Abstract
The role of Groucho/transducin-like Enhancer of split (Gro/TLE) family members as corepressors of transcription is well documented. TLX1 is a homeodomain transcription factor involved in splenogenesis and neuron formation, and its aberrant expression gives rise to T-cell acute lymphoblastic leukemia. We demonstrate by glutathione-S-transferase pull-down assays, in vivo biotinylation tagging and confocal laser microscopy that TLX1 interacts with TLE1 via an Eh1-like motif. Paradoxically, we found that this motif is essential for optimal transcriptional activation of two TLX1 target genes, Aldh1a1 and Fhl1. Using a well characterized target of the Hairy/Enhancer of split 1 (HES1)·TLE1 repressor complex, the ASCL1 gene, we show that TLX1 counteraction of ASCL1 repression by HES1 in SK-N-BE(2) neuroblastoma cells is associated with dismissal of TLE1 from the ASCL1 promoter and requires the Eh1-like motif for maximal effect. Collectively, these results indicate that TLX1-mediated target gene activation can occur in part via derepression strategies involving Gro/TLE corepressors.
Keywords: TLX1 homeodomain transcription factor, Gro/TLE corepressors, Eh1-like motif, Derepression strategies
TLX1 (T-cell leukemia homeobox 1, previously known as HOX11 or TCL3) is an evolutionarily conserved member of the dispersed NKL (NK-Like or NK-Linked) subclass of homeobox genes which is essential for splenogenesis and required for the development of certain neurons [1; 2]. Although TLX1 is not expressed in the hematopoietic system, its inappropriate activation is a recurrent event in human T cell acute lymphoblastic leukemia (TALL) [3]. While several lines of evidence indicate that TLX1 functions as a transcription factor, the mechanism by which deregulated TLX1 expression induces neoplastic conversion remains to be fully elucidated [4–8]. Moreover, it remains unclear how TLX1 activates transcription of any of the downstream target genes identified to date [4–11].
The Groucho/transducin-like Enhancer of split (Gro/TLE) proteins are regulated by multiple signaling cascades and serve as corepressors for many developmental transcription factors including various homeodomain proteins [12–17]. The transcription factors that interact with Gro/TLE corepressors contain short peptide sequences related to either WRPW or to FSIDNIL, the latter referred to as the Engrailed homology 1 (Eh1) motif, a repression domain first identified in the Drosophila Engrailed homeodomain protein [12]. The peptide sequences interacting with Gro/TLE exhibit differential in vitro binding affinity [17], suggesting that context-dependent competition for Gro/TLE between different transcription factors may dictate transcriptional outcome, which might have oncogenic consequences [12].
We report here that TLX1 interacts with TLE1 in vitro and in vivo through an Eh1-like motif. This motif is required for optimal induction of expression of two previously described TLX1 target genes, aldehyde dehydrogenase 1a1 (Aldh1a1) and four and a half LIM domains 1 (Fhl1) [9–11]. Additional studies using a well characterized TLE1-dependent transcriptional repressor model in which the achaete-scute complex-like 1 (ASCL1) gene is negatively regulated by the transcription factor Hairy/Enhancer of split 1 (HES1) indicate that TLX1-mediated target gene activation can occur at least in part via derepression strategies involving repression of intermediary transcriptional repressors and/or competitive sequestration of Gro/TLE corepressors [13–15].
