Abstract
Voltage-dependent calcium channels (CaV) open in response to changes in membrane potential, but their activity is modulated by Ca2+ binding to calmodulin (CaM). Structural studies of this family of channels have focused on CaM bound to the IQ motif; however, the minimal differences between structures cannot adequately describe CaM's role in the regulation of these channels. We report a unique crystal structure of a 77-residue fragment of the CaV1.2 α1 subunit carboxyl terminus, which includes a tandem of the pre-IQ and IQ domains, in complex with Ca2+·CaM in 2 distinct binding modes. The structure of the CaV1.2 fragment is an unusual dimer of 2 coiled-coiled pre-IQ regions bridged by 2 Ca2+·CaMs interacting with the pre-IQ regions and a canonical CaV1-IQ–Ca2+·CaM complex. Native CaV1.2 channels are shown to be a mixture of monomers/dimers and a point mutation in the pre-IQ region predicted to abolish the coiled-coil structure significantly reduces Ca2+-dependent inactivation of heterologously expressed CaV1.2 channels.
Keywords: structure, function, voltage-gated calcium channel
Excitation-contraction coupling and other important cellular processes are controlled by the voltage-gated Ca2+ channels (CaV). The ubiquitous Ca2+ sensor and regulator molecule, calmodulin, is an essential component of CaV regulation by Ca2+, and several regions of the cytoplasmic carboxyl terminus of the CaV α1 subunit have been identified as critical molecular determinants for CaM's regulation of CaV. Ca2+·CaM bound to the IQ motif of the carboxyl terminus of the α1 subunit of L-type Ca2+ channels is required for both a feed-forward regulation, Ca2+-dependent facilitation (CDF), and a feed-back regulation, Ca2+-dependent inactivation (CDI) (1, 2). CaM acts as the Ca2+ sensor for CDI in CaV1.2, CaV2.1, CaV2.2 and CaV2.3, and CDF in CaV1.2 and CaV2.1. This duality of CaV regulation by CaM suggests that there are either multiple binding sites or alternative interactions exist to regulate the channel based on different functional states of the channel.
Regions upstream of the IQ motif, designated the pre-IQ region, have been implicated in Ca2+·CaM regulation of the channel (3–5). Consistent with this, 2 different segments within the pre-IQ motif (1606–1627 and 1618–1652, identified as the A and C sequences, respectively) have been shown to bind CaM (4). Moreover, both the IQ motif and amino acids within the pre-IQ region (N1630-E1631) have been indicated to be critical for CaV1.2 CDI (6).
Recent crystal structures of CaM in complex with the IQ motifs from CaV1.2 (7, 8), CaV2.2 and CaV2.3 (5) reveal few structural differences that could account for the differences in regulation of CaV1.2 and CaV2.2 by CaM. However, very little is known about the structure of the pre-IQ region or the molecular basis for its interactions with CaM.
Here, we report the isolation and determination of the crystal structure at 2.1 Å resolution of a 77-residue (1609–1685) fragment of the carboxyl terminus of the α1 subunit of CaV1.2, which is composed of a tandem of the pre-IQ and IQ domains, in complex with Ca2+·CaM. The structure is an unusual dimer of 2 fragments in complex with 4 fully Ca2+-saturated CaMs. The Ca2+·CaMs are bound to 2 structurally distinct regions of the CaV1.2 carboxyl termini, the pre-IQ (T1609 to Q1651) and IQ (K1665 to Q1685). The pre-IQ region reveals a unique coiled-coil structure of 2 antiparallel, crossed, 11 turn α-helices, with 2 Ca2+·CaMs bridging the 2 pre-IQ regions followed by the Ca2+·CaM-IQ structure, the latter of which is equivalent to published structures (7, 8). In support of the dimeric crystal, we provide evidence that [3H]PN200–110 labeled, digitonin solubilized CaV1.2 from cardiac microsomes are a mixture of monomers and dimers. Moreover, the strength of CDI in CaV1.2 is significantly reduced after introduction of a mutation in the pre-IQ region predicted to abolish the coiled-coil structure. This new dimeric architecture, combined with the identification of dimeric channels in cardiac microsomes and finding that this structure impacts the strength of CDI, provides a deeper structural understanding of the regulation of these channels.
Results
Crystal Structure.
