Summary
Postnatal cartilage development and growth are regulated by key growth factors and signaling molecules. To fully understand the function of these regulators, an inducible and chondrocyte-specific gene deletion system needs to be established to circumvent the perinatal lethality. In this report, we have generated a transgenic mouse model (Col2a1-CreERT2) in which expression of the Cre recombinase is driven by the chondrocyte-specific col2a1 promoter in a tamoxifen-inducible manner. To determine the specificity and efficiency of the Cre recombination, we have bred Col2a1-CreERT2 mice with Rosa26R reporter mice. The X-Gal staining showed that the Cre recombination is specifically achieved in cartilage tissues with tamoxifen-induction. In vitro experiments of chondrocyte cell culture also demonstrate the 4-hydroxy tamoxifen-induced Cre recombination. These results demonstrate that Col2a1-CreERT2 transgenic mice can be used as a valuable tool for an inducible and chondrocyte-specific gene deletion approach.
Keywords: chondrocyte, Cre-mediated recombination, conditional knockout, tamoxifen, X-Gal staining
Chondrocyte maturation and cartilage formation involves multiple steps that are regulated by numerous growth factors, their downstream signaling molecules and transcription factors. Conventional and tissue-specific gene deletions provide powerful tools to investigate roles of specific genes in chondrocyte maturation. However, embryonic lethality is often encountered because of the essential role of various genes in early embryonic development (Akiyama et al., 2004; Karaplis et al., 1994; Lanske et al., 1996, 1999; Razzaque et al., 2005; Sakamoto et al., 2005; St-Jacques et al., 1999). Furthermore, some gene deletions result in such severe skeletal malformation that interpretation of the phenotype is challenging even if the animal survive birth. An inducible conditional gene deletion approach is an exquisite method to determine the function of a gene that is either embryonic lethal or associated with marked abnormalities of morphology and tissue architectures. In this report, we present a chondrocyte-specific and tamoxifen-inducible Cre transgenic mouse model.
Type II collagen is a chondrocyte-specific protein and its expression is detected in growth plate and articular chondrocytes in long bones and other cartilage tissues in the body. Type II collagen promoter (col2a1) has been used in a variety of animal models to achieve tissue-specific gene expression in chondrocytes (Schipani et al., 1997; Stricker et al., 2002; Takeda et al., 2001; Ueta et al., 2001; Weir et al., 1996). In the present studies, we generated transgenic mouse lines in which the Cre recombinase was fused to a mutated ligand-binding domain of human estrogen receptor (ER) driven by the col2a1 promoter (Col2a1-CreERT2; Metzger et al., 2005). The fusion protein has been reported to be translocated into the nuclei in response to estrogen antagonist tamoxifen or 4-hydroxy tamoxifen (4-OH tamoxifen), an active metabolite of tamoxifen in vivo (Indra et al., 1999). This transgenic mouse model will serve as a valuable tool for gene deletion in a chondrocyte-specific and temporally-controlled manner.
The CreERT2 cDNA was cloned into the plasmid PKN-185 (Tanaka et al., 2000; Tsuda et al., 2003). The Col2a1-CreERT2 transgene consists of the 1.0-kb mouse col2a1 promoter (nucleotide 1940-2971, GenBank accession number: M65161) linked to CreER with mutations of G400V, M543A, and L544A in the ligand-binding domain of human ER (Feil et al., 1997; Indra et al., 1999; Metzger et al., 2005) followed by the col2a1 enhancer (nucleotide 4930-5571, GenBank accession number: M65161), as shown in Figure 1 (Krebsbach et al., 1996; Metsaranta et al., 1991).
FIG. 1.
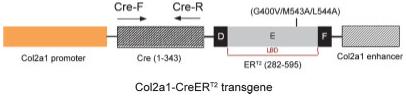
Col2a1-CreERT2 transgene construct. The Cre recombinase cDNA fused with cDNA encoding the ER ligand-binding domain containing three mutation sites was cloned into the PKN-185 vector. The expression of the fusion protein is under control of the 1.0-kb type II collagen promoter. The primer positions for genotyping transgenic mice have been indicated by arrows.
