The endocannabinoid signaling system (eCBSS) is composed of cannabinoid (CB) receptors, their endogenous ligands (the endocannabinoids, eCB) and the enzymes that produce and inactivate these ligands. Neurons use this signaling system to communicate with each other and Δ9-tetrahydrocannabinol (THC), the main psychotropic ingredient of Cannabis sativa, induces profound behavioral effects by impinging on this communication. Evidence now shows that microglia, the macrophages of the brain, also express a functional eCBSS and that activation of CB receptors expressed by activated microglia controls their immune-related functions. This review summarizes this evidence, discusses how microglia might use the eCBSS to communicate with each other and neighboring cells, and argues that compounds selectively targeting the eCBSS expressed by microglia constitute valuable therapeutics to manage acute and chronic neuroinflammation, without inducing the psychotropic effects and underlying addictive properties commonly associated with THC.
Cannabis sativa contains over 60 phytocannabinoids, at least three of which are bioactive: THC, cannabinol (CBN) and cannabidiol (CBD) (Howlett et al., 2002; Turner et al., 1980). This plant is the most famous of the Cannabis family, but represents only one of the many subspecies containing bioactive cannabinoids. THC induces psychotropic effects and modifies essential physiological processes by interacting with CB receptors expressed by neurons and other cell types (Howlett et al., 2002; Huestis et al., 2001). CBN does not induce psychoactive effects, but reduce inflammatory responses by interacting with CB receptors expressed by immune (Herring and Kaminski, 1999; Perez-Reyes et al., 1973). CBD is anxiolytic, reduces blood pressure and inflammation, most likely by interacting with CB receptors expressed in the brain, vascular endothelial cells and immune cells, respectively (Campos and Guimaraes, 2008; Costa et al., 2004; Járai et al., 1999; Malfait et al., 2000; Perez-Reyes et al., 1973). Research in this field has focused on understanding the molecular mechanisms mediating the action of cannabinoids – be they endogenous, plant-derived or synthetic – in specific cell types, and has developed powerful pharmacological and genetic tools targeting the eCBSS that allowed for a better understanding of its involvement in physiological functions and pathological processes.
This review presents evidence for the existence of a functional eCBSS in one such cell type, the microglial cells, and describes how cannabinoids control their immune-related responses and role in the initiation and propagation of neuroinflammation. But before reviewing this evidence, I will summarize our current understanding of the basic components that compose the eCBSS: namely the CB receptors, the eCBs and their metabolic enzymes.
Cannabinoid receptors
At least five subtypes of CB receptors have been identified: the two cloned receptors (CB1 and CB2), GPR55, and two receptors that have been pharmacologically pinpointed but remain to be identified at the molecular level.
CB1 receptors are expressed throughout the brain by many different subtypes of neurons and at lower levels by other types of cells (Howlett et al., 2002; Matsuda et al., 1990; Tsou et al., 1998). In brain, they are most abundant in GABAergic interneurons, being between three to ten times less abundant in glutamatergic principal neurons (Kawamura et al., 2006; Uchigashima et al., 2007). A series of elegant experiments demonstrated that despite exhibiting a lower expression in glutamatergic principal neurons, selective genetic deletion of CB1 receptors in this neuronal subpopulation blocks the classic behavioral tetrad induced by THC, namely catalepsy, locomotion impairment, analgesia and changes in body temperature, all of which are normally induced by cannabinoid-like compounds (Monory et al., 2007).
CB1 receptors couple to Gi/o proteins, and modulate the activity of specific ion channels and second messengers. It is likely that their acute versus sustained stimulation differentially modulates cell functions. For example, acute stimulation of neuronal CB1 receptors for milliseconds to seconds inhibits presynaptic N-type calcium channels and activates inwardly rectifying potassium channels, thereby reducing neurotransmisson (Henry and Chavkin, 1995; Mackie and Hille, 1992; Mackie et al., 1995). Their sustained stimulation for minutes to hours regulates effector proteins such as PKA and Erk (Chevaleyre et al., 2007; Derkinderen et al., 2003; Marsicano et al., 2003), thereby modifying enzymatic activities and gene expression. A particularly relevant example in the context of neuroinflammation is the CB1-mediated increase in the expression of brain derived neurotrophic factor (BDNF) in neurons, a factor known to modify synaptic plasticity and to enhance cell survival (Khaspekov et al., 2004; Marsicano et al., 2003).
CB2 receptors couple to Gi proteins, but most likely not to Go proteins (Glass and Northup, 1999). They share 44% protein identity with CB1 and display a distinct pharmacological profile and expression pattern (Felder et al., 1995; Galiègue et al., 1995; Munro et al., 1993). Many laboratories have reported that healthy brain tissue does not contain CB2 receptors, with the exception of a small population of neurons located in the brain stem (Carlisle et al., 2002; Derocq et al., 1995; Galiègue et al., 1995; Munro et al., 1993; Schatz et al., 1997; Sugiura et al., 2000; Van Sickle et al., 2005). The consistency of these reports suggests that the “resting” microglia that are present in healthy brain also lack CB2 receptors. However, the assumption that CB2 receptors are never expressed in brain is oversimplified, since the expression of this receptor can be induced in immune cells, be they resident microglia (see below) or invading immune cells. Note that a couple of reports claimed that almost all neurons present in healthy brain express CB2 receptors, but these studies did not include critical controls, including the parallel immunostaining of the analyzed brain areas, but this time using tissue from CB2 knockout mice to ascertain for staining specificity (Gong et al., 2006; Onaivi et al., 2008).
Recent studies suggest that the orphan receptor GPR55 might represent a third CB receptor (Johns et al., 2007; Lauckner et al., 2008; Oka et al., 2007; Ryberg et al., 2007; Waldeck-Weiermair et al., 2008). However, each study reported distinct – in some cases opposite – pharmacological profiles for classic cannabinoids at this receptor, most likely because of pharmacodynamic differences that such a receptor can exhibit when expressed in different cell-based systems. For example, one study claimed that the potency of CP55,940 at GPR55 lies within the nanomolar range, while another claimed that it act with a potency in the micromolar range, and yet another study claimed that it is inactive (Johns et al., 2007; Lauckner et al., 2008; Oka et al., 2007; Ryberg et al., 2007). The inconsistency of these studies indicates that more work is required for the field to establish GPR55 as a true component of the eCBSS.