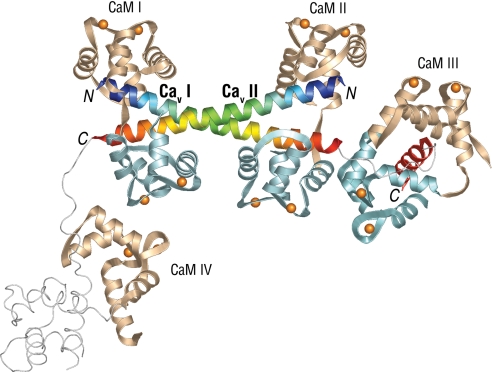
The bacterially expressed and purified 77-residue carboxyl-terminal fragment of the human sequence of CaV1.2 (SI Materials and Methods), designated pre-IQ-IQ, has the sequence TL1610 FALVRTALRI1620 KTEGNLEQAN1630 EELRAIIKKI1640 WKRTSMKLLD1650 QVVPPAGDDE1660 VTVGKFYATF1670 LIQEYFRKFK1680 KRKEQ with the IQ domain comprising residues 1665–1680. The fragment forms a stable complex with CaM in the presence of Ca2+ with an apparent mass of 81.1 kDa as determined by size exclusion chromatography (Fig. S1). This complex was crystallized and the structure was determined by MAD technique of a Pb2+-soaked crystal and refined to 2.1 Å resolution (Table S1). Consistent with the mass of the complex, the structure is comprised of 2 pre-IQ-IQ fragments with 4 bound Ca2+·CaMs, 1 of which has only 1 lobe visible in the electron density. Hereafter, the 2 CaV1.2 pre-IQ-IQ regions are identified as CaV I and II, and the 4 Ca2+·CaMs are named CaM I, II, III and IV.
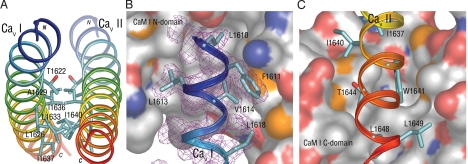
The most striking structural feature of the dimeric CaV1.2 fragment is the arrangement of the 2 crossed 64 Å, 11 turn pre-IQ α-helices bridged by 2 extended Ca2+·CaM molecules (CaMs I and II), creating a vertical pseudo 2-fold rotation axis passing through the center of the crossed helices at residue E1631 (Fig. 1). These very long antiparallel crossed helices (T1609 to Q1651) exhibit a slight but smooth curvature throughout their length, with no obvious kinks or irregularities, resulting in a coiled-coil structure (Fig. 2A). This feature allows numerous close contacts between hydrophobic residues in both pre-IQ helices (Fig. 2).
Fig. 1.
Crystal structure of the C-terminal pre-IQ-IQ fragment from the CaV1.2 α1 subunit. CaM N-lobes/domains are tan; C-lobes light blue; Ca2+ ions are gold spheres. CaV1.2 fragments (I and II) are blue to orange rainbow starting from the N termini (marked with N); the IQ domain for fragment I is red. Loop regions, the IQ domain for fragment II, and the C-domain of CaM IV are not seen in the density and are modeled, by analogy with fragment I and CaM III, as thin light gray lines for reference. The N- and C-lobes of the extended form of CaMs I and II bridging the pre-IQ segments are separated by ≈15 Å, whereas those of CaM III bound to the IQ motif of CaVI fragment make contacts. Figs. 1 and 2 were made with PyMOL (www.pymol.org).
Fig. 2.
Complementary dimer interface of the CaV1.2 pre-IQ segments and CaM contacts. (A) End view of the dimer interface showing major hydrophobic contact residues. (B) Major hydrophobic contacts made by CaVI fragment residues close to the N terminus with the CaM I N-lobe represented as van der Waals surface. The CaM N-lobe surface is white for nonpolar areas, red for oxygen, blue for nitrogen, and yellow for sulfur. Shown in magenta mesh is a portion of the 2Fo–Fc electron density surrounding the CaVI peptide (contoured at 1σ) with only selected hydrophobic residues displayed. The CaM N-lobe hydrophobic pocket harbors L1610. (C) Major hydrophobic CaVII residues close to the pre-IQ C terminus contacting the C-lobe of CaM I. The CaM C-lobe hydrophobic pocket contains W1641.
The contacts that the 2 pre-IQ-IQ fragments make with each other are solely between the pre-IQ helices, which could facilitate dimer formation. As depicted in Figs. 1 and 2A, the interactions between the pre-IQ helices involve mostly hydrophobic residues, 17 of a total of 28 interhelix residues. At the ends furthest from the crossover point E1631 the peptides are divergent and do not make contact except for a K1615-D1650 charge-coupling interaction. Moving closer to the crossover point, the residues most closely approaching the other peptide become hydrophobic (Fig. 2A), with the methyl group of T1622 and L1626 contacting I1640 of the other peptide, and I1626 and A1629 contacting I1636 and L1633. This nonpolar contact area between the 2 peptides spans the crossover point such that a hydrophobic interface region is created that is 26 Å in length, or >1/3 of the total length of the peptide dimer (Fig. 2A). Thus, temporarily disregarding the calmodulin molecules, the peptides display a rather weak polar bond near the divergent ends and show an extensive hydrophobic contact area across the region of closest approach. The finding that the interaction between the 2 pre-IQ helices buries ≈1,500 Å2 area further attests to the stability of the interaction.