Two independent transgenic mouse lines have been established from 15 founder mice that exhibited similar patterns and levels of Cre expression and show tamoxifen-dependent induction of Cre-recombination activity. Detail analysis was performed in one line of the transgenic mice and presented in this manuscript.
To assess recombination in vivo, Col2a1-CreERT2 transgenic mice were crossed with Rosa26R reporter mice (R26R strain). The Rosa26R mice have the lacZ gene inserted into the ubiquitously expressed Rosa locus that is preceded by a transcriptional stop cassette flanked by loxP sites (Mao et al., 1999; Soriano, 1999). In Col2a1-CreERT2;R26R transgenic mice, β-Gal activity was observed only in chondrocytes in mice treated with tamoxifen to activate Cre-recombinase.
To analyze the activity of tamoxifen-induced Cre recombination during embryonic development, a single dose of tamoxifen was administered to pregnant female Col2a1-Cre+/-ERT2;R26R+/- mice with embryos at the E15.5 stage. E18.5 embryos were collected and analyzed by X-Gal staining. Areas of cartilage showed high levels of X-Gal staining including long bones, ribs, and the spine. In contrast, the calvariae and clavicles, which undergo intra-membranous bone formation, were negative for X-Gal staining. Weak or no staining was observed in cartilage tissues of control embryos that did not receive tamoxifen treatment (Fig. 2a). X-Gal staining in knee joint sections of E18.5 embryos showed over 80% of the recombination efficiency in chondrocytes of articular cartilage and patella tissues but some epiphyseal and growth plate cartilage showed weaker X-Gal staining (Fig. 2b), suggesting the insufficient fixation of the samples or relative poor access of tamoxifen in these areas. The results indicate that Cre recombination is activated in a tamoxifen-dependent manner in Col2a1-Cre+/-ERT2 embryos.
FIG. 2.
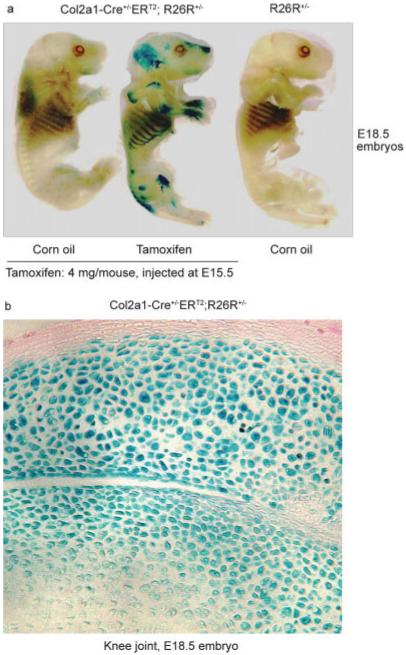
Tamoxifen-induced lacZ expression in Col2a1-CreERT2 embryos. The male Col2a1-Cre+/-ERT2 mice were mated with female Rosa26R reporter mice. The pregnant female mice with E15.5 embryos were injected intraperitoneally with tamoxifen (4 mg/mouse) or corn oil (control). Three days later, the E18.5 embryos were prepared and stained with X-Gal. Only the double transgenic mice exposed to tamoxifen showed X-Gal positive staining. The ribs, spine, and long bones showed positive X-Gal staining (a). A histology section from the knee joint showed positive X-Gal staining of chondrocytes in articular cartilage and patella tissues (b).
