Abstract
We previously reported that the plasma level of endotoxin and colonic expression of IgA in the mouse increased with obstructive jaundice induced by bile duct ligation (BDL). To elucidate the mechanism of the BDL-induced increase, we analyzed the effect of BDL on intestinal flora in wild type and inducible nitric oxide synthase (iNOS)-deficient mice (iNOS−/−) using the terminal restriction fragment length polymorphism analysis (T-RFLP) and 16S rDNA clone libraries. The amounts of bacterial DNA detected in fecal samples from both animal groups pretreated with antibiotics were extremely low as compared with untreated groups. We found that the profiles of enteric bacteria changed markedly after BDL. The bacterial composition is significantly different between not only wild type and iNOS−/− mice but also those before and after BDL, respectively. Among enteric bacteria examined, Lactobacillus murinus was found to increase markedly after BDL in rectum of both animal groups. However, Escherichia coli markedly increased after BDL in the iNOS−/− mice. These findings suggest that profiles of enteric flora change markedly in animals during obstructive jaundice by some mechanism that is affected by bile constituents and iNOS-derived NO.
Keywords: intestinal flora, endotoxemia, obstructive jaundice, nitric oxide, T-RFLP
Introduction
The composition of the intestinal microbial flora is relatively stable and unique for each species and individual [1]. The commensal microbial flora in gastrointestinal tract plays important roles in the maintenance and regulation of animal health and nutrition, respectively. Under physiological conditions, the presence of enteric microbiota is important for the protection of animals against potential pathogens [2]. Hence, disruption of the ecological balance in intestinal lumen gives rise to clinical complications [3, 4].
It has been suggested that bile acids are one of the important factors that affect the ecological balance of intestinal flora. In fact, the translocation of intestinal bacteria across the mucosal layer increases markedly and sometimes causes lethal endotoxemia in patients with obstructive jaundice [5–8]. Changes in intestinal flora sometimes modulate the population of lymphocytes that secrete Th2-type cytokines and antibodies, such as IL-10 and IgA, respectively [9]. We previously demonstrated that obstructive liver injury was inhibited by pretreatment of animals with antibiotics [10]. Kinetic analysis revealed that disturbance of the ecological balance of enteric bacteria played critical roles in the regulation of immunological network among liver, spleen and intestine, and in the pathogenesis of liver injury during obstructive jaundice. However, the pathological significance of the bile duct ligation (BDL)-induced changes in the profiles of bacterial flora remains unknown. We also reported that BDL increased endotoxin levels in plasma more markedly with inducible nitric oxide synthase deficient (iNOS−/−) mice than with wild-type animals and this increase was strongly inhibited by antibiotics [10]. Since nitric oxide (NO) has a potent antibacterial activity, bacterial composition in the intestinal lumen would be affected by luminal occurrence of NO. To examine the role of iNOS-derived NO in the profiles of bacterial flora, we used iNOS−/− mice in this study.
It has been practically difficult for a long time to characterize the composition of intestinal microbiota predominantly due to its large variations among individuals and a lack of appropriate methods. For example, classic methods based on cell culture are useful for the detection of less than 40% of the microorganisms predominantly due to the difficulty to handle anaerobic bacteria in the intestine [11]. However, the recent development of molecular techniques for the analysis of complex microbial communities bypassed the necessity of cell culture and made considerable progress in the characterization of intestinal bacteria [1, 3, 12, 13]. The terminal restriction fragment length polymorphism analysis (T-RFLP) is a useful method for the assessment of the differences in the composition of intestinal microbial communities [3, 14–16].
To elucidate the mechanism underling the pathological changes induced by BDL, we analyzed the profiles of fecal bacteria in C57BL and iNOS−/− mice before and after BDL using TRFLP and 16S rDNA clone libraries.