Materials and methods
Expression Constructs
FLAG-tagged TLX1 wild-type and mutant (TLX1 ΔN50, TLX1 ΔN119, TLX1 ΔHD, TLX1 ΔH3and TLX1 ΔC70) coding regions as well as the glutathione S-transferase (GST)-fusion constructs have been described [4; 7]. The TLX1 F19E mutant was generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). Coding sequences for the 23-amino acid biotinylation peptide tag and a fusion protein consisting of GFP and the bacterial BirA biotin ligase (GFP-BirA) were obtained from the pTRIP/BPcter/IRES/EGFPBirA plasmid (provided by John Strouboulis, Erasmus Medical Center, Rotterdam, The Netherlands) [18]. A construct containing the TLX3 coding region (I.M.A.G.E. ID: 4906239) was purchased from the ATCC. FLAG-tagged TLE1 was obtained from pCMV2-FLAGTLE1 (provided by Padma-Sheela Jayaraman, University of Bristol, Bristol, UK) [14]. The intracellular domain of NOTCH1 (ICN1; codons 1770–2555) was obtained from Mig ICN1 (provided by Warren Pear, University of Pennsylvania School of Medicine, Philadelphia, PA) [19]. Mammalian expression constructs were created by cloning PCR-amplified coding regions into a pCL20cSLFR-MSCV-GFP-based lentiviral vector backbone (provided by Arthur Nienhuis, St. Jude Children’s Research Hospital, Memphis, TN), which was modified to coexpress a downstream yellow fluorescent protein (YFP) gene or a red fluorescent protein gene via an encephalomyocarditis virus internal ribosome entry site [20]. Vesicular stomatitis virus (VSV)-G pseudotyped lentiviral vector particles were produced by transient transfection of 293T cells with vector and packaging (pCAG-SIVgprre and pCAG4-RTR-SIV) plasmids (provided by Arthur Nienhuis) plus the VSV-G envelope plasmid pMD.G as described previously [21]. All constructs were confirmed by DNA sequencing. NIH3T3, SupT1 and SK-N-BE(2) cells were transduced with lentiviral vector conditioned medium supplemented with 8 μg/ml of polybrene, cultured for 10 days and then sorted for fluorescent protein expression on a FACSAria instrument (BD Biosciences) as described previously [20; 21].
Streptavidin affinity pulldowns
To prepare nuclear extracts, cells (3 × 109) were washed once in PBS and once in hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM PMSF, 0.5 mM DTT), incubated in hypotonic buffer with protease and phosphatase inhibitor cocktails (Cat. nos. 11836153001 and 04906837001, respectively; Roche) on ice for 15 min and centrifuged at 10,000 × g at 4°C for 10 min. Pelleted nuclei were extracted with NE buffer (20 mM HEPES pH 7.9, 25% glycerol, 0.25 M NaCl, 0.1% NP-40, 5 mM EDTA, 1 mM PMSF, 0.5 mM DTT with protease and phosphatase inhibitor cocktails). The hypotonic and NE fractions were subjected to streptavidin affinity precipitation as described [18]. After in-gel tryptic digestion (Cat. no. PP0100, Sigma-Aldrich), mass spectrometry analysis was performed using a MALDI-TOF instrument in reflectron mode equipped with Kompact software (Kratos Axima CFR/Plus, Shimadzu Biotech). Protein database searches were performed using Mascot software (www.matrixscience.com).
Chromatin immunoprecipitation
ChIP analysis of the ASCL1 promoter was performed following the Chromatin Immunoprecipitation Assay Protocol (Affymetrix) except that the phosphatase inhibitor cocktail was included in addition to the protease inhibitor cocktail (see above). Formaldehyde crosslinked SK-N-BE(2) cells were sonicated five times for 15 s each with 1–5 min resting intervals using a Branson Sonifier 250 set at constant duty and microtip output control limited to 6. For each immunoprecipitation, 6 μg of antibody was used per 2 × 106 cells. Primers were designed using OligoPerfect Designer software (Invitrogen). The region from −294 to −214 was detected with the 5′-TGTTTATTCAGCCGGGAGTC-3′ and 5′-CTTGCAAACTCTCCATTCAGC-3′ primer set; the region from −384 to −214 was detected with a different forward primer, 5′-CAATTCCTAGAGCCATTTGTCC-3′. The PCR reaction was performed with 4% of immunoprecipitated chromatin and 2.5 units of Taq DNA polymerase (Roche) per reaction.
Antibodies and qRT-PCR reagents
The following antibodies were used: anti-TLE1 (M-101), anti-PP1 (E-9), anti-TLX1 (C-18) and anti-GST (Z-5) for GST pulldowns [6; 7]: rabbit anti-TLX1 (C-18) followed by Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Invitrogen) and goat anti-TLE1 (N-18) followed by Alexa Fluor 647-conjugated donkey anti-goat IgG (Invitrogen) for immunofluorescent staining [6; 7]: and goat anti-HES1 (H-20), rabbit anti-Gro/TLE (H-321) and rabbit anti-TLX1 (C-18) for ChIP. All primary antibodies were from Santa Cruz. The following TaqMan primers and probe sets were used for real time qRT-PCR (Applied Biosystems) [6; 7]: ASCL1, Hs00269932_m1; HES1, Hs00172878_m1; human GAPDH, 4352934E; Aldh1a1, Mm00657317_m1; Fhl1, Mm00515772_m1; and mouse GAPDH, 4352339E.
