Abstract
A major involvement of IFNα in the etiopathogenesis of systemic lupus erythematosus has been suggested by clinical observations, including the increase of serum levels of this cytokine in patients with active disease. Supporting this hypothesis, we have shown that expression of IFNα from a recombinant adenovirus (IFNα Adv) precipitates lupus manifestations in genetically susceptible New Zealand Black (NZB) × New Zealand White (NZW)F1 mice (NZB/W) but not in BALB/c mice. In the present investigation, we have prepared an IFNα immunogen, termed IFNα kinoid, which, appropriately adjuvanted, induces transient neutralizing antibodies (Abs) but no cellular immune response to the cytokine and without apparent side effects. Using this preparation, we also showed that, in kinoid-vaccinated NZB/W mice, lupus manifestations, including proteinuria, histological renal lesions, and death triggered by IFNα Adv challenge were delayed/prevented as long as an effective threshold of anti-IFNα inhibitory capacity was present in the serum.
Keywords: anti-IFNα immunization, anti-SLE immune therapy, systemic lupus erythematosous
Systemic lupus erythematosus (SLE) is a frequent and life-threatening chronic autoimmune disease characterized by a break of tolerance to nuclear components and profound alterations of the immune system (1). Dysregulated activation of many cells of the immune system involving several cytokines has been studied extensively in both human and murine lupus. Recently, type I IFNs (IFNα and β) have gained considerable interest as candidates in the etiopathogenic process of lupus (2–4). Involvement of IFNα in SLE has initially been suggested by clinicians as early as 1979 based on the findings of (i) elevated levels of this cytokine in the serum of SLE patients (5–7); (ii) IFNα treatment of cancer or hepatitis C induced in some patients' autoantibodies (autoAbs), including anti-nucleus Abs and anti-native DNA, and more rarely SLE (<1%) (8, 9); (iii) gene expression characteristic of an IFNα signature in blood cells of SLE patients, revealed by microarray assays (10, 11); (iv) animal experiments in which type I IFN receptor deficiency significantly reduced the expression of the lupus hallmarks in lupus-prone mice (12). More recently, we (13) developed an SLE-like murine flare model in which IFNα5 supplied by recombinant adenovirus-expressing IFNα5 (IFNα Adv) drives the early expression of lupus manifestations, including autoAbs, glomerulonephritis, and death.
SLE patients are currently treated with the antimalarial agents chloroquine and hydroxychloroquine, steroids, and/or immunosuppressive drugs (14). More recently, biological therapies targeting immune cells involved in lupus pathogenesis have been developed, such as CD20 monoclonal antibodies (mAbs) (rituximab) that inhibit B cell activation (15). Monoclonal Abs have also been produced to counteract pathogenic effects of cytokines in SLE. Given the key role of IFNα in the SLE pathogenesis (3), anti-BDCA2/4 mAbs blocking IFNα production by plasmacytoid dendritic cells were prepared (16) on the one hand, and fully human mAb (Medi545) and recombinant humanized mAbs directed to IFNα are currently under clinical trial (http://clinicaltrials.gov/ct2/show/NCT00657189?term=anti+interferon&rank=3). We hypothesized that high-affinity polyclonal autoAbs induced by active immunization to IFNα may represent an alternative and likely improved therapeutic strategy. In the present work, we first show that an IFNα derivative, termed IFNα kinoid, prepared by the same procedure as that applied to produce other cytokine immunogens, including TNFα (17), VEGF (18), and IL4 kinoids (19), can be used as an effective immunogen to mount a neutralizing anti-IFNα Ab response. When administered to animals with an appropriate adjuvant, this immunogen proved to be safe and induced high titers of polyclonal autoAbs but no cellular immune response to the cytokine. We further show that kinoid vaccination inducing a high serum anti-IFNα inhibitory capacity contained manifestations of SLE in the New Zealand Black (NZB) × New Zealand White (NZW)F1 mice (NZB/W) challenged with IFNα Adv. Successful repression of SLE-related phenomena occurred as long as an effective threshold of serum inhibitory capacity was maintained.
Results
IFNα Kinoid Immunobiological Properties.
An optimally effective anti-IFNα immunogen was obtained by treating a mixture of keyhole limpet hemocyanin (KLH) and murine IFNα (mIFNα) with aldehydes, as described in Materials and Methods. The kinoid is devoid of the IFNα antiviral bioactivity, as tested by the standard biological assay for IFN cytopathic effect (CPE) inhibition assay [supporting information (SI) Fig. S1] (20).
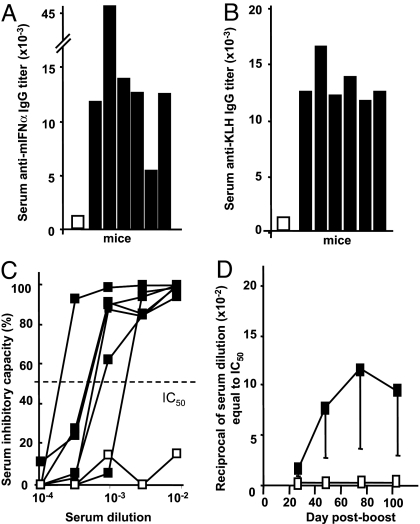
Kinoid vaccination triggers a strong but transient Ab response to IFNα in BALB/c mice. Immunization of BALB/c mice with mIFNα kinoid induced high titers of serum anti-mIFNα Abs (Fig. 1A). Kinoid immunization also resulted in the generation of anti-KLH Abs (Fig. 1B). Hyperimmune sera from kinoid-immunized BALB/c mice inhibited mIFNα bioactivity (Fig. 1C). Serum anti-mIFNα inhibitory capacity (IC50) peaked 2–3 weeks after boosting and declined over 3 months (Fig. 1D).