Because the crystal structure revealed a coiled-coil fold, we used the coiled-coil prediction algorithm, COILS (9) to further examine the carboxyl termini of CaV1 channels. The pre-IQ region of CaV1.2 and CaV1.3 are predicted to have a high probability of forming a coiled-coil interaction, whereas CaV1.1 and CaV1.4 have a lower coiled-coil probability (Fig. S2A). The helical wheel diagram of the CaV1.2 pre-IQ region (Fig. S2B) shows that the predicted “a” and “d” core positions in the coiled-coil motif mainly correspond to hydrophobic residues with mostly polar residues located at the other sites in the motif. This coiled-coil motif creates a stripe of hydrophobic residues on one side of the helix consistent with what is observed in the crystal structure (Fig. 2A). The carboxyl termini of the CaV2 and CaV3 families are not predicted to form coiled-coils based on the COILS algorithm (see Fig. S3). The coiled-coil motif in the pre-IQ region appears to be unique to the CaV1s, particularly CaV1.2 and CaV1.3. The functional significance of this coiled-coil domain is unknown, but in a number of the identified CaV1.2 splice variants this interaction is predicted to be totally disrupted (Figs. S2 C and D).
Each Ca2+·CaM (I or II) binds the pair of pre-IQ segments in the same complementary way, with the N-lobe of CaM binding to the N-terminal region of 1 pre-IQ helix and the C-lobe of CaM binding to the C-terminal region of the opposite pre-IQ helix (Figs. 1B and 3). CaMs I and II involved in the interactions with the pre-IQ helix each display an intradomain separation of ≈15 Å with 2 pre-IQ helices in between, the central linkers of both CaMs having expanded to a well ordered loop between residues M76 and S81. The observation that the binding of both CaMs I and II bury ≈7,470 Å2 (1.4 Å probe), which is almost the entire accessible surface area (≈7,590 Å2) of the 2 combined pre-IQ helices, indicates that considerable additional stability of the Ca2+ channel polypeptide dimer is provided by CaM binding.
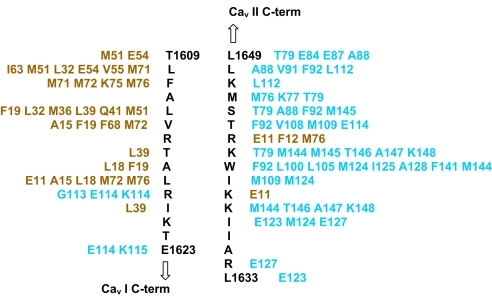
Fig. 3.
Schematic diagram of the residue contacts (<4.5 Å distance) of the Cav I N-terminal region (T1609–1623; left column) with primarily the N-lobe of CaM I (residues in tan) and Cav II C-terminal region (1633–1649; right column) with mainly the C-lobe of CaM I (residues in light blue). The total contacts of nonhydrogen atoms between Cav I N-terminal region and CaM I N-lobe/C-lobe is ≈200 and that between Cav II C-terminal region and CaM I C-lobe/N-lobe is ≈260. Similar contacts are made with CaM II lobes.
The residues following the pre-IQ segments emerge at opposite sides of the dimeric structure, with the electron density for CaV I becoming disordered at residue V1653 and reappearing at residue V1663 in proximity to the Ca2+·CaM (CaM III) bound to the IQ helix (residues V1663-E1684) (Fig. 1B). The disordered 10-residue loop between the pre-IQ and IQ helices indicates a region of high flexibility. The wrap-around or engulfing mode of Ca2+·CaM binding to the IQ segment of CaV I (Fig. 1) is very similar to that reported for the binding of Ca2+·CaM to the IQ helix in isolation, with Ca2+·CaM binding to the IQ region in an unusual parallel fashion (7, 8).
The CaV II peptide is disordered beyond the pre-IQ region, after residue Q1651, and remains disordered throughout the remainder of its length, including the IQ region (Fig. 1B). An isolated N-lobe of Ca2+·CaM (CaM IV) is located in good density in the area where this second CaM-IQ complex would be expected by analogy with the channel segment I; although some experimental density is present for the expected position of the channel segment II peptide it could not be fitted reliably. The C-lobe of CaM IV is also not seen and thus is disordered. Both the IQ domain of the CaV II fragment and the C-lobe of CaM IV are assumed to be present in multiple conformations. To determine why this complex is not seen for the IQ domain of CaV II, a crystal packing analysis was carried out. The analysis shows that a complete IQ-CaM complex such as that seen for CaV I cannot be accommodated in the space provided given the location of the visible CaM IV N-lobe, suggesting that this second IQ-CaM complex interaction has been disrupted by crystal packing forces. The absence of reliable electron density for the IQ domain of fragment II and the C-lobe of CaM IV indicates their high flexibility.