Results and discussion
TLX1 binds TLE1 via an Eh1-like motif
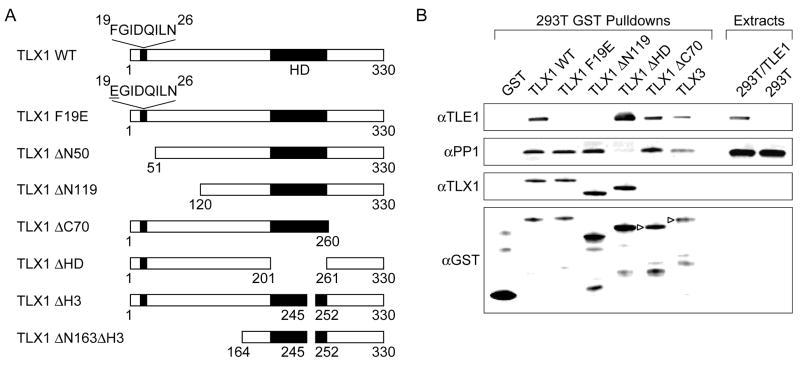
Prior work by others identified Aldh1a1 as a TLX1-inducible gene in NIH3T3 fibroblasts [9; 10]. Optimal activation of Aldh1a1 by ectopic expression of TLX1 was found to be dependent on an 8 amino acid sequence (FGIDQILN) encompassing amino acids 19 to 26 (Fig. 1A). Paradoxically, upon close inspection of this sequence, we noticed similarity to a consensus Eh1 motif, FXIXXIL (where X can be any amino acid) [17]. We first investigated whether TLX1 interacted with TLE1 by performing in vitro pulldown experiments with GST-TLX1 fusion proteins. As seen in Fig. 1B, full-length GST-TLX1 but not a GST-TLX1 mutant containing a 119-amino acid NH2-terminal deletion (TLX1 ΔN119) was capable of coprecipitating exogenous TLE1 from 293T cell extracts although, as expected, both TLX1 forms interacted with endogenous protein serine-threonine phosphatase 1 (PP1) [6]. TLE1 could also be pulled down with a GST fusion protein containing the full-length coding region of the TLX3 gene, a closely related paralog of TLX1. The only conserved sequence homology between TLX1 and TLX3 in their 119-amino acid NH2-terminal regions encompasses the putative Eh1 motif. It was previously shown that an invariant Phe in the Eh1 motif is required for efficient recruitment of Gro/TLE by other homeodomain transcription factors; accordingly, mutation of this amino acid to Glu significantly reduced the interaction [14]. To examine whether the FGIDQILN sequence in TLX1 was likewise responsible for the interaction with Gro/TLE, we mutated Phe 19 to Glu (TLX1 F19E). This amino acid change was found to largely abolish in vitro TLX1 binding to TLE1 whereas the TLX1-PP1 interaction was unaffected. These data demonstrated that TLX1 is capable of interacting with Gro/TLE proteins via an Eh1-like motif.
Fig. 1.
TLX1 interacts with TLE1 in vitro via an Eh1-like motif. (A) TLX1 mutants. Abbreviations: ΔN, NH2-terminal deletion; ΔC, COOH-terminal deletion; ΔHD, homeodomain deletion; ΔH3, deletion of the third helix of the homeodomain. (B) GST-TLX1 pulldowns in 293T cells transiently transfected with a TLE1 expression vector (293T/TLE1). Due to the absence of an epitope recognized by the anti-TLX1 antibody, GST-TLX1ΔC70 and GST-TLX3 fusion proteins were detected by an anti-GST antibody (▹).