To determine the specificity and efficiency of the Cre-mediated recombination in cartilage tissues of postnatal mice, 2- and 8-week-old Col2a1-Cre+/-ERT2;R26R+/- mice were treated with tamoxifen (1 mg/mouse/day, ×5days). Strong X-Gal staining was observed in regions of cartilage. Histological sections showed staining in growth plate chondrocytes including cells from the resting, proliferating, and hypertrophic zones (Fig. 3a,b). Quantification of percentage of X-Gal-positive cells showed that 78% recombination efficiency was achieved in the growth plate in 2-week-old mice. Slight reduction in the numbers of X-Gal-positive cells was observed in 8-week-old mice (Fig. 3b). Tamoxifen-induced Cre recombination was also observed in articular chondrocytes at a similar level of recombination efficiency (Fig. 3a,b). Although some positive X-Gal staining was found in corn oil treated control mice, the staining was mainly observed in bone matrix instead of chondrocytes. It seems that there is a very limited leakiness for Cre expression in these transgenic mice. In ribs, strong X-Gal staining was also observed (Fig. 3c). In vertebral tissue, the efficiency of tamoxifen-induced Cre-recombination was little bit lower (75%) (Fig. 3d). In the nucleus pulposus of the intervertebral discs, we did not detect the X-Gal staining (Fig. 3d, lower panel). Although cells in the nucleus pulposus produce type II collagen, the major protein component in this tissue is the proteoglycans (over 80%). Another possibility is the low tamoxifen penetration into the nucleus pulposus. No positive X-Gal staining was detected in noncartilage tissues, such as heart, kidney, and liver (Fig. 3d).
FIG. 3.
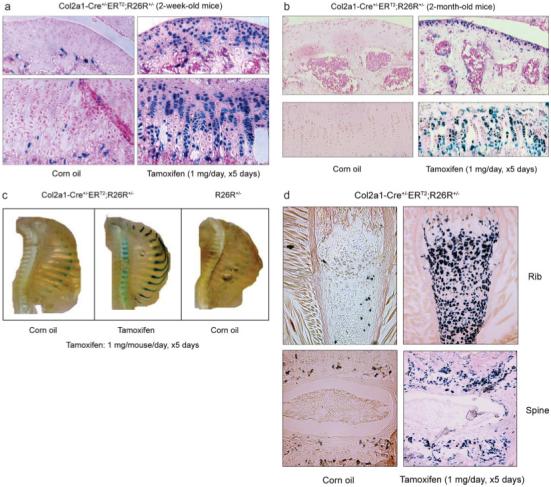
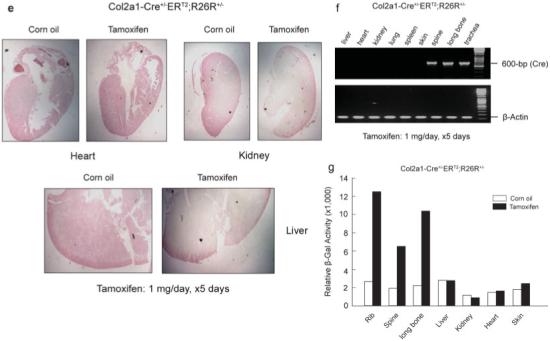
Histological analyses of the Col2a1-Cre+/-ERT2;R26R+/- mice. Two- and eight-week-old double transgenic mice were injected with tamoxifen (1 mg/mouse/day for 5 consecutive days). Mice were killed 5 days after injections were completed. Bone samples and other tissues were fixed, decalcified, and processed for frozen section preparation followed by X-Gal staining. Sections were then counterstained by Nuclear Fast Red. Growth plate and articular chondrocytes from 2- and 8-week-old transgenic mice (a,b) and ribs and spine from 2-week-old transgenic mice (c) received tamoxifen showed X-Gal positive staining. X-Gal-positive chondrocytes were only identified in histological sections of ribs (d, upper panel) and spine (d, lower panel) of transgenic mice received tamoxifen (right panels). Recombination efficiency was analyzed from multiple histological sections of three transgenic mice (n = 3). Other organs, such as heart, kidney, and liver did not show X-Gal positive staining (e). RNA was extracted from multiple tissues including liver, heart, kidney, lung, spleen, skin, spine, long bone, and trachea of the 2-week-old Col2a1-Cre+/-ERT2;R26R+/- mice. The Cre expression was detected in spine, long bone, and trachea but not in other tissues by RT-PCR using the Cre-specific primers (f). The β-Gal activity from tissues rib, spine, long bone, liver, kidney, heart, and skin were also measured and normalized to protein contents. The induction of β-Gal activity by tamoxifen was observed in rib, spine, and long bone but not in other tissues (g).
