Abstract
The aim of the current study was to determine if induction of metallothionein (MT) via acute or chronic dietary zinc supplementation attenuates intestinal inflammation, and to investigate the relationship with site-specific intestinal MT determined by immunolocalization. Growing rats were assigned to zinc-deficient (ZD), acute zinc-treated (ZT), pair-fed, control or chronic Zn-supplemented (ZS) groups. Half the rats in each dietary group received 5% dextran sulphate sodium (DSS) in their drinking water for 4 days. DSS treatment produced acute intestinal inflammation in the colon only, however, dietary zinc deficiency, acute zinc treatment or chronic zinc supplementation did not alter the severity of ulceration. Serum zinc concentrations were attenuated in the DSS-challenged ZT and ZS groups suggesting that zinc was being utilized in some capacity in response to inflammation. DSS-challenge induced MT immunostaining in the colonic submucosa, however, MT was not associated with histological improvements in the present study. The site-specific MT induction in colonic submucosa during intestinal inflammation requires further clarification as a component of the host defense.
Keywords: zinc, metallothionein, intestine, inflammation
Introduction
Zinc (Zn) is an important mineral for both gut immunity and free radical protection, as both immune and antioxidant defense systems are impaired in Zn deficiency [1, 2]. Zn status is compromised in patients with acute inflammatory bowel disease (IBD) and this may contribute to compromised defense systems during states of intestinal inflammation [3–7]. In the small intestine, Zn deficiency promotes ulceration and inflammation and supplemental dietary Zn reverses this effect [8]. Oral and intra-rectal Zn treatments have been shown to reduce colonic inflammation and disease severity in experimental colitis [9–12].
The mechanisms for the possible protective effects of Zn during acute intestinal inflammation have not yet been established. The Zn-ligand, metallothionein (MT) plays a key role in Zn uptake, distribution, release, storage and absorption, as well as in free radical scavenging [6, 13, 14]. We have previously demonstrated that MT immunolocalization and concentration in the small intestine is responsive to dietary Zn and localization is cell-type specific [15], suggesting a role for Zn and MT in gut immunity and intestinal mucosal turnover. Others have demonstrated that MT is also located in mucosal epithelium of the colon, responsive to dietary Zn level, and that MT levels are attenuated during acute colonic inflammation in both rats and people [16–18]. Protective effects of Zn, possibly through MT induction, could reduce apoptosis [19] and Kruidenier et al. [18] have demonstrated compromised MT status in inflamed colon from IBD patients, but this was not necessarily related to the severity of inflammation.
The dextran sulfate sodium (DSS)-induced colitis model exhibits many morphological and pathophysiological features that resemble human acute intestinal inflammation, including mucosal damage, superficial ulceration, leukocyte infiltration and production of cytokines and other inflammatory mediators [20–23]. Our hypothesis is that acute and chronic dietary Zn supplementation will attenuate, and conversely dietary Zn deficiency will exacerbate, intestinal inflammation and injury, and that site-specific induction of MT in response to Zn may play a protective role. The objectives were to determine the effects of dietary Zn deficiency, repletion or supplementation on mucosal injury and inflammation, hematology, trace mineral status, and immunolocalization of MT and caspase-3 (apoptosis marker) in small intestine and colon during acute DSS-challenge in growing rats. We evaluated the effects of supplemental dietary Zn in two ways: chronic Zn supplementation before and during DSS challenge, and acute Zn treatment of Zn deficient rats after induction of inflammation (i.e. mimicking clinical practice whereby Zn treatment may be initiated after the diagnosis of inflammation).
Materials and Methods
Animals, diet and study design
Sixty-four 3-week old male Sprague Dawley rats (Charles River, St Constant, PQ) were acclimatized for 5–7 days and randomly assigned to one of four groups: Zn-deficient (ZD, n = 32, 3 mg Zn/kg diet), control (C, n = 16, 30 mg Zn/kg diet), Zn-supplemented (ZS, n = 16, 300 mg Zn/kg diet), or pair-fed (PF, n = 16, 30 mg Zn/kg diet) for 17 days. The PF group was given the same amount of feed as consumed by the ZD rats daily but were provided with the control diet. The PF group was included to delineate the effects of Zn deficiency per se, from the malnutrition associated with Zn deficiency. On day 17, half the rats in each dietary group (n = 8) received 5% DSS (M.W. 40 KD; MP Biomedicals) in their drinking water for 4 days. On day 18, (one day after DSS induction) half of the rats fed the ZD diet were given the ZS diet for the remaining 3 days and designated the Zn-treated (ZT) group. Thus, there were n = 8 rats/group either receiving DSS treatment (ZD+, PF+, ZT+, C+, ZS+) or not receiving DSS treatment (ZD−, PF−, ZT−, C−, ZS−). All diets were based on the AIN-93G diet (Table 1) [24]. Feed cups were refilled 3 times weekly for all groups, except PF rats were fed daily. Feed consumption was measured and corrected for spillage.
Table 1.
Diet Formulations
| Diet Ingredientsa | ZD | C | ZS |
|---|---|---|---|
| (g/kg diet) | (3 mg Zn/kg diet) | (30 mg Zn/kg diet) | (300 mg Zn/kg diet) |
| Dextrose (cerelose) | 603.8 | 594.6 | 504.6 |
| Egg white | 212.5 | 212.5 | 212.5 |
| Fiber (cellulose) | 50 | 50 | 50 |
| Mineral mix (AIN-93M-MX Zn-free) | 35 | 35 | 35 |
| Potassium phosphateb | 5.4 | 5.4 | 5.4 |
| Vitamin mix (AIN-93-VX) | 10 | 10 | 10 |
| Choline | 2.5 | 2.5 | 2.5 |
| Biotin mixc | 10 | 10 | 10 |
| Zn premixd | 0.8 | 10 | 100 |
| Soybean oil | 70 | 70 | 70 |
aDiet ingredients were obtained from Harlan Teklad, except the dextrose (Moonshiners) and potassium phosphate (Fisher Scientific)
bAdditional potassium phosphate was added to make the AIN-93M-MX mineral mix equivalent to the AIN-93G-MX mineral mix
c200 mg biotin/kg dextrose; additional biotin due to avidin in egg white, and egg white as the protein source to formulate zinc deficient diet
d5.775 g zinc carbonate/kg dextrose
All rats were housed individually in a controlled environment (21–23°C; humidity 55%) in a 14:10-h light-dark cycle. Rats were housed in stainless steel wire-bottom cages to avoid mineral contamination and to collect feed spillage. Deionized water was provided in plastic bottles with stainless steel sipper tubes to avoid mineral contamination. After DSS induction, water intake was measured daily. Body weight was measured weekly, and after DSS induction, body weight and the absence or presence of diarrhea and bloody stool were measured daily. Animal care was provided in accordance with a protocol approved by the University of Manitoba Protocol Management and Review Committee.