TLX1 colocalizes and physically interacts with TLE1 in vivo
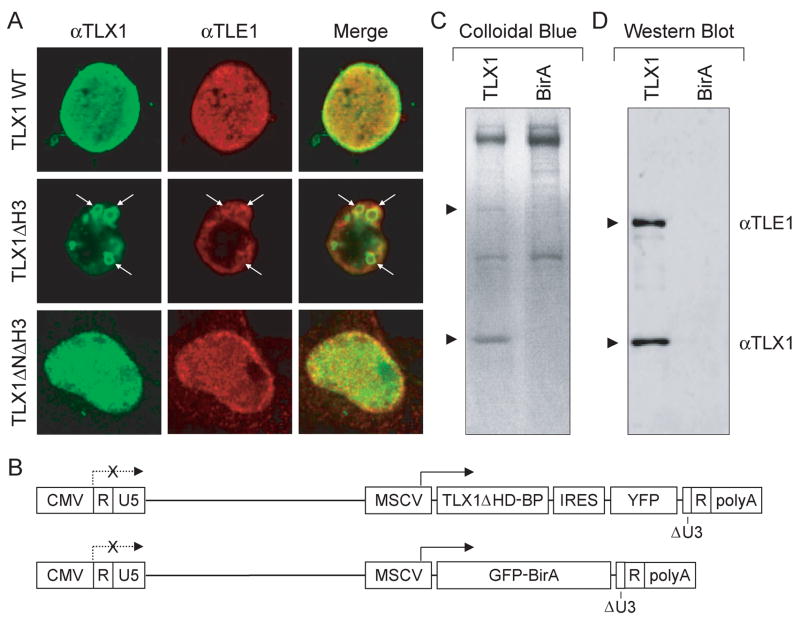
To examine whether TLX1 interacted with Gro/TLE proteins in vivo, we investigated their intracellular distribution by immunofluorescent staining. We reported previously that TLX1 is localized selectively within the nucleus with strong staining observed at the nuclear periphery [7]. When 293T cells were cotransfected with TLX1 and TLE1 expression vectors, significant nuclear colocalization was observed, especially at the nuclear periphery (Fig. 2A). Unexpectedly, when a DNA binding-defective mutant of TLX1 missing helix 3 of the homeodomain (TLX1 ΔH3) was contransfected with the TLE1 expression vector, variably-sized ring-like structures were formed where colocalization of the two proteins could be detected (Fig. 2A). Notably, deletion of the NH2 terminus of the TLX1 DNA binding-defective mutant (TLX1 ΔN163ΔH3) disrupted the formation of the ring-like structures. We conclude that TLX1 interacts with TLE1 in vivo and the interaction occurs predominantly within the nucleus. While binding of TLX1 to DNA is not required for its interaction with TLE1, an intact homeodomain prevents formation of the artificial ring-like structures, possibly by tethering TLE1 to chromatin or the nuclear matrix (see below) [15].
Fig. 2.
TLX1 interacts with TLE1 in vivo. (A) 293T cells transiently expressing TLX1 and TLE1 (indicated to the left of the panels) were labeled with anti-TLX1 (green) and anti-TLE1 (red) antibodies, and immunofluorescent staining was analyzed by confocal laser scanning microscopy. Overlapping regions of protein distribution appear yellow. Ring-like structures are indicated by white arrows. (B) Schematic representation of the self-inactivating lentiviral vectors used to produce biotinylated TLX1. Abbreviations: CMV, cytomegalovirus immediate early enhancer-promoter; R, direct repeat; U5, unique 5′ region of long terminal repeat; MSCV, murine stem cell virus promoter; TLX1ΔHD-BP, TLX1 homeodomain deletion mutant with COOH-terminal biotinylation peptide tag; IRES, internal ribosome entry site; YFP, yellow fluorescent protein; GFP-BirA, green fluorescent protein-BirA biotin ligase fusion protein; ΔU3, deleted unique 3′ region of long terminal repeat; polyA, polyadenylation signal. (C) SDS-PAGE gel stained with Colloidal blue after streptavidin pulldown of extracts from SupT1 cells expressing the TLX1ΔHD-BP protein (TLX1) or the GFP-BirA protein alone. Arrows point to the positions of TLX1 and TLE1 (identified by mass spectrometry). (D) Western blot analysis of the same material described in (C).