To further confirm the specificity of tamoxifen-induced Cre recombination, the Cre expression was examined by RT-PCR in multiple tissues including liver, heart, kidney, lung, spleen, skin, spine, long bone and trachea in 2-week-old transgenic mice. The Cre expression was detected in spine, long bone and trachea tissues but not in liver, heart, kidney, lung, spleen, and skin tissues (Fig. 3f). β-Gal activity was also examined in some of these tissues including rib, spine, long bone, liver, kidney, heart, and skin. The induction of the β-Gal activity by tamoxifen was detected in rib, spine, and long bone but not in the liver, kidney, heart, and skin of the transgenic mice (Fig. 3g).
To determine the efficiency of tamoxifen-induced recombination in chondrocytes in vitro, primary sternal chondrocytes were isolated from 3-day-old neonatal Col2a1-Cre+/-ERT2;R26R+/- mice and treated with 4-OH tamoxifen. X-Gal staining and β-Gal activity measurements showed that 4-OH tamoxifen-induced efficient Cre-mediated recombination (Fig. 4a,b). In contrast, estrogen did not induce β-Gal activity at the same concentration (Fig. 4b). Tamoxifen is metabolized into its active form, 4-OH tamoxifen in vivo (Reed et al., 2005) and 4-OH tamoxifen has been shown to bind specifically to the mutant ER (Feil et al., 1997). These results suggest that expression of CreERT2 transgene can only be induced by exogenous 4-OH tamoxifen treatment but not by endogenous estrogen in chondrocytes derived from Col2a1-Cre+/-ERT2 transgenic mice. These findings show the specificity of tamoxifen-induced gene deletion. The nonuniformed X-Gal staining in the cell culture is probably due to the impurity of the isolated primary chondrocytes. Low percentage of fibroblasts or other cells are usually present in the primary cell culture.
FIG. 4.
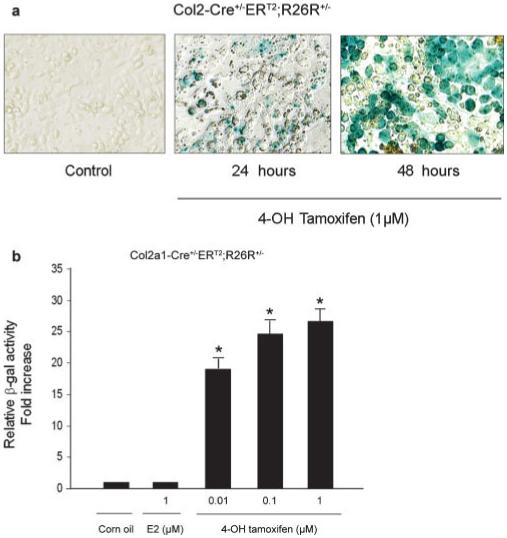
Tamoxifen-induced β-Gal activity in chondrocytes. (a) The sternal chondrocytes were isolated from 3-day-old neonatal Col2a1-Cre+/-ERT2;R26R+/- mice. Cells were seeded in 12 well-plates and treated with 4-OH tamoxifen for 24 and 48 h followed by X-Gal staining. Chondrocytes isolated from double transgenic mice were treated with 1 μM 4-OH tamoxifen for 24 and 48 h. Weak X-Gal staining was observed at 24 h time point and strong X-Gal staining was observed when cells were treated with 4-OH tamoxifen for 48 h (n = 3). (b) Primary chondrocytes isolated from Col2a1-Cre+/-ERT2;R26R+/- mice were treated with different concentrations of 4-OH tamoxifen and 1 μM estradiol (E2), the β-Gal activity was measured. 4-OH tamoxifen stimulated β-Gal activity in chondrocytes and estradiol had no significant effect on β-Gal activity. *P < 0.01, compared to corn oil control group; **P < 0.05, compared to 0.01 μM 4-OH tamoxifen group; one way analysis of variance followed by Dunnett’s test, n = 4.
