Abstract
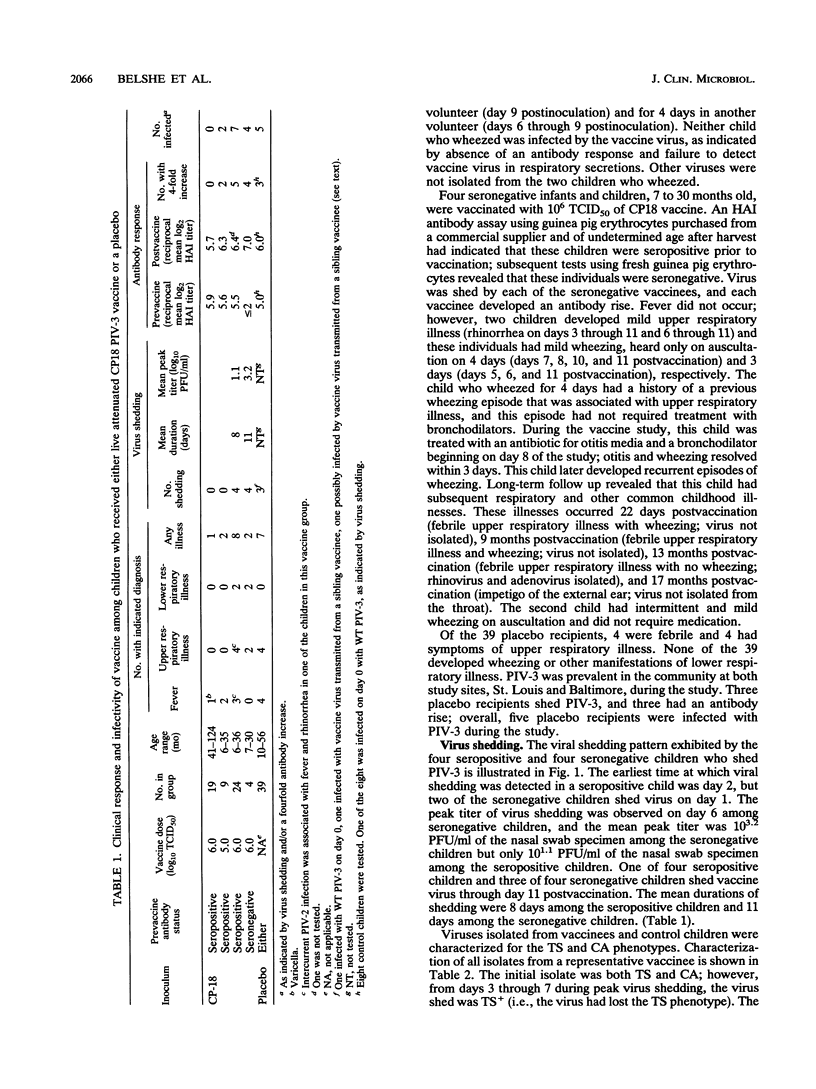
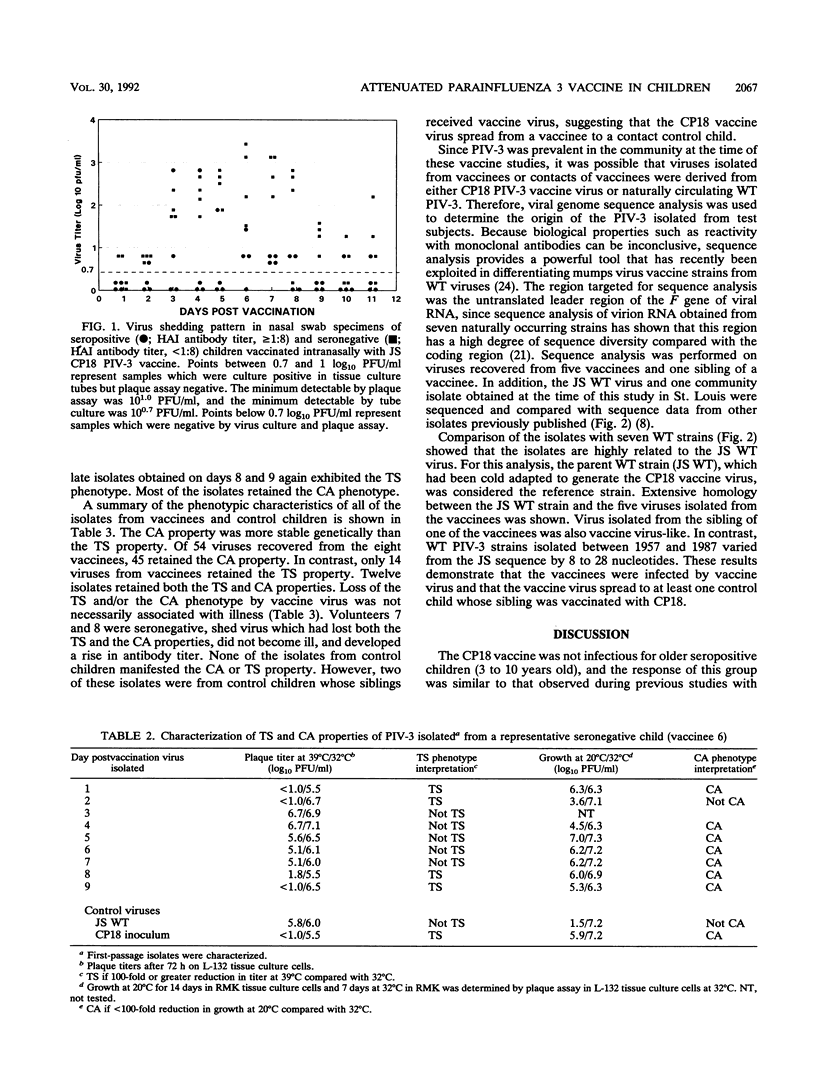
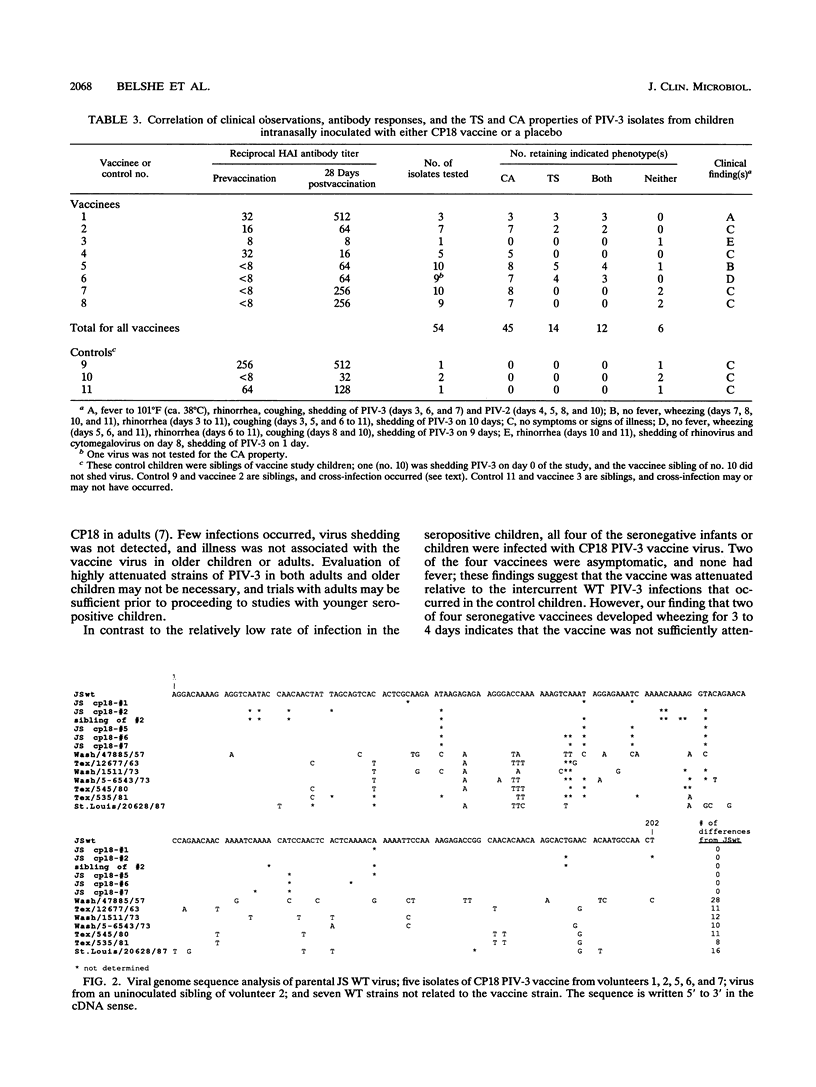
Cold passage 18 (CP18) parainfluenza virus type 3 (PIV-3) vaccine was evaluated in a double-blind, randomized, placebo-controlled study of 95 infants and young children. None of 19 seropositive older children 41 to 124 months old became infected when 10(6) 50% tissue culture infective doses (TCID50) of vaccine virus was administered intranasally. Two of nine and seven of twenty-four young seropositive children given 10(5) or 10(6) TCID50 of CP18 PIV-3, respectively, became infected. Each of four seronegative young children became infected, as indicated by virus shedding and antibody response, when given 10(6) TCID50 of CP18 PIV-3 intranasally. Illness was not observed in seropositive children. Two of the four seronegative children developed a mild illness characterized by rhinorrhea and wheezing on auscultation; none had fever. In one case, vaccine virus spread from a vaccine to a sibling control but did not cause illness. The vaccine is attenuated relative to wild-type PIV-3, but additional attenuation will be required to achieve a satisfactory PIV-3 vaccine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belshe R. B., Hissom F. K. Cold adaptation of parainfluenza virus type 3: induction of three phenotypic markers. J Med Virol. 1982;10(4):235–242. doi: 10.1002/jmv.1890100403. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Richardson L. S., London W. T., Sly D. L., Camargo E., Prevar D. A., Chanock R. M. Evaluation of five temperature-sensitive mutants of respiratory syncytial virus in primates: II. Genetic analysis of virus recovered during infection. J Med Virol. 1978;3(2):101–110. doi: 10.1002/jmv.1890030203. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P., Bartram J., Crookshanks F. K. Live attenuated influenza A virus vaccines in children: results of a field trial. J Infect Dis. 1984 Dec;150(6):834–840. doi: 10.1093/infdis/150.6.834. [DOI] [PubMed] [Google Scholar]
