol. 1986 May;23(5):954–955. doi: 10.1128/jcm.23.5.954-955.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach S. L., Borriello S. P., Gaya H., Barclay F. E., Welch A. R. Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol. 1986 Sep;39(9):1013–1018. doi: 10.1136/jcp.39.9.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Holter J. J., Shanholtzer C. J., Garrett C. R., Gerding D. N. Detection of Clostridium difficile toxins A (enterotoxin) and B (cytotoxin) in clinical specimens. Evaluation of a latex agglutination test. Am J Clin Pathol. 1986 Aug;86(2):208–211. doi: 10.1093/ajcp/86.2.208. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Olson M. M., Shanholtzer C. J., Gerding D. N. Results of a prospective, 18-month clinical evaluation of culture, cytotoxin testing, and culturette brand (CDT) latex testing in the diagnosis of Clostridium difficile-associated diarrhea. Diagn Microbiol Infect Dis. 1988 Jun;10(2):85–91. doi: 10.1016/0732-8893(88)90045-4. [DOI] [PubMed] [Google Scholar]
- Sherertz R. J., Sarubbi F. A. The prevalence of Clostridium difficile and toxin in a nursery population: a comparison between patients with necrotizing enterocolitis and an asymptomatic group. J Pediatr. 1982 Mar;100(3):435–439. doi: 10.1016/s0022-3476(82)80455-1. [DOI] [PubMed] [Google Scholar]
- Sherman M. E., DeGirolami P. C., Thorne G. M., Kimber J., Eichelberger K. Evaluation of a latex agglutination test for diagnosis of Clostridium difficile-associated colitis. Am J Clin Pathol. 1988 Feb;89(2):228–233. doi: 10.1093/ajcp/89.2.228. [DOI] [PubMed] [Google Scholar]
- Tichota-Lee J., Jaqua-Stewart M. J., Benfield D., Simmons J. L., Jaqua R. A. Effect of age on the sensitivity of cell cultures to Clostridium difficile toxin. Diagn Microbiol Infect Dis. 1987 Dec;8(4):203–214. doi: 10.1016/0732-8893(87)90051-4. [DOI] [PubMed] [Google Scholar]
- Varki N. M., Aquino T. I. Isolation of Clostridium difficile from hospitalized patients without antibiotic-associated diarrhea or colitis. J Clin Microbiol. 1982 Oct;16(4):659–662. doi: 10.1128/jcm.16.4.659-662.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. C., Ruane P. J., Rosenblatt J. E., Lyerly D. M., Gleaves C. A., Smith T. F., Pierce P. F., Jr, Wilkins T. D. Comparison of culture, cytotoxicity assays, and enzyme-linked immunosorbent assay for toxin A and toxin B in the diagnosis of Clostridium difficile-related enteric disease. Diagn Microbiol Infect Dis. 1986 May;5(1):61–69. doi: 10.1016/0732-8893(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Welkon C. J., Long S. S., Thompson C. M., Jr, Gilligan P. H. Clostridium difficile in patients with cystic fibrosis. Am J Dis Child. 1985 Aug;139(8):805–808. doi: 10.1001/archpedi.1985.02140100067032. [DOI] [PubMed] [Google Scholar]
- Wu T. C., Gersch S. M. Evaluation of a commercial kit for the routine detection of Clostridium difficile cytotoxin by tissue culture. J Clin Microbiol. 1986 Apr;23(4):792–793. doi: 10.1128/jcm.23.4.792-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