Using CB1 knockout mouse brain tissue, several laboratories identified a unique subtype of CB receptor that inhibits glutamatergic transmission in the hippocampus, but does not control adenylyl cyclase activity (Breivogel et al., 2001; Hájos et al., 2001; Hoffman et al., 2005; Monory et al., 2002). This receptor is stimulated by the non-selective cannabinoid agonists WIN55,212-2 and CP55,940, as well as by the TRPV1 agonist capsaicin (Hájos and Freund, 2002). SR141716A, which antagonizes CB1 receptors at nanomolar concentrations, antagonizes this receptor as well, but only at micromolar concentrations (Hájos and Freund, 2002). These intriguing pharmacological results beg for the molecular identification of such receptor so that the field can directly test its relevance in the eCBSS.
Using CB1/CB2 receptor double knockout mice, the group of George Kunos identified the abnormal-cannabidiol (abn-CBD) receptors (also known as anandamide receptors) (Járai et al., 1999). These Gi/o protein-coupled receptors are expressed by endothelial cells of blood vessels, increase cGMP production and regulate blood pressure (Begg et al., 2003; Offertáler et al., 2003). They are activated by abn-CBD and are antagonized by CBD and O-1918 (Járai et al., 1999; Offertáler et al., 2003). Our laboratory found that microglia also express this receptor subtype (see below) (Franklin and Stella, 2003; Walter et al., 2003). Here too, the field is waiting for the molecular identification of this receptor to test its relevance in the eCBSS.
In summary, two CB receptors, CB1 and CB2, have been identified at the molecular level and unambiguously shown to belong to the eCBSS. Additional receptors might also be stimulated by cannabinoids, but many basic questions, including their molecular identification and their true belonging to the eCBSS, remain open.
Endocannabinoid production, pharmacology and inactivation
In 1992, Raphael Mechoulam and coworkers identified arachidonoylethanolamide, which was named anandamide, as the first endogenous compound that binds to CB1 receptors with high affinity (Devane et al., 1992). Anandamide is considered a bona fide eCB because (I) it is produced by cells in an activity-dependent manner, (II) it activates CB1 and CB2 receptors and (III) it is enzymatically inactivated. Depolarization- and agonist-induced increase in anandamide production was initially shown in neurons in primary culture and then in rat brains (Bequet et al., 2007; Caille et al., 2007; Di Marzo et al., 1994; Giuffrida et al., 1999; Stella and Piomelli, 2001; Walker et al., 1999). A rise in intracellular calcium concentration is often required to stimulate anandamide production and multiple calcium-dependent lipases and acyltransferases have been implicated in this process (for review, see (Di Marzo, 2008)). Whether distinct enzymatic pathways generating anandamide are recruited by different physiopathological stimuli remains unknown. Anandamide binds CB1 and CB2 receptors with relatively high affinity and regulates the activities of their effector proteins as a partial agonist (Bouaboula et al., 1995; Davis et al., 2003; Derkinderen et al., 2003; Felder et al., 1993; Vogel et al., 1993). Biological inactivation of anandamide occurs in two steps: a rapid uptake into cells, the molecular identity of which is still unknown, followed by intracellular hydrolysis, which is primarily mediated by fatty acid amide hydrolase (FAAH) (Cravatt et al., 2001). A large amount of data is available on FAAH: from its crystal structure to the chemical platform required for its selective inhibition (McKinney and Cravatt, 2005).
In 1995, two laboratories identified a second endogenous ligand, 2-arachidonoylglycerol (2-AG), which is more abundant than anandamide in brain (Mechoulam et al., 1995; Sugiura et al., 1995). Many laboratories commonly measure anandamide and 2-AG levels in brain, but depending on the analytical method that is used, amounts of either anandamide or 2-AG range from nanomoles to micromoles per gram of protein (for review see (Muccioli and Stella, 2007)). 2-AG is produced by diacylglycerol lipase (DGL) and a rise in intracellular calcium concentration increases this production (Beltramo and Piomelli, 2000; Dinh et al., 2002; Stella and Piomelli, 2001; Witting et al., 2004a). Alternative pathways for 2-AG synthesis have been suggested (Di Marzo, 2008). 2-AG binds to CB1 and CB2 with lower affinity than anandamide, but stimulates the activity of effector proteins as a full agonist (Mechoulam et al., 1995; Stella et al., 1997; Sugiura et al., 2000; Sugiura et al., 1995; Walter et al., 2003). In fact, 2-AG has a higher intrinsic efficacy than anandamide, because both anandamide and 2-AG can appear to be full agonists under appropriately high levels of CB1 (CB2) expression and/or efficient post-receptor coupling to signal transduction cascades. My laboratory found that 2-AG might also act as a full agonist at abn-CBD receptors, one of the cannabinoid receptor that style needs to be identified at the molecular level (Franklin and Stella, 2003; Walter et al., 2003).
Similarly to anandamide, inactivation of 2-AG occurs in two steps: a rapid uptake into cells, probably involving the same unknown molecular entity responsible for anandamide uptake (Beltramo and Piomelli, 2000), followed by its intracellular hydrolysis by monoacylglycerol lipase (MGL). MGL was initially purified and its cDNA isolated and cloned from adipose tissue (Karlsson et al., 1997; Tornqvist and Belfrage, 1976). This enzyme is abundant in brain tissue, particularly at presynaptic terminals (Gulyas et al., 2004; Karlsson et al., 1997). Adenovirus-mediated expression of MGL in cultured neurons enhances 2-AG hydrolysis and reduces activity-dependent accumulation of 2-AG, suggesting that MGL constitutes a rate-limiting step of 2-AG accumulation (Dinh et al., 2002). Accordingly, pharmacological inhibition of MGL leads to 2-AG accumulation in brain tissue and enhancement of its cannabimimetic effects (Makara et al., 2005). Additional enzymes have been shown to metabolize 2-AG, including cyclooxygenases, lipooxygenases, DGL and FAAH, as well as several newly identified serine hydrolases (Blankman et al., 2007; Dinh et al., 2004; Goparaju et al., 1998; Kozak et al., 2000; Mentlein et al., 1984; Somma-Delpéro et al., 1995). Thus, 2-AG hydrolysis appears to be more complex than that of anandamide, involving more enzymes, the respective roles of which still need to be defined.