Understanding the regulation of cartilage during growth and development is facilitated by conventional or conditional gene deletion strategies. However, the global and conditional gene deletions often result in lethality. Furthermore, some genes do not have effect in specific tissues until postnatal or adult stage. To circumvent these problems, we have generated the CreERT2 mice driven by the 1.0-kb col2a1 promoter, which are highly specific for chondrocytes. In this report, we have shown that tamoxifen-induced Cre recombination occurs specifically in chondrocytes with high efficiency. The utility of these mice is that it will allow us to carry out inducible gene knockout studies in cartilage tissues in a stage-specific manner. Since we have detected col2 mRNA expression in 6-month-old mice by in situ hybridization, it is possible that the col2a1 promoter will remain active in older mice and we can specifically knockout chondrocyte-specific genes in adult mice. Further studies are still required to examine the activity of inducible Cre recombinase for different ages of adult mice.
Although our present work validates Cre expression by breeding with the Rosa26R mice and analyzing the heterozygous offspring for both the Cre and R26R alleles, the ultimate test for this system is to knockout floxed genes in chondrocyte-specific fashion in both alleles. Currently, we are breeding these transgenic mice with β-catenin-loxP mice to determine the role of β-catenin in postnatal cartilage growth and development.
A chondrocyte-specific doxycline-inducible system has been recently reported (Grover and Roughley, 2006), which also offers a valuable tool for the gene deletion at various stages. Similar approach was also reported recently by Nakamura et al. (2006) during the revision of our manuscript. In these Col2-CreERT transgenic mice, the CreERT cDNA was driven by the col2a1 promoter. The recombination efficiency was evaluated during later stages of embryogenesis till the early postnatal stage. Cartilage-specific Cre expression was demonstrated. The doxycline-inducible and tamoxifen-inducible systems should provide complementary and alternative approaches for stage-specific gene deletion in chondrocytes.
MATERIALS AND METHODS
Construction of the Col2a1-CreERT2 Transgene
The CreERT2 cDNA was released from pCreERT2 plasmid by EcoRI and the fragment was cloned into the PGEM-EZT vector. The DNA fragment was then released by NotI digestion and cloned into the plasmid PKN-185 plasmid. The transgene Col2a1-CreERT2 cDNA was released by PvuII and HindIII digestion.
Generation of Col2a1-CreERT2 Transgenic Mice
The Col2a1-CreERT2 transgene was microinjected into pronuclei of fertilized eggs (C57BL/6). Positive transgenic mice were identified by PCR using primers Cre-F and Cre-R and confirmed by Southern blot analysis. Cre-specific primers for genotyping the transgenic mice are as follows: 5′-ATCCGAAAAGAAAACGTTGA-3′ (upper primer) and 5′-ATCCAGGTTACGGATATAGT-3′ (lower primer).
Analysis of Recombination Activity Induced by Tamoxifen
Col2a1-CreERT2 transgenic mice were crossed with reporter mice (Rosa26R) in which expression of Escherichia coli β-galactosidase can be induced by Cre-mediated recombination (Mao et al., 1999; Soriano, 1999). Offspring were genotyped by PCR using Cre-specific primers and primers for detecting R26R alleles, respectively. PCR primers for genotyping Rosa26R mice are as follows: R1295, 5′-GCGAAGAGTTTGTCCTCAACC-3′; R523, 5′-GGAGCGGGAGAAATGGATATG-3′ and R26F2, 5′-AAAGTCGCTCTGAGTTGTTAT-3′. Using these primers 600-bp PCR product was detected in wild-type mice and 325-bp PCR product was detected in homozygous Rosa26R mice. In heterozygous Rosa26R mice, both 600 and 325-bp PCR products were detected.
