Abstract
Currently, a standard third-line therapy for Helicobacter pylori (H. pylori) eradication remains to be established. Quinolones show good oral absorption, no major side effects, and marked activity against H. pylori. Several authors have studied quinolone-based third-line therapy and reported encouraging results, with the reported H. pylori cure rates ranging from 60% to 84%. Resistance to quinolones is easily acquired, and the resistance rate is relatively high in countries with a high consumption rate of these drugs. We recently reported a significant difference in the eradication rate obtained between patients infected with gatifloxacin-susceptible and gatifloxacin-resistant H. pylori, suggesting that the selection of quinolones for third-line therapy should be based on the results of drug susceptibility testing. As other alternatives of third-line rescue therapies, rifabutin-based triple therapy, high-dose proton pump inhibitor/amoxicillin therapy and furazolidone-based therapy have been suggested.
Keywords: Helicobacter pylori, quinolone, third-line therapy
Introduction
The first-line regimen for the treatment of Helicobacter pylori (H. pylori) infection in Japan is triple therapy with a proton pump inhibitor (PPI), amoxicillin and clarithromycin [1, 2]. Failure of eradication of H. pylori infection with this first-line therapy has been reported in approximately 20% of infected patients [3, 4]. With the increase in the frequency of clarithromycin-resistant H. pylori, there is rising concern about the potential decline in the eradication rate of this infection [5]. Although 7-days’ therapy with PPI-amoxicillin-metronidazole has been found to be effective as a second-line regimen in patients showing failure of the first-line regimen, approximately 10% of patients fail to respond to even the second-line treatment [6, 7]. We recently developed a useful predictor of the response to metronidazole-containing second-line regimens based on the minimal inhibitory concentrations (MICs) of both amoxicillin and metronidazole, and the results of the urea breath test [8]. Since a high eradication resistance index signals a poor response to second-line eradication therapy, patients with a high eradication resistance index may benefit from a change to other treatment regimens selected based on the results of drug susceptibility testing.
Currently, a standard third-line therapy still remains to be established, and European guidelines recommend culture before the selection of a third-line treatment based on the microbial sensitivity to the antibiotics [9]. H. pylori isolates after two eradication failures are often resistant to both metronidazole and clarithromycin. Therefore, these two drugs are not recommended for inclusion in third-line regimens. The alternative candidates for third-line therapy are quinolones, tetracycline, rifabutin, furazolidone; high-dose PPI/amoxicillin therapy is also promising [10, 11]. Quinolones show good oral absorption, no major side effects and marked activity against H. pylori [12]. A recent in vitro study showed synergistic effects of a quinolone and PPI against H. pylori strains [13]. Some studies have evaluated the efficacy of quinolones for use in a third-line regimen. Herein, we discuss the usefulness of quinolone-based therapy as a third-line regimen.
Quinolone Resistance and gyrA Mutation
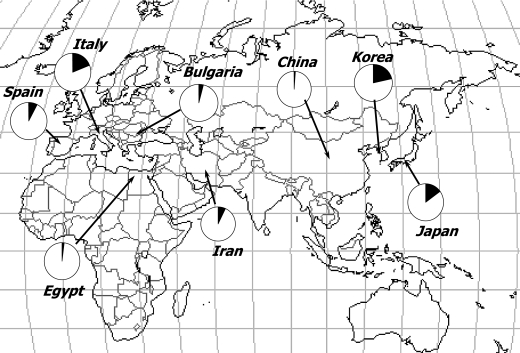
Primary resistance of H. pylori to quinolones has been reported to range between 2%–22% in different countries or regions [14–21]. The prevalence of quinolone resistance is higher in Japan, Korea and Italy (15–22%), and the prevalence is very low in China and Egypt (about 2%, Fig. 1). We reported ahigh resistance rate (47.9%) togatifloxacin (8-methoxy quinolone) after eradication failure in Japan [22]. Resistance to quinolones is easily acquired, and the resistance rate is relatively high in countries with a high consumption rate of these drugs [23].
Fig. 1.
Primary resistance of H. pylori to quinolone in different countries.