The balance between eCB production and inactivation determines the extent of eCB accumulation in tissue and resulting activation of CB receptors (Figure 1). Thus, compounds inhibiting enzymes responsible for either anandamide or 2-AG hydrolysis will lead to their respective accumulation, and partial or full activation of CB receptors, respectively. Inhibitors that lead to the accumulation of either anandamide or 2-AG have been reported. Systemic injection of the FAAH inhibitor URB597 leads to anandamide accumulation in brain without affecting 2-AG levels (Fegley et al., 2005). This selective accumulation of anandamide relieves signs of anxiety and depression in mice undergoing distress, and reduces inflammation-induced pain and autoimmune-mediated cell damage, without inducing CB1-mediated psychotropic effects (Baker et al., 2001; Cravatt et al., 2004; Gobbi et al., 2005; Kathuria et al., 2003). Systemic injection of the MGL inhibitor URB602 leads to 2-AG accumulation in the dorsal midbrain without affecting anandamide levels. This selective accumulation of 2-AG mimics stress-induced analgesia, also without producing psychotropic effects (Hohmann et al., 2005). A recent study showed that combined inhibition of FAAH and MGL with a non-selective organophosphorus agent mimics full-blown CB1-mediated effects, emphasizing the need to selectively inhibit either of the two eCB inactivation pathways if one desires a therapeutic approaches that lack untoward side effects (Nomura et al., 2008).
Figure 1. Endocannabinoid signaling in healthy brain.
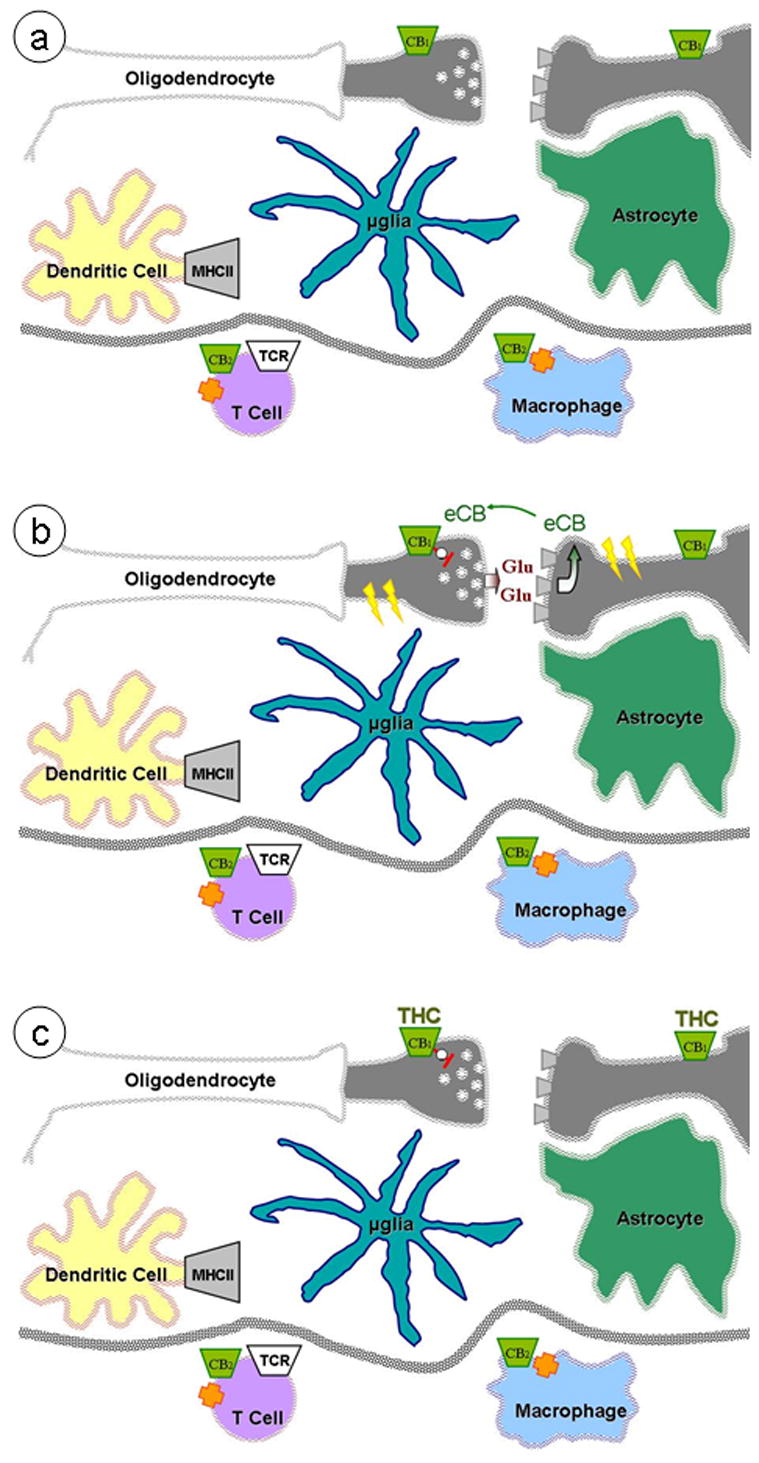
(a) CB1 receptors are expressed by neurons and CB2 receptors by peripheral immune cells. (b) Neuronal depolarization and neurotransmitter release (e.g. glutamate, Glu) leads to post-synaptic rise in calcium, which increases endocannabinoid (eCB) production. eCB act as retrograde signals onto presynaptic CB1 receptors, reducing neurotransmitter release. (c) Δ9-tetrahydrocannabinol (THC) acts as a high-affinity partial agonist at CB1 receptors, impinging on this eCB signaling.
In summary, two eCBs, anandamide and 2-AG, have been extensively studied. They are produced by independent enzymatic pathways, act at CB receptors with distinct pharmacological profiles and are inactivated by uptake followed by independent hydrolysis. Evidence suggests that the selective inhibition of distinct enzymes responsible for eCB hydrolysis constitutes a promising therapeutic approach to relieve specific symptoms without producing overt side effects.