For analyzing the Cre induction during embryonic stage, pregnant female mice with embryos at the E15.5 stage were injected with 4 mg of tamoxifen in corn oil once intraperitoneally. Previous report showed that this is an optimal concentration for tamoxifen-induction at embryonic stage (Hayashi and McMahon, 2002). Three days later, mice were killed and the embryos were processed for whole-mount X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining to detect β-galactosidase (β-Gal) activity. Corn oil was injected into the control mice.
For analyzing the postnatal activity of the Cre recombination, 2-week-old mice received intraperitoneal injection of 1 mg of tamoxifen for 5 consecutive days. Five days after the last injection, mice were killed and various tissue samples were fixed in 0.2% glutaraldehyde, decalcified, and processed for frozen sections followed by X-Gal staining. The recombination efficiency was determined by counting the X-Gal-positive cells divided by the total number of cells in the entire growth plate (proliferating zone and hypertrophic zone) and in cells on the articular surface (n = 3 mice).
For RNA extraction, multiple tissues were collected from mice and adjacent tissues were removed. Tissue samples were then frozen in liquid nitrogen and homogenized with the mortar. The RNA was extracted by Trizol solution using the standard protocol. The first strand cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Laboratories, Hercules, CA). To measure β-Gal activity from different tissues, tissue samples were collected and adjacent tissues were removed. For long bone preparation, the epiphyseal regions of the distal femora and proximal tibiae were collected. Tissue samples were then cut into 2 mm pieces and homogenized in 1 ml of AMPER buffer (Pierce, Rockford, IL) using a Polytron blender. After centrifugation, the supernatant was collected and used for the measurement of β-Gal activity, which was normalized by the protein content of cell lysates.
Measurement of Tamoxifen-Induced β-Gal Activity in Vitro
Primary sternal chondrocytes were isolated from 3-day-old neonatal mice and treated with 4-OH tamoxifen (1 μM) or estrogen (1 μM). After 48 h treatment, cells were lysed using passive lysis buffer. Cell lysates (20 μl) were used to measure the β-Gal activity using BD Luminacent β-Gal kit. The β-Gal activity was normalized to the protein content, which was determined using the Bio-Rad protein assay reagents (Bio-Rad). For X-Gal staining of primary chondrocyte cultures, cells were washed with phosphate-buffered saline (PBS) three times and fixed with 0.2% glutaraldehyde for 5 min at room temperature. Cells were then stained with X-Gal solution at 37°C overnight.
X-Gal Staining and Histological Analysis
X-Gal staining for β-Gal activity in embryos was performed as reported (Kohn et al., 2004). For the embryo whole-mount staining, the pregnant female mice were killed and the embryos were isolated in PBS and prefixed in 0.2% glutaraldehyde solution containing 2 mM MgCl2, 5 mM EGTA, and 0.02% Nonidet P-40 at 4°C for 90 min. Samples were washed three times with 0.02% Nonidet P-40 solution at room temperature for 30 min followed by staining in X-Galsolution containing 1 mg/mlX-Gal, 5 mM potassium ferricyanide, 2 mM potassium ferrocyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40 at 30°C for 3 h. For bone tissue staining, samples were rinsed twice with PBS, fixed in 0.25% glutaraldehyde for 4 days at 4°C, and washed three times with PBS. Samples were decalcified with buffered EDTA for 3 weeks, and then embedded and processed for frozen sections. X-Gal staining for β-Gal activity was carried out in X-Gal solution containing 30 mM potassium ferricyanide, 30 mM potassium ferrocyanide, and 1 mM MgCl2 at 30°C overnight.