Quinolones exert their antimicrobial activity by inhibiting the enzyme DNA gyrase. This enzyme, in addition to relaxing supercoiled DNA, introduces negative supercoils into the DNA causing the bacterial chromosome to be maintained in a negatively supercoiled state. In addition, the enzyme is involved in DNA replication, recombination and transcription. The bacterial enzyme gyrase is a tetramer consisting of two A and two B subunits encoded by the gyrA and gyrB genes, respectively. Quinolones exert their antimicrobial activity at the level of the A subunit of the DNA gyrase. This subunit, responsible for DNA cleavage and rejoining, is also the site of action of quinolones. Several studies have shown that the “quinolone resistance-determining region” (QRDR) of the gyrA gene plays a critical role in the resistance of H. pylori to quinolones [24]. H. pylori does not possess a gene encoding topoisomerase IV, an important quinolone target in other bacteria. We recently demonstrated a significant association between the MICs of gatifloxacin equal to or greater than 1 µg/mL against H. pylori and mutations of the QPDR of the gyrA [22]. Furthermore, we recently designed a rapid test based on an allele-specific polymerase chain reaction (PCR) to detect gyrA mutations [25]. Because the traditional culture test for bacterial susceptibility to antibiotics is expensive and requires 10–14 days, this test is not feasible in routine clinical practice. However, the genotyping by allele-specific PCR takes less than 3–4 h and the allele-specific-PCR method is useful for easy identification of quinolone-resistant strains of H. pylori.
A few years ago, we showed the in vitro development of gatifloxacin resistance of H. pylori strains in gatifloxacin-containing agar culture. In these studies, four gatifloxacin and clarithromycin-susceptible strains were serially plated onto gatifloxacin-containing agar or clarithromycin-containing agar, respectively, of increasing agar density. None of the strains plated on the clarithromycin-containing agar developed resistance to clarithromycin, until the 10th generation of repeated culture. In contrast, all four strains plated on the gatifloxacin-containing agar developed gatifloxacin resistance, and three of these four strains had mutations of gyrA [22]. These data suggest that resistance to quinolones is easily acquired and widespread prescription of quinolones may lead to the spread of resistance of H. pylori to quinolones.
Quinolone-Based Triple Therapy
Zullo et al. [26] evaluated the efficacy of a combination of levofloxacin-amoxicillin in 36 patients with a history of failure of two or more therapeutic attempts. A 10-day regimen of rabeprazole (20 mg b.i.d.), levofloxacin (250 mg b.i.d.), and amoxicillin (1 g b.i.d.) was administered. H. pylori was successfully eradicated in 30 patients, representing an eradication rate of 83.3% (95% confidence interval, 71.2–95.5) and 88.2% (95% confidence interval, 77.4–99) by intention-to-treat (ITT) and per protocol (PP) analysis, respectively. This study demonstrated that levofloxacin-amoxicillin triple therapy administered for 10 days is a successful third-line therapeutic approach for H. pylori eradication (Table 1).
Table 1.
Quinolone-based regimens for third-line therapy
| Author, year | No. of patients | Third-line therapy | Duration of therapy days | Adverse effect (%) | Eradication rate (%) |
|
|---|---|---|---|---|---|---|
| ITT | PP | |||||
| Zullo et al., 2003 | 36 | Rabeprazole 20 mg b.i.d. | 10 | 20.1 | 83.3 (30/36) | 88.2 (30/34) |
| Levofloxacin 250 mg b.i.d. | ||||||
| Amoxicillin 1 g b.i.d. | ||||||
| Gisbert et al., 2006 | 100 | Omeprazole 20 mg b.i.d. | 10 | 25 | 60 (60/100) | 66 (60/91) |
| Levofloxacin 500 mg b.i.d. | ||||||
| Amoxicillin 1 g b.i.d. | ||||||
| Hsu et al., 2008 | 37 | Rabeprazole 20 mg b.i.d. | 10 | 19 | 84 (31/37) | 84 (31/37) |
| Levofloxacin 500 mg o.d. | ||||||
| Bismuth subcitrate 300 mg q.d.s. | ||||||
| Amoxicillin 500 mg q.d.s. | ||||||
| Nishizawa et al., 2008 | 11 | Rabeprazole 10 mg q.d.s. | 7 | 27.2 | 63.6 (7/11) | 63.6 (7/11) |
| Gatifloxacin 400 mg o.d. | ||||||
| Amoxicillin 500 mg q.d.s. | ||||||
Gisbert et al. [27], in a prospective multicenter study, managed 100 patients with a history of two consecutive H. pylori eradication failures with a third-line levofloxacin-based regimen. Patients with failure of a first trial of omeprazole-clarithromycin-amoxicillin and a second trial of omeprazole-bismuth-tetracycline-metronidazole (or ranitidine bismuth citrate with these antibiotics) were enrolled. A 10-day regimen consisting of omeprazole (20 mg b.i.d.), levofloxacin (500 mg b.i.d.), and amoxicillin (1 g b.i.d.) was administered. The eradication rates determined by PP and ITT analyses were 66% (95% confidence interval, 56–75%) and 60% (95% confidence interval, 50–70%), respectively, suggesting that levofloxacin-based rescue therapy may represent an encouraging empirical third-line strategy after multiple previous H. pylori eradication failures.