Microglial cells
Microglia derive from the monocytic lineage, entering the developing brain before the establishment of a functional blood-brain barrier and distribute uniformly throughout the parenchyma (Streit, 2001). In healthy brain, microglia display a “resting” phenotype responsible for the continuous immune surveillance of their environment (Fetler and Amigorena, 2005; Raivich, 2005). Studies carried out with GFP-expressing microglia suggest that thorough surveillance of the CNS environment is linked to the rapid extension and retraction of the microglial cellular processes, which occurs without evident movement of their cell bodies and is independent of neuronal activity (Davalos et al., 2005; Nimmerjahn et al., 2005; Wu and Zhuo, 2008). When neural cells are damaged, neighboring “resting” microglia rapidly change their phenotype and behavior, a process referred to as “microglial cell activation”. The molecular steps involved in this activation process implement distinct cellular functions aimed at repairing damaged neural cells and eliminating toxins and pathogens from the area (Garden and Moller, 2006). Damaged cells release chemoattractants that both increase the overall motility (i.e. chemokinesis) and stimulate the directed migration (i.e. chemotaxis) of microglia, the combination of which recruits the microglia much closer to the damaged cells (Trapp et al., 2007). Microglia express membrane receptors that recognize toxins, pathogens, and molecules released by damaged cells, including fractalkine, ATP, glutamate and eCBs (see below). Depending on the combination and extent of stimulation of such receptors, expression of specific genes is induced and their respective products tailor the phenotype of microglia toward becoming pro-inflammatory (also referred to as a M1 phenotype) or anti-inflammatory (also referred to as a M2 phenotype) (Garden and Moller, 2006; Gordon, 2003). Thus, pharmacological tools that target the molecular mechanisms controlling microglial cell migration and phenotype may be therapeutically useful for diseases associated with excessive pro-inflammatory responses. Ideally, such tools would prevent the accumulation of pro-inflammatory microglia while enhancing the recruitment of anti-inflammatory microglia at injury sites. The evidence summarized below suggests that compounds targeting the eCBSS expressed in microglia might represent such pharmacological tools.
Microglial cells express functional cannabinoid receptors
Whether resting microglia found in healthy brain parenchyma express CB1 and/or CB2 receptors has not been addressed directly, but one can presume – as stated at the beginning of this review – that these cells do not express CB2 receptors (because the mRNA encoding for these receptors is undetectable in healthy brain tissue) (Carlisle et al., 2002; Derocq et al., 1995; Galiègue et al., 1995; Griffin et al., 1999; McCoy et al., 1999; Munro et al., 1993; Schatz et al., 1997; Sugiura et al., 2000). On the other hand, a rapidly growing number of studies performed on both microglial cells in culture and activated microglia found in diseased brain tissue suggest that CB2 receptor expression may be up-regulated as part of their activation process.
Microglial cells in primary cultures are intrinsically activated or “primed” because of the procedure involved in transferring these cells into culture (Becher and Antel, 1996). Multiple laboratories have shown that “primed” microglia prepared from human, rat or mouse tissue express CB2 receptors (Carlisle et al., 2002; Facchinetti et al., 2003; Klegeris et al., 2003; Ramirez et al., 2005; Rock et al., 2007; Walter et al., 2003). The expression level of these receptors can be further up- or down-regulated by certain pathogens and cytokines (Carayon et al., 1998; Derocq et al., 2000; Gardner et al., 2002; Lee et al., 2001; Rodríguez et al., 2001; Waksman et al., 1999). For example, lipopolysaccharide (LPS) reduces CB2 receptor expression in primed microglia and combinations of GM-CSF plus IFNγ increase it (Carlisle et al., 2002; Maresz et al., 2005) Although IFNγ activates microglia toward an M1 phenotype, this cytokine does not significantly affect CB2 receptor expression in primed microglia (Carlisle et al., 2002; Maresz et al., 2005). Several rodent microglial cell lines, which intrinsically exhibit high rates of cell proliferation, also express CB2 receptors (Carrier et al., 2004; Walter et al., 2003) In summary, CB2 expression by microglia in culture is well accepted since this result was found by many independent laboratories that use different in vitro models.
Depending of the neuropathology or type of brain insult, the phenotype of activated microglia will vary, as well as the presence or not of CB2 receptors. One remarkable example is found in rat spinal cord, where neuropathic pain up-regulates CB2 receptor in microglia, but chronic inflammatory pain does not (Zhang et al., 2003). Note that this study used in situ hybridization to detect the presence of CB2 receptor mRNA in microglia, most likely because of the well-known difficulty of obtaining antibodies that unambiguously recognize CB2 receptor protein in tissue. This latter point is noteworthy because many studies used polyclonal antibodies to claim CB2 receptor expression by activated microglia in diseased tissue, but these studies often lack the required control of parallel immunostaining of similar tissue isolated from CB2 knockout mice. One study reported CB2 receptor expression by perivascular microglia in healthy human brain tissue (Nunez et al., 2004). This particular subpopulation of microglia has a higher turn-over rate with circulating peripheral monocytes compared to the microglia residing in brain parenchyma. The specificity of the antibodies that were used in this study is more difficult to assess because of the obvious lack of knockout tissue control, but if this result holds true, it would imply that healthy human brain constitutively expresses CB2 receptors (not on neurons but on perivascular microglia). Activated microglia found in brain tissue from patients suffering from Alzheimer's disease, multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) also express CB2 receptors, especially when these cells are associated with the plaques that accumulate in these diseases (Benito et al., 2003; Benito et al., 2007; Yiangou et al., 2006). One study also reported CB2 receptor expression by activated microglia found in a simian model of AIDS dementia (Benito et al., 2005).
In summary, CB2 receptor up-regulation in activated microglia may occur as a result of specific neuroinflammatory responses, and this up-regulation most likely depends on the combination of toxins, pathogens, cytokines and molecules encountered by the microglia. However, many of the reports claiming the presence of CB2 receptors in activated microglia will have to be confirmed with more reliable tools and – when possible – the systematic use of CB2 knockout tissue and cells.