Fig. 1.
Humoral response to IFNα kinoid immunization in BALB/c mice. (A and B) Ab titers to mIFNα and to KLH were determined by ELISA. Results were expressed by the reciprocal of the serum dilution at a cutoff of OD = 0.300. Each bar represents one serum sample. (C and D) Serum samples from 6 mIFNα kinoid-immunized mice were collected before immunization (open symbols) and 10 ± 2 days after the last immunization (filled symbols). (C) Serum inhibitory capacity, as assessed by CPE assay. Dotted line represents the IC50. (D) Kinetics of serum capacity to inhibit mIFNα bioactivity (IC50) in kinoid-immunized mice after the last boost.
Kinoid Vaccination of NZB/W Mice Delayed/Prevented Lupus Manifestations in the NZB/W Lupus Flare Model.
Immune response to kinoid vaccination of NZB/W mice. Anti-mIFNα antibody response.
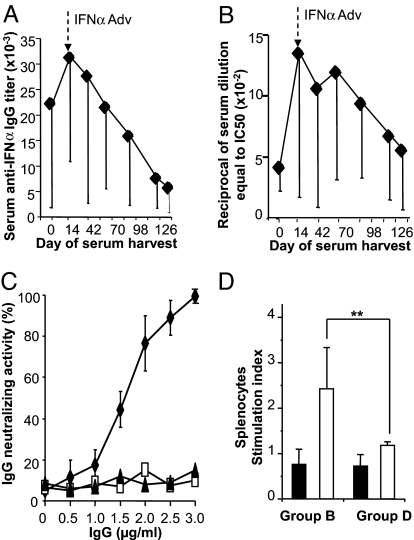
In the NZB/W mice experimental lupus model (Table 1), mIFNα kinoid immunization elicited high anti-mIFNα Ab titers (Fig. 2A), whereas immunization with a modified KLH, henceforth written as *KLH, failed to trigger an antibody response to mIFNα Abs. Anti-mIFNα Abs belonged mainly to IgG1, IgG2b, and IgG2a classes. Of note, IgM isotype was also present but at a low concentration, and IgG3 Abs were present at unexpected high titers (see Table S1). Kinoid immunization of NZB/W mice resulted in varying anti-IFNα antibody responses from animal to animal.
Table 1.
Experimental protocol: Immunization and Adv challenge in NZB/W mice
| Groups | Animal no. | Vaccine preparations | i.m. injections on days before challenge | Adv challenge (109 particles) at day 0 |
|---|---|---|---|---|
| A: Positive control | 12 | PBS | 51, 43, 22, 2 | IFNα Adv |
| B: Kinoid-immunized | 12 | IFNα kinoid | 51, 43, 22, 2 | IFNα Adv |
| C: KLH-immunized | 12 | KLH | 51, 43, 22, 2 | IFNα Adv |
| D: Reference | 9 | PBS | 51, 43, 22, 2 | Null Adv |
Study end points were anti-IFNα antibody titers and serum inhibitory capacity (IC50), proteinuria, survival, and kidney histopathology at sacrifice. Sera were collected 1 week after the 2nd boost (day 14 before challenge) and periodically thereafter (see Materials and Methods).
Fig. 2.
Humoral and cellular response to IFNα kinoid immunization in NZB/W mice. (A and B) Sera from kinoid-immunized mice were collected 1 week after the 2nd boost [day 0 in abscissa corresponding to 14 days before IFNα Adv challenge (↓)] and at various times during the study. Sera were assessed for their anti-IFNα Ab titer by ELISA (A) and for their anti-mIFNα inhibitory capacity by CPE assay (B). (C) IgG purified from a pool of sera collected after the 2nd boost from group B (mIFN-K, filled losanges), group C (*KLH, open squares) and group D (PBS, filled triangles) were tested for their anti-mIFNα neutralizing activity by CPE assay. (D) Lack of cellular immune response to IFNα kinoid. Splenocytes collected from kinoid- (group B) and PBS- (group D) immunized mice at sacrifice were purified and cultured for 96 h in presence of mIFNα (10 μg/mL, filled bars) and KLH (10 μg/mL, open bars). Immune cell response to mIFNα was evaluated by specific cell proliferation by [3H]thymidine incorporation, the positive threshold for response being 1.5 in reference to the control group response. **, P < 0.01.
Serum anti-mIFNα inhibitory capacity.
Sera from kinoid-immunized but not from *KLH-immunized or from control group animals strongly inhibited IFNα bioactivity as tested by the standard biological assay (Fig. 2B). This effect is to be ascribed to the Abs, given that purified IgG from hyperimmune sera but not from sera of other animal groups also neutralized IFNα bioactivity (Fig. 2C). As shown in Fig. 2B, serum IC was transiently effective, and its kinetics of decline paralleled that of the Ab levels (Fig. 2B).