Beginning from the N-terminal end of the pre-IQ-IQ fragment (CaV I), the contacts made by CaM I with the fragments will now be described in more detail (Figs. 2B and 3). The contacts made by the N-terminal end of the pre-IQ helix (T1609-K1620) with CaM I are overwhelmingly hydrophobic in nature, with L1610, L1613, V1614, A1617, L1618, and I1620 making extensive contacts with the CaM I N-lobe, and F1611 making the greatest number of contacts (27 total) with CaM I residues M71, M72 and K75 and M76 in the N-lobe portion of the linker region. Polar contacts are made by T1609 with CaM N-lobe E54, and R1619 and E1623 form a hydrogen bonding network with the CaM C-lobe residues G113 and K115 main chain.
The CaM I C-lobe is engaged in a greater number of hydrophobic contacts with the C-terminal portion of the pre-IQ helix of CaV II (L1633-L1649) (Figs. 2C and 3), interacting with mostly I1637, I1640, W1641, T1644, L1648 and L1649, and the long aliphatic chain of K1642. Of the identified contacts, those made by W1641 are especially numerous (58 total) because its side chain resides in the hydrophobic pocket of the CaM I C-lobe (Fig. 2C). The polar contacts made by the CaV II C-terminal binding region with CaM I are, K1642 in a hydrogen bond with the M145 main chain oxygen, and K1639 with R1643 in a salt link with E11 of the CaM N-lobe (Fig. 3). Analogous contacts are made by CaM II with both fragments to form the cross bridged structure.
CaV1.2 Channel Dimer.
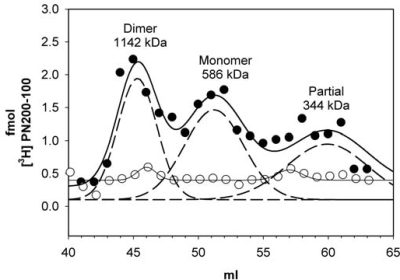
The presence of the coiled-coil in the crystal structure predicts that 2 CaV1.2 channels in close proximity have the ability to form dimers even in the absence of CaM. To test this, cardiac microsomes were labeled with [3H]PN200–110, a specific L-type channel ligand, detergent solubilized with 1% digitonin, and proteins were separated by size exclusion chromatography (see Methods in SI Materials and Methods). Under these conditions the CaV1.2 elutes as 3 major species at 45.3, 51.2 and 58.9 mL with apparent molecular masses of 1,142, 673, and 344 kDa, respectively (Fig. 4). The first peak elutes at 43.7 mL and correlates to a dimer of CaV1.2 and the second peak is the monomer CaV1.2. A third peak is also detected and appears to correspond to a partially dissociated form of CaV1.2 because of the subunit dissociation in digitonin (10). Specificity of the [3H]PN200–110 binding was shown by incubating the cardiac microsomes with 1 μM isradipine, a nonradiolabeled dihydropyridine (Fig. 4). The predicted mass of macromolecular complex of CaV1.2, is ≈443–426 kDa. This, however, does not take into account the glycosylation of the α2 subunit (11), detergent binding, or the presence of modulatory proteins. We were unable to detect either PKA or CaMKII by Western Blot in peak fractions, suggesting that these enzymes are not present in stoichiometric amounts and are, therefore, unlikely to account for the major peaks of CaV1.2.
Fig. 4.
Isolation of native CaV1.2 as mixtures of monomers and dimers. Cardiac microsomes were labeled with 20 nM [3H]PN200–110, solubilized with 1% digitonin and proteins were separated on a Superdex 200 column. Total bound [3H]PN200–110 is plotted for each 1-mL fraction in the absence (filled circle) or presence of isradipine (open circle). Gaussian fit for the data are plotted as a solid line and the individual Gaussians are plotted as dashed lines.
Disruption of the C-Terminal Coiled-Coil Interaction Reduces CDI of CaV1.2.
The coiled-coil algorithm, COILS (9), predicts that the pre-IQ coiled-coil interaction is disrupted by a substitution of the glutamate residue at position 1631 with a proline residue (E1631P), creating a “kink” within the coils (Fig. S2A). We also surmise that this substitution would introduce a kink in the pre-IQ helix, resulting in the disruption of its slight smooth curvature in the coiled coil structure (Fig. 2A). Therefore, we determined the effect of this substitution in rabbit CaV1.2 (E1613P), generated as described in the SI Materials and Methods, on channel function. For these experiments, the magnitude, kinetics, and voltage dependence of whole cell Ca2+ and Ba2+ currents were measured after transient transfection of HEK-293 cells with either wild-type or E1613P CaV1.2 together with the auxiliary subunits β2a and α2-δ1. We found that the magnitude and voltage dependence of macroscopic Ca2+ channel conductance were not significantly different between wild-type- and E1613P-expressing cells (see Table S2). These results indicate that the pre-IQ coiled-coil interaction is not required for proper channel folding, targeting to the plasma membrane or function as a voltage-gated, Ca2+-permeable ion channel.