Tissue collection
At the end of the study period, rats were euthanized by CO2 asphyxiation. Body weight, body length and tail length were recorded. Trunk blood was collected in tubes without coagulant for serum collection, and in heparinized tubes for complete blood count (CBC) analysis of plasma (Hematology Laboratory, Health Sciences Centre, Winnipeg, MB Canada), and both tubes were immediately placed on ice. Blood in tubes without coagulant was centrifuged for 8 min at 1400 rpm to separate serum, and the serum was stored at −80°C.
Small intestine and colon were dissected and weighed, and length of the small intestine and colon were recorded. Sections from the mid-point of the small intestine and the proximal colon were placed in phosphate buffered formalin and sent to the Pathology Laboratory (Health Sciences Centre, Winnipeg, MB, Canada) for preparation of paraffin-embedded blocks and tissue sections for immunohistochemistry and histology. Livers were dissected, weighed, immediately frozen in liquid nitrogen and stored at −80°C.
Haptoglobin and trace mineral analyses
Haptoglobin was measured using a kit (Tridelta Development Ltd). Zn, copper (Cu) and iron (Fe) were assessed using atomic absorption spectrometry (Varian Spectra AA-30, Georgetown, ON) after wet-ashing of liver tissue and appropriate dilution of serum samples.
Histology
Hemotoxylin and eosin stained tissue sections were prepared from small intestine and colon tissue (Pathology Laboratory, Health Sciences Centre Winnipeg, MB, Canada). All slides were blinded, examined microscopically and scored for inflammation.
MT localization
The procedure for MT immunostaining has been previously described [15, 25]. Briefly, slides for small intestine and colon were incubated for 1 h at room temperature with monoclonal mouse anti-MT antibody (clone E9; Dako, Carpinteria, CA; diluted 1:25 in phosphate buffered saline). Prediluted Dako Envision System goat anti-mouse/anti-rabbit peroxidase labeled polymer was added for 30 min. The reaction product was visualized with 3,3'-diaminobenzidine tetrahydrochloride (DAB.4HCL; Polysciences, Warrington, PA). Tissues were counterstained with Harris hematoxylin and eosin. Computer images of immunostained sections were obtained using Nikon Coolscope. Slides were blinded and viewed at 10× magnification under the microscope. The intensity of MT staining was estimated using an arbitrary subjective scale of minuses and pluses according to Jasani & Elmes [26] as described in the figure legends. Staining was specific to MT, as staining was absent in all negative controls.
Apoptosis
Signalstain Cleaved Caspase-3 (ASP 175) IHC Detection kit (Cell Signaling) was used according to the manufacturer’s protocol. The slides were blinded and viewed by 10×–40× magnification. The number of apoptotic cells was counted in the whole tissue section of small intestine and colon for each animal. Apoptosis was graded per tissue section as the number of apoptotic-positive cells: 1–5 cells = low, 5–15 cells = moderate, > 15 cells = elevated apoptosis. The grading included the mucosa, submucosa and serosa of the small intestine and the submucosa and crypts of the colon. Staining was specific to caspase-3, as staining was absent in all negative controls.
Statistical analysis
Data were analyzed with the Statistical Analysis System (SAS V9.1 for Windows, SAS Institute Inc., Cary, NC) using a two-way ANOVA for the main effects of DSS challenge and diet, and the interaction of DSS x diet. Duncan’s Multiple Range test was used for means testing. Chi-sqared analysis was used to detect differences in the frequency of bloody stool and diarrhea and severity of inflammation in colon tissue among the DSS-treated groups. The level of significance was p<0.05. All values were reported as means ± standard error of the mean (SEM).
Results
Body weight
The animals challenged with DSS had a 5% lower final body weight compared to the untreated animals (Table 2). The final body weight of C and ZS groups was ~30% higher than PF and ZT groups and ~40% higher than the ZD group which had the lowest final body weight. Even though the ZT group had the same diet as the ZD group until the last 3 days of the study when it was changed to Zn-supplemented diet, the ZT rats had an 8% higher final body weight than the ZD rats.
Table 2.
Effect of DSS challenge or diet on anthropometric and oral intake dataa
| DSS Treatmentb |
Dietc |
DSS × Dietd |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DSS+ | DSS− | p | ZD | PF | ZT | C | ZS | p | p | |
| Final body weight (g) | 244 ± 6 | 256 ± 7 | 0.0003 | 208 ± 4c | 230 ± 5b | 225 ± 5b | 295 ± 4a | 294 ± 6a | <0.0001 | NS |
| Feed intake before DSS challenge (g) | 419 ± 9 | 417 ± 9 | NS | 380 ± 10b | 390 ± 11b | 378 ± 10b | 474 ± 10a | 468 ± 6a | <0.0001 | NS |
| Feed intake after DSS challenge (g) | 51 ± 3 | 68 ± 4 | <0.0001 | 46 ± 3b | 41 ± 2b | 69 ± 8a | 69 ± 6a | 72 ± 5a | <0.0001 | NS |
| Water intake (g) | 118 ± 7 | 124 ± 9 | NS | 110 ± 9b | 94 ± 5b | 113 ± 7b | 151 ± 8a | 139 ± 8a | <0.0001 | NS |
| Water intake (g/g body weight) | 0.48 ± 0.02 | 0.48 ± 0.02 | NS | 0.52 ± 0.04a | 0.4 ± 0.02b | 0.51 ± 0.03a | 0.51 ± 0.03a | 0.48 ± 0.03ab | 0.0464 | NS |
| Small intestine weight (g) | 9.61 ± 0.32 | 8.02 ± 0.30 | <0.0001 | 6.96 ± 0.37c | 7.58 ± 0.47c | 8.81 ± 0.37b | 10.07 ± 0.33a | 10.64 ± 0.43a | <0.0001 | NS |
| Small intestine weight (g/g body weight) | 0.0393 ± 0.0008 | 0.0312 ± 0.0007 | <0.0001 | 0.0332 ± 0.0013b | 0.0330 ± 0.0019b | 0.0392 ± 0.0015a | 0.0343 ± 0.0013b | 0.0364 ± 0.0015ab | 0.0006 | NS |
| Small intestine length (cm) | 52.7 ± 0.6 | 52.2 ± 0.7 | NS | 49.5 ± 0.5b | 51.6 ± 0.9b | 49.4 ± 0.8b | 55.3 ± 0.7a | 56.4 ± 0.9a | <0.0001 | NS |
| Colon weight (g) | 1.71 ± 0.07 | 2.22 ± 0.08 | <0.0001 | 1.83 ± 0.14 | 2.14 ± 0.12 | 2.03 ± 0.14 | 1.8 ± 0.11 | 2.03 ± 0.16 | NS | NS |
| Colon weight (g/g body weight) | 0.0072 ± 0.0004 | 0.0089 ± 0.0004 | 0.0004 | 0.0089 ± 0.0006a | 0.0094 ± 0.0005a | 0.0090 ± 0.0005a | 0.0061 ± 0.0003b | 0.0068 ± 0.0005b | <0.0001 | NS |
| Colon length (cm) | 13.7 ± 3.1 | 12.9 ± 0.3 | NS | 10.8 ± 0.4 | 12.1 ± 0.5 | 11.7 ± 0.6 | 11.7 ± 0.5 | 12.5 ± 0.5 | NS | NS |
aValues are means ± SEM, significant p values <0.05.