Determining how CB2 receptor regulates microglial cell function is often guided by the vast literature available on the role of the eCBSS in peripheral monocytes and macrophages. Typical immune functions that are assessed include: cell migration, cell proliferation, cytokine and free radical release, and phagocytosis. Stimulation of CB2 receptors by 2-AG increases Erk activity in monocytes, resulting in increased cell migration (Bouaboula et al., 1996; Derocq et al., 2000; Jordà et al., 2002; Klemke et al., 1997). A similar 2-AG response has been replicated in BV-2 cells, a widely used mouse microglial cell line (Dirikoc et al., 2007; Eljaschewitsch et al., 2006; Walter et al., 2003). Multiple lines of evidence show that local eCB production is increased in response to tissue damage (and allied increase in intracellular calcium) (for review, see (Stella, 2004)). When considering local increases in eCB levels with the fact that 2-AG increases microglial cell migration, it is tempting to propose that one role of the eCBSS is to recruit microglia and peripheral monocytes at lesion sites (Figure 2). Note that stimulation of CB2 receptors also increases microglial cell proliferation (Carrier et al., 2004), while reducing their ability to release detrimental factors, including TNFα and free radicals (Eljaschewitsch et al., 2006; Ramirez et al., 2005). Thus, the overall result of stimulating CB2 receptors in microglia would be that higher numbers of anti-inflammatory (or less harmful) microglia will accumulate at lesion sites. While this hypothesis is testable, a major outstanding question is whether stimulation of CB2 receptors expressed by microglia also enhances their beneficial properties, including the release of trophic factors, such as of BDNF, and their ability to eliminate cell debris through silent phagocytosis (Coull et al., 2005; Green and Beere, 2000).
Figure 2. Endocannabinoid signaling in diseased brain.
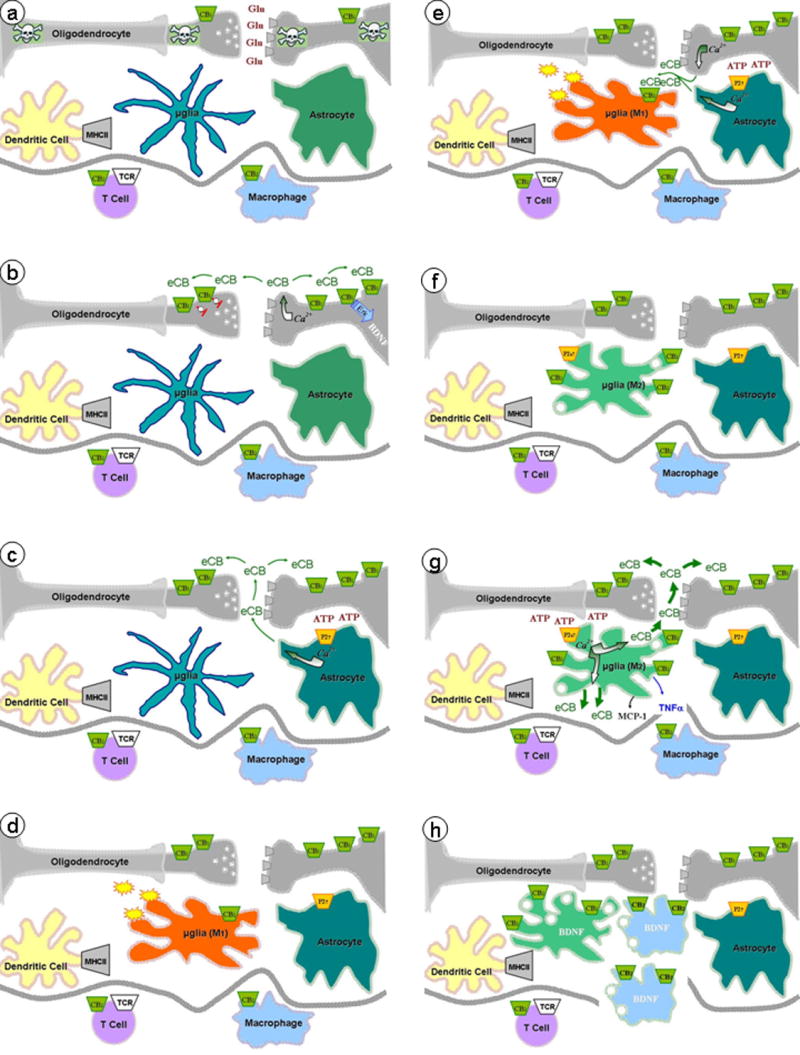
(a) Neurons damaged by injury, toxins or pathogens release large amounts of Glu, (b) resulting in strong neuronal depolarization and Glu receptor activation and sustained rise in post-synaptic calcium and enhanced eCB production. CB1 receptor expression is up-regulated in damaged neurons. eCB acting at pre-synaptic CB1 receptors reduce neurotransmitter release and at post-synaptic CB1 receptors increase Erk activity and allied gene expression (e.g. BDNF). (c) ATP, released from damaged cells, stimulates purinergic receptors expressed by astrocytes and enhances eCB production, which may participate in stimulating pre- and post-synaptic CB1 receptors. (d) Neuronal damage is associated with microglial cell activation (M1, proinflammatory phenotypes), resulting in free radicals and toxin release, as well as up-regulation of CB2 receptor expression. (e) eCB produced by damaged neurons and stimulated astrocytes act on CB2 receptors expressed by microglial cells, (f) leading to a switch in their phenotype (M2, anti-inflammatory phenotype) and further up-regulation of CB2 and P2X7 receptor expression. (g) ATP released by damaged cell enhances the abundant and sustained production of eCB from microglia, which participates in stimulating pre- and post-synaptic CB1 receptors, (h) as well as in recruiting peripheral monocytes/macrophages (in concert with the chemokine MCP-1 and the cytokine TNFα). The overall result is to limit the propagation of cell damage and favor cell repair (e.g. through the release of BDNF).
A fascinating series of studies showed that stimulation of CB2 receptors expressed by microglia reduces HIV-1 expression in these cells (Peterson et al., 2004). A molecular mechanism that could be responsible for this effect is the CB2-mediated down-regulation of CCR5, a chemokine receptor subtype involved in the docking and entry of HIV-1 into microglial cells (Rock et al., 2007). Thus, an additional function of the eCBSS in microglia might be to temper viral-induced activation of microglia, which has major therapeutic relevance in the context of AIDS dementia.