- Brunell P. A., Brickman A., Steinberg S. Evaluation of a live attenuated mumps vaccine (Jeryl Lynn). With observations on the optimal time for testing serologic response. Am J Dis Child. 1969 Sep;118(3):435–440. doi: 10.1001/archpedi.1969.02100040437004. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Murphy B. R. Use of temperature-sensitive and cold-adapted mutant viruses in immunoprophylaxis of acute respiratory tract disease. Rev Infect Dis. 1980 May-Jun;2(3):421–432. doi: 10.1093/clinids/2.3.421. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Belshe R. B., King J., Newman F., Westblom T. U., Tierney E. L., London W. T., Murphy B. R. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991 Jun;29(6):1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. V., Winter C. C. Naturally occurring human parainfluenza type 3 viruses exhibit divergence in amino acid sequence of their fusion protein neutralization epitopes and cleavage sites. J Virol. 1990 Mar;64(3):1329–1334. doi: 10.1128/jvi.64.3.1329-1334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookshanks-Newman F. K., Belshe R. B. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J Med Virol. 1986 Feb;18(2):131–137. doi: 10.1002/jmv.1890180205. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Downie A. W., Kempe C. H. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967 Dec 18;202(12):1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Pyles G., Brandt C. D., Camargo E., Chanock R. M., Parrott R. H. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics. 1971 Nov;48(5):745–755. [PubMed] [Google Scholar]
- Steinhoff M. C., Halsey N. A., Wilson M. H., Burns B. A., Samorodin R. K., Fries L. F., Murphy B. R., Clements M. L. Comparison of live attenuated cold-adapted and avian-human influenza A/Bethesda/85 (H3N2) reassortant virus vaccines in infants and children. J Infect Dis. 1990 Aug;162(2):394–401. doi: 10.1093/infdis/162.2.394. [DOI] [PubMed] [Google Scholar]
- Tolpin M. D., Clements M. L., Levine M. M., Black R. E., Saah A. J., Anthony W. C., Cisneros L., Chanock R. M., Murphy B. R. Evaluation of a phenotypic revertant of the A/Alaska/77-ts-1A2 reassortant virus in hamsters and in seronegative adult volunteers: further evidence that the temperature-sensitive phenotype is responsible for attenuation of ts-1A2 reassortant viruses. Infect Immun. 1982 May;36(2):645–650. doi: 10.1128/iai.36.2.645-650.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolpin M. D., Massicot J. G., Mullinix M. G., Kim H. W., Parrott R. H., Chanock R. M., Murphy B. R. Genetic factors associated with loss of the temperature-sensitive phenotype of the influenza A/Alaska/77-ts-1A2 recombinant during growth in vivo. Virology. 1981 Jul 30;112(2):505–517. doi: 10.1016/0042-6822(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Bhargava M., Johnson P. R., Thompson J., Karzon D. T. Simultaneous administration of live, attenuated influenza A vaccines representing different serotypes. Vaccine. 1985 Sep;3(3):305–308. doi: 10.1016/s0264-410x(85)90140-9. [DOI] [PubMed] [Google Scholar]
- Yamada A., Takeuchi K., Tanabayashi K., Hishiyama M., Takahashi Y., Sugiura A. Differentiation of the mumps vaccine strains from the wild viruses by the nucleotide sequences of the P gene. Vaccine. 1990 Dec;8(6):553–557. doi: 10.1016/0264-410x(90)90007-9. [DOI] [PubMed] [Google Scholar]