bDSS Challenge: DSS− = no dextran sulfate sodium challenge, DSS+ = 5% dextran sulfate sodium challenge, n = 40/group.
cDiet: ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc), n = 16/group.
dInteraction effects of DSS challenge × diet were not significant, therefore the data are presented as main effects for DSS Treatment and Diet.
Feed and water intake
Before DSS challenge, the C and ZS rats consumed more feed than the ZD, PF and ZT rats (Table 2). DSS challenge had an inhibitory effect on feed intake, as the DSS+ rats consumed 33% less feed than the DSS− rats. After DSS challenge, the ZT group did not have a different feed intake compared to the C and ZS groups, while the feed intake of the ZD and PF groups remained lower.
There was no effect of DSS on water intake (Table 2). The ZD, PF and ZT rats had lower water intake compared to the C and ZS rats, however, when water intake was corrected for body weight, only the PF group had reduced water intake.
Stool characteristics
The groups exposed to DSS challenge had diarrhea and bloody stool starting one day after giving the rats 5% DSS in the drinking water (data not shown). By day 2, the PF and ZS groups had the worst bloody stool and diarrhea, respectively, and by day 3, these groups both had more bloody stool and diarrhea than the other groups. By day 4, all groups had the same severity of bloody stool and diarrhea.
Small intestine and colon weights and lengths
The DSS challenged rats had 1.3 fold greater small intestine/body weight ratio but no difference in small intestine length (Table 2) compared to the unchallenged rats. The small intestine/body weight ratio of ZD, PF and C rats was not different. However, the ZT rats had a 1.3 fold higher small intestine/body weight ratio than ZD, PF and C rats. The small intestine of C and the ZS groups was longer than ZD, PF, and ZT rats.
The DSS challenged group had a 1.2 fold lower colon weight/body weight ratio than the untreated rats (Table 2). The ZD, PF and ZT groups had a higher colon weight/body weight ratio than C and ZS rats. However, colon length was less in the DSS challenged rats than the unchallenged rats.
Complete blood count
The DSS-challenged rats had lower red blood cells (RBC), hemoglobin (Hgb), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) counts and higher white blood cell (WBC), lymphocyte and neutrophil counts than the unchallenged rats (Table 3). Hematocrit (Hct), mean cell volume (MCV), monocytes and platelets were not affected by DSS challenge. The ZD and PF rats had a higher RBC and Hgb counts than ZT, C and ZS groups. The ZD rats had higher Hct levels than ZT and ZS rats. The hematocrit of ZD rats was not different from PF or C rats. The hematocrit of ZT groups was lower than ZD, PF and C groups. ZD, PF and ZT rats had a lower MCV than the C and ZS rats. The C group had the highest MCH, while the ZD, PF, and ZT rats had higher MCHC values compared to C and ZS rats. The lymphocyte counts were higher in the ZS group compared to PF and ZT groups. There were no differences in WBC, neutrophils, monocytes or platelets due to diet.
Table 3.
Effect of DSS challenge or diet on complete blood count of ratsa
| DSS Challengeb |
Dietc |
DSS × Dietd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DSS+ | DSS− | p | ZD | PF | ZT | C | ZS | p | p | |
| RBC (1012/L) | 6.64 ± 0.15 | 6.94 ± 0.10 | 0.0393 | 7.61 ± 0.11a | 7.19 ± 0.14a | 6.45 ± 0.20b | 6.43 ± 0.11b | 6.31 ± 0.17b | <0.0001 | NS |
| Hemoglobin (g/L) | 138 ± 3 | 146 ± 2 | 0.0069 | 157 ± 3a | 148 ± 4a | 133 ± 4b | 139 ± 2b | 132 ± 4b | <0.0001 | NS |
| Hematocrit (L) | 0.464 ± 0.009 | 0.472 ± 0.009 | NS | 0.507 ± 0.013a | 0.486 ± 0.010ab | 0.431 ± 0.015c | 0.471 ± 0.013ab | 0.45 ± 0.014bc | 0.0009 | NS |
| MCV (109/L) | 69.5 ± 0.8 | 68.2 ± 0.4 | 0.0584 | 66.9 ± 0.5b | 67 ± 0.6b | 67.2 ± 0.4b | 72.7 ± 1.1a | 70.6 ± 1.1a | <0.0001 | NS |
| MCH (109/L) | 20.7 ± 0.1 | 21 ± 0.1 | 0.0324 | 20.7 ± 0.2b | 20.7 ± 0.2b | 20.6 ± 0.2b | 21.5 ± 0.1a | 21 ± 0.2b | 0.0009 | NS |
| MCHC (109/L) | 298.8 ± 2.2 | 308.8 ± 1.3 | <0.0001 | 308.9 ± 2.0a | 308.6 ± 2.5a | 306.3 ± 2.3a | 296.8 ± 3.4b | 297.9 ± 3.8b | 0.0007 | NS |
| WBC (109/L) | 22.7 ± 1.3 | 12.7 ± 0.7 | <0.0001 | 16.7 ± 1.9 | 15.7 ± 2.5 | 17.7 ± 2.3 | 17.1 ± 1.7 | 20.9 ± 2.0 | NS | NS |
| Lymphocytes (109/L) | 17.9 ± 1.2 | 11.1 ± 0.7 | <0.0001 | 14.3 ± 1.5ab | 12.4 ± 2.3b | 12.2 ± 1.5b | 16.2 ± 1.2ab | 17.7 ± 1.9a | 0.0352 | NS |
| Neutrophils(109/L) | 3.74 ± 0.24 | 1.18 ± 0.26 | <0.0001 | 2.28 ± 0.52 | 2.84 ± 0.73 | 2.47 ± 0.52 | 2.07 ± 0.41 | 2.57 ± 0.40 | NS | NS |
| Monocytes (109/L) | 0.46 ± 0.01 | 0.30 ± 0.05 | NS | 0.34 ± 0.08 | 0.16 ± 0.07 | 0.42 ± 0.14 | 0.5 ± 0.14 | 0.49 ± 0.15 | NS | NS |
| Platelets (109/L) | 717 ± 20 | 778 ± 25 | NS | 759 ± 33 | 771 ± 42 | 661 ± 37 | 766 ± 23 | 792 ± 38 | NS | NS |
aValues are means ± SEM, significant p values <0.05.
bDSS Challenge: DSS– = no dextran sulfate sodium challenge, DSS+ = 5% dextran sulfate sodium challenge, n = 40/group.
cDiet: ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc), n = 16/group.
dInteraction effects of DSS challenge × diet were not significant, therefore the data are presented as main effects for DSS Treatment and Diet
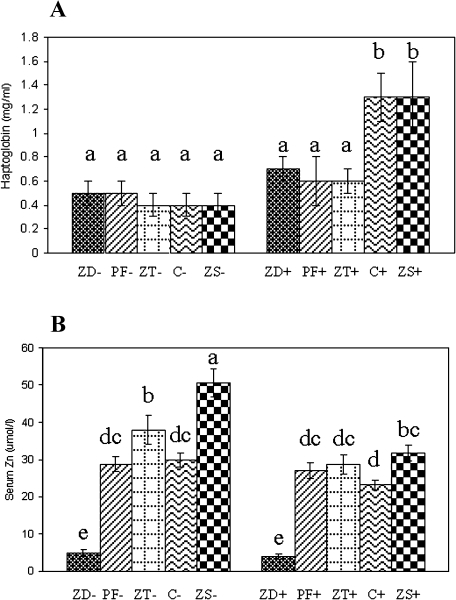
Serum haptoglobin and Zn
DSS-challenged C and ZS rats had 2-fold greater serum haptoglobin concentrations compared to the other groups (Fig. 1A). In unchallenged rats, the ZD group had a 84% lower serum Zn concentration compared to PF and C rats, and the serum Zn of ZS rats was 69% higher than C rats (Fig. 1B). The 3 day Zn repletion of ZT rats elevated serum Zn 8-fold compared to ZD rats and 1.3-fold compared to C rats. With DSS challenge, ZD rats still had the lowest serum Zn compared to all other groups. However, the elevation of serum Zn in the ZT+ group was attenuated compared to the ZT− group, and the elevation of serum Zn in the ZS+ group was attenuated compared to the ZS− group.
Fig. 1.
Effects of DSS challenge and dietary zinc on the serum haptoglobin (A) and zinc (B) status of rats. Values are means ± SEM. − = no dextran sulfate sodium challenge, + = 5% dextran sulfate sodium challenge, n = 8/DSS × diet group; ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc); different letters indicate significant differences between DSS × diet groups (p<0.05).
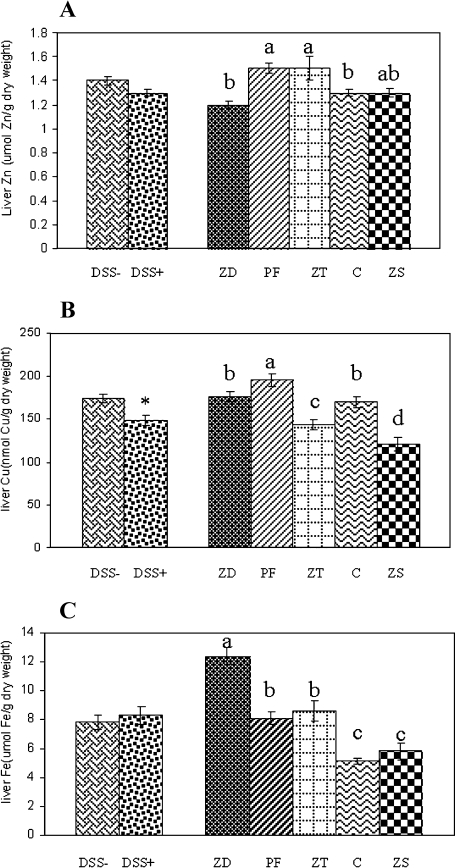
Liver trace mineral assessment
DSS challenge did not affect liver Zn and Fe concentration (Fig. 2A, C) but DSS challenged rats had an 18% lower liver Cu concentration than the unchallenged rats (Fig. 2B). The ZD and C groups had a ~15% lower liver Zn concentration compared to PF and ZT groups, while the ZS group was not different from any of the other groups. The PF rats had the highest liver Cu concentration while the ZS rats had the lowest. The ZD and C rats had a similar liver Cu concentration that was elevated compared to the ZT group. Liver Fe was elevated ~1.5 fold in ZD rats compared to PF and ZT rats, and was elevated 1.6 fold in PF compared to C. The C and ZS rats had similar liver Fe concentrations. The 3 days of Zn supplementation reduced liver Fe of the ZT group to a level equivalent to the PF group.
Fig. 2.
Effects of DSS challenge or dietary zinc on the liver trace mineral status of rats. Liver zinc (A), copper (B) and iron (C). Values are means ± SEM. − = no dextran sulfate sodium challenge, + = 5% dextran sulfate sodium challenge, n = 40/group DSS group; ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc), n = 16/group; * = DSS− different from DSS+; different letters indicate significant differences between diet groups (p<0.05).
Histology
(i) small intestine.
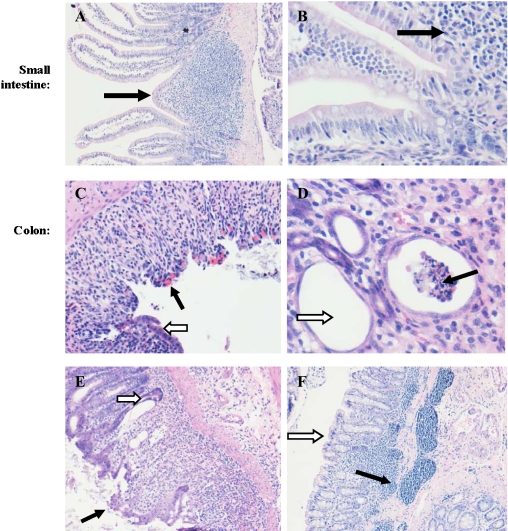
In the small intestine, neither DSS or dietary treatment affected the histology (Fig. 3A, B).
Fig. 3.