To independently verify the results of the cell culture colocalization assay and demonstrate physical interaction of TLX1 with endogenous Gro/TLE proteins, we used an approach based on purification of transcription factor complexes after in vivo biotinylation tagging [18]. SupT1 cells, which are negative for TLX1 expression but which are arrested at the same stage of T-cell differentiation as TLX1+ T-ALL cells, were transduced with lentiviral vectors expressing tagged wild-type (TLX1-BP) and homeodomain-deleted (TLX1 ΔHD-BP) TLX1 genes and sorted for YFP expression. However, these cells did not tolerate high levels of wild-type TLX1 protein [9]. Because we demonstrated that the homeodomain is not involved in Gro/TLE binding, we focused on TLX1 ΔHD-BP-expressing SupT1 cells and transduced them with a second lentiviral vector encoding a GFP-BirA fusion protein (Fig. 2B) [18]. SupT1 cells expressing GFP-BirA alone served as control for background binding of endogenous biotinylated proteins to the streptavidin beads. Interestingly, besides a band corresponding to TLX1 ΔHD-BP, only one other prominent band was identified in SupT1 TLX1 ΔHD-BP/GFP-BirA double transductants, which was not present in extracts of SupT1 cells expressing only GFP-BirA (Fig. 2C). The band corresponding to this protein (~100 kDa) was subjected to MALDI-TOF mass spectrometry analysis. Six out of ten peptides submitted matched human TLE1 trypsin fragments. Western blotting with an anti-TLE1 antibody confirmed pulldown of endogenous TLE1 specifically from SupT1 cells coexpressing TLX1 ΔHD-BP and GFP-BirA (Fig. 2D).
Mechanism of Eh1-dependent activation of target gene expression by TLX1 involves Gro/TLE
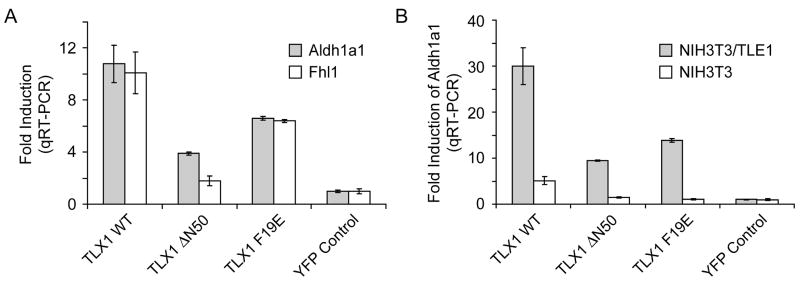
NIH3T3 cells were stably transduced with lentiviral vectors coding for wild-type or mutant TLX1 proteins. Significant induction of Aldh1a1 expression was detected by qRT-PCR analysis of RNA from NIH3T3 cells expressing wild-type TLX1 compared to NIH3T3 cells transduced with the empty lentiviral vector backbone (Fig. 3A). Deletion of the NH2-terminal 50 amino acids of TLX1 (TLX1 ΔN50) resulted in an ~2.8-fold reduction in Aldh1a1 expression (Fig. 3A). By comparison, the TLX1 F19E mutant induced Aldh1a1 at ~1.6-fold lower levels than wild-type TLX1 (Fig. 3A). These results indicated that TLX1 activates Aldh1a1 via a mechanism that involves the Eh1-like motif which functions as a Gro/TLE-binding site.
Fig. 3.
TLE1-mediated induction of Aldh1a1 and Fhl1 expression by TLX1 in NIH3T3 cells. (A) The effect of various TLX1 mutations on the ability to induce expression relative to a reporter gene only control (YFP) is shown. The Eh1-like motif is required for optimal transcriptional activation of Aldh1a1 and Fhl1 expression in NIH3T3 cells. (B) Coexpression of exogenous TLE1 augmented the transcriptional up-regulation of Aldh1a1 by TLX1, and the Eh1-like motif was required for maximal effect.
A second gene, Fhl1, was identified along with Aldh1a1 as being TLX1-inducible in NIH3T3 cells [10]. In a recent study, Fhl1 activation was also found to be dependent on the NH2-terminal 50 amino acids of TLX1 [11]. Unlike Aldh1a1, which is induced by a wide range of TLX1 concentrations, Fhl1 was only activated by a high level of TLX1, leading the investigators to propose that TLX1 regulates Aldh1a1 and Fhl1 by distinct mechanisms [11]. As described above for Aldh1a1, we found that the TLX1 F19E mutant was compromised in its ability to fully activate Fhl1 expression (~1.6-fold lower levels than wild-type TLX1) (Fig. 3A). The combined results thus demonstrated that TLX1 activates both Fhl1 and Aldh1a1 in NIH3T3 cells with similar domain specificity through a mechanism(s) that involves the FGIDQILN Gro/TLE-binding sequence.
Although TLX1 up-regulates Aldh1a1 expression in NIH3T3 cells, it represses Aldh1a1 expression in the developing spleen [10]. Since NIH3T3 cells express very low levels of Gro/TLE, we next asked whether overexpression of TLE1 in NIH3T3 cells would convert TLX1-mediated transcriptional activation of Aldh1a1 into repression. To our surprise, coexpression of exogenous TLE1 augmented the transcriptional up-regulation of Aldh1a1 by wild-type TLX1, and the Eh1-like motif was important for this effect (Fig. 3B). This experiment provided further evidence that optimal activation of Aldh1a1 by TLX1 in NIH3T3 cells requires functional interaction with Gro/TLE. Studies of homeodomain proteins expressed during neuronal fate specification—in particular, some evolutionarily-related members of the NKL subclass—have revealed transcriptional activation of downstream target genes by derepression strategies involving Gro/TLE-dependent repression of intermediary transcriptional repressors [13]. Increased induction of Aldh1a1 expression by TLX1 in the presence of TLE1 overexpression could likewise suggest the existence of an intermediary transcriptional repressor of Aldh1a1 that is more effectively repressed by TLX1•Gro/TLE complexes in the presence of excess TLE1 [14].
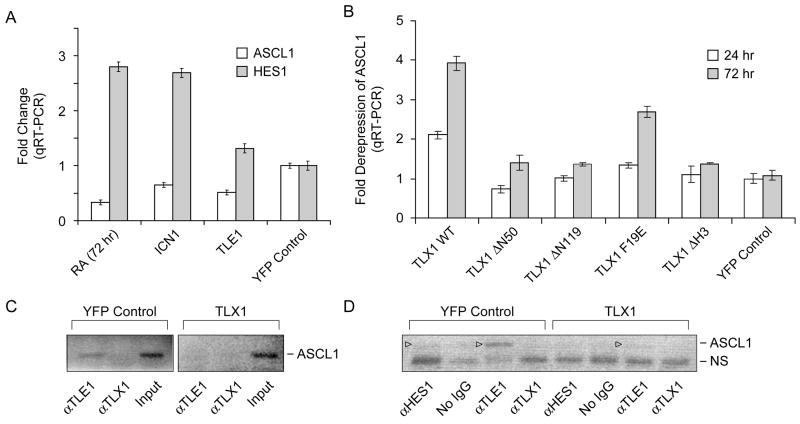
Another possible derepression strategy is one in which TLX1 competes with transcriptional repressor complexes associated with the Aldh1a1 and Fhl1 promoters for available Gro/TLE [15]. In an attempt to further explore the generality of the above observations, we took advantage of a well established Gro/TLE-dependent transcriptional repressor model involving the proneural gene ASCL1 [16]. Previously it was shown that ASCL1 levels are down-regulated in the SK-N-BE(2) neuroblastoma cell line in response to retinoic acid treatment via transient activation of HES1 [22]. Consistent with previous work [22], SK-N-BE(2) cells (in our case, expressing a YFP reporter gene) showed increased HES1 expression and markedly reduced ASCL1 expression following treatment with retinoic acid for 72 hours (Fig. 4A; and data not shown). Transient expression of a constitutively active form of NOTCH1 encoding the intracellular domain of the human NOTCH1 receptor (ICN1) induced elevated HES1 expression levels as previously described [22], and we demonstrated that these also coincided with decreased ASCL1 expression (Fig. 4A). Further, ectopic expression of TLE1 in SK-N-BE(2) cells resulted in a marked decrease in ASCL1 expression, while exerting minimal effects on HES1 levels (Fig. 4A). In addition, we confirmed the involvement of TLE1 in ASCL1 down-regulation by performing ChIP assays on SK-N-BE(2) cells treated with retinoic acid for 72 hours, finding that both HES1 and TLE1 were bound to the N box in the ASCL1 promoter (see Fig. 4D). Taken together, these results corroborated and extended the earlier studies implicating Gro/TLE in HES1-mediated repression of the ASCL1 gene in this experimental system [22].
Fig. 4.