Materials and Methods
Reagents
A Kit for the isolation of DNA from mouse feces and primers 7F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3') were obtained from MO BIO laboratories Inc. (Carlsbad, CA) and SIGMA Genosis (Japan), respectively. TaKaRa Ex TaqTM (1,000 U) and TA Cloning® Kit and One Shot TOP 10 Chemically Competent E. coli were obtained from Takara Bio (Shiga, Japan) and InvitrogenTM (Carlsbad, CA), respectively. Wizard Plus SV Minipreps DNA Purification System and MontageTM PCR was obtained from Promega (Madison, WI) and Millipore (Bedford, MA), respectively. Mouse monoclonal antibody against iNOS (610432), rabbit anti-iNOS polyclonal antibody (KAS-NO001) were obtained from BD Transduction Laboratories (San Jose, CA) and Stressgen Biotechnologies (Victoria, Canada), respectively. Other reagents used were of the highest grade commercially available.
Animal experiments
Male C57BL/6J mice (20–25 g) and their iNOS−/− strain were purchased from SLC (Shizuoka, Japan) and Jackson Laboratories (Coldspring Harbor, MA), respectively. Animals were allowed free access to laboratory chow (CE-2, Oriental Yeast Co., Tokyo) and water ad libitum during the experiments. All experiments were approved by the Institutional Animal Care and Use Committee of Osaka City University Medical School. Obstructive jaundice was elicited by the common BDL as described previously [17]. Under light ether anesthesia, animals were subjected to BDL. In the control sham group, sham-operation was performed. At indicated times after performing BDL, animals were sacrificed to obtain blood and feces. The collected fecal samples were stored at −80°C until analysis.
DNA extraction from fecal samples
Extraction and purification of DNA were carried out according to the methods described by Clement and Kitts [18] using an Ultra Clean Soil DNA isolation kit as described previously [19].
PCR amplification for TRFLP analysis
A pair of universal primers, 27F and 1492R [20] was used for PCR amplification and 27F was labeled with 6-carboxyfluorescein (6-FAM). PCR was performed with a Thermocycler T Gradient (Biometra, Göttingen, Germany) in 50 µl of reaction mixture containing 5 µl of dissolved DNA (100 ng), 1.25 U of TaKaRa Ex Taq, 10 × Ex Taq buffer, 4 µl of dNTP mixture (2.5 mM each), and 10 pmol of each primer. The amplification program used was as follows: preheating at 95°C for 3 min; 30 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 1.5 min; terminal extension at 72°C for 10 min [19, 21]. The amplified DNA was verified by 1.5% agarose gel electrophoresis. PCR products were purified using a MontageTM PCR (Millipore). The purified PCR products were stored at −20°C until analysis.
TRFLP analysis
The restriction enzymes used were selected according to the methods of Liu et al. [15] and Moyer et al. [22]. Purified PCR products (4 µl) were digested with 20 U of either HhaI or MspI (Takara Bio) in a total volume of 10 µl at 37°C for 24 h. The lengths of the terminal restriction fragments (T-RFs) were determined using a standard size marker GS500 ROX and 1000 ROX (Applied Biosystems, Carlabad, CA) in an ABI PRISMTM 3100 genetic analyzer (Applied Biosystems) and GeneScan® Analysis Software (Applied Biosystems). The peak of T-RFs was determined using TRFLP analysis program (TAP) [23].
PCR amplification of 16S rDNA gene sequences and cloning
The samples used for cloning and sequencing were obtained from mice before and 7 day after BDL. The 16S rDNA gene was amplified using two universal primers 27F and 1492R [19, 20]. PCR was performed as described previously [24] and [19]. The amplified 16S rDNA genes were purified using an Ultra Clean PCR Clean-up Kit (Mo Bio Laboratories). The purified amplicons were cloned into pCR® 2.1 vectors (Invitrogen, San Diego, CA), and One Shot® INVaF’ competent cells were transformed [19]. The transformants were randomly picked and purified with Wizard® Plus SV Minipreps DAN Purification System (Promega).
DNA sequencing and phylogenetic analysis
Cycle sequencing was performed with a Big Dye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), 27F or 1492R primers, and ABI PRISMTM 3100 Genetic Analyzer (Applied Biosystems). All sequences were compared with similar sequences of the reference organisms by using BLAST search [25].
Histological analysis
The colon specimens were rapidly frozen in an OCT embedding medium (Tissue-Tek, Elkhart, IN) and stored at −80°C until use. Cryostat sections (6 µm thickness) were fixed in ice-cold acetone for 10 min. The expression of iNOS was evaluated immunohistochemically under a fluorescent microscope as described previously [26, 27]. Rabbit anti-iNOS polyclonal antibody (KAS-NO001) was obtained from Stressgen Biotechnologies (Victoria, Canada).