Small intestine and colon histology. In the small intestine note normal histology (A–B, 10× and 40× magnification, respectively). In the colon note ulceration (C black arrow), congestion (C white arrow, 20× magnification), crypt abscesses (D black arrow), goblet cell depletion (D white arrow, 10× magnification), loss of crypts (E black arrow), goblet cell depletion, infiltration of inflammatory cells, regenerative hyperplasia of crypt epithelium, and flattening of crypt lining (E white arrow, 10× magnification), lymphocytic aggregates in the submocusa extending to the mucosa (F black arrow) and loss of crypts (F white arrow, 10× magnification).
(ii) colon.
In the colon, all rats challenged with DSS showed colonic inflammation and there were no differences in the degree of inflammation among dietary groups (data not shown). Unexpectedly in the colon, some of the unchallenged rats had indications of quiescent nonspecific chronic inflammation represented by focal submucosal infiltration by mononuclear inflammatory cells, mainly lymphocytes, which sometimes extended to the deeper parts of the mucosa leading to atrophy of the overlying mucosa. However, mucosal changes indicative of active inflammation, namely, significant neutrophilic and eosinophilic infiltration, crypt abscesses and ulceration were not seen in untreated rats. The DSS-challenged cases showed focal mucosal changes of variable severity, consisting of edema, congestion and significant infiltration of the lamina propria by neutrophils and eosinophils, in addition to mononuclear inflammatory cells (Fig. 3C). The inflammatory infiltrate in the mucosa extended to the submucosa in the more severe cases. Variable numbers of crypt abscesses (Fig. 3D black arrow) were also seen, appearing as dilated crypts lined by flattened epithelium devoid of goblet cells, and containing intraluminal neutrophilic aggregates. There was also variable focal goblet cell depletion (Fig. 3D white arrow), as well as crypt loss and/or mucosal ulcers that varied in extent (Fig. 3E). The crypt epithelium adjoining the ulceration often showed regenerative hyperplasia. The submucosa showed an extensive, dense, predominantly lymphocytic mononuclear inflammatory cell infiltrate that focally extended to the mucosa, a finding comparable to that noted in control and untreated animals (Fig. 3F).
MT localization
(i) Small intestine.
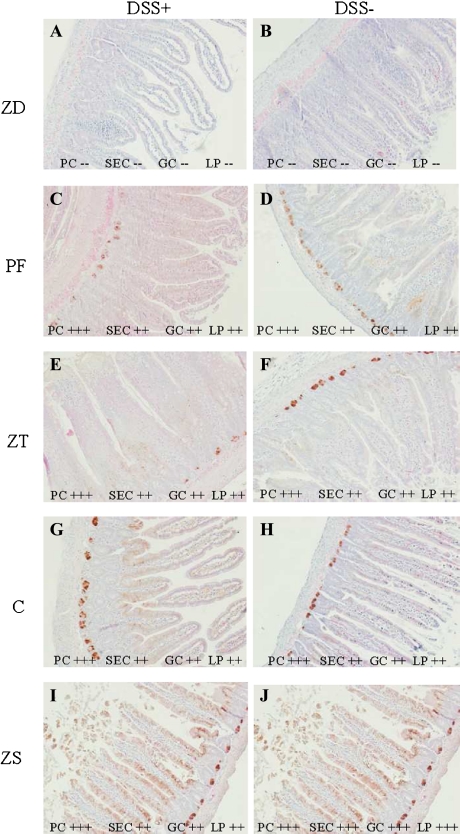
Moderate to strong cytoplasmic and nuclear MT staining of Paneth cells (PC), surface epithelial cells (SEC), goblet cells (GC) and lamina propria (LP) was present in all groups with the exception of the ZD group which had no to very weak (one to two cells only) MT staining (Fig. 4A, B). The strongest and the most consistent MT immunostaining of Paneth cells, surface epithelial cells, goblet cells and lamina propria was seen in the unchallenged ZS rats (Fig. 4J). There was strong MT immunostaining in Paneth cells and moderate immunostaining in surface epithelial cells, goblet cells and lamina propria in all other groups except the ZD rats which had very weak to no detectable MT immunostaining (Fig. 4).
Fig. 4.
Small intestinal metallothionein localization. Metallothionein was absent in the ZD groups (A–B) and present in all other groups (C–J) as indicated by scoring: (−−) none to very few cells, (+) mild, (++) moderate, (+++) high. PC = Paneth cells, SEC = surface epithelial cells, GC = goblet cells and LP = lamina propria. DSS– = no dextran sulfate sodium challenge, DSS+ = 5% dextran sulfate sodium challenge; ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc). All slides 10× magnification.
(ii) Colon.
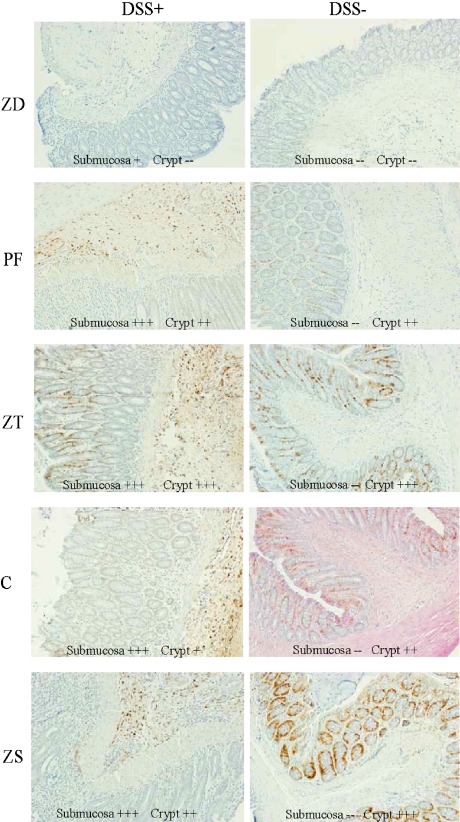
MT immunostaining was nondetectable in the unchallenged ZD group whereas it was very weak in the submucosa of the DSS-challenged ZD rats (Fig. 5A, B). All other DSS-challenged rats had MT staining in the crypts and submucosa (Fig. 5C, E, G, I), while the non-challenged rats had MT immunostaining only in the crypts (Fig. 5D, F, H, J). The strongest crypt staining was seen in the unchallenged ZT rats (Fig. 5F) and the unchallenged ZS rats (Fig. 5J). There was strong submucosal staining in the DSS-challenged PF, ZT, C and ZS rats (Fig. 5C, E, G, I, respectively).
Fig. 5.