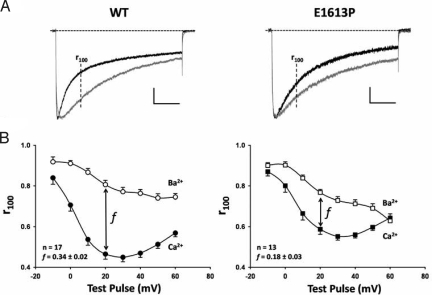
Because the pre-IQ coiled-coil structure binds 4 calmodulin molecules, which are implicated in CDI of CaV1.2 (1, 2), we determined the effect of the E1613P mutation on CDI. Fig. 5A compares normalized and superimposed Ca2+ (Fig. 5A black trace) and Ba2+ (Fig. 5A gray trace) currents elicited by a 500-ms step depolarization to + 20 mV obtained from representative HEK-293 cells expressing either wild-type CaV1.2 (Fig. 5A Left) or E1613P CaV1.2 (Fig. 5A Right). Although the Ca2+ current trace decays significantly faster than the Ba2+ current trace in both cases, this difference is noticeably smaller for E1613P. On average, the fraction of peak Ca2+ current remaining 100 ms after depolarization (r100) exhibited a prominent U-shaped dependence on test pulse voltage, consistent with a Ca2+ permeation-dependent inactivation process (Fig. 5B). The corresponding Ba2+ current r100 value decayed approximately monotonically with voltage, reflecting a slower voltage-dependent mechanism. The difference between r100 in Ca and Ba (f) provides a quantitative index of the strength of CDI. As shown in Fig. 5B Inset, the value of f at + 20 mV is significantly (P < 0.01) reduced for E1631P. A similar significant reduction in f is observed over a broad range of test potentials (0–50 mV). These results indicate that E1613P mutation in CaV1.2 reduces, but does not eliminate, CDI. The lack of complete elimination of CDI could reflect either disruption of dimer formation only partially affects CDI or that the mutation does not completely disrupt the structure required for CDI.
Fig. 5.
Inhibition of CDI by the E1613P mutation in CaV1.2. (A) representative CaV1.2 current inactivation of wild-type (Left) and E1613P (Right) CaV1.2 channels elicited by a 500-ms depolarization to +20 mV in either 10 mM Ca2+ (black traces) or 10 mM Ba2+ (gray traces). Ca2+ current traces were normalized to the peak of the corresponding Ba2+ current trace. The vertical scale bar represents 230 pA and 200 pA for the wild-type Ca2+ and Ba2+ currents, respectively and 470 pA and 260 pA for the E1613P Ca2+ and Ba2+ currents, respectively. (B) average (± SE) ratio of current remaining 100 ms after depolarization (r100) to the corresponding peak Ca2+ (filled symbols) and Ba2+ (open symbols) current plotted as a function of test potential for wild-type (Left) and E1613P (Right) CaV1.2. (Inset) (f) the difference between r100 in Ca2+ and Ba2+ and measure of CDI strength, is shown for the test potential of +20 mV (P < 0.01).
Discussion
CaM serves as a Ca2+ sensor/regulator for a number of different ion channels that respond to very different types of Ca2+ signals. Adding to this complexity, CaM plays a critical role in 2 opposing functions in CaV1.2 channels, Ca2+-dependent facilitation (CDF) and Ca2+-dependent inactivation (CDI), and CaM binding to the IQ motif is crucial for both processes. Mori et al. (12) proposed that a single molecule of CaM, bound to CaV1.2, is both necessary and sufficient for CDI of this channel. Whether a single molecule of CaM is required for CDF is not known. Other potential CaM binding sites have been identified in both the N terminus, intracellular loops, and carboxyl terminus of CaV1.2 (4, 6, 13). A major question is whether these additional sites contribute, along with the IQ motif, to a CaM binding pocket, where a single CaM is able to move to engage different sequence elements in response to different Ca2+ signals, thereby allowing a single molecule of CaM to perform different functions. Another possibility is that these other sites bind additional molecules of CaM that participate in totally different functions. We now provide new data to indicate that >1 molecule of CaM can simultaneously bind to the carboxyl terminus of CaV1.2 and, in doing so, participate in the formation of novel dimers between neighboring channels.
