Abstract
1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], the most active form of vitamin D3, and its analogues have therapeutic benefits for prostate cancer treatment. However, the development of hypercalcemia is an obstacle to clinical applications of 1α,25(OH)2D3 for cancer therapy. In this study, we provide evidence that menthol, a key component of peppermint oil, increases an anti-proliferation activity of 1α,25(OH)2D3 in LNCaP prostate cancer cells. We found that menthol per se does not exhibit antiproliferative activity, but it is able to enhance 1α,25(OH)2D3-mediated growth inhibition in LNCaP cells. Fluorometric assays using Fura-2 showed that 1α,25(OH)2D3 does not induce acute Ca2+ response, whereas menthol evokes an increase in [Ca2+]i, which suggests that cross-talks of menthol-induced Ca2+ signaling with 1α,25(OH)2D3-mediated growth inhibition pathways. In addition, Western blot analysis revealed that 1α,25(OH)2D3 and menthol cooperatively modulate the expression of bcl-2 and p21 which provides the insight into the molecular mechanisms underlying the enhanced 1α,25(OH)2D3-mediated growth inhibition by menthol. Thus, our findings suggest that menthol may be a useful natural compound to enhance therapeutic effects of 1α,25(OH)2D3.
Keywords: 1α,25(OH)2D3; menthol; anti-proliferation; prostate cancer
Introduction
Prostate cancer, the most commonly diagnosed non-cutaneous cancer, is one of the main causes of cancer death in men [1]. It is a heterogeneous disease with a highly varied clinical course ranging from asymptomatic to fatal malignancy [2]. Initial growth of prostate cancer depends on androgen, and thereby responds to androgen-deprivation therapy [2]. However, almost all of the patients eventually become refractory to androgen-deprivation therapy and die of recurrent androgen-independent cancer for which no effective therapy is available [2].
Many epidemiologic studies have identified the low level of circulating 25-hydroxyvitamin D3, the most commonly used index of vitamin D status, as a significant risk factor for prostate cancer [3]. Recent clinical trials have proven that 1α, 25-dihydroxyvitamin D3 [1α,25(OH)2D3] and its analogues have therapeutic benefits for cancer treatment [4, 5]. 1α,25(OH)2D3 exerts antitumor effects through the transcriptional regulation of the genes involved in cell cycle, differentiation, and apoptosis [6]. 1α,25(OH)2D3-mediated transcription is achieved by its binding to vitamin D receptor (VDR), of which knockout mice are vulnerable to chemical carcinogenesis in several tissues [5, 7]. In addition, 1α,25(OH)2D3 can elicit transcription-independent nongenomic responses, such as acute Ca2+ influx and its resultant signaling cascade activation, which can in turn elevate VDR activity [4, 8]. Thus, an understanding of vitamin D signaling pathways can help to devise novel approaches to prostate cancer therapy. However, administration of 1α,25(OH)2D3 is limited by hypercalemic toxicity [4, 5]. Thus, the development of safe and effective strategies improving anticancer efficacy and reducing toxicity is required for successful use of 1α,25(OH)2D3.
Menthol, 2-isopropyl-5-methylcyclohexanol, has been widely used as an active ingredient of food, cosmetical, and pharmaceutical products [9]. It is a key component of peppermint oil that has a variety of biological activities, including antitumor activity and chemopreventive potential [10]. Because menthol was found to increase [Ca2+]i in prostate cancer cell lines [11, 12], we questioned whether menthol can enhance an antiproliferative activity of 1α,25(OH)2D3. In this study, we demonstrated that menthol per se little affects cell growth but enhances an antiproliferative activity of 1α,25(OH)2D3 in LNCaP prostate cancer cells. Our findings suggest that a combination of 1α,25(OH)2D3 with menthol could be a promising therapeutic strategy for prostate cancer.
Materials and Methods
Cell culture
LNCaP cells were supplied by Korean Cell Line Bank (KCLB, Seoul, Korea) and maintained in RPMI media plus 10% FBS. All cell culture agents used were obtained from Invitrogen. 1α,25(OH)2D3 and (−)-menthol (Sigma, St. Louis, MO) in ethanol were added to the culture medium as the indicated concentrations or times.
Cell growth assay
LNCaP cells were grown in 12-well or 24-well culture plates (Nunc, Roskilde, Denmark). MTT assay was used to assess cell growth according to the manufacturer’s instruction (Sigma). Assays were quantitated by measuring the absorbance at 570 nm on a microplate spectrophotometer (Asys Hitech, Cambridge, UK).
Intracellular Ca2+ measurement
The detached cells were incubated with 5 µM Fura-2-AM (Molecular probes, Eugene, OR) in normal Tyrode’s solution for 20 min at 37°C. After washing twice, the cells were resuspended with normal Tyrode’s solution consisting of 10 mM HEPES, 145 mM NaCl, 3.6 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, and 5 mM glucose. Fluorescence emission at 510 nm was measured with excitation at 340/380 nm in a stirred quartz-microcuvette (1 ml volume at 37°C) of fluorescence spectrophotometer (Photon Technology Instrument, Birmingham, NJ). Maximum and minimum fluorescence values at 380 nm (Fmax and Fmin) were calibrated with 0.2% Triton X-100 and 10 mM EGTA, respectively. The [Ca2+]i was calculated from the equation, [Ca2+] = Kd × β × (R − Rmin) / (Rmax − R) where Kd is the dissociation constant for Fura-2 (224 nM), β is Fmin/Fmax, and R is F340/F380.
Western blot analysis
The total proteins were prepared by incubation with RIPA buffer containing protease inhibitor (Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Calbiochem, Darmstadt, Germany). The proteins were resolved in 8–12% SDS-PAGE and analyzed with antibodies specific for caspase-3 (Cell Signaling, Danvers, MA), PARP (Cell Signaling), Bcl-2 (SantaCruz, Santa Cruz, CA), p21 (SantaCruz), and p27 (SantaCruz). Antibody to GAPDH (SantaCruz) was used as a loading control.
Statistical analysis
Data was expressed as mean ± SD. Statistical significance was assessed by paired or unpaired t test using GraphPad Prism. Differences resulting in p values <0.05 were considered to be statistically significant.
Results
Menthol increases antiproliferative activity of 1α,25(OH)2D3
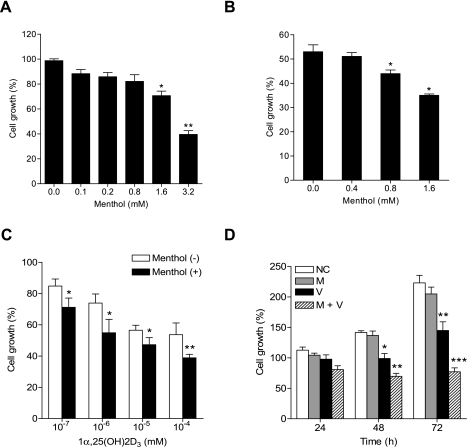
To assess the antiproliferation activity of menthol, we performed MTT assays using LNCaP cells. Cell growth was gradually decreased depending on menthol concentration (Fig. 1A). At high menthol concentrations above 1.6 mM, the cells began to detach from the culture dish. Although only a few cells were detached immediately after treatment with menthol at 0.8 mM, overall cell growth was not significantly reduced (Fig. 1A). The antiproliferative or cytotoxic effect of menthol was evident only in the presence of the supramillimolar concentration ranges, which indicates that menthol per se has little antitumor activity. We then investigated whether menthol can increase an antiproliferative activity of 1α,25(OH)2D3 in LNCaP cells. The combination of 1α,25(OH)2D3 with menthol above 0.8 mM suppressed significantly cell growth, compared to 1α,25(OH)2D3 alone (Fig. 1B). Dose-response relationship study confirmed that menthol markedly enhances an antiproliferative activity of 1α,25(OH)2D3 (Fig. 1C). These results were further corroborated by quantitating cell growth over time (Fig. 1D). While 1α,25(OH)2D3 alone attenuated cell growth, 1α,25(OH)2D3 combined with menthol almost completely inhibited the growth. Under the conditions, the considerable cell death, as examined by LDH release assay, did not occur and little apparent morphological changes were observed (data not shown).
Fig. 1.