Colonic metallothionein localization. Metallothionein staining was weak in the submucosa of the ZD+ group (A), absent in the ZD− group (B); all other DSS+ groups had staining in the submucosa and crypts (C, E, G, I) while the DSS− groups had staining only in the crypts (D, F, H, J) as indicated by scoring: (−−) none to very weak, (+) mild, (++) moderate, (+++) high of submocosa and crypts. − = no dextran sulfate sodium challenge, + = 5% dextran sulfate sodium challenge; ZD = zinc deficient (3 mg/kg zinc), PF = pair fed (30 mg/kg zinc) to match energy intake of ZD group, ZT = zinc deficient (3 mg/kg zinc) and repleted (300 mg/kg zinc), C = control (30 mg/kg zinc), ZS = zinc supplemented (300 mg/kg zinc).
Apoptosis (caspase-3) immunohistochemistry
(i) Small intestine.
The DSS-challenged ZD, PF and ZT rats had elevated apoptosis based on caspase-3 immunostaining compared to their respective unchallenged counterparts (images not shown). Caspase-3 immunostaining was lowest in ZS rats and moderate in C rats, regardless of DSS treatment.
(ii) Colon.
DSS challenge increased caspase-3 immunostaining in ZT, PF and C rats but had no effect in ZD rats, while caspase-3 immunostaining was lower in DSS-challenged ZS rats compared to unchallenged ZS rats.
Discussion
Administration of 5% DSS in the drinking water of rats for 4 days produced moderate to severe colonic inflammation but contrary to our hypothesis, it was not exacerbated by dietary Zn deficiency, nor was it reduced by acute Zn treatment or chronic Zn supplementation via the diet. Others providing Zn dose-dependently by oral gavage or by enema have noted protective effects of zinc on colitis disease activity index and tissue damage [9, 11, 12]. In rats with dinitrobenzene-sulphonic acid (DNBS)-induced colitis, intra-rectal zinc was a more effective treatment than oral zinc [11]. These findings indicate that the route of zinc administration appears important, with local administration being more effective and dietary supplementation least effective in experimental models of acute intestinal inflammation.
MT immunostaining was responsive to dietary zinc. In the small intestine, MT immunostaining was absent or very weak in the ZD rats and highest in ZS rats (Fig. 4). This is in agreement with other studies assessing MT by immunohistochemistry and cadmium hemoglobin affinity assay [15, 26, 27]. Furthermore, dietary Zn induced MT in small intestine as early as 24-h after Zn repletion [15] and thus if colonic tissue responds similarly, MT would be present for protection against DSS-challenge in ZT and ZS rats in the present study. In the colon, the site of inflammation, MT immunostaining was strongest in the ZT and ZS groups (Fig. 5), showing that acute and chronic Zn supplementation enhances MT localization in the colon. Interestingly, only the DSS-challenged animals had MT localization in the submocosa of the colon (Fig. 5), suggesting the type of injury and inflammation caused by DSS induces a site-specific response of MT in the submucosa. Conversely, DiLeo et al. [16] showed an attenuated MT concentration in the colonic mucosa of DNBS-induced colitis. However, neither the current study or others, including those using MT-null, MT-knockout or MT-transgenic mice have been able to demonstrate a beneficial effect of MT on macroscopic inflammation or colitis severity with administration of zinc by oral or intra-rectal routes [12, 16, 28]. Although zinc may be an effective treatment in models of colitis, it does not appear that MT is central to the protective mechanism, and the question remains as to why MT was induced in the colonic submucosa of DSS-treated rats.
Dietary Zn did not affect DSS dose, however, calorie restriction (PF group) resulted in lower water intake corrected for body weight and thus DSS dose (Table 2). DSS challenge produced reddish anus and bloody diarrhea, reduced feed intake, 5% lower body weight (Table 2), and colonic (but not small intestinal) inflammation consisting of significant infiltration of white blood cells, crypt abscess, mucosal ulcers, goblet cell depletion, edema and congestion (Fig. 3) compared to the untreated rats, in agreement with other studies [6, 29–33]. Although both DSS- and non-challenged rats had quiescent inflammatory cell infiltrates in the small intestine and colon, the overall level of active inflammation was significantly elevated in the DSS-challenged rats. The greater weight loss in the DSS-challenged rats may have been due to the intestinal inflammation and diarrhea causing dehydration, along with reduced feed intake, consistent with other reports of DSS-induced inflammation [21, 34]. The rats challenged with DSS also had lower red blood cell and hemoglobin levels (Table 3) compared to the non-challenged groups, likely due to the diarrheal blood loss, again consistent with previous reports using similar DSS treatment [35]. Fewer red blood cells and a lower concentration of hemoglobin could reduce the amount of oxygen transported to the cells to metabolize energy, thus, making it more difficult to maintain growth. Furthermore, MCH and MCHC were slightly reduced in the DSS-challenged rats which indicated that the rats were hypochromic compared to the control rats. White blood cell count, lymphocyte count and neutrophils were significantly higher in the DSS challenged rats (Table 3) obviously due to inflammation.
Serum Zn concentrations were responsive to dietary Zn level, including in those rats with intestinal inflammation (Fig. 1B). It is interesting to point out, however, that the serum Zn response to supplementation (ZT and ZS groups) was attenuated during DSS-challenge, suggesting that Zn was utilized in some capacity in response to acute inflammation. It is possible that there was less zinc absorption during the inflammatory challenge in these groups, however zinc is mostly absorbed in the small intestine where there was not inflammation and all the DSS-treated groups displayed inflammation while only the ZT and ZS groups had lower serum zinc concentrations compared to their untreated counterparts.
Zn status also affected the CBC; the red blood cell count and the hemoglobin levels were higher due to Zn and energy deficiency (ZD and PF rats, respectively) compared to the control and Zn supplemented groups (Table 3) and in agreement with previous work, suggesting altered hematological development due to Zn deficiency in young rats [36]. In contrast, El-Hendy et al. [37] found that longer term Zn deficiency in growing rats produced a 17% decline in the hemoglobin levels and they attributed it to a reduction in the rate of red blood cell formation. Short term Zn treatment (ZT group) was not adequate to protect against declines in MCV and MCH. There did not seem to be differences in inflammation between dietary Zn treatment groups based on WBC and neutrophils, although lymphocytes were higher in ZS rats compared to ZT and PF rats.
There are similarities in the absorption and transport of Zn, Cu and Fe, thus it was not surprising that liver concentrations of these minerals were affected by both DSS challenge and dietary Zn manipulation. DSS-challenge resulted in 15% lower liver Cu but did not affect liver Zn or Fe (Fig. 2B, A, C, respectively). Acute and chronic Zn supplementation produced lower levels of liver Cu in the ZS and ZT rats presumably due to reduced intestinal Cu absorption [38]. PF rats had elevated liver Zn, Cu and Fe compared to C rats, likely due to the energy restriction and consequential developmental delay. Liver Fe was highest in ZD rats compared to both energy restricted and control rats, as shown previously [39], implying that Zn deficiency increases Fe absorption. Even short-term zinc supplementation (3 days) reduced liver Fe concentration in the ZT group but long-term Zn-supplementation did not alter liver Fe levels. From this we conclude that supplementation with 300 mg Zn/kg did not adversely affect iron absorption, however, Zn deficiency may be associated with increased Fe absorption.