Furthermore, Gisbert et al. [10] compared rifabutin and levofloxacin rescue regimens in patients with two consecutive H. pylori eradication failures. Patients who failed two eradication attempts received 10 days’ treatment with either rifabutin (150 mg b.i.d.) or levofloxacin (500 mg b.i.d.), plus amoxicillin (1 g b.i.d.) and omeprazole (20 mg b.i.d.). Twenty patients received rifabutin, and 20 received levofloxacin. The cure rate obtained by per-protocol analysis was 45% (95% confidence interval, 26–66%) in the rifabutin group and 81% (57–93%) in the levofloxacin group (p<0.05). The eradication rates obtained by ITT analysis were 45% (26–66%) and 85% (64–95%), respectively (p<0.01). This study demonstrated that a triple levofloxacin-based rescue regimen administered for 10 days is more effective than a rifabutin-based triple regimen after two previous H. pylori eradication failures.
Hsu et al. [28] designed the prospective study to assess the efficacy of levofloxacin, amoxicillin, bismuth and rabeprazole quadruple therapy as a third-line treatment for H. pylori infection. The patients were 37 consecutive H. pylori-infected patients with a history of failure of standard first-line and second-line treatments and received a 10-day quadruple therapy comprising rabeprazole (20 mg b.i.d.), bismuth subcitrate (300 mg q.d.s.), amoxicillin (500 mg q.d.s.) and levofloxacin (500 mg o.d.). H. pylori was successfully eradicated in 84% of the patients, as determined by both ITT analysis and PP analysis. This study suggested that a 10-day regimen of levofloxacin and amoxicillin-based quadruple therapy is well tolerated and yields a high eradication rate as a third-line empirical treatment regimen for H. pylori infection [28].
Recently novel quinolones were developed, and superior in vitro activity of gatifloxacin over that of levofloxacin against H. pylori was reported. Furthermore, garenoxacin (des-fluoro(6) quinolone) and sitafloxacin show activities four-fold or greater than those of gatifloxacin [29–31]. Sharara et al. evaluated the efficacy of a 7-day regimen of gatifloxacin (400 mg o.d.), amoxicillin (1 g b.i.d.) and rabeprazole (20 mg b.i.d.) in 45 patients as a second-line treatment. H. pylori was successfully eradicated in 84.4% of the patients, as determined by both ITT analysis and PP analysis (95% confidence interval, 74–95%) [12]. Although the meta-analysis of Gisbert et al. showed that 10-day regimens were more effective than 7-day regimens in the case levofloxacin-based triple therapy, (81% vs 73%; p<0.01) [32], gatifloxacin-based triple therapy was sufficiently effective even when administered for only 7 days.
We recently investigated the efficacy of gatifloxacin-based triple therapy as a third-line treatment for H. pylori eradication, administered after assessment of the susceptibility of the organisms to gatifloxacin and the presence of gyrA mutations. A 7-day regimen of gatifloxacin (400 mg o.d.), amoxicillin (500 mg q.d.s.) and rabeprazole (10 mg q.d.s.) was administered in 11 patients. Successful eradication of H. pylori was achieved in 63.6% of the patients, as assessed by both ITT analysis and PP analysis. The eradication rate was 100% in the patients infected with gatifloxacin-susceptible bacteria and/or bacteria without gyrA mutations, but only 33.3% in those infected with gatifloxacin-resistant bacteria or bacteria with gyrA mutations. This difference in the eradication rate between patients infected with gatifloxacin-susceptible and gatifloxacin-resistant bacteria was statistically significant (p<0.05). Our data suggest that the selection of gatifloxacin for third-line therapy should be based on the results of drug susceptibility testing or gyrA analysis [33].
Adverse Effects
Gisbert et al. [27] reported that adverse effects occurred in 25% of the patients (23/91) administered the 10-day regimen of levofloxacin, rabeprazole and amoxicillin, consisting mainly of metallic taste (8%), nausea (8%), myalgia/arthralgia (5%), and diarrhea (4%). No severe side effects were reported.
Sharara et al. [12] reported that adverse effects occurred in 8.9% of the patients (4/45) treated with a 7-day regimen of gatifloxacin, rabeprazole and amoxicillin mainly consisting of nausea, headache and mild diarrhea, none of which necessitated discontinuation of therapy. Compliance with the prescribed treatment was excellent. However, they mention that clinicians should be aware of possible alterations in the blood glucose levels, which finally leads to the withdrawal of gatifloxacin [34], QTc interval prolongation, seizures and phototoxicity with the use of fluoroquinolones, especially in patients with other risk factors for these conditions.
Conclusion
Quinolone-based rescue therapy serves as an encouraging third-line strategy after multiple previous H. pylori eradication failures. Novel quinolones, including sitafloxacin or garenoxacin, are more potent against H. pylori than levofloxacin and gatifloxacin, and switching to these quinolones may improve third-line eradication efficacy [35]. European guidelines recommend culture before the selection of a third-line treatment based on microbial sensitivity to the antibiotics. The selection of quinolones for third-line therapy should be based on the results of drug susceptibility testing or gyrA analysis. As other alternatives for third-line rescue therapy, rifabutin-based triple therapy [36, 37], high-dose PPI/amoxicillin therapy and furazolidone-based therapy, if available, have been suggested.
References
- 1.Suzuki H., Hibi T., Marshall B.J. Helicobacter pylori: present status and future prospects in Japan. J. Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki H., Masaoka T., Nomura S., Hoshino Y., Kurabayashi K., Minegishi Y., Suzuki M., Ishii H. Current consensus on the diagnosis and treatment of H. pylori-associated gastroduodenal disease. Keio J. Med. 2003;52:163–173. doi: 10.2302/kjm.52.163. [DOI] [PubMed] [Google Scholar]
- 3.Asaka M., Sugiyama T., Kato M., Satoh K., Kuwayama H., Fukuda Y., Fujioka T., Takemoto T., Kimura K., Shimoyama T., Shimizu K., Kobayashi S. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254–261. doi: 10.1046/j.1523-5378.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 4.Masaoka T., Suzuki H., Kurabayashi K., Kamiya A.G., Ishii H. Second-line treatment of Helicobacter pylori infection after dilution agar methods and PCR-RFLP analysis. Aliment. Pharmacol. Ther. 2004;20 Suppl 1:68–73. doi: 10.1111/j.1365-2036.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 5.Adamek R.J., Suerbaum S., Pfaffenbach B., Opferkuch W. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin—influence on treatment outcome. Am. J. Gastroenterol. 1998;93:386–389. doi: 10.1111/j.1572-0241.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsuhisa T., Kawai T., Masaoka T., Suzuki H., Ito M., Kawamura Y., Tokunaga K., Suzuki M., Mine T., Takahashi S., Sakaki N. Efficacy of metronidazole as second-line drug for the treatment of Helicobacter pylori Infection in the Japanese population: a multicenter study in the Tokyo Metropolitan Area. Helicobacter. 2006;11:152–158. doi: 10.1111/j.1523-5378.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 7.Masaoka T., Suzuki H., Kurabayashi K., Nomoto Y., Nishizawa T., Mori M., Hibi T. Could frameshift mutation in the frxA and rdxA genes of Helicobacter pylori be a marker for metronidazole resistance? Aliment. Pharmacol. Ther. 2006;24 (Suppl. 4):81–87. [Google Scholar]