HES1•TLE1-dependent repression of ASCL1 in retinoic acid-induced SK-N-BE(2) neuroblastoma cells is counteracted by enforced expression of TLX1 and requires the Eh1-like motif for maximal effect. (A) ASCL1 and HES1 mRNA levels after the indicated treatments relative to untreated YFP-expressing SK-N-BE(2) cells. Abbreviations: RA, 1 μM retinoic acid; ICN1, intracellular NOTCH1 lentiviral expression vector. TLE1, TLE1 lentiviral expression vector. (B) ASCL1 mRNA levels in the presence of the indicated TLX1 lentiviral expression vectors during treatment with 1 μM retinoic acid for 24 or 72 hours. Data are presented as relative expression levels to a reporter gene only control (YFP). (C) Dismissal of TLE1 from the N box of the ASCL1 promoter (−294 to −214) by TLX1 during treatment with 1 μM retinoic acid for 72 hours as determined by ChIP assay. (D) ChIP analysis of the same material as in (C) using a different primer combination covering the −384 to −214 region of the ASCL1 promoter. ASCL1 promoter-specific bands are indicated (▹). NS, nonspecific PCR amplification product. Note that we did not detect TLX1 occupancy of the N-box region of the ASCL1 promoter (above background levels) in the anti-TLX1 ChIP experiments although the anti-TLX1 antibody efficiently immunoprecipitates TLX1 under the conditions used. Also, HES1 occupancy of the ASCL1 promoter in TLX1-expressing cells was below the limit of detection with the anti-HES1 antibody used.
We next transduced SK-N-BE(2) cells with a series of lentiviral vectors coding for wild-type TLX1 or the mutant TLX1 proteins described above (Fig. 1A) and subjected the cells to retinoic acid induced differentiation. Importantly, TLX1 levels were maintained during this process and no differences in endogenous HES1 or TLE1 levels or in HES1 in vitro DNA binding activity were observed compared to control cells expressing the YFP reporter vector (data not shown). The results, summarized in Fig. 4B, demonstrated that enforced expression of TLX1 was capable of counteracting ASCL1 down-regulation by ~2-fold after 24 hours of treatment and ~4-fold after 72 hours of treatment. As predicted, concomitant with maintenance of ASCL1 expression, ChIP analysis of TLX1-expressing SK-N-BE(2) cells treated with retinoic acid for 72 hours revealed reduced presence of TLE1 on the N box of the ASCL1 promoter (Fig. 4C,D). Moreover, the ability of TLX1 to interfere with HES1•TLE1-mediated repression of ASCL1 exhibited exactly the same domain specificity as observed for TLX1-mediated activation of Aldh1a1 and Fhl1 expression in NIH3T3 cells: i.e., an intact homeodomain was required and deletion of the NH2-terminal 50 or 119 amino acids of TLX1 (TLX1 ΔN50 and TLX1 ΔN119 mutants, respectively) largely prevented the TLX1-mediated block of ASCL1 down-regulation, with the TLX1 F19E Eh1-like motif mutant being likewise compromised in its ability to carry out this function. Within the context of this competition model, the requirement of the TLX1 homeodomain for transcriptional activation might be explained by sequestration of TLX1•TLE1 complexes elsewhere in the genome [23], which would be in line with previous observations that a fraction of TLX1 localizes at heterochromatic regions [7; 24] possibly through binding to satellite DNA [23; 24].
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01HL66305 and R01HL65519, and by an Elaine H. Snyder Cancer Research Award and a King Fahd Endowed Professorship (to R.G.H.) from The George Washington University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts CW, Shutter JR, Korsmeyer SJ. Hox11 controls the genesis of the spleen. Nature. 1994;368:747–749. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q. Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev. 2002;16:1220–1233. doi: 10.1101/gad.982802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawley RG, Fong AZC, Reis MD, Zhang N, Lu M, Hawley TS. Transforming function of the HOX11/TCL3 homeobox gene. Cancer Res. 1997;57:337–345. [PubMed] [Google Scholar]
- 4.Owens BM, Zhu YX, Suen TC, Wang PX, Greenblatt JF, Goss PE, Hawley RG. Specific homeodomain-DNA interactions are required for HOX11-mediated transformation. Blood. 2003;101:4966–4974. doi: 10.1182/blood-2002-09-2857. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann K, Dixon DN, Greene WK, Ford J, Taplin R, Kees UR. A microarray model system identifies potential new target genes of the proto-oncogene HOX11. Genes Chromosomes Cancer. 2004;41:309–320. doi: 10.1002/gcc.20104. [DOI] [PubMed] [Google Scholar]