Western blot analysis
The colon was rinsed with saline and homogenized in a lysis buffer containing 0.5% Nonidet P-40, 10% glycerol, 137 mM NaCl, 2 mM ethylendiamine-tetraacetic acid, and 50 mM Tris-HCl buffer (pH 8.0). After centrifugation at 3,000 × g for 10 min, the supernatant was separated and stored at −80°C. The stored specimens were subjected to 7.5% polyacrylamide gel electrophoresis (PAGE) in the presence of 0.1% SDS. The electrophoresed proteins in the gel were transferred to an Immobilon membrane (Millipore). The membrane was blocked with 5% skim milk at 4°C for overnight, subsequently incubated with primary antibodies at 25°C for 1 h and then with horseradish peroxidase-conjugated secondary antibodies. Immune complexes thus formed were detected with ECL reagents (Amersham Bioscience, Buckinghamshire, UK).
Results
Analysis of fecal bacterial community by TRFLP
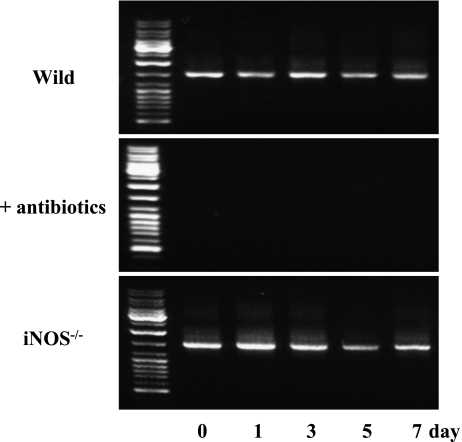
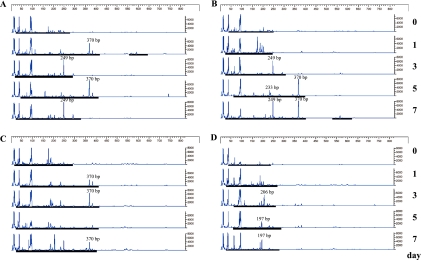
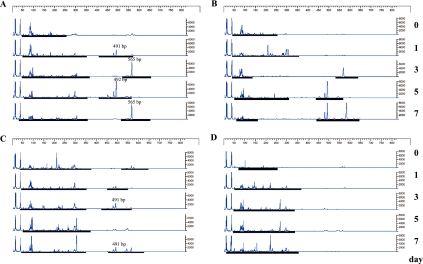
Although bacterial DNA was easily detected in fecal samples from wild and iNOS−/− mice, it was practically difficult to detect DNA from both animal groups pretreated with antibiotics (Fig. 1). TRFLP patterns of the wild and iNOS−/− mice were compared using two different restriction enzymes before and after BDL (Fig. 2 and 3). The profiles of bacterial community in the feces from wild and iNOS−/− mice changed markedly after BDL. The profiles of bacterial distribution in the feces from the rectum and the cecum also differed significantly after BDL. After digestion with HhaI, terminal restriction fragments (T-RFs) at bp 249 and 370, and bp 370 were detected in the feces from rectum and cecum of wild type mice after BDL. In contrast, HhaI digestion revealed T-RFs at bp 233, 249, and 370, and bp 197 and 208 in the feces from the rectum and cecum of iNOS−/− mice that had been subjected to BDL (Fig. 2). MspI digestion revealed T-RFs at bp 491, 492 and 565, and bp 491 in the feces from the rectum and cecum of BDL-treated wild type mice. MspI digestion revealed T-RFs at bp 492, 568 and 583, and bp 223 and 269 in the fecal sample from the rectum and cecum of BDL-treated iNOS−/− mice (Fig. 3).
Fig. 1.
Bacterial DNA extraction from fecal samples. DNA was extracted from 50 mg of the fecal sample. A pair of universal primers, 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3'), were used for PCR amplification. The amplified DNA was verified by 1.5% agarose gel electrophoresis.