Antiproliferation effect of 1α,25(OH)2D3 and menthol in LNCaP cells. (A–C) Dose-response effect. The cells were cultured with menthol alone (A), 1α,25(OH)2D3 at 10−4 mM plus menthol at the indicated concentrations (B), or menthol at 0.8 mM plus 1α,25(OH)2D3 at the indicated concentrations (C) for 72 h prior to MTT assays. (D) Time-dependent effect. Cell growth is expressed as a relative value to that of the untreated cells or that of cells harvested at zero time. NC, negative control (ethanol as a vehicle); M, menthol; V, 1α,25(OH)2D3; M+V, menthol plus 1α,25(OH)2D3. The figures show mean ± SD (n = 3-6). *p<0.05, **p<0.01, ***p<0.005.
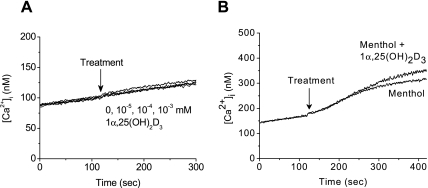
Menthol, but not 1α,25(OH)2D3, evokes an increase in [Ca2+]i
1α,25(OH)2D3 is known to increase [Ca2+]i through its nongenomic action [4, 8]. Also, menthol can increase [Ca2+]i via transmembrane influx or store release pathways [12, 13]. We thus examined whether 1α,25(OH)2D3 and/or menthol can induce the change of [Ca2+]i in LNCaP cells. Fluorescence-based ratiometric assays with Fura-2 showed that 1α,25(OH)2D3 does not increase [Ca2+]i in our assay conditions (Fig. 2A), which indicates that no nongenomic Ca2+ response occurs in LNCaP cells. By contrast, menthol elevated [Ca2+]i, as expected [11], which is comparable to the [Ca2+]i increase by the combination of 1α,25(OH)2D3 with menthol (Fig. 2B). Peak increase in [Ca2+]i was 102.3 ± 39 nM (n = 3) in menthol alone and 124.5 ± 51 nM (n = 3) in combination of 1α,25(OH)2D3 with menthol, respectively.
Fig. 2.
Intracellular Ca2+ change in LNCaP cells exposed to 1α,25(OH)2D3 and menthol. The [Ca2+]i was measured using Fura-2 as described in Materials and Methods (A) The effect of 1α,25(OH)2D3 on [Ca2+]i. (B) The effect of menthol alone (0.8 mM) or 1α,25(OH)2D3 (10−4 mM) plus menthol (0.8 mM) on [Ca2+]i. Data shown are a representative result of at least three independent experiments. Arrows indicates the point of treatments with 1α,25(OH)2D3 and/or menthol.
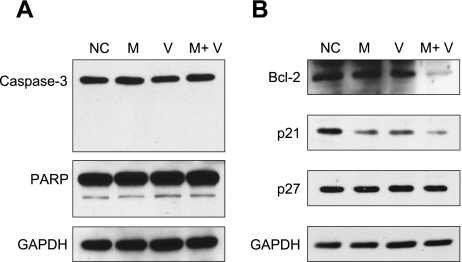
Combination of 1α,25(OH)2D3 with menthol cooperatively modulates bcl-2 and p21 expression
To get a clue to the molecular mechanisms underlying the enhanced antiproliferation effect of the combination of 1α,25(OH)2D3 with menthol, we performed Western blot analyses with LNCaP cells. Neither caspase-3 nor PARP, a caspase-3 substrate, was cleaved in the experimental conditions used (Fig. 3A) which indicates that 1α,25(OH)2D3 plus menthol does not induce caspase-3-dependent apoptosis. We then examined the expression levels of an anti-apoptotic gene bcl-2 and cell cycle inhibitors p21 and p27. The expression of bcl-2 was markedly reduced by the combined treatment of 1α,25(OH)2D3 with menthol, which may permit the cells to be vulnerable to apoptotic stimuli. The expression level of bcl-2 appeared to be unaffected by either alone (Fig. 3B). However, of five independent experiments, we once observed the reduced expression of bcl-2 by 1α,25(OH)2D3 alone but not by menthol alone. The expression of p21 was reduced by treatment with either alone, but the reduction of p21 was more remarkable when 1α,25(OH)2D3 was combined with menthol (Fig. 3B). The expression of p27 was not affected by 1α,25(OH)2D3 or menthol, either alone or in combination (Fig. 3B).
Fig. 3.
The expression of apoptosis- or cell cycle-related genes in LNCaP cells exposed to 10−4 mM 1α,25(OH)2D3 and 0.8 mM menthol. Western blot analyses of proteins following treatment with 1α,25(OH)2D3 and menthol for 72 h. Data shown are a representative result of at least four independent experiments. GAPDH was used as a loading control.
Discussion
In the present study we described the advantageous effect of 1α,25(OH)2D3 combined with menthol. We showed that menthol per se exhibits little antiproliferative or pro-apoptotic activity (Fig. 1A), but it is able to enhance an antiproliferative activity of 1α,25(OH)2D3 in LNCaP cells (Fig. 1B–D). These results suggest that menthol is helpful to improve the anti-cancer efficacy and to reduce the hypercalcemic toxicity of 1α,25(OH)2D3. In addition, our findings suggest that menthol is a valuable probe to identify the novel pathways that determine 1α,25(OH)2D3 reactivity.
1α,25(OH)2D3 is known to up-regulate the expression of transient receptor potential vanilloid 6 (TRPV6), a Ca2+-selective cation channel [14], TRPV6 mediates transcellular Ca2+ transport at the apical membrane of the duodenal and renal epithelial cells [15]. TRPV6 ablation mice showed aberrant Ca2+ handling, such as reduced intestinal Ca2+ absorption and increased urinary Ca2+ excretion [15]. These results suggest that TRPV6 is an crucial transcriptional target of 1α,25(OH)2D3 for maintaining body Ca2+ homeostasis. In addition, 1α,25(OH)2D3 mediates Ca2+ influx via membrane-type VDR and unidentified Ca2+ channel activation that is transcription-independent processes, which is well exemplified in intestinal epithelial cells [4]. It has of interest been shown that 1α,25(OH)2D3 increases the expression of TRPV6 in LNCaP cells. [14, 16]. Thus, it is likely that 1α,25(OH)2D3 mediates Ca2+ transport via the genomic action involving the transcriptional induction of TRPV6. However, it has been undetermined whether 1α,25(OH)2D3-mediated TRPV6 induction is a crucial mechanism for 1α,25(OH)2D3-induced growth inhibition. Little has been known as to whether acute Ca2+ responses of 1α,25(OH)2D3 occurs in prostate cancer cells like those in intestinal epithelial cells. In this study, we did not find that 1α,25(OH)2D3 elicits acute Ca2+ response in LNCaP cells (Fig. 2A). When [Ca2+]i was measured for 30 min, we still did not see acute [Ca2+]i increase (Data not shown). These results suggest that membrane-type VDR or Ca2+ transport proteins are impaired in LNCaP cells, permitting tumor cells to be resistant to 1α,25(OH)2D3-induced growth inhibition, which may be a survival strategy of tumor cells. In addition, it is likely that the acute Ca2+ responsiveness to 1α,25(OH)2D3 is crucial for maximizing its therapeutic effect.
Menthol binds and activates transient receptor potential melastatin 8 (TRPM8), a Ca2+-permeable nonselective cation channel, to increase [Ca2+]i [12]. Compared to 1α,25(OH)2D3 (Fig. 2A), menthol evoked an increase in [Ca2+]i in LNCaP cells (Fig. 2B). TRPM8 activation can be sufficiently achieved by menthol at submillimolar concentrations [12, 13]. By contrast, our results showed that growth inhibition or cell death occurs only in the presence of supramillimolar concentrations of menthol (Fig. 1A), which indicates that antiproliferative or cytotoxic activity of menthol is independent of TRPM8 in LNCaP cells. However, it is still unsolved question whether TRPM8 is a key mediator in the enhanced cellular responses to 1α,25(OH)2D3 by menthol. Currently, answering this question is not easy due to technical limitations, such as lack of TRPM8-specific inhibitors. In addition, siRNA-mediated TRPM8 knockdown may induce cell death; despite the underlying molecular mechanisms are unclear [11]. Furthermore, a recent study showed that menthol increases [Ca2+]i through TRPM8-independent pathways [13]. Interestingly, both TRPV6 and TRPM8 are up-regulated in the early-stage of prostate cancer [17], which suggests that the simultaneous or sequential activation of two different Ca2+ pathways contributes to growth inhibition of prostate cancer. However, a causal relationship between these channels and tumorigenesis or tumor progression is uncertain. Moreover, little is known about the regulatory mechanisms of both channels concerning pathophysiologically relevant conditions, such as proliferative inflammatory atrophy [18].