Absence of anti-mIFNα autoreactive T cells.
The cellular immune response to mIFNα has been investigated by culturing purified splenocytes from kinoid-immunized NZB/W mice. In vitro stimulation with mIFNα failed to activate these splenocytes, as evaluated by T cell proliferation (Fig. 2D) and by the absence of IL2 and IFNγ release in culture supernatants (Fig. S2). By contrast, the KLH-stimulated splenocytes of kinoid-immunized animals mounted a cellular response to KLH (Fig. 2D). The absence of autoreactive T cell response to the targeted cytokine after kinoid immunization was also observed in previous experimental studies carried out in mice immunized against other cytokines, including TNFα, VEGF, and IL4 (17–19).
Safety.
After kinoid immunization, NZB/W mice remained free of any short- or long-term clinical adverse complications, and their behavior was normal all through the follow-up period of 123 days. At sacrifice, macroscopic examination failed to reveal any alteration of visceral organs in kinoid-immunized mice.
Follow-up of SLE-like manifestations triggered by IFNα challenge in kinoid-immunized NZB/W mice.
IFNα Adv was administered to 3 groups of 12 NZB/W mice receiving PBS (positive control group A), kinoid (experimental group B), or KLH (carrier control group C) (Table 1). Of note, as reported in ref. 13, circulating IFNα, as assessed by ELISA, was detected prior to and after challenge at low levels in the 3 groups of mice that were given IFNα Adv (Table S2). As representative of the natural evolution of NZB/W mice, we additionally included in the study 9 IFNα nontreated mice that received null Adv in place of IFNα Adv to confirm that the Adv vector per se had no impact on the lupus evolution (reference group D). Survival evolution of these reference mice was similar to that of untreated animals of the same age originating from our NZB/W breeding (13).
SLE-like manifestations in control mice.
Regarding the lupus clinical evolution in IFNα Adv-challenged NZB/W mice (positive control group A), as shown in Table S3, we found endpoint results similar to those described in ref. 13. Indeed, at the end of the study follow-up period (day 123 after challenge), all IFNα-treated mice from the control group A died, whereas 4 of the 9 mice of the reference group D survived. Proteinuria and death in control group A mice occurred rapidly compared with those in IFNα nontreated mice (group D), the median time for onset being, respectively, 32 versus 95 days and 47 versus 112 days after Adv challenge (Table S3).
SLE-like manifestations in kinoid-immunized mice.
Lupus clinical manifestations (as evaluated by proteinuria, survival, and histopathology end points) were markedly improved in the kinoid-immunized mice (group B) compared with those observed in other IFNα-treated mice from groups A and C (Table S3).
Proteinuria.
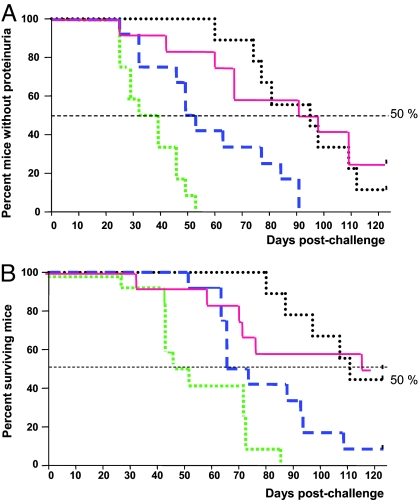
As shown in Fig. 3A, the median time of occurrence of proteinuria was 95 days after IFNα Adv challenge in the kinoid-immunized mice (group B). These changes were significantly delayed compared with those of control group A (day 32, P < 0.0001) and KLH-immunized group C (day 53, P < 0.01) mice.
Fig. 3.
Kaplan–Meier graphs of proteinuria occurrence and survival in immunized NZB/W mice, expressed as days after IFNα Adv challenge. Group A, positive control (green dashed line); group B immunized with IFNα kinoid (red solid line); group C, immunized with KLH (blue large dashed line); group D reference mice that received null Adv in place of IFN adv (black dotted line). (A) mIFNα kinoid immunization delays the proteinuria onset. P < 0.01 kinoid versus KLH and P < 0.001 kinoid versus positive control. (B) mIFNα kinoid immunization prolongs survival of NZB/W mice. P < 0.001 kinoid versus challenged control, and P < 0.05 kinoid versus KLH group.
Survival.
Four months after challenging the mice with IFNα, 50% of kinoid-immunized mice (6 of 12 in group B) survived, a survival rate comparable with that of reference mice (group D) (Fig. 3B). This is in strong contrast to the 100% death of the control mice (12 of 12 in group A) and the 90% death in the KLH-immunized mice (11 of 12 in group C). Fig. 3 also shows that death was delayed in mice of the KLH-immunized group C compared with those of the control group A. The clinical benefit effect conferred by KLH vaccination in this study, although at a significantly lower level than that caused by kinoid immunization, might be the consequence of the known effects of KLH on innate immunity (21).
Histology.
Microscopic abnormalities of the kidneys collected from surviving mice at the end of the study were analyzed on the basis of renal lesions and quantified by the histopathologic scores initially defined for lupus NZB/W mice (22). In our initial study, tissue sections of NZB/W mouse kidneys collected 40 days after an IFNα Adv challenge as that administered to the positive control group A animals showed a diffuse proliferative glomerulonephritis with severe glomerular sclerosis and large numbers of immune complex deposits in the subendothelial regions as illustrated in Fig. S3 (13).