Anandamide also binds to CB2 receptors, where it acts as a partial agonist or antagonist (Gonsiorek et al., 2000; Griffin et al., 2000; Shire et al., 1996). Understanding how anandamide interacts with CB2 receptors expressed by microglia is important since neuropathologies are frequently associated with increases in both 2-AG and anandamide (Baker et al., 2001; Berger et al., 2004; Ferrer et al., 2003; Franklin et al., 2003; Hansen et al., 2001; Marsicano et al., 2003; Panikashvili et al., 2001; Schäbitz et al., 2002; Witting et al., 2004b). Thus, depending on the relative quantities of 2-AG and anandamide that accumulate in specific brain areas, their competition at CB2 receptors will determine the extent to which CB2 receptors will regulate microglial cell behavior and phenotype.
CB1 receptors are expressed by microglial cells in culture prepared from mollusk, mouse and rat, but not human (Carlisle et al., 2002; Facchinetti et al., 2003; Klegeris et al., 2003; Molina-Holgado et al., 2002; Sinha et al., 1998; Stefano et al., 1996; Waksman et al., 1999; Walter et al., 2003). How these receptors regulate microglial cell function is controversial. For example, CP55,940 acting at CB1 receptors increases nitric oxide (NO) production from mollusk microglia (Stefano et al., 1996), but this ligand inhibits the LPS-induced release of NO from rat microglia (Waksman et al., 1999). The latter effect of CP55,940 on NO production is only partially blocked by micromolar concentrations of SR141716A, which calls into question the true involvement of CB1 receptors in this response (Stefano et al., 1996; Waksman et al., 1999). My laboratory has revisited the role of CB1 receptors in microglia by using BV-2 cells and found that WIN55212-2 at 1 μM does not affect basal release of NO, nor does it modulate the LPS/IFNγ-induced production of NO (Franklin et al., 2003). Other reports have shown that only high concentrations of cannabinoids affect microglial cell function. For example, only micromolar concentrations of the three most commonly used synthetic cannabinoid agoinsts, CP55,940, WIN55,212-2 and HU210, regulate cytokine release from cultured microglia (Facchinetti et al., 2003; Puffenbarger et al., 2000). This effect is not stereoselective and only partially blocked by micromolar concentrations of CB receptor antagonists (Facchinetti et al., 2003; Puffenbarger et al., 2000). Could it be that the relevance of CB1 receptors in regulating microglial cell function is difficult to assess because these receptors are expressed at low levels and are predominately located in the intracellular compartments of these cells (Walter et al., 2003)? Could variable responses to CB1 agonists on microglial cells in cultures be due to changes in the expression of this receptor linked to culture conditions (changes that might not occur in situ)? Or is it that microglia express orphan CB receptors and that only high concentrations of commonly used synthetic cannabinoids stimulate these receptors? Supporting this third possibility, my laboratory found pharmacological evidence for the presence of abn-CBD receptors in BV-2 cells (Franklin and Stella, 2003; Walter et al., 2003). Clearly, the presence and function of CB1 and orphan CB receptors in microglia requires further investigation.
Microglial cells produce and inactivate endocannabinoids
Microglial cells in culture produce anandamide and 2-AG, with ionomycin and millimolar concentrations of ATP selectively increasing 2-AG production (Carrier et al., 2004; Walter et al., 2003). The molecular mechanism underlying ATP-induced 2-AG production involves the activation of P2X7 ionotropic receptors, which are highly permeable to calcium and induce sustained rises in intracellular calcium that directly increase DGL activity while inhibiting MGL activity (Witting et al., 2004a). This inverse sensitivity of DGL and MGL to calcium constitutes an original and efficient modality for sustained increased production of 2-AG by microglia, a modality recently extended to neurons (Maccarrone et al., 2008).
Two pieces of evidence suggest that microglia likely constitute the main cellular source of eCBs measured under neuroinflammatory conditions. First, microglial cells in culture produce approximately 20-fold more eCBs than neurons and astrocytes in culture (Walter et al., 2002; Walter et al., 2003). Second, 2-AG accumulation measured in experimental autoimmune encephalomyelitis (EAE)-inflamed brains from P2X7 knockout mice is significantly lower than that measured in EAE-inflamed brains from wild-type mice mouse brains, which is remarkable when considering that P2X7 receptors are only expressed by activated microglia (Witting et al., 2006).
Microglia efficiently inactivate both anandamide and 2-AG. Accordingly, primary microglia in culture express FAAH and MGL (Witting et al., 2004a). My laboratory found that BV-2 cells express a novel 2-AG hydrolyzing activity that is pharmacologically distinct from MGL and FAAH (Muccioli and Stella, 2008). Furthermore, a screen for novel inhibitors of eCB hydrolysis identified several compounds that differentially reduce MGL, FAAH and the novel 2-AG hydrolyzing activity expressed by BV-2 cells (Muccioli and Stella, 2008). A recent elegant study that was performed by the laboratory of Ben Cravatt (which used a functional proteomics approach) led to the identification of novel enzymes capable of hydrolyzing 2-AG (Blankman et al., 2007), one of which might be responsible for 2-AG hydrolysis by BV-2 cells. This result is noteworthy because the existence of a novel, pharmacologically distinct 2-AG hydrolyzing activity expressed by microglia opens promising therapeutic avenues. Indeed, the chemical platform required to selectively inhibit this novel enzyme will likely be different from the one required to selectively inhibit MGL and FAAH. It is also likely that selective inhibition of this novel enzyme expressed by microglia will lead to different cannabimimetic effects in vivo compared to those induced by selective FAAH and MGL inhibitors. Directly testing these possibilities will require the molecular identification and characterization of this novel enzyme expressed by microglia, as well as the identification of specific inhibitors of its activity.