- 6.Riz I, Hawley RG. G1/S transcriptional networks modulated by the HOX11/TLX1 oncogene of T-cell acute lymphoblastic leukemia. Oncogene. 2005;24:5561–5575. doi: 10.1038/sj.onc.1208727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riz I, Akimov SS, Eaker SS, Baxter KK, Lee HJ, Mariño-Ramírez L, Landsman D, Hawley TS, Hawley RG. TLX1/HOX11-induced hematopoietic differentiation blockade. Oncogene. 2007;26:4115–4123. doi: 10.1038/sj.onc.1210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riz I, Hawley TS, Johnston H, Hawley RG. Role of TLX1 in T-cell acute lymphoblastic leukaemia pathogenesis. Br J Haematol. doi: 10.1111/j.1365-2141.2008.07556.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masson N, Greene WK, Rabbitts TH. Optimal activation of an endogenous gene by HOX11 requires the NH2-terminal 50 amino acids. Mol Cell Biol. 1998;18:3502–3508. doi: 10.1128/mcb.18.6.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene WK, Bahn S, Masson N, Rabbitts TH. The T-cell oncogenic protein HOX11 activates Aldh1 expression in NIH 3T3 cells but represses its expression in mouse spleen development. Mol Cell Biol. 1998;18:7030–7037. doi: 10.1128/mcb.18.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice KL, Kees UR, Greene WK. Transcriptional regulation of FHL1 by TLX1/HOX11 is dosage, cell-type and promoter context-dependent. Biochem Biophys Res Commun. 2008;367:707–713. doi: 10.1016/j.bbrc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Buscarlet M, Stifani S. The ‘Marx’ of Groucho on development and disease. Trends Cell Biol. 2007;17:353–361. doi: 10.1016/j.tcb.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 14.Swingler TE, Bess KL, Yao J, Stifani S, Jayaraman PS. The proline-rich homeodomain protein recruits members of the Groucho/Transducin-like enhancer of split protein family to co-repress transcription in hematopoietic cells. J Biol Chem. 2004;279:34938–34947. doi: 10.1074/jbc.M404488200. [DOI] [PubMed] [Google Scholar]
- 15.McLarren KW, Theriault FM, Stifani S. Association with the nuclear matrix and interaction with Groucho and RUNX proteins regulate the transcription repression activity of the basic helix loop helix factor Hes1. J Biol Chem. 2001;276:1578–1584. doi: 10.1074/jbc.M007629200. [DOI] [PubMed] [Google Scholar]
- 16.Ju BG, Solum D, Song EJ, Lee KJ, Rose DW, Glass CK, Rosenfeld MG. Activating the PARP-1 sensor component of the groucho/TLE1 corepressor complex mediates a CaMKinase IIδ-dependent neurogenic gene activation pathway. Cell. 2004;119:815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Jennings BH, Pickles LM, Wainwright SM, Roe SM, Pearl LH, Ish-Horowicz D. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol Cell. 2006;22:645–655. doi: 10.1016/j.molcel.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Goardon N, Lambert JA, Rodriguez P, Nissaire P, Herblot S, Thibault P, Dumenil D, Strouboulis J, Romeo PH, Hoang T. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 2006;25:357–366. doi: 10.1038/sj.emboj.7600934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 20.Hawley TS, Telford WG, Ramezani A, Hawley RG. Four-color flow cytometric detection of retrovirally expressed red, yellow, green and cyan fluorescent proteins. BioTechniques. 2001;30:1028–1034. doi: 10.2144/01305rr01. [DOI] [PubMed] [Google Scholar]
- 21.Ramezani A, Hawley TS, Hawley RG. Combinatorial incorporation of enhancer blocking components of the chicken β-globin 5′HS4 and human T-cell receptor α/δ BEAD-1 insulators in self-inactivating retroviral vectors reduces their genotoxic potential. Stem Cells. 2008;26:3257–3266. doi: 10.1634/stemcells.2008-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelson H. The Notch signaling cascade in neuroblastoma: role of the basic helix-loop-helix proteins HASH-1 and HES-1. Cancer Lett. 2004;204:171–178. doi: 10.1016/S0304-3835(03)00453-1. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Wu B, Szary J, Kofoed EM, Schaufele F. Functional sequestration of transcription factor activity by repetitive DNA. J Biol Chem. 2007;282:20868–20876. doi: 10.1074/jbc.M702547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidari M, Rice KL, Phillips JK, Kees UR, Greene WK. The nuclear oncoprotein TLX1/HOX11 associates with pericentromeric satellite 2 DNA in leukemic T-cells. Leukemia. 2006;20:304–312. doi: 10.1038/sj.leu.2404071. [DOI] [PubMed] [Google Scholar]