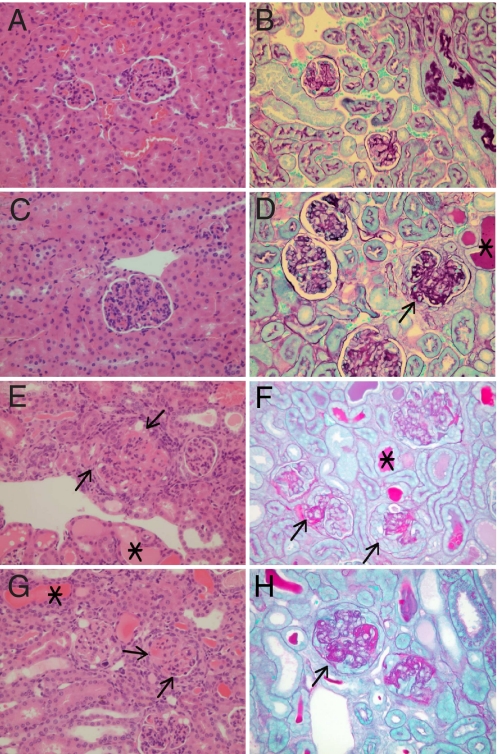
In the 6 surviving kinoid-immunized mice (group B), the 3 animals free of proteinuria showed no glomerular changes (Fig. 4 A and B). The 3 others presenting with proteinuria exhibited minimal to slight deposits of immune complexes affecting less than half of the glomeruli, and none showed glomerular sclerosis (Fig. 4 C and D). The overall score mean allotted to the tested animals of group B was graded 1.33. In the only surviving KLH-immunized mouse, renal lesions including glomerular sclerosis and immune complex deposits were scored grade 5 (Fig. 4 E and F). In the 4 surviving mice from the reference group (natural evolution), histological lesions paralleled with proteinuria (Table S4).
Fig. 4.
Representative tissue sections of kidneys from NZB/W mice stained with H&E (Left) or periodic acid/Schiff light green (Right). (Original magnification, 40×.) (A and B) Animal B1 from group B (IFNα kinoid-immunized). Note negligible changes in the kidney, graded as 0 associated with absence of proteinuria (Table 2). (C and D) Animal B4 from group B (IFNα kinoid-immunized) exhibiting proteinuria: kidneys with protein casts in tubules (*), increased cellularity of glomeruli (H&E), and only slight glomerular deposits (arrow) overall graded as 2 (Table 2). (E and F) Animal from group C (KLH-immunized mice) exhibiting proteinuria (Table S4) with sclerosis (arrow) and glomerular deposits and protein casts (*) overall graded as 5. (G and H) Animal D1 from reference group D (Adv challenge) with moderate glomerular deposit (arrow) in the majority of glomeruli, with some sclerosis (arrow) and protein casts (*) overall graded as 4 (Table S4).
Role of Serum Inhibitory IFNα Bioactivity in the Prevention/Delay of SLE in IFNα Kinoid-Vaccinated Mice.
Impact of the serum anti-mIFNα IC50 response on the lupus manifestations.
As shown in Tables 2 and 3, the 6 kinoid-immunized mice still surviving at the end of the study (123 days after challenge) were those whose IC50 peaked repeatedly over the threshold value for efficacy, which turn out to be >800 in this work. In these responder mice, serum inhibitory capacity to IFNα decreased variably during the follow-up period. In the 3 surviving mice (B1, B2, B3), whose inhibitory capacity remained over the background value, no clinical signs or histopathological lesions were detected, whereas in the 3 other mice (B4, B5, B6) whose serum inhibitory capacity declined to background levels (<400), late proteinuria and mild kidney alterations occurred.
Table 2.
Follow-up of clinical end points in IFNα kinoid-immunized mice (group B)
| Kinoid-immunized mice | Serum IC50 to IFNα |
Proteinuria onset, day after challenge | Death onset, day after challenge | Histology, score mean | |
|---|---|---|---|---|---|
| Peak/subsequent sample* | Last sample† | ||||
| B1 | 3,100/2,400 | 2,000 | 0 | ||
| B2 | 2,400/1,700 | 700 | 1 | ||
| B3 | 3,300/2,000 | 500 | 1 | ||
| B4 | 5,600/1,800 | Bg‡ | +(d109) | 2 | |
| B5 | 1,700/800 | Bg | +(d109) | 2 | |
| B6 | 1,400/1,300 | Bg | +(d98) | 2 | |
| B7 | 800/Bg | Bg | +(d91) | +(d119) | ND§ |
| B8 | 2,000/Bg | Bg | +(d67) | +(d79) | ND |
| B9 | Bg/Bg | Bg | +(d60) | +(d73) | ND |
| B10 | 600/Bg | Bg | +(d49) | +(d74) | ND |
| B11 | 400/Bg | Bg | +(d42) | +(d61) | ND |
| B12 | 400/Bg | Bg | +(d25) | +(d34) | ND |
End points dependent on serum inhibitory capacity to mIFNα.
*Subsequent samples correspond to the first serum collected after the peak.
†Last serum sample collected prior to death or at sacrifice (day 123 after challenge) at a stage when the serum IC was still detectable in 3 of 12 mice.