Isolation of Primary Chondrocytes
Three-day-old neonatal mice were killed and genotyped using tail tissues obtained at the time of death. The anterior rib cage and sternum were harvested, washed with sterile PBS, and then digested with Pronase (Roche Applied Science) dissolved in PBS (2 mg/ml) in a 37°C water bath, with continuous shaking for 60 min. This was followed by incubation in a solution of collagenase D (3 mg/ml dissolved in serum-free Dulbecco’s modified Eagle’s medium, Roche Applied Science) for 90 min at 37°C. The remaining sterna and costosternal junctions were further digested in fresh collagenase D solution in Petri dishes in a 37°C incubator for 5 h, with intermittent shaking. This step allows remnant fibroblasts to attach to the Petri dish while the chondrocytes remain afloat in the medium. The digestion solution was filtered through Swinex to remove all residual bone fragments. The solution was centrifuged, and the cells were resuspended in complete medium (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 1% penicillin/streptomycin, 100 mM-l-glutamine, and 50 μg/ml ascorbic acid, pH 7.1). To remove any remaining fibroblasts, 24-h cultures were treated with 0.05% trypsin for 1 min to lift the fibroblasts from the culture dish while allowing the chondrocytes to remain attached.
ACKNOWLEDGMENTS
We thank Dr. Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Université Louis Pasteur de Strasbourg, Communauté Urbaine de Strasbourg, France) for providing us the pCreERT2 plasmid.
Contract grant sponsor: National Institute of Health, Contract grant numbers: R01 AR051189, K02 AR052411.
LITERATURE CITED
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Grover J, Roughley PJ. Generation of a transgenic mouse in which Cre recombinase is expressed under control of the type II collagen promoter and doxycycline administration. Matrix Biol. 2006;25:158–165. doi: 10.1016/j.matbio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Nakata K, Bernier SM, Hatano O, Miyashita T, Rhodes CS, Yamada Y. Identification of a minimum enhancer sequence for the type II collagen gene reveals several core sequence motifs in common with the link protein gene. J Biol Chem. 1996;271:4298–4303. doi: 10.1074/jbc.271.8.4298. [DOI] [PubMed] [Google Scholar]
- Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsaranta M, Toman D, de Crombrugghe B, Vuorio E. Mouse type II collagen gene: Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991;266:16862–16869. [PubMed] [Google Scholar]
- Metzger D, Li M, Chambon P. Targeted somatic mutagenesis in the mouse epidermis. Methods Mol Biol. 2005;289:329–340. doi: 10.1385/1-59259-830-7:329. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2 α1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Reed CA, Berndtson AK, Nephew KP. Dose-dependent effects of 4-hydroxytamoxifen, the active metabolite of tamoxifen, on estrogen receptor-α expression in the rat uterus. Anticancer Drugs. 2005;16:559–567. doi: 10.1097/00001813-200506000-00012. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS. Chondrocyte-specific knockout of the G protein G (s) α leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res. 2005;20:663–671. doi: 10.1359/JBMR.041210. [DOI] [PubMed] [Google Scholar]
- Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs C, Lee K, Pirro A, Kronenberg H, Jüppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci USA. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker S, Fundele R, Vortkamp A, Mundlos S. Role of Runx genes in chondrocyte differentiation. Dev Biol. 2002;245:95–108. doi: 10.1006/dbio.2002.0640. [DOI] [PubMed] [Google Scholar]
- Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Matsumoto Y, Nakatani F, Iwamoto Y, Yamada Y. A zinc finger transcription factor, αA-crystallin binding protein 1, is a negative regulator of the chondrocyte-specific enhancer of the 1α(II) collagen gene. Mol Cell Biol. 2000;20:4428–4435. doi: 10.1128/mcb.20.12.4428-4435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tsuda M, Takahashi S, Takahashi Y, Asahara H. Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem. 2003;278:27224–27229. doi: 10.1074/jbc.M303471200. [DOI] [PubMed] [Google Scholar]
- Ueta C, Iwamoto M, Kanatani N, Yoshida C, Liu Y, Enomoto-Iwamoto M, Ohmori T, Enomoto H, Nakata K, Takada K, Kurisu K, Komori T. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol. 2001;153:87–100. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA. 1996;93:10240–10245. doi: 10.1073/pnas.93.19.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]