Zn deficiency is associated with more apoptosis, particularly in organs with elevated cell turnover [40–42], thus we wanted to explore whether DSS-challenge and dietary Zn manipulation was affecting caspase-3 immunostaining in small intestine and colon. However, due to the nature of the apoptotic process, there are relatively few cells undergoing apoptosis at any one time and this makes detection and interpretation difficult. The pattern of caspase-3 immunostaining differed in the small intestine and the colon in response to dietary zinc and DSS challenge. Although our immunostaining results in the small intestine may indicate a reduction of caspase-3 with Zn supplementation and enhancement with Zn deficiency, we did not observe this pattern in the colon which was the site of inflammation. These preliminary results require further investigation with additional measures of apoptosis.
In summary, 5% DSS treatment in rats produced acute colonic inflammation but dietary Zn deficiency, acute treatment or chronic supplementation did not alter the severity of ulceration in the colon. MT immunostaining in the colon responded to dietary Zn manipulations similarly to the small intestine. Furthermore, DSS-challenge induced MT immunostaining in the colonic submucosa. Site-specific MT induction in colonic submucosa during acute intestinal inflammation may be an important component of the host defense and requires further investigation to determine its potential protective role in various intestinal inflammatory diseases.
Acknowledgements
We would like to also acknowledge Jennifer Jamieson, Heather Hosea-Blewett, Laura Burr and Natasha Ryz, University of Manitoba, for assistance during the experimental phase; and Dr. Reem Al-Jindan, Saudi Arabia, for assistance in the interpretation of the hematology.
Abbreviations
- CBC
complete blood count
- Cu
copper
- C
control
- DSS
dextran-sodium sulfate
- Fe
Iron
- GC
goblet cells
- IBD
inflammatory bowel disease
- LP
lamina propria
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean cell volume
- MT
metallothionein
- PC
paneth cells
- PF
pair fed
- RBC
red blood cells
- SEC
surface eptithelial cells
- WBC
white blood cells
- ZD
zinc deficient
- ZS
zinc supplemented
- ZT
zinc treatment
- Zn
zinc
References
- 1.Perry D., Smyth M., Stennicke H., Salvesen G., Duriez P., Poirier G., Hannun Y. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. Biochem. Molec. Biol. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard R.K., Moore J.B., Green C.L., Cousins R.J. Modulation of intestinal gene expression by dietary zinc status: effectiveness of cDNA arrays for expression profiling of a single nutrient deficiency. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13507–13513. doi: 10.1073/pnas.251532498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-Bañares F., Mingorance M.D., Esteve M., Cabré E., Lachica M., Abad-Lacruz A., Gil A., Humbert P., Boix J., Gassull M.A. Serum zinc, copper, and selenium levels in inflammatory bowel disease: effect of total enteral nutrition on trace element status. Am J Gastroenterol. 1990;85:1584–1589. [PubMed] [Google Scholar]
- 4.Naveh Y., Lee-Ambrose L.M., Samuelson D.A., Cousins R.J. Malabsorption of zinc in rats with acetic acid-induced enteritis and colitis. J. Nutr. 1993;123:1389–1395. doi: 10.1093/jn/123.8.1389. [DOI] [PubMed] [Google Scholar]
- 5.Mulder T.P., van der Sluys Veer A., Verspaget H.W., Griffioen G., Peña A.S., Janssens A.R., Lamers C.B. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 1994;9:472–477. doi: 10.1111/j.1440-1746.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 6.Kruidenier L., Kuiper I., Lamers C.B., Verspaget H.W. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003a;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 7.Walker C.F., Black R.E. Zinc and the risk for infectious disease. Ann. Rev. Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 8.Duff M., Ettarh R. Crypt cell production rate in the small intestine of the zinc-supplemented mouse. Cell Tis. Org. 2002;172:21–28. doi: 10.1159/000064383. [DOI] [PubMed] [Google Scholar]
- 9.Rohweder J., Runkel N., Fromm M., Schulzke J.D., Buhr H.J. Zinc acts as a protective agent on the mucosal barrier in experimental TNBS colitis. Langenbeacs. Arch. Chir. Suppl. Kogressbd. 1998;115 Suppl. I:223–227. [PubMed] [Google Scholar]
- 10.Chen B.W., Wang H.H., Liu J.X., Liu X.G. Zinc sulphate solution enema decreases inflammation in experimental colitis in rats. Gastroenterol. Hepatol. 1999;14:1088–1092. doi: 10.1046/j.1440-1746.1999.02013.x. [DOI] [PubMed] [Google Scholar]
- 11.Luk H.H., Ko J.K., Fung H.S., Cho C.H. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur. J. Pharmacol. 2002;443:197–204. doi: 10.1016/s0014-2999(02)01592-3. [DOI] [PubMed] [Google Scholar]
- 12.Tran C.D., Ball J.M., Sundar S., Coyle P., Howarth G.S. The role of zinc and metallothionein in the dextran suflate sodium-induced colitis mouse model. Dig. Dis. Sci. 2007;52:2113–2121. doi: 10.1007/s10620-007-9765-9. [DOI] [PubMed] [Google Scholar]
- 13.Cousins R.J. Absorption, transport and hepatic metabolism of copper and zinc special reference to metallothionein and caeruloplasmin. Physiol. Rev. 1985;65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- 14.Vasak M., Hasler D.W. Metallothionein: new functional and structural insights. Curr. Opin. Chem. Biol. 2000;4:177–183. doi: 10.1016/s1367-5931(00)00082-x. [DOI] [PubMed] [Google Scholar]
- 15.Szczurek E.I., Bjornsson C.S., Taylor C.G. Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats. J. Nutr. 2001;131:2132–2138. doi: 10.1093/jn/131.8.2132. [DOI] [PubMed] [Google Scholar]
- 16.Di Leo V., D’Inca R., Barollo M., Tropea A., Fries W., Mazzon E., Irato P., Cecchetto A., Sturniolo G.C. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig. Liver Dis. 2001;33:135–139. doi: 10.1016/s1590-8658(01)80068-2. [DOI] [PubMed] [Google Scholar]
- 17.Kruidenier L., Kuiper I., Van Duijn W., Mieremet-Ooms M.A., van Hogezand R.A., Lamers C.B., Verspaget H.W. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J. Pathol. 2003b;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 18.Kruidenier L., van Meeteren M.E., Kuiper I., Jaarsma D., Lamers C.B., Zijlstra F.J., Verspaget H.W. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free. Radic. Biol. Med. 2003c;34:753–765. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- 19.Penkowa M., Hidalgo J. Metallothionein treatment reduces proinflammatory cytokines IL-6 and TNF-alpha and apoptotic cell death during experimental autoimmune encephalomyelitis (EAE) Exp. Neurol. 2001;170:1–14. doi: 10.1006/exnr.2001.7675. [DOI] [PubMed] [Google Scholar]
- 20.Korkina L., Suprun M., Petrova A., Mikhalchik E., Luci A., De Luca C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors. 2003;18:255–264. doi: 10.1002/biof.5520180228. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T., Suzuki M., Fujimura J., Hisada K., Yoshikazu O., Obinata K., Yamashiro Y. The relationship between the concentration of DSS and the degree of induced experimental colitis in weanling rats. J. Pediatr. Gastroenterol. Nutr. 2003;37:481–486. doi: 10.1097/00005176-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Masubuchi Y., Horie T. Endotoxin-mediated disturbance of hepatic cytochrome P450 function and development of endotoxin tolerance in the rat model of dextran sulfate sodium-induced experimental colitis. Drug Metab. Dispos. 2004;32:437–441. doi: 10.1124/dmd.32.4.437. [DOI] [PubMed] [Google Scholar]
- 23.Osman N., Adawi D., Ahrne S., Jeppsson B., Molin G. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig. Dis. Sci. 2004;49:320–327. doi: 10.1023/b:ddas.0000017459.59088.43. [DOI] [PubMed] [Google Scholar]
- 24.Reeves P.G., Nielsen F.H., Fahey C.G. Jr. AIN-93G purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson J.A., Stringer D.M., Zahradka P., Taylor C.G. Dietary zinc attenuates renal lead deposition but metallothionein is not directly involved. Biometals. 2008;21:29–40. doi: 10.1007/s10534-007-9090-y. [DOI] [PubMed] [Google Scholar]
- 26.Jasani B., Elmes M.E. Immunohistochemical detection of metallothionein. Methods Enzymol. 1991;205:95–107. doi: 10.1016/0076-6879(91)05091-9. [DOI] [PubMed] [Google Scholar]
- 27.Paski S., Covery L., Kummer A., Xu Z. Role of metallothionein in regulating the abundance of histochemically reactive zinc in rat tissue. Can. J. Physiol. Pharmacol. 2003;81:815–824. doi: 10.1139/y03-076. [DOI] [PubMed] [Google Scholar]
- 28.Oz H.S., Chen T., deVilliers W.J., McClain C.J. Metallothionein expression does not protect against inflammatory bowel disease in a murine colitis model. Med. Sci. Monit. 2005;11:BR69–73. [PubMed] [Google Scholar]
- 29.Tamaru T., Kobayashi H., Kishimoto S., Kajiyama G., Shimamoto F., Brown W. Histochemical study of colonic cancer in experimental colitis of rats. Dig. Dis. Sci. 1993;38:529–537. doi: 10.1007/BF01316510. [DOI] [PubMed] [Google Scholar]
- 30.Gaudio G., Taddei A., Vetuschi R., Sferra G., Frieri G., Ricciardi S., Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural and ultrastructural aspects. Dig. Dis. Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- 31.Kitajima S., Takuma S., Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp. Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 32.Erichsen K., Milde A., Arslan G., Helgeland L., Gudbrandsen O., Ulvik R., Berge R., Hausken T., Berstad A. Low-dose oral ferrouse fumarate aggravated intestinal inflammation in rats with DSS-induced colitis. Inflamm. Bowel Dis. 2005;11:744–748. doi: 10.1097/01.mib.0000174374.83601.86. [DOI] [PubMed] [Google Scholar]
- 33.Vicario M., Crespi M., Franch A., Amat C., Pelegri C., Moreto M. Induction of colitis in young rats by dextran sulfate sodium. Dig. Dis. Sci. 2005;50:143–150. [PubMed] [Google Scholar]
- 34.Geier M., Butler R., Giffard P., Howarth G. Prebiotic and symbiotic fructooligosaccharide administration fails to reduce the severity of experimental colitis in rats. Dis. Colon Rectum. 2007;50:1–9. doi: 10.1007/s10350-007-0213-x. [DOI] [PubMed] [Google Scholar]
- 35.Vowinkel T., Kalogeris T.J., Mori M., Krieglstein C.F., Granger D.N. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Digest. Dis. Sci. 2004;94:556–564. doi: 10.1023/b:ddas.0000026298.72088.f7. [DOI] [PubMed] [Google Scholar]
- 36.Paterson G., Bettger W. Effect of dietary zinc intake on the haematological profile of the rat. Comp. Biochem. Physiol. 1985;83A:721–725. doi: 10.1016/0300-9629(86)90716-4. [DOI] [PubMed] [Google Scholar]
- 37.El-Hendy H., Yousef M., El-Naga A. Effect of dietary zinc deficiency on haematological and biochemical parameters and concentrations of zinc, copper, and iron in growing rats. Toxicol. 2001;167:163–170. doi: 10.1016/s0300-483x(01)00373-0. [DOI] [PubMed] [Google Scholar]
- 38.L’Abbé M.R., Fischer P.W. The effects of high dietary zinc and copper deficiency on the activity of copper-requiring metalloenzymes in the growing rat. J. Nutr. 1984;14:813–822. doi: 10.1093/jn/114.5.813. [DOI] [PubMed] [Google Scholar]
- 39.Takashi S., Fumie M., Masanobu W., Shigeru Y. Comparative study of zinc, copper, manganese, and iron concentrations in organs of zinc deficient rats and rats treated neonatally with L-monosodium glutamate. Biol. Trace Elem. Res. 2004;97:163–182. doi: 10.1385/BTER:97:2:163. [DOI] [PubMed] [Google Scholar]
- 40.Zalewski P.D., Forbes I.J., Betts W.H. Correlation of apoptosis with change in intracellular labile Zn(II) using zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy) acetic acid], a new specific fluorescent probe for Zn(II) Biochem. J. 2003;296:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 42.Didier A., Windisch W., Pfaffl M. Elevated caspase-3 and Fas mRNA expression in jejunum of adult rats during subclinical zinc deficiency. J. Trace Elem. Med. Biol. 2004;18:41–45. doi: 10.1016/j.jtemb.2004.04.006. [DOI] [PubMed] [Google Scholar]