‡Bg, background.
§ND, not done.
Table 3.
Correlation between the serum IC kinetics and lupus manifestations
| Lupus manifestations | Serum inhibitory capacity to IFNα |
||
|---|---|---|---|
| Peak | Subsequent sample* | Last sample† | |
| Proteinuria occurrence, day | r = 0.85, P < 0.01 | r = 0.86, P < 0.01 | r = 0.529, P > 0.05 |
| Histological score | r = 0.87, P < 0.01 | r = 0.89, P < 0.01 | r = 0.75, P < 0.01 |
| Death, day | r = −0.78, P < 0.01 | r = −0.97, P < 0.001 | NA‡ |
*Subsequent samples correspond to the first serum collected after the peak.
†Last serum sample collected prior to death or at sacrifice (day 123 after challenge) at a stage when the serum IC was still detectable in 3 of 12 mice.
‡NA, not available.
Among the 6 animals that died during the study, 2 mice (B7 and B8) exhibited on one functional assay high serum IC50 (>800) that was followed in the subsequent tests by a persistent decline down to background levels. In these mice, death was preceded by late proteinuria (days 67 and 91). In the 4 other animals (B9, B10, B11, B12), the serum inhibitory capacity never reached an effective value (>800) and declined rapidly during the study to background levels.
Correlation between serum anti-mIFNα IC50 kinetics and lupus manifestations.
Spearman correlation coefficients were used to evaluate the association between kinetics of inhibitory capacity and lupus manifestations. As shown in Table 3, a longer duration of an effective serum IC is associated with the prevention/delay of proteinuria occurrence, renal histological lesions, and death.
Discussion
SLE etiopathogenesis involves genetic susceptibility, environmental factors, and clinical manifestations that are mediated by immunoregulatory cytokine expression including APC-released IFNα (23). Indeed, SLE flares are most often associated with IFNα overexpression (4) and/or the presence of IFNα-inducing factors (24). Moreover, IFNα treatment prescribed for other pathologies in susceptible individuals may cause autoimmune diseases, including SLE. These latter observations led us to studies with a lupus animal model, by using NZB/W mice treated with IFNα (13). Briefly prolonged expression of IFNα elicited by IFNα Adv challenge precipitated lupus-like disease in genetically prone NZB/W animals (13) but not in BALB/c mice. Applying this model to IFNα kinoid-immunized NZB/W mice, we showed here that lupus manifestations were delayed/prevented as long as the transient serum IFNα inhibitory capacity remained above an effective threshold.
A murine IFNα kinoid immunogen has been successfully produced by coupling mIFNα3 to KLH by aldehyde treatment that led to the formation of a heterocomplex in which each KLH carrier molecule is linked to a high number of IFNα molecules by covalent and noncovalent bonds (25). The heterocomplex is devoid of the biological activity of native IFNα and proved to be immunogenic in mice (Figs. 1 and 2). Whereas in control groups A and C mice receiving incomplete Freund adjuvant (IFA) (group A) or immunized with and IFA-KLH*, mIFNα-released by mIFNα Adv challenge, as expected, did not elicit anti-mIFNα Abs, kinoid-immunized mice receiving the same challenge mounted a strong and transient neutralizing Ab response (see Fig. 2D). Of note, in this work, the polyclonal Ab response triggered by mIFNα3 kinoid inhibited the biological activity of the mIFNα5 isoform released by IFNα Adv challenge. B cells directed to circulating self-antigens are silent under physiological conditions, but these cells can be activated to produce polyclonal autoantibodies under pathologic circumstances, including AIDS (26) or under experimental conditions, such as stimulation with lipopolysaccharide (27), CpG (28), or after kinoid immunization, as shown in this work, by using IFNα kinoid and elsewhere by using kinoids of other specificities (17–19). We assumed that in the latter instances the physiologic mechanisms of B cell tolerance were overwhelmed by the conjunction of the foreign T cell help provided by the KLH partner and the highly concentrated and conformationally native IFNα B epitopes cross-linking Ig receptors (capping) on specific B cells.
The absence of T cell-mediated immunity to self-IFNα observed in this work is not surprising given the negative T cell clonal selection to self-antigens in the thymus and the presence of immune suppressive Treg cells controlling autoreactive T cells to self-antigens in the periphery. Taking into account the short life of B cell memory in the absence of specific T cell help (29), the lack of immune T cell response to self-IFNα may explain the observed rapid decline of circulating Abs (Fig. 2 A and B) and the need for repeated kinoid boostering to maintain effective threshold levels of neutralizing Abs to the cytokine (30).