- 8.Nishizawa T., Suzuki H., Masaoka T., Iwasaki E., Hibi T. A New Eradication Resistance Index as a Predictor of Metronidazole-Containing Second-Line Treatment of Helicobacter pylori. Digestion. 2007;76:215–220. doi: 10.1159/000112649. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P., Megraud F., O’Morain C., Hungin A.P., Jones R., Axon A., Graham D.Y., Tytgat G. Current concepts in the management of Helicobacter pylori infection—the Maastricht 2-2000 Consensus Report. Aliment. Pharmacol. Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 10.Gisbert J.P., Gisbert J.L., Marcos S., Moreno-Otero R., Pajares J.M. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment. Pharmacol. Ther. 2006;24:1469–1474. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 11.Cianci R., Montalto M., Pandolfi F., Gasbarrini G.B., Cammarota G. Third-line rescue therapy for Helicobacter pylori infection. World J. Gastroenterol. 2006;12:2313–2319. doi: 10.3748/wjg.v12.i15.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharara A.I., Chaar H.F., Aoun E., Abdul-Baki H., Araj G.F., Kanj S.S. Efficacy and safety of rabeprazole, amoxicillin, and gatifloxacin after treatment failure of initial Helicobacter pylori eradication. Helicobacter. 2006;11:231–236. doi: 10.1111/j.1523-5378.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M., Isogai E., Isogai H., Hayashi S., Hirose K., Kimura K., Sugiyama T., Sato K. Synergic effect of quinolone antibacterial agents and proton pump inhibitors on Helicobacter pylori. J. Antimicrob. Chemother. 2002;49:1039–1040. doi: 10.1093/jac/dkf055. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.M., Kim J.S., Kim N., Kim S.G., Jung H.C., Song I.S. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int. J. Antimicrob. Agents. 2006;28:6–13. doi: 10.1016/j.ijantimicag.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Miyachi H., Miki I., Aoyama N., Shirasaka D., Matsumoto Y., Toyoda M., Mitani T., Morita Y., Tamura T., Kinoshita S., Okano Y., Kumagai S., Kasuga M. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter. 2006;11:243–249. doi: 10.1111/j.1523-5378.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 16.Rafeey M., Ghotaslou R., Nikvash S., Hafez A.A. Primary resistance in Helicobacter pylori isolated in children from Iran. J. Infect. Chemother. 2007;13:291–295. doi: 10.1007/s10156-007-0543-6. [DOI] [PubMed] [Google Scholar]
- 17.Toro C., Garcia-Samaniego J., Carbo J., Iniguez A., Alarcon T., Lopez-Brea M., Baquero M. [Prevalence of primary Helicobacter pylori resistance to eight antimicrobial agents in a hospital in Madrid] Rev. Esp. Quimioter. 2001;14:172–176. [PubMed] [Google Scholar]
- 18.Zou J., Yang Z.X., Qin Z.M. [Laboratory and clinical study of levofloxacin against Helicobacter pylori] Zhonghua Yi Xue Za Zhi. 2003;83:1778–1781. [PubMed] [Google Scholar]
- 19.Zullo A., Perna F., Hassan C., Ricci C., Saracino I., Morini S., Vaira D. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment. Pharmacol. Ther. 2007;25:1429–1434. doi: 10.1111/j.1365-2036.2007.03331.x. [DOI] [PubMed] [Google Scholar]
- 20.Boyanova L., Stancheva I., Spassova Z., Katzarov N., Mitov I., Koumanova R. Primary and combined resistance to four antimicrobial agents in Helicobacter pylori in Sofia, Bulgaria. J. Med. Microbiol. 2000;49:415–418. doi: 10.1099/0022-1317-49-5-415. [DOI] [PubMed] [Google Scholar]
- 21.Sherif M., Mohran Z., Fathy H., Rockabrand D.M., Rozmajzl P.J., Frenck R.W. Universal high-level primary metronidazole resistance in Helicobacter pylori isolated from children in Egypt. J. Clin. Microbiol. 2004;42:4832–4834. doi: 10.1128/JCM.42.10.4832-4834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishizawa T., Suzuki H., Kurabayashi K., Masaoka T., Muraoka H., Mori M., Iwasaki E., Kobayashi I., Hibi T. Gatifloxacin resistance and mutations in gyrA after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob. Agents. Chemother. 2006;50:1538–1540. doi: 10.1128/AAC.50.4.1538-1540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrita J., Oleastro M., Matos R., Manhente A., Cabral J., Barros R., Lopes A.I., Ramalho P., Neves B.C., Guerreiro A.S. Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990–1999) J. Antimicrob. Chemother. 2000;46:1029–1031. doi: 10.1093/jac/46.6.1029. [DOI] [PubMed] [Google Scholar]
- 24.Moore R.A., Beckthold B., Wong S., Kureishi A., Bryan L.E. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob. Agents Chemother. 1995;39:107–111. doi: 10.1128/aac.39.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishizawa T., Suzuki H., Umezawa A., Muraoka H., Iwasaki E., Masaoka T., Kobayashi I., Hibi T. Rapid detection of point mutations conferring resistance to fluoroquinolone in gyrA of Helicobacter pylori by allele-specific PCR. J. Clin. Microbiol. 2007;45:303–305. doi: 10.1128/JCM.01997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zullo A., Hassan C., De Francesco V., Lorenzetti R., Marignani M., Angeletti S., Ierardi E., Morini S. A third-line levofloxacin-based rescue therapy for Helicobacter pylori eradication. Dig. Liver Dis. 2003;35:232–236. doi: 10.1016/s1590-8658(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 27.Gisbert J.P., Castro-Fernandez M., Bermejo F., Perez-Aisa A., Ducons J., Fernandez-Bermejo M., Bory F., Cosme A., Benito L.M., Lopez-Rivas L., Lamas E., Pabon M., Olivares D. Third-line rescue therapy with levofloxacin after two H. pylori treatment failures. Am. J. Gastroenterol. 2006;101:243–247. doi: 10.1111/j.1572-0241.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 28.Hsu P.I., Wu D.C., Chen A., Peng N.J., Tseng H.H., Tsay F.W., Lo G.H., Lu C.Y., Yu F.J., Lai K.H. Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur. J. Clin. Invest. 2008;38:404–409. doi: 10.1111/j.1365-2362.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez J.E., Saenz N.G., Rincon M.R., Martin I.T., Sanchez E.G., Martinez M.J. Susceptibility of Helicobacter pylori to mupirocin, oxazolidinones, quinupristin/dalfopristin and new quinolones. J. Antimicrob. Chemother. 2000;46:283–285. doi: 10.1093/jac/46.2.283. [DOI] [PubMed] [Google Scholar]
- 30.Rhomberg P.R., Biedenbach D.J., Jones R.N. Activity of BMS284756 (T-3811) tested against anaerobic bacteria, Campylobacter jejuni, Helicobacter pylori and Legionella spp. Diagn. Microbiol. Infect. Dis. 2001;40:45–49. doi: 10.1016/s0732-8893(01)00247-4. [DOI] [PubMed] [Google Scholar]
- 31.Otani T., Tanaka M., Ito E., Kurosaka Y., Murakami Y., Onodera K., Akasaka T., Sato K. In vitro and in vivo antibacterial activities of DK-507k, a novel fluoroquinolone. Antimicrob. Agents Chemother. 2003;47:3750–3759. doi: 10.1128/AAC.47.12.3750-3759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gisbert J.P., Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment. Pharmacol. Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishizawa T., Suzuki H., Nakagawa I., Iwasaki E., Masaoka T., Hibi T. Gatifloxacin-based triple therapy as third-line regimen for Helicobacter pylori eradication. J. Gastroenterol. Hepatol. 2008;23 (Suppl. 2):167–170. doi: 10.1111/j.1440-1746.2008.05407.x. [DOI] [PubMed] [Google Scholar]
- 34.Haerian H., McHugh P., Brown R., Somes G., Solomon S.S. Gatifloxacin produces both hypoglycemia and hyperglycemia: a retrospective study. Am. J. Med. Sci. 2008;335:95–98. doi: 10.1097/MAJ.0b013e31812f65fc. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H., Nishizawa T., Muraoka H., Hibi T. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of H. pylori with gyrA mutation. Antimicrob. Agents Chemother. 2009;53 doi: 10.1128/AAC.00049-09. Feb 2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miehlke S., Hansky K., Schneider-Brachert W., Kirsch C., Morgner A., Madisch A., Kuhlisch E., Bastlein E., Jacobs E., Bayerdorffer E., Lehn N., Stolte M. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment. Pharmacol. Ther. 2006;24:395–403. doi: 10.1111/j.1365-2036.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S., Suzuki H., Nishizawa T., Kaneko F., Ootani S., Muraoka H., Saito Y., Kobayashi I., Hibi T. Past rifampicin dosing determines rifabutin resistance of H. pylori. Digestion. 2009;79:1–4. doi: 10.1159/000191204. [DOI] [PubMed] [Google Scholar]