Our data suggest that 2 closely positioned CaV1.2 channels are capable of dimerizing via a coiled-coil interaction that is stabilized by bridging CaMs. The coiled-coil structure is predicted by the COILS algorithm to occur in CaV1.2 and CaV1.3, but not in CaV2 and CaV3 channels, because of the absence of a homologous pre-IQ sequence in the P/Q, R, and N channels (3). If the structure we observe reflects a structure that native CaV1.2 can assume in vivo, the most obvious question is what is the functional significance of CaV1.2 dimers with multiple associated CaMs? Several situations for the need of a CaV1.2 dimer are possible including a modulatory role in CDI and/or CDF, a chaperone-like role in helping the channels transit to the membrane (14), a stabilizing role to prevent channel turnover, and/or a targeting role to sort channels to regions such as caveolae or other specialized locations within a cell. Our functional data supports a role for the coiled-coil dimer to be involved in the modulation of channel activity by reducing the effects of CDI. Whether this mutation also alters CDF requires additional study.
We have previously shown that a peptide containing the sequence (1627–1652) of the C-lobe CaM binding site within the pre-IQ sequence, which as revealed by our structure, is part of the region involved in the coiled-coil pre-IQ domains (Figs. 1 and 2), enhances CDF (3), suggesting that dimer formation may limit CDF. In addition, Kim et al. (15), using alanine scanning mutagenesis, found that mutation of residues within the region that we show is involved in the coiled-coil and stabilizes the dimer, decreases CDI. The mutations 1609TLF → AAA, 1613LVR → AAA, and 1620IKT → AAA displayed reduced CDI and each contains large hydrophobic residues that contact Ca2+·CaM (Figs. 1 and 3). The 1609TLF → AAA mutant in particular showed a strong effect, consistent with it containing the largest hydrophobic CaM-binding residues within this motif (Figs. 2B and 3). The 1636IIK → AAA and 1645SMK → AAA mutants did not affect CDI, but the very large W1641 residue within this CaM-binding region remained intact in these mutants, so it is likely that Ca2+·CaM binding is not strongly affected. These studies suggest that the dimer with its channel bridging CaMs may be more efficient than the monomer for CDI. Consistent with this, CDI is stronger for the dimer than for the channel with the mutation predicted to interfere with dimer formation.
Several CaV1.2 (SwissProt catalog no. Q13936) splice variants exist that are not predicted to form dimers. Splice variants 9 and 26 have the following substitution: 1618LRIKTEGNLEQANEELRAIIKKIWKRTSMKLLDQVVPPAGDDEVTVGKFYATFLIQEYFRKFKKRKEQGLVGKPSQRNALSL1699 → LRETELSSQVQYQAKEASLLERRRKSSHPKSSTKPNKLLSSGGSTGWVEDARALEGQVLARGCGWLGSLEERERGPHHPPLGF. In isoforms 10, 13, 14, 15, 24, 25, 27 and 29, the glutamic residue 1623 is replaced with the sequence EEGPSPSEAHQGAEDPFRPA. In all of these splice variants, the coiled-coil interaction is expected to be totally disrupted (Fig. S2). Localization of these splice variants within the cell and careful analysis of their ability to undergo CDI and CDF is needed to assess the functional consequences of these changes that are predicted to disrupt both dimer formation and the interaction of these channels with bridging CaMs.
Our pre-IQ-CaM structure of CaV1.2 shows that both the N- and C- lobes of CaM bind to the pre-IQ region in a parallel manner, albeit spanning 2 separate segments of the region. Pitt et al. (4) proposed that the N-lobe of CaM engages the pre-IQ-A sequence (F1606-E1627), leaving the C-lobe of CaM to interact with the IQ motif. The proposal is partially supported by our structure that shows that the N-lobe of Ca2+·CaM interacts with a shorter span of residues (1610–1620) within the A sequence located at the N terminus of one pre-IQ helix (Figs. 1 and 2). However, contrary to the proposal, the C-lobe interacts with I1637-L1649 at the C terminus of the other pre-IQ helix (Figs. 1 and 2).
Several determinants outside of the IQ motif have been shown to be necessary for Ca2+-dependent inactivation, including the sequences 1620IKTEG and 1648LLDQV in the pre-IQ domain (6) and the 2 polar residues (N1630-E1631) within the pre-IQ (6). The potential importance of these sequences and residues can be seen in our structure. Among the residues in the 2 sequences, I1620 of one pre-IQ interacts with the N-lobe of CaM, T1622 engages in the coiled coil interaction and L1648 and L1649 of the other pre-IQ interact with the C-lobe of the same CaM (Figs. 1, 2A, and 3). N1630 and E1631 occur at the crossover point of the coiled coil pre-IQ regions (Figs. 1 and 2A).