Safety of immunization to self-cytokines has been initially investigated for kinoids targeting cytokines of different specificities, including human TNFα currently under clinical trial (17). In agreement with the absence of toxicity reported for kinoid vaccines (17–19), mIFNα kinoid immunization in mice proved to be safe. As in other kinoid immunizations, the lack of specific autoimmune disorders was expected from the absence of autoreactive T cells to IFNα (Fig. 2D). Moreover, it could be anticipated that there was a minimal risk of hampering physiologic cytokine bioactivities that occur in healthy tissues within cytokine fields comprising activated effector–target cell synapses (31). Indeed, under this tissue compartmentalization, anti-mIFNα Abs, even of high avidity (Kd = 10−8 to 10−9 M) (32) as observed after immunization, present in the thin film of poorly renewed stromal lymph surrounding cytokine fields, cannot efficiently compete for IFNα with target cell receptors of a much higher Kd value (affinity ranging from 1 to 5.10−11 M) (33, 34). By contrast, in pathologic tissues, ectopic IFNα abnormally released in the abundant stromal lymph compartment characterizing inflammation (edema) can be inhibited by Abs of high avidity present in the body fluid of kinoid-immunized animals. These considerations should account for why after more than a decade of animal experimentations or clinical trials, using high-affinity Abs supplied by passive administration (mAb immunotherapy) (35) or triggered by kinoid immunization, as performed in this and other studies (17–19, 26), adverse effects caused by the direct impact of Abs on the physiologic cytokine reactions occurring in healthy tissues were not reported.
In this work, not all kinoid-immunized mice mounted an immune response of the same magnitude, and they could be separated in responders exhibiting an IC50 ≥800 and low/nonresponders (IC50 <800) (Table S3). The Ab response was transient (Fig. 2A), and serum IC50 declined rapidly (Fig. 2B). Importantly, the Ab level paralleled the lupic evolution as assessed by the clinical end points (Tables 2 and 3). Thus, we hypothesize that the protective effect against proteinuria and death is associated with antibodies levels above a certain threshold, and the early occurrence of proteinuria and death is associated with the absence or low Ab response to IFNα. The survival and absence of proteinuria at the end of the study are likely to be transient and as long as serum IC50 remained over the background levels (>400) and the delayed proteinuria is predicted by a rapid fall of serum IC from protective to background levels. To maintain a long-term Ab-dependent clinical benefit triggered by the kinoid immunization, periodic booster injections (2–4 per year) will very likely be required (17–19). In fact, frequency of kinoid boosters should be optimally determined by monitoring serum-neutralizing capacity to maintain an effective Ab threshold, keeping with the logic of metronomic scheduled treatments (36).
The present work supports the use of anti-mIFNα Ab therapy in SLE currently under trial (http://clinicaltrials.gov/ct2/show/NCT00657189?term=anti+interferon&rank=3), at least for patients in whom, as in our experimental model, the disease has been triggered by IFNα treatment (8, 9) or for patients whose blood also exhibits an IFNα signature (10, 11). In view of these specific indications, we have prepared a human IFNα kinoid exhibiting immunological characteristics similar to those described for huTNFα kinoid currently under clinical investigation and here for mIFNα kinoid. After its validation in humans, the vaccine could represent an anti-SLE medication in addition to current therapies and particularly an alternative in case of resistance to passive mAb therapy under clinical investigation (37). The kinoid vaccine provides substantial therapeutic advantages compared with passive immunization: (i) a generation of polyclonal autoAbs excludes the formation of Abs directed to themselves, the source of relapse; (ii) a serological monitoring of the frequency of vaccine boosters allows maintenance of an effective Ab threshold; and (iii) the low frequency of boosters fosters patient compliance.
In conclusion, the present work, showing that an IFNα kinoid triggers a strong and transient Ab response to IFNα, which prevents the pathogenic effects of this cytokine in an experimental lupus murine model, represents an initial step in the development of a novel therapeutic vaccine targeting IFNα to be applied to SLE and perhaps to other systemic autoimmune diseases mediated by IFNα, such as psoriasis or diabetes mellitus (3).
Materials and Methods
Reagents.
KLH was purchased from Intracel, murine IFNα3 from PBL, and ISA-51 adjuvant from Seppic.
Animals.
BALB/c mice were purchased from Charles River Breeding Laboratory. Female New Zealand Black (NZB) and the phenotypically normal New Zealand White (NZW) male were purchased from Harlan, and a colony of NZB/W mice was established. Female NZB/W mice were treated at 9–11 weeks of age.
Preparation of Immunogen.
Murine IFNα kinoid was prepared by complexing mIFNα3 with KLH with aldehyde treatment, as reported in ref. 18. In brief, the KLH–mIFNα heterocomplex was prepared by treating a mixture of KLH and mIFNα at a ratio of 1:40, with glutaraldehyde (22.5 mM). Glutaraldehyde excess was removed by dialysis against PBS, and the dialysate was formulated. After quenching with glycine and subsequent dialysis against PBS, the kinoid was stored at 4 °C. Native KLH treated as described above was used as control and referenced in the text as *KLH.
Adenovirus Preparation.
Adenovirus (Adv) and IFNα5 expressing adenovirus (IFNα Adv) preparations were obtained by applying a published procedure (13) and administered i.v. into retroorbital plexus at 109 viral particles per mouse.
Cell Cultures.
Mouse L929 cell line (American Tissue Culture Collection, CCL 1) and mouse L cells (a gift from Pierre Lebon, Unité Virologie, Saint-Vincent de Paul Hospital, Paris, France) were cultured in RPMI medium 1640 containing 10% FCS (culture medium). Purified murine splenocytes were resuspended in RPMI medium 1640 containing 5% FCS and 50 μM 2-mercaptoethanol and incubated in culture medium with or without KLH (10 μg/mL) and murine IFNα (10 μg/mL) antigens.
T Cell Proliferation Assay.
Antigen-activated splenocytes were cultured for 4 days and then pulsed for 18 h with [3H]thymidine (0.5 μg per well). Cells were then harvested on filter mats, and thymidine incorporation was measured with a beta counter. Stimulation index (SI) was expressed as [(mean of cpm from stimulated cells) − (mean of cpm from unstimulated cells)]/(mean of cpm from unstimulated cells). SI values >1.5 were considered as positive. Culture supernatants were collected at 48 and 72 h to measure release of IL-2 and IFNγ, respectively.
Circulating mIFNα Quantification.
Serum IFNα levels were measured by ELISA (PBL Biomedical Laboratories) following the manufacturer's instructions.
Anti-mIFNα/KLH Ab Titer Assay.
Serum anti-mIFNα and anti-KLH antibody titers were determined by ELISA. Briefly, precoated ELISA plates with 100 ng of mIFNα per well or 100 ng of KLH per well were incubated with serial dilutions of sera from preimmunized and kinoid-immunized mice. Specific IgG were detected by using peroxidase rabbit anti-mouse IgG (Zymed). Endpoint titers were expressed as the reciprocal of the highest sample dilution giving an OD of 0.3, which is the equivalent of twice the OD mean preimmune serum samples at 1/50 dilution.
mIFNα Bioactivity Assay.
The antiviral activity of mIFNα was assessed by using the standard L929 cells with encephalomyocarditis virus (EMCV) or L cells with vesicular stomatitis virus (VSV) CPE inhibition assay (20). Briefly, the cells were seeded in flat-bottomed 96-well plates and grown to 95% confluence. Serial dilutions of either mIFNα or the kinoid were added to the wells. After 24 h of incubation at 37 °C, the medium was removed and replaced with medium containing EMCV or VSV at a predetermined optimal concentration. The cells were then incubated at 37 °C overnight and the number of surviving cells determined by the MTT dying assay. Antiviral activity was expressed as IC50. The IC50 was defined as the concentration required to achieve 50% protection against EMCV- or VSV-induced cytopathic effects. The ability of hyperimmune and control sera to inhibit mIFNα bioactivity was determined by incubating serum dilutions with 10 ng of mIFNα per ml. The inhibitory capacity (IC50) was expressed as the reciprocal of the serum dilution that inhibited 50% of mIFNα bioactivity. Mice were considered Ab responders if their serum IC50 peaked at 800 or more at 2 consecutive tests, and IC50 <400 was scored as background responses. Purified IgG s from hyperimmune and control sera were obtained by using the kit from Pierce, and serial dilutions of IgG were tested for anti-IFNα neutralizing activity by the standard L cells with VSV CPE inhibitory assay in the presence of mIFNα.
Immunizations.
BALB/c mice.
Six BALB/c mice were immunized by 3 i.m. injections of mIFNα kinoid in IFA (ISA 51, Seppic, France) at 0, 21, and 42 days. Sera were collected 10 ± 2 days after the last injection, and the anti-IFNα and anti-KLH antibody titers in the sera were determined by ELISA and the capacity of the serum to inhibit IFNα as assessed with the antiviral activity of mIFNα. To evaluate the serum anti-IFNα IC50 kinetics, another group of 3 animals received 2 injections of mIFNα kinoid in ISA 51 at days 0 and 21. Serum samples were collected at days 28, 49, 75, and 104.
NZB/W mice.
Three groups of 12 mice and 1 group of 9 mice were used for the experimentation. All groups received 4 i.m. injections of PBS (group A), mIFNα kinoid (group B), modified KLH (group C), and PBS (group D) in ISA 51. At the end of the immunization, all 3 groups A to C were challenged with IFNα Adv, whereas group D received only null Adv. From all animals, serum samples were collected before the challenge and afterward periodically after challenge up to their death or at sacrifice (day 123 after challenge). The corresponding antibody titers of the serum samples and their IC50 values were assessed.
NZB/W Mice Follow-Up.
Proteinuria and survival.
Mice were observed for proteinuria once until day 10 and at least twice weekly from day 15 to day 123 after IFNα Adv or Adv administration. Urine was tested for proteinuria by using a dipstick (Chemstrip 2 GP; Roche Diagnostics). Mice were considered to have proteinuria if 2 consecutive urine samples exhibited titers of at least 5 mg/mL or more as determined in our previous study (13). Death of animals was daily recorded.
Histology.
At day 123 (animal sacrifice), tissue examinations of kidneys, liver, and spleen were performed in accordance with literature (22). Kidneys, thymus, and spleen samples from the surviving mice at this time were fixed in buffered formalin for 18–24 h and transferred to 70% alcohol. Tissues fixed in formalin were sampled, processed, and embedded in paraffin wax. The blocks were sectioned at 4 μm, two sections of kidneys were prepared, and one was stained with H&E and the other with periodic acid/Schiff light green. The spleen and thymus sections were stained with H&E. The slides were identified with the animal number. The slides were examined microscopically without knowledge of the treatment given or protein levels in the urine. The glomerular changes were evaluated for both severity and extent. Both kidneys for each animal were evaluated, and abnormalities including severity and distribution of each lesion were graded on a scale of 1–5 (minimal, slight, moderate, marked, and severe).
Statistical Analysis.