Conclusions
The studies outlined in this review were carried out over the last decade and convincingly show that microglia express many of the components required for functional eCB paracrine and autocrine signaling. Microglia express CB2 receptors and non-psychotropic CB2 agonists regulate their immune-related functions, including migration, proliferation and cytotoxin release. Microglia produce large amounts of eCBs that most likely contribute to the long-lasting increase in eCB levels measured under neuropathological conditions. Such sustained increases in eCB production may contribute to the orchestration of a defense mechanism typified by the accumulation of anti-inflammatory microglia at lesion sites. Thus, the pharmacological stimulation of CB2 receptors, as well as the pharmacological inhibition of eCB hydrolysis, in microglia should result in boosting this defense mechanism, a working hypothesis that has already been tested in some mouse models of neurodegeneration (Bilsland et al., 2006; Lastres-Becker et al., 2003).
In closing, I would like to highlight an exception to this pattern that is found in mice undergoing EAE. In this case, neural cell damage does not lead to a pronounced increase in 2-AG at lesion sites (Witting et al., 2006) (Maresz et al., 2005) even though CB2 receptors on microglia are functional (synthetic CB2 agonists confine EAE-induced lesions (Arévalo-Martín et al., 2003)). This lack of increase in 2-AG production could be due to IFN-γ released by T-cells invading the CNS. Indeed, we found that IFN-γ disrupts the functionality of purinergic P2X7 receptors expressed in microglia (the main receptor subtype involved in ATP-mediated increase in eCB production) (Witting et al., 2006; Witting et al., 2004a). IFN-γ also down-regulates the expression of DGL, the enzyme responsible for producing 2-AG in microglia (Witting et al., 2006; Witting et al., 2004a). Accordingly, we found that induction of EAE in P2X7 knockout mice results in even lower 2-AG levels at lesion sites and more pronounced cell damage than in wild type mice (Witting et al., 2006). Thus, the pro-inflammatory properties of IFN-γ, which play a central role in both EAE and MS pathogenesis, may also disrupt what one could refer to as the “help me” signal carried by 2-AG, while not affecting the functionality of CB2 receptors expressed by microglia. If this working hypothesis holds true, it would provide strong support for the use of both non-psychotropic CB2 agonists and inhibitors of 2-AG hydrolysis as medicine to treat MS patients.
Rationale for cannabinoid-based therapies relies on a unique characteristic of these compounds: their curative properties do not overlap with currently available medicines and thus cannabinoids constitute a novel therapeutic platform. Yet, in order to gain broad public support, cannabinoid-based therapies will need to “kick to the curb” the drug of abuse stigma by remaining devoid of THC-related adverse effects, which include psychotropic effects, memory impairment, anxiety, weight gain and potential addiction. Based on our understanding of the dependence that can be developed by patients using morphine as a painkiller, it is now important to rapidly increase our understanding of the medicinal potential of targeting the eCBSS so that we can exploit their desirable properties while trying to avoid grim scenarios.
Acknowledgments
to the members of my laboratory (Eiron Cudaback, Eric Horne, William Marrs, Aaron Miller, Poulami Mitra, Faith Reyes and Michelle Sexton) for critical reading of this manuscript, and to NIDA (DA 14486, DA21285 and DA22469) for grant support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arévalo-Martín Á, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. The Journal of Neuroscience. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford LJ, Brown P, Pertwee RG, Makriyannis A, Knanolkar A, Layward L, Fezza F, Bisogno T, DiMarzo V. Endocannabinoids control spasticity in a multiple sclerosis model. The FASEB journal. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertáler L, Bátkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. The Journal of Biological Chemistry. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuropharmacology. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Núnez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaques-associated glia in Alzheimer's disease brains. The Journal of Neuroscience. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, Guaza C, Romero J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bequet F, Uzabiaga F, Desbazeille M, Ludwiczak P, Maftouh M, Picard C, Scatton B, Le Fur G. CB1 receptor-mediated control of the release of endocannabinoids (as assessed by microdialysis coupled with LC/MS) in the rat hypothalamus. Eur J Neurosci. 2007;26:3458–3464. doi: 10.1111/j.1460-9568.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo V, Baker D, Greensmith L. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. Faseb J. 2006;20:1003–1005. doi: 10.1096/fj.05-4743fje. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinase by stimulation of the central cannabinoid receptor CB1. Biochemical Journal. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrié B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. European Journal of Biochemistry. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Molecular Pharmacology. 2001;60:155–163. [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, Bouaboula M, Galiègue S, Mondière P, Pénarier G, LeFur G, Defrance T, Casellas P. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharm. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, Giagnoni G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:74–79. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BJ, Demarest K, Patricelli M, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Davis MI, Ronesi J, Lovinger DM. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J Biol Chem. 2003;278:48973–48980. doi: 10.1074/jbc.M305697200. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derocq JM, Jbilo O, Bouaboula M, Ségui M, Clère C, Casellas P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. The Journal of Biological Chemistry. 2000;275:15621–15628. doi: 10.1074/jbc.275.21.15621. [DOI] [PubMed] [Google Scholar]
- Derocq JM, Ségui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS letters. 1995;369:177–182. doi: 10.1016/0014-5793(95)00746-v. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA Interference Suggests a Primary Role for Monoacylglycerol Lipase in the Degradation of the Endocannabinoid 2-Arachidonoylglycerol. Mol Pharmacol. 2004 doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Dirikoc S, Priola SA, Marella M, Zsurger N, Chabry J. Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J Neurosci. 2007;27:9537–9544. doi: 10.1523/JNEUROSCI.1942-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′ -carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proceedings of the National Academy of Sciences. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Molecular Pharmacology. 1995;48:443–450. [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- Fetler L, Amigorena S. Neuroscience. Brain under surveillance: the microglia patrol. Science. 2005;309:392–393. doi: 10.1126/science.1114852. [DOI] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cells motility. The Journal of Neuroscience. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. European Journal of Pharmacology. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gardner B, Zu LX, Sharma S, Liu Q, Makriyannis A, Tashkin D, Dubinett SM. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-β. Biochemical and biophysical research communication. 2002;290:91–96. doi: 10.1006/bbrc.2001.6179. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signalling in dorsal striatum. Nature Neuroscience. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Molecular Pharmacology. 2000;57:1045–1050. [PubMed] [Google Scholar]
- Goparaju K, Natsuo U, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS letters. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Green DR, Beere HM. Apoptosis. Gone but not forgotten. Nature. 2000;405:28–29. doi: 10.1038/35011175. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. The Journal of Pharmacology and Experimental Therapeutics. 2000;292:886–894. [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, Martin BR, Abood ME. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hájos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzanares J, Ikonomidou C, Schmid HHO, Fernández-Ruiz, Hansen H. Anandamide, but not 2-arachidonoylglycerol, accumulates during IN VIVO neurodegeneration. The Journal of Neurochemistry. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neuroscience Lettres. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- Herring AC, Kaminski NE. Cannabinol-mediated inhibition of nuclear factor-κB, cAMP response element-binding protein, and interleukin-2 secretion by activated thymocytes. The Journal of Pharmacology and Experimental Therapeutics. 1999;291:1156–1163. [PubMed] [Google Scholar]
- Hoffman AF, Macgill AM, Smith D, Oz M, Lupica CR. Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus. Eur J Neurosci. 2005;22:2387–2391. doi: 10.1111/j.1460-9568.2005.04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CBl-selective cannabinoid receptor antagonist SR141716. Arc Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proceedings of the National Academy of Sciences. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordà MA, Verbakel SE, Valk PJM, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agrò A, Löwenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci. 2004;19:1691–1698. doi: 10.1111/j.1460-9568.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. British Journal of Pharmacology. 2003;139:775–786. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. The Journal of Cell Biology. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, de Miguel R, De Petrocellis L, Makriyannis A, Di Marzo V, Fernandez-Ruiz J. Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington's disease. J Neurochem. 2003;84:1097–1109. doi: 10.1046/j.1471-4159.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulation and following B cell stimulation. European Journal of Pharmacology. 2001;423:235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proceedings of the National Academy of Sciences. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptors. The Journal of Neuroscience. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proceedings of the National Academy of Sciences. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005 doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Grazia Cascio M, Ortega Gutiérrez S, Van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. The Journal of Pharmacology and Experimental Therapeutics. 1999;289:1620–1625. [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Suttorp M, Heymann E. Specificity of purified monoacylglycerol lipase, palmitoyl-CoA hydrolase, palmitoyl-carnitine hydrolase, and nonspecific carboxylesterase from rat liver microsomes. Arch Biochem Biophys. 1984;228:230–246. doi: 10.1016/0003-9861(84)90064-x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arévalo-Martin A, Almazán G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptor and phosphatidyllinositol-3 kinase/Akt signaling. The Journal of Neuroscience. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J. Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun. 2002;292:231–235. doi: 10.1006/bbrc.2002.6635. [DOI] [PubMed] [Google Scholar]
- Muccioli GG, Stella N. An optimized GC-MS method detects nanomolar amounts of anandamide in mouse brain. Anal Biochem. 2007 doi: 10.1016/j.ab.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG, Stella N. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology. 2008;54:16–22. doi: 10.1016/j.neuropharm.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat Chem Biol. 2008 doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo F, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective Ligands And Cellular Effectors Of A G Protein-Coupled Endothelial Cannabinoid Receptor. Molecular Pharmacology. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Timmons MC, Davis KH, Wall ME. A comparison of the pharmacological activity in man of intravenously administrered Ð9-tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia (Basel) 1973;29:1368–1369. doi: 10.1007/BF01922823. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J Neuroimmunol. 2004;147:123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cabral GA, Martin BR, Peterson PK. WIN55,212-2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors. J Neuroimmune Pharmacol. 2007;2:178–183. doi: 10.1007/s11481-006-9040-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in μ-opioid receptor patches of the rat caudate putamen nucleus. The Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäbitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke. Stroke. 2002;33:2112–2114. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessègue B, Bonnin-Cabanne O, Le Fur G, Caput D, Ferrara P. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochimica et Biophysica acta. 1996;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Sinha D, Bonner TI, Bhat NR, Matsuda LA. Expression of the CB1 cannabinoid receptor in macrophage-like cells from brain tissue: immunochemical characterization by fusion protein antibodies. Journal of Neuroimmunology. 1998;82:13–21. doi: 10.1016/S0165-5728(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Somma-Delpéro C, Valette A, Lepetit-Thévenin J, Nobili O, Boyer J, Vérine A. Purification and properties of a monoacylglycerol lipase in human erythrocytes. Biochemical Journal. 1995;312:519–525. doi: 10.1042/bj3120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. The Journal of Biological Chemistry. 1996;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid signaling in glial cells. Glia. 2004;48:267–277. doi: 10.1002/glia.20084. [DOI] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. European Journal of Pharmacology. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonylglycerol but not N-palmitpylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. The Journal of Biological Chemistry. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and biophysical research communication. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. The Journal of Biological Chemistry. 1976;251:813–819. [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vogel Z, Barg J, Levy R, Saya D, Heldman E, Mechoulam R. Anandamide, a brain endogenous compound, interacts specifically with the cannabinoid receptors and inhibits adenylate cyclase. The Journal of Neurochemistry. 1993;61:352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GY. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. The Journal of Pharmacology and Experimental Therapeutics. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proceedings of the National Academy of Sciences. 1999:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Möller T, Stella N. Astrocytes in culture produce anandamide and other acylethanolamides. The Journal of Biological Chemistry. 2002;277:20869–20876. doi: 10.1074/jbc.M110813200. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Non-psychotropic cannabinoid receptors regulate microglial cell migration. The Journal of Neuroscience. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Chen L, Cudaback E, Straiker A, Walter L, Rickman B, Moller T, Brosnan C, Stella N. Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103:6362–6367. doi: 10.1073/pnas.0510418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Walter L, Wacker J, Moller T, Stella N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A. 2004a;101:3214–3219. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Weydt P, Hong S, Kliot M, Moller T, Stella N. Endocannabinoids accumulate in spinal cord of SOD1G93A transgenic mice. Journal of Neurochemistry. 2004b;89:1555–1557. doi: 10.1111/j.1471-4159.2004.02544.x. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in Mammalian brain. J Neurophysiol. 2008;99:2026–2032. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu K, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptors expression in the rat spinal cord of neuropathic but not inflammatory chonic pain model. European Journal of Neuroscience. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]