Assuming that the presence of the coiled-coil region does indeed lead to dimer formation, is this a more general phenomenon? That is, do channel dimers (with or without CaM) exist in other ion channels? Ca2+·CaM in association with dimers of both the NMDA receptor (16) and the Ca2+ activated K+ channel (17) have been reported. In addition, a coiled-coil dimer interface has been identified for the Hv1 proton channel (18). Although 2 reports have suggested that L-type calcium channels are dimers (19, 20), at least 2 laboratories find primarily monomers (21, 22). However, purification protocols, especially those that involve sucrose gradient sedimentation or size exclusion chromatography, are likely to inadvertently select against dimers. The structure presented here reveals that multiple CaMs are able to bind simultaneously to the carboxyl terminus of the CaV1.2 α subunit and, under these conditions, no structural interactions are observed between the Ca2+·CaMs bound to the pre-IQ and IQ regions or between the pre-IQ and IQ regions themselves.
The crystal structure of the CaV.1.2 carboxyl terminus is the first to show a coiled-coil structure of 2 pre-IQ domains and the simultaneous binding of 2 CaMs in 2 structurally distinct modes: 2 extended Ca2+·CaMs bridging 2 coiled-coil pre-IQ helices and 1 compact Ca2+·CaM binding in a canonical fashion to the IQ motif. This structure is physiologically relevant because the strength of CDI of CaV1.2 is significantly reduced after molecular disruption of the coiled-coil pre-IQ interaction. Our structural, biochemical, and cellular findings provide a sound basis for further study of the regulation and function of CaVs.
Materials and Methods
Expression and Purification of the Cardiac pre-IQ-IQ/CaM Complex.
The methods for expression, purification and crystallization of the cardiac pre-IQ-IQ/CaM complex are described in SI Materials and Methods.
Crystallization and Structure Determination of the pre-IQ-IQ/CaM Complex.
The original complex crystals were obtained by sending a sample to the Hauptmann–Woodward Medical Institute high-throughput crystallization screening program (23). The final optimized crystals were grown by mixing 6 μL of the complex (10 mg/mL) with 6 μL of the well solution (20% wt/vol polyethylene glycol 6000, 1.0 M LiCl, 3% vol/vol 2,4-methylpentane diol and 100 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.0, in a hanging drop format. To prepare a heavy atom derivative solid, Pb(NO3)2 was added to saturation to drops containing crystals. The crystals were mounted and flash-cooled in liquid nitrogen after 3 days of exposure to this solution. Multi wavelength anomalous scattering derivative data to 2.8 Å resolution were collected at the Center for Advance Microstructures and Devices protein crystallography beamline in Baton Rouge, Louisiana. The data were processed with HKL2000 and scaled with SCALEPACK (24). Analysis of an anomalous differences Patterson map calculated from the peak data with the CNS (25) software package gave several possibilities for a strong heavy atom site, one of which was used for phasing and calculation of anomalous difference Fourier maps to complete the heavy atom model. The final phasing run included 26 heavy atom sites, with the figure of merits shown in Table S1. An electron density map calculated with the experimental phases showed many right-handed helices with some well-defined side chains, and fitting of the density and refinement of the model began with iterations between the graphics program CHAIN (26) and CNS (25). A native dataset to 2.1 Å was collected at APS beamline 19ID and processed with HKL (24), which was used for the final stages of model building. The statistics for the diffraction data used in our structure analysis and the refinement are shown in Table S1.
Measurement of Macroscopic Ca2+ and Ba2+ Currents.
The whole cell patch clamp technique was used to record macroscopic Ca2+ and Ba2+ currents from expressing HEK-293 cells. Patch pipettes were pulled and fire-polished to a final resistance of 1–2 MΩ when filled with an internal solution containing (in mM): 140 Cs-aspartate, 5 MgCl2, 10 Cs2-EGTA, and 10 Hepes (pH to 7.4 with CsOH). The external solution contained (in mM): 10 CaCl2 or 10 BaCl2, 145 tetraethylammonium-Cl, and 10 Hepes (pH to 7.4 with tetraethylammonium-OH). Ca2+ and Ba2+ currents sampled at 10 kHz and filtered at 2 kHz were elicited by 500-ms step depolarizations applied from a holding potential of −80 mV to test potentials ranging from −50 mV to +70 mV in 10 mV increments. Series resistance was typically ≈1–2 MΩ after >70% compensation. Leak and capacitive currents were subtracted by a P/4 protocol. For all experiments, a family of Ca2+ current traces was first generated >5 min after establishing whole cell access. The dish was then perfused with 15–20 mL of Ba2+-containing external solution and a family of Ba2+ currents were then collected. Peak Ca2+ and Ba2+ currents were normalized to total cell capacitance (determined from integrating the capacity transient resulting from a 10-mV depolarization delivered from the holding potential), plotted as a function of membrane potential (Vm), and fit according to the following equation:
where Gmax is the maximal L channel conductance, VG1/2 is the voltage required for half-maximal activation of Gmax, Vrev is the reversal potential, and kG is a slope factor. Fractional inactivation of the current at 100 ms from the time of depolarization (r100) was determined by dividing the current remaining at 100 ms by the peak current. The strength of Ca2+-dependent inactivation (CDI) was quantified by the parameter f, defined as the difference between the r100 values obtained in Ca2+ versus Ba2+. All data are presented as mean ± SEM with significance accepted at P < 0.01 (Student's t test).
Supplementary Material
Acknowledgments.
We thank Dr. Henry Bellamy at the CAMD GCPCC beamline for assistance with the MAD data collection, Dr. Stephan Ginell for assistance with the native diffraction data at APS, Linda Groom for generating the E1613P CaV1.2 mutant, and Dr. Kurt Beam and Joshua Ohrtman for kindly providing the wild-type CaV1.2 channel subunits used in this study. This work was supported by National Institutes of Health Grants AR44864 (to S.L.H.), AR44657 (to R.T.D.), GM68826 (to F.A.Q.), HL087099 (to F.A.Q., Co-I), National Institutes of Health Dental and Craniofacial Training Grant (to R.E.L.), and Welch Foundation Grant Q-581 (to F.A.Q.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have deposited in the Protein Data Bank (PDB ID code 3G43).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807487106/DCSupplemental.
References
- 1.Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 2.Zühlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the alpha 1C subunit. J Biol Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]
- 3.Pate P, et al. Determinants for calmodulin binding on voltage-dependent Ca2+ channels. J Biol Chem. 2000;275:39786–39792. doi: 10.1074/jbc.M007158200. [DOI] [PubMed] [Google Scholar]
- 4.Pitt GS, et al. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- 5.Mori MX, Vander Kooi CW, Leahy DJ, Yue DT. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: High-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zühlke RD, Reuter H. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc Natl Acad Sci USA. 1998;95:3287–3294. doi: 10.1073/pnas.95.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fallon JL, Halling DB, Hamilton SL, Quiocho FA. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1.2 calcium channel. Structure. 2005;13:1881–1886. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton SL, Hawkes MJ, Brush K, Cook R. Subunit composition of the purified dihydropyridine binding protein from skeletal muscle. Biochemistry. 1989;28:7820–7828. doi: 10.1021/bi00445a044. [DOI] [PubMed] [Google Scholar]
- 11.Jay SD, et al. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 12.Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Yu K, McCoy KL, Lee A. Molecular mechanism for divergent regulation of Cav1.2 Ca2+ channels by calmodulin and Ca2+-binding protein-1. J Biol Chem. 2005;280:29612–29619. doi: 10.1074/jbc.M504167200. [DOI] [PubMed] [Google Scholar]
- 14.Gao T, Bunemann M, Gerhardstein BL, Ma H, Hosey MM. Role of the C terminus of the alpha 1C (CaV1.2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem. 2000;275:25436–25444. doi: 10.1074/jbc.M003465200. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–754. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Wang HG, Xie H, Pitt GS. Ca2+/CaM controls Ca2+-dependent inactivation of NMDA receptors by dimerizing the NR1 C termini. J Neurosci. 2008;28:1865–1870. doi: 10.1523/JNEUROSCI.5417-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MC, et al. 3D Structure of the Skeletal Muscle Dihydropyridine Receptor. J Mol Biol. 2002;323:85–98. doi: 10.1016/s0022-2836(02)00890-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, et al. The three-dimensional structure of the cardiac L-type voltage-gated calcium channel: Comparison with the skeletal muscle form reveals a common architectural motif. J Biol Chem. 2004;279:7159–7168. doi: 10.1074/jbc.M308057200. [DOI] [PubMed] [Google Scholar]
- 21.Serysheva II, Ludtke SJ, Baker MR, Chiu W, Hamilton SL. Structure of the voltage-gated L-type Ca2+ channel by electron cryomicroscopy. Proc Natl Acad Sci USA. 2002;99:10370–10375. doi: 10.1073/pnas.162363499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J Mol Biol. 2003;332:171–182. doi: 10.1016/s0022-2836(03)00899-4. [DOI] [PubMed] [Google Scholar]
- 23.Luft JR, et al. A deliberate approach to screening for initial crystallization conditions of biological macromolecules. J Structural Biol. 2003;142:170–179. doi: 10.1016/s1047-8477(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 24.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in the oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 25.Brünger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 26.Sack JS, Quiocho FA. CHAIN-A Crystallographic Modeling Program. Methods Enzymol. 1997;277:158–173. doi: 10.1016/s0076-6879(97)77011-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.