Mean were compared by using nonparametric Mann–Whitney U test. Survival and proteinuria data were analyzed by using Kaplan–Meier graphs and log-rank tests. Spearman correlation coefficients were used to evaluate the association between serum IC kinetics and lupus manifestations.
Supplementary Material
Acknowledgments.
We thank the Neovacs management staff for its technical contribution and for reviewing the manuscript and Ms. B. Drouet for skilled secretarial assistance. Seppic generously supplied the ISA 51 adjuvant. This work was supported by Neovacs SA.
Footnotes
Conflict of interest statement: P.L. is a scientist and D.Z. a shareholder of Neovacs SA.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900615106/DCSupplemental.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 3.Stewart TA. Neutralizing interferon α as a therapeutic approach to autoimmune diseases. Cytokine Growth Factor Rev. 2003;14:139–154. doi: 10.1016/s1359-6101(02)00088-6. [DOI] [PubMed] [Google Scholar]
- 4.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: All roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Hooks JJ, et al. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 6.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25:401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 7.Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: Presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982;216:429–431. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- 8.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 9.Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago-Raber ML, et al. Type I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 14.D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 15.Goldblatt F, Isenberg DA. Anti-CD20 monoclonal antibody in rheumatoid arthritis and systemic lupus erythematosus. Handb Exp Pharmacol. 2008;181:163–181. doi: 10.1007/978-3-540-73259-4_8. [DOI] [PubMed] [Google Scholar]
- 16.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Rönnblom L. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-α by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2524–2532. doi: 10.1002/art.11225. [DOI] [PubMed] [Google Scholar]
- 17.Le Buanec H, et al. TNFα kinoid vaccination-induced neutralizing antibodies to TNFα protect mice from autologous TNFα-driven chronic and acute inflammation. Proc Natl Acad Sci USA. 2006;103:19442–19447. doi: 10.1073/pnas.0604827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rad FH, et al. VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc Natl Acad Sci USA. 2007;104:2837–2842. doi: 10.1073/pnas.0611022104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Buanec H, et al. Control of allergic reactions in mice by an active anti-murine IL-4 immunization. Vaccine. 2007;25:7206–7216. doi: 10.1016/j.vaccine.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Familletti PC, Rubinstein S, Pestka S. A convenient and rapid cytopathic effect inhibition assay for interferon. Methods Enzymol. 1981;78:387–394. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- 21.Presicce P, Taddeo A, Conti A, Villa ML, Della Bella S. Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Mol Immunol. 2008;45:1136–1145. doi: 10.1016/j.molimm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Burnett R, Ravel G, Descotes J. Clinical and histopathological progression of lesions in lupus-prone (NZB × NZW) F1 mice. Exp Toxicol Pathol. 2004;56:37–44. doi: 10.1016/j.etp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Koutouzov S, Mathian A, Dalloul A. Type I interferons and systemic lupus erythematosus. Autoimmun Rev. 2006;5:554–562. doi: 10.1016/j.autrev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Batteux F, Palmer P, Daeron M, Weil B, Lebon P. FCgammaII (CD32)-dependent induction of interferon-alpha by serum from patients with lupus erythmatosus. Eur Cytokine Netw. 1999;10:509–514. [PubMed] [Google Scholar]
- 25.Neovacs SA. Stable immunogenic product comprising heterocomplexes, composition thereof, and preparation method. 2004 FR0211455 patent WO 04024189. [Google Scholar]
- 26.Gringeri A, et al. Active anti-IFNα immunization: A European–Israeli, randomized, double-blind, placebo-controlled clinical trial in 242 HIV-1-infected patients (the EURIS study) J Acquired Immune Defic Syndr Hum Retrovirol. 1999;20:358–370. doi: 10.1097/00042560-199904010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 α, β, and tumor necrosis factor during experimental endotoxemia: Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J Leukocyte Biol. 2000;68:455–463. [PubMed] [Google Scholar]
- 29.Gray D, Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988;336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 30.Zagury D, et al. Active versus passive anti-cytokine antibody therapy against cytokine-associated chronic diseases. Cytokine Growth Factor Rev. 2003;14:123–137. doi: 10.1016/s1359-6101(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 31.Kourilsky P, Truffa-Bachi P. Cytokine fields and the polarization of the immune response. Trends Immunol. 2001;22:502–509. doi: 10.1016/s1471-4906(01)02012-9. [DOI] [PubMed] [Google Scholar]
- 32.Zagury D, Gallo RC. Anti-cytokine Ab immune therapy: Present status and perspectives. Drug Discov Today. 2004;9:72–81. doi: 10.1016/S1359-6446(03)02955-6. [DOI] [PubMed] [Google Scholar]
- 33.Zagury D, Burny A, Gallo RC. Toward a new generation of vaccines: The anti-cytokine therapeutic vaccines. Proc Natl Acad Sci USA. 2001;98:8024–8029. doi: 10.1073/pnas.141224798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau JY, et al. Interferon-α receptor expression and regulation in chronic hepatitis B virus infection. Hepatology. 1991;13:332–338. [PubMed] [Google Scholar]
- 35.Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med. 2005;72:250–256. [PubMed] [Google Scholar]
- 36.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 37.Friedberg JW. Unique toxicities and resistance mechanisms associated with monoclonal antibody therapy. Hematology Am Soc Hematol Educ Program. 2005;2005:329–334. doi: 10.1182/asheducation-2005.1.329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.