The effect of 1α,25(OH)2D3 on the expression level of bcl-2 or p21 is controversial, probably due to the cell-types and experimental conditions used [4, 19]. In this study, we found that the expression level of bcl-2 was reduced only by the combination of 1α,25(OH)2D3 with menthol (Fig. 3B). In addition, we showed that combined treatment more remarkably reduces the expression level of p21 than treatment with either 1α,25(OH)2D3 or menthol alone. Previous preclinical studies showed that the reduction of p21 sensitizes cancer cells to anticancer drugs [19]. Thus, these results raise the possibility that 1α,25(OH)2D3 plus menthol can sensitize the cancer cells to chemotherapeutic agents. Unfortunately, here we did provide the detailed molecular mechanisms by which menthol enhances 1α,25(OH)2D3 activity. Our preliminary results from reporter assays showed that menthol does not activate 1α,25(OH)2D3-mediated transcription (data not shown), which suggests that the effect of menthol is unrelated to the modulation of VDR activity. In several cancers, 24-hydroxylase, a 1α,25(OH)2D3 catabolic enzyme, is known to be up-regulated to inactivate 1α,25(OH)2D3 rapidly [4], which may be a tumor escaping mechanism conferring vitamin D resistance. Thus, the effect of menthol on 1α,25(OH)2D3 metabolic enzymes appears to be a challengeable issue to be determined.
Acknowledgement
This study was supported by a grant of the Korea Health 21R&D project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (A060058) and by Seoul National University Hospital Research Fund (09-2006-005-0). P.E.J., K.S.H., and K.B.J were supported by graduate program of BK21 project from Ministry of Ministry of Education, Science and Technology.
References
- 1.Jemal A., Siegel R., Ward E., Murray T., Xu J., Smigal C., Thun M.J. Cancer statistics 2006. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Feldman B.J., Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Deeb K.K., Trump D.L., Johnson C.S. Vitamin D signalling pathways in cancer, potential for anticancer therapeutics. Nat. Rev. Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 5.Trump D.L., Hershberger P.A., Bernardi R.J., Ahmed S., Muindi J., Fakih M., Yu W.D., Johnson C.S. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J. Steroid Biochem. Mol. Biol. 2004;89–90:519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 6.Wang T.T., Tavera-Mendoza L.E., Laperriere D., Libby E., MacLeod N.B., Nagai Y., Bourdeau V., Konstorum A., Lallemant B., Zhang R., Mader S., White J.H. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 7.Welsh J. Vitamin D and breast cancer: insights from animal models. Am. J. Clin. Nutr. 2004;80:1721S–1724S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 8.Norman A.W. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 9.Patel T., Ishiuji Y., Yosipovitch G. Menthol: a refreshing look at this ancient compound. J. Am. Acad. Dermatol. 2007;57:873–878. doi: 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother. Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Barritt G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 12.Voets T., Owsianik G., Nilius B. TRPM8. Handb. Exp. Pharmacol. 2007;179:329–344. doi: 10.1007/978-3-540-34891-7_20. [DOI] [PubMed] [Google Scholar]
- 13.Mahieu F., Owsianik G., Verbert L., Janssens A., De Smedt H., Nilius B., Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J. Biol. Chem. 2007;282:3325–3336. doi: 10.1074/jbc.M605213200. [DOI] [PubMed] [Google Scholar]
- 14.Meyer M.B., Watanuki M., Kim S., Shevde N.K., Pike J.W. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol. Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 15.Bianco S.D., Peng J.B., Takanaga H., Suzuki Y., Crescenzi A., Kos C.H., Zhuang L., Freeman M.R., Gouveia C.H., Wu J., Luo H., Mauro T., Brown E.M., Hediger M.A. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J. Bone Miner. Res. 2007;22:274–285. doi: 10.1359/jbmr.061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehen’kyi V., Flourakis M., Skryma R., Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380–7385. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- 17.Monteith G.R., McAndrew D., Faddy H.M., Roberts-Thomson S.J. Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 18.De Marzo A.M., Platz E.A., Sutcliffe S., Xu J., Grönberg H., Drake C.G., Nakai Y., Isaacs W.B., Nelson W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger P.A., Yu W.D., Modzelewski R.A., Rueger R.M., Johnson C.S., Trump D.L. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin. Cancer Res. 2001;7:1043–1051. [PubMed] [Google Scholar]