Fig. 2.
T-RFLP analysis of intestinal microbiota before and after BDL. Data shows T-RFLP patterns of 16S rDNA from mouse feces (day 0~7) digested with HhaI. 16S rDNA were amplified using universal primers 27F and 1492R. The minimum and maximum values of the ordinate of each T-RFLP pattern are 0 to 800 fluorescence intensity (arbitary units) for HhaI. Fecal samples from the rectum of (A) wild and (B) iNOS−/− mice, and the cerum of (C) wild and (D) iNOS−/− mice
Fig. 3.
T-RFLP analysis of intestinal microbiota before and after BDL. Data shows T-RFLP patterns of 16S rDNA from mouse feces (day 0~7) digested with MspI. Other conditions were same as in Fig. 2.
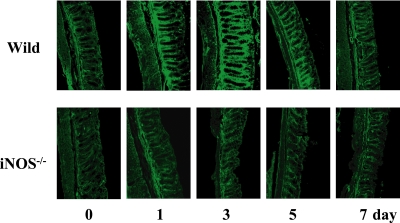
BDL induced expression of iNOS in the colon
Colonic expression of iNOS markedly increased after BDL. As shown in Fig. 4 and 5, the expression of iNOS in the wild type mice increased after BDL, peaked on day 3, and decreased thereafter. Naturally, no iNOS was induced by BDL in the knockout mice.
Fig. 4.
Histological examination of the expression of iNOS in the colon. At the indicated times after BDL, colon specimens were frozen, cut into thin sections, treated with anti-iNOS antibody and then stained with FITC-conjugated second antibody. Scale bar = 50 µm
Fig. 5.
Western blotting analysis of expression of iNOS in the colon. At the indicated times after BDL, colon specimens were collected. Each amounts of protein (30 µg/lane) from colon was subjected to a 7.5% SDS-PAGE, and the electrophoresed proiteins were transferred onto Immobilon-P membranes, and analyzed using anti-iNOS antibody. The bands with 130 kDa responsible for iNOS were seen in colon specimens from BDL mice. Data show one typical result out of 3 representative experiments.
Identification of bacterial 16S rDNA gene
The sequences of 16S rDNA clones were used to identify enteric bacterial profiles in the feces from the wild type and iNOS−/− mice before and after BDL (Table 1). Among fifty clones from the inoculum analyzed, no exact 16S rDNA gene similarity limits exist to define specific taxa, such as genus and species. The definition of species generaly requires sequence similarities of greater than 98% [28]. The compositions of enteric bacteria from wild type mice differed significantly from that of iNOS−/− mice; both of them changed markedly after BDL. Among various bacteria observed, Lactobacillus murinus in the rectum markedly increased after BDL both in wild type and iNOS−/− mice. On day 7 after BDL, total numbers of Lactobacillus murinus in the rectum were found to be about 35.0% and 30.2% of the flora in wild type and iNOS−/− mice, respectively. Bacteroides acidofaciens in the rectum of wild animals showed no detectable changes even after BDL. In contrast, Escherichia coli in the rectum of iNOS−/− mice markedly increased after BDL (Table 1). On day 7 after BDL, Bacteroides intestinalis, Clone aab24h05, and Bacteroides acidofaciens in the cecum of wild animals increased to 30%, 12%, and 10% of the total number of enteric bacteria, respectively (Table 1).
Table 1.
Identification by 16S rDNA sequences analysis
| Wild | (%) | iNOS−/− | (%) | ||
|---|---|---|---|---|---|
| rectum | Day 0 | Clone Adhufec 33 ml | 10.4 | Clone R-1264 (2) | 16.7 |
| Uncultured rumen bacterium gene | 5.1 | Clone aab23g09 | 11.1 | ||
| Bacteroides acidofaciens | 5.1 | Clone F3 | 11.1 | ||
| Clone R-1264(2) | 5.1 | Clone aab21h12 | 8.3 | ||
| Clone ratBN020109C | 5.1 | Clone M1-g02-3 | 8.3 | ||
| The others (each less than 4%) | 69.2 | Clone aab42c05 | 5.1 | ||
| Lactobacillus johnsonii NCC 533 | 5.1 | ||||
| The others (each less than 4%) | 33.3 | ||||
| Day 7 | Lactobacillus murinus | 35.0 | Lactobacillus murinus | 30.2 | |
| Clone aab24h05 | 7.5 | Escherichia coli | 25.6 | ||
| Clone M3-c08-3 | 5.0 | Shigella boydii strain 5216-70 | 7.0 | ||
| Bacteroides acidofaciens | 5.0 | The others (each less than 4%) | 37.2 | ||
| The others (each less than 4%) | 47.5 | ||||
| cecum | Day 0 | Clone aab22a11 | 7.3 | Clone M2-a05 | 11.4 |
| Lactobacillus johnsonii NCC 533 | 7.3 | Clone aab23f05 | 8.6 | ||
| Bacteroides acidofaciens | 4.9 | Mucispirillum schaedleri strain HRI I 17 | 5.7 | ||
| Clone M1-b06-1 | 4.9 | The others (each less than 4%) | 74.3 | ||
| Clone aab21h12 | 4.9 | ||||
| The others (each less than 4%) | 70.7 | ||||
| Day 7 | Bacteroides intestinalis | 30.0 | Clone 28-2 small subunit | 12.9 | |
| Clone aab24h05 | 12.0 | Clone aab 24a06 | 6.5 | ||
| Bacteroides acidofaciens | 10.0 | The others (each less than 4%) | 80.6 | ||
| Uncultured Gram-positive bacterium | 6.0 | ||||
| Lactobacillus murinus | 4.0 | ||||
| Escherichia coli | 4.0 | ||||
| Clone abc 38b10.x1 | 4.0 | ||||
| The others (each less than 4%) | 30.0 | ||||
Values show the percentage of bacterial species obtained from the rectum and cecum of wild and iNOS−/− mice.
Discussion
The present work shows that bacterial composition in the intestinal lumen was different in wild type and iNOS−/− mice, and both of them changed markedly after BDL. Bile acids have been known to inhibit bacterial overgrowth and their translocation across the intestinal mucosa [5, 6, 29]. Since NO has a potent antibacterial activity, bacterial composition in the intestinal lumen would be affected by luminal occurrence of NO.
The population of E. coli in the intestine increased after BDL in iNOS−/− mice but not in wild type mice. Although, the lifetime of NO has been postulated to be extremely short (several seconds) particularly under air atmospheric conditions, it is fairly stable under low oxygen tensions [30–32]. It should be noted that oxygen is the substrate for NOS and, hence, the rate of NO synthesis may decrease under physiological low oxygen tensions in the intestinal lumen. Thus, the enhancement of NO action by low oxygen tensions may occur minimally in anaerobic compartments, such as in the large intestinal lumen. In this context, the Km value of iNOS for molecular oxygen has been reported to be about 6.3 ± 0.9 µM [33]. Therefore, macrophages and neutrophils not only in the circulation but also in the intestinal mucosa might generate reactive oxygen species including NO [34, 35]. Because of a gaseous nature of NO, it easily diffuses from a mucosal site of generation into intestinal lumen. Thus, NO generated by the activated leukocytes in an inflammatory lesion of the intestine might reach to its lumenal space where a large number of enteric bacteria live. We previously reported that endotoxin levels in mouse plasma increased after BDL; its increase was more marked in iNOS−/− than in the wild mice [10]. The increased endotoxin in BDL-treated iNOS−/− mice might reflect the change in intestinal flora, such as increased E. coli.
Interestingly, Lactobacillus murinus markedly increased after BDL both in wild type and iNOS−/− mice. Since the growth of Lactobacilli and Bifidobacteria were inhibited by bile acids [36], it is not surprising that L. murinus increased markedly after BDL. Lactobacilli are a major component of the commensal microbial flora in the intestine both of humans and animals [37]. Such probiotic bacteria have been shown to give beneficial effects including improvement of intestinal health, enhancement of the immunological activity, synthesis of nutrients and reduction of lactose intolerance, allergic reactions, and risk of cancers [37–39]. Although the mechanisms by which such probiotics exert their beneficial effects are largely unknown, it may involve regulation of lumenal pH, and competition for available nutrients and growth factors with pathogens. We previously reported that obstructive liver injury was inhibited by the administration of antibiotics and that enteric bacteria play critical roles in the pathogenesis of liver injury induced by obstructive jaundice [10]. However, the role of L. murinus in the pathological events in the intestine of BDL-treated animals remains obscure.
It should be noted that large numbers of unknown bacteria not cultured successfully are living in the intestine and play a role in the modulation of intestinal diseases. Thus, TRFLP is a useful method for the assessment of the changes in intestinal microbial flora under physiological as well as pathological conditions.
The present work suggests that bacterial composition in the intestinal lumen changes significantly after BDL by a mechanism that is affected by iNOS-derived NO.
Recent studies revealed that metagenome analysis is also an efficient and convenient method for the analysis of enteric bacteria. Pathophysiological significance of BDL-induced changes in intestinal flora should be studied further using more convenient methods.
Acknowledgements
The authors thank to Prof. Yoshimi Benno in RIKEN BioResource Center (Japan Collection of Microorganisms) for providing the methods of T-RFLP and 16S rDNA clone libraries.
Abbreviations
- T-RFLP
terminal restriction fragment length polymorphism analysis
- BDL
bile duct ligation
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
References
- 1.Zoetendal E.G., Akkermans A.D., De Vos W.M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 3.Jernberg C., Sullivan A., Edlund C., Jansson J.K. Monitoring of antibiotic-induced alterations in the human intestinal microflora and detection of probiotic strains by use of terminal restriction fragment length polymorphism. Appl. Environ. Microbiol. 2005;71:501–506. doi: 10.1128/AEM.71.1.501-506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock G.W. Analysis of the intestinal microflora: a renaissance. Antonie Van Leeuwenhoek. 1999;76:265–278. [PubMed] [Google Scholar]
- 5.Ding J.W., Andersson R., Soltesz V., Willen R., Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur. Surg. Res. 1993;25:11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- 6.Gouma D.J., Coelho J.C., Schlegel J.F., Li Y.F., Moody F.G. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch. Surg. 1987;122:731–734. doi: 10.1001/archsurg.1987.01400180113022. [DOI] [PubMed] [Google Scholar]
- 7.Diamond T., Thompson R.L., McGlone E., Rowlands B.J. Endotoxin concentrations following internal and external biliary drainage for obstructive jaundice. Curr. Surg. 1989;46:311–313. [PubMed] [Google Scholar]
- 8.Roughneen P.T., Kumar S.C., Pellis N.R., Rowlands B.J. Endotoxemia and cholestasis. Surg. Gynecol. Obstet. 1988;167:205–210. [PubMed] [Google Scholar]
- 9.Fagarasan S., Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 10.Hong J.-Y., Sato E.F., Hiramoto K., Nishikawa M., Inoue M. Mecanism of Liver Injury during Obstructive Jaundice: Role of Nitric Oxide, Splenic Cytokines, and Intestinal Flora. J. Clin. Biochem. Nutr. 2007;40:184–193. doi: 10.3164/jcbn.40.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suau A., Bonnet R., Sutren M., Godon J.J., Gibson G.R., Collins M.D., Dore J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks A.H., Harmsen H.J., Raangs G.C., Jansen G.J., Schut F., Welling G.W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furrie E. A molecular revolution in the study of intestinal microflora. Gut. 2006;55:141–143. doi: 10.1136/gut.2005.081695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leser T.D., Lindecrona R.H., Jensen T.K., Jensen B.B., Moller K. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Appl. Environ. Microbiol. 2000;66:3290–3296. doi: 10.1128/aem.66.8.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W.T., Marsh T.L., Cheng H., Forney L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mai V. Dietary modification of the intestinal microbiota. Nutr. Rev. 2004;62:235–242. doi: 10.1301/nr2004.jun235-242. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi H., Rust C., Roberts P.J., Burgart L.J., Gores G.J. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 18.Clement B.G., Kitts C.L.Isolating PCR-quality DNA from human feces with a soil DNA kit Biotechniques 28640–642, 644, 6462000 [DOI] [PubMed] [Google Scholar]
- 19.Kibe R., Sakamoto M., Hayashi H., Yokota H., Benno Y. Maturation of the murine cecal microbiota as revealed by terminal restriction fragment length polymorphism and 16S rRNA gene clone libraries. FEMS Microbiol. Lett. 2004;235:139–146. doi: 10.1016/j.femsle.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Lane D.J. In: 16S/23S rRNA sequencing, in Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt E., Goodfellow M., editors. John Wiley and Sons; New York: 1991. pp. 115–175. [Google Scholar]
- 21.Sakamoto M., Hayashi H., Benno Y. Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol. Immunol. 2003;47:133–142. doi: 10.1111/j.1348-0421.2003.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 22.Moyer C.L., Tiedje J.M., Dobbs F.C., Karl D.M. A computer-simulated restriction fragment length polymorphism analysis of bacterial small-subunit rRNA genes: efficacy of selected tetrameric restriction enzymes for studies of microbial diversity in nature. Appl. Environ. Microbiol. 1996;62:2501–2507. doi: 10.1128/aem.62.7.2501-2507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsh T.L., Saxman P., Cole J., Tiedje J. Terminal restriction fragment length polymorphism analysis program, a web-based research tool for microbial community analysis. Appl. Environ. Microbiol. 2000;66:3616–3620. doi: 10.1128/aem.66.8.3616-3620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson K.H., Blitchington R.B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 1996;62:2273–2278. doi: 10.1128/aem.62.7.2273-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M., Kweon M.N., Rennert P.D., Hiroi T., Fujihashi K., McGhee J.R., Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J. Immunol. 2004;173:762–769. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- 27.Yagnik G.P., Takahashi Y., Tsoulfas G., Reid K., Murase N., Geller D.A. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573–581. doi: 10.1053/jhep.2002.35058. [DOI] [PubMed] [Google Scholar]
- 28.Stackebrandt E., Goebel B.E. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 1994;44:846–849. [Google Scholar]
- 29.Ogata Y., Nishi M., Nakayama H., Kuwahara T., Ohnishi Y., Tashiro S. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J. Surg. Res. 2003;115:18–23. doi: 10.1016/s0022-4804(03)00308-1. [DOI] [PubMed] [Google Scholar]
- 30.Takehara Y., Kanno T., Yoshioka T., Inoue M., Utsumi K. Oxygen-dependent regulation of mitochondrial energy metabolism by nitric oxide. Arch. Biochem. Biophys. 1995;323:27–32. doi: 10.1006/abbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa M., Sato E.F., Utsumi K., Inoue M. Oxygen-dependent regulation of energy metabolism in ascites tumor cells by nitric oxide. Cancer Res. 1996;56:4535–4540. [PubMed] [Google Scholar]
- 32.Tomita M., Sato E.F., Nishikawa M., Yamano Y., Inoue M. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis. Rheum. 2001;44:96–104. doi: 10.1002/1529-0131(200101)44:1<96::AID-ANR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Rengasamy A., Johns R.A. Determination of Km for oxygen of nitric oxide synthase isoforms. J. Pharmacol. Exp. Ther. 1996;276:30–33. [PubMed] [Google Scholar]
- 34.Miyoshi M., Kasahara E., Park A.M., Hiramoto K., Minamiyama Y., Takemura S., Sato E.F., Inoue M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic. Res. 2003;37:85–90. doi: 10.1080/1071576021000086632. [DOI] [PubMed] [Google Scholar]
- 35.Yu H., Sato E.F., Nagata K., Nishikawa M., Kashiba M., Arakawa T., Kobayashi K., Tamura T., Inoue M. Oxygen-dependent regulation of the respiration and growth of Escherichia coli by nitric oxide. FEBS Lett. 1997;409:161–165. doi: 10.1016/s0014-5793(97)00494-8. [DOI] [PubMed] [Google Scholar]
- 36.Kurdi P., Kawanishi K., Mizutani K., Yokota A. Mechanism of Growth Inhibition by Free Bile Acids in Lactobacilli and Bifidobacteria. J. Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahrne S., Nobaek S., Jeppsson B., Adlerberth I., Wold A.E., Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 1998;85:88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- 38.Marcinkiewicz J., Ciszek M., Bobek M., