Abstract
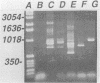
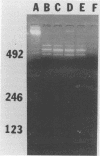
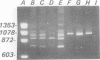
Traditional ribotyping detects genomic restriction fragment length polymorphisms by probing chromosomal DNA with rRNA. Although it is a powerful method for determining the molecular epidemiology of bacterial pathogens, technical difficulties limit its application. As an alternative, polymorphisms were sought in the 16S-23S spacer regions of bacterial rRNA genes by use of the polymerase chain reaction (PCR). Chromosomal DNA from isolates of Pseudomonas cepacia was used as a template in the PCR with oligonucleotide primers complementary to highly conserved sequences flanking the spacer regions of the rRNA genes. Length polymorphisms in the amplified DNA distinguished unrelated isolates of P. cepacia. Isolates of P. cepacia previously implicated in person-to-person transmission were shown to have identical amplification patterns. These data demonstrate the utility of this new PCR ribotyping method for determining the molecular epidemiology of bacterial species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Penner S., Slaney L., Brunton J. Biochemical characteristics of Haemophilus influenzae in relationship to source of isolation and antibiotic resistance. J Clin Microbiol. 1978 Jun;7(6):519–523. doi: 10.1128/jcm.7.6.519-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. J., Kuhns J. S., Vasil M. L., Gerding D. N., Janoff E. N. DNA fingerprinting by pulsed field gel electrophoresis and ribotyping to distinguish Pseudomonas cepacia isolates from a nosocomial outbreak. J Clin Microbiol. 1991 Mar;29(3):648–649. doi: 10.1128/jcm.29.3.648-649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacot C. M., Reeves R. H. Novel tRNA gene organization in the 16S-23S intergenic spacer of the Streptococcus pneumoniae rRNA gene cluster. J Bacteriol. 1991 Jul;173(13):4234–4236. doi: 10.1128/jb.173.13.4234-4236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry T., Powell R., Gannon F. A general method to generate DNA probes for microorganisms. Biotechnology (N Y) 1990 Mar;8(3):233–236. doi: 10.1038/nbt0390-233. [DOI] [PubMed] [Google Scholar]
- Boros I., Kiss A., Venetianer P. Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1979;6(5):1817–1830. doi: 10.1093/nar/6.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchko J., Klassen G. R. Detection of length heterogeneity in the ribosomal DNA of Pythium ultimum by PCR amplification of the intergenic region. Curr Genet. 1990 Oct;18(3):203–205. doi: 10.1007/BF00318381. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Kaplan E. L., Livdahl C., Skjold S. DNA fingerprints of Streptococcus pyogenes are M type specific. J Infect Dis. 1988 Dec;158(6):1317–1323. doi: 10.1093/infdis/158.6.1317. [DOI] [PubMed] [Google Scholar]
- Gilligan P. H., Gage P. A., Bradshaw L. M., Schidlow D. V., DeCicco B. T. Isolation medium for the recovery of Pseudomonas cepacia from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol. 1985 Jul;22(1):5–8. doi: 10.1128/jcm.22.1.5-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Schnare M. N., Gray M. W. A compilation of large subunit (23S-like) ribosomal RNA sequences presented in a secondary structure format. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2319–2330. doi: 10.1093/nar/18.suppl.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens J. Z., Hall L. M. Characterisation of methicillin-resistant Staphylococcus aureus isolates by restriction endonuclease digestion of chromosomal DNA. J Med Microbiol. 1988 Oct;27(2):117–123. doi: 10.1099/00222615-27-2-117. [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., van Alphen L., Leenders E., Zanen H. C. Typing of Aeromonas strains by DNA restriction endonuclease analysis and polyacrylamide gel electrophoresis of cell envelopes. J Clin Microbiol. 1989 Jun;27(6):1280–1285. doi: 10.1128/jcm.27.6.1280-1285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Dasen S. E., Nielson D. W., Stern R. C., Stull T. L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990 Nov 3;336(8723):1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Fisher M. C., Dasen S. E., Mortensen J. E., Stull T. L. Ribotype stability of serial pulmonary isolates of Pseudomonas cepacia. J Infect Dis. 1991 Jul;164(1):133–136. doi: 10.1093/infdis/164.1.133. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Mortensen J. E., Dasen S. E., Edlind T. D., Schidlow D. V., Burns J. L., Stull T. L. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J Pediatr. 1988 Nov;113(5):859–862. doi: 10.1016/s0022-3476(88)80018-0. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Stull T. L., Dasen S. E., Pidcock K. A., Kaye D., Korzeniowski O. M. DNA polymorphisms among Escherichia coli isolated from bacteriuric women. J Infect Dis. 1989 Mar;159(3):526–532. doi: 10.1093/infdis/159.3.526. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- Parisi J. T. Coagulase-negative staphylococci and the epidemiological typing of Staphylococcus epidermidis. Microbiol Rev. 1985 Jun;49(2):126–139. doi: 10.1128/mr.49.2.126-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérolat P., Grimont F., Regnault B., Grimont P. A., Fournié E., Thevenet H., Baranton G. rRNA gene restriction patterns of Leptospira: a molecular typing system. Res Microbiol. 1990 Feb;141(2):159–171. doi: 10.1016/0923-2508(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Regnery R. L., Spruill C. L., Plikaytis B. D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991 Mar;173(5):1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Currie-McCumber C. A. Plasmid analysis in molecular epidemiology: a summary and future directions. Rev Infect Dis. 1986 Sep-Oct;8(5):738–746. doi: 10.1093/clinids/8.5.738. [DOI] [PubMed] [Google Scholar]
- Snipes K. P., Hirsh D. C., Kasten R. W., Hansen L. M., Hird D. W., Carpenter T. E., McCapes R. H. Use of an rRNA probe and restriction endonuclease analysis to fingerprint Pasteurella multocida isolated from turkeys and wildlife. J Clin Microbiol. 1989 Aug;27(8):1847–1853. doi: 10.1128/jcm.27.8.1847-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spreadbury C. L., Bainbridge B. W., Cohen J. Restriction fragment length polymorphisms in isolates of Aspergillus fumigatus probed with part of the intergenic spacer region from the ribosomal RNA gene complex of Aspergillus nidulans. J Gen Microbiol. 1990 Oct;136(10):1991–1994. doi: 10.1099/00221287-136-10-1991. [DOI] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Mechanism and regulation of bacterial ribosomal RNA processing. Annu Rev Microbiol. 1990;44:105–129. doi: 10.1146/annurev.mi.44.100190.000541. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Vaudry W. L., Tierney A. J., Wenman W. M. Investigation of a cluster of systemic Candida albicans infections in a neonatal intensive care unit. J Infect Dis. 1988 Dec;158(6):1375–1379. doi: 10.1093/infdis/158.6.1375. [DOI] [PubMed] [Google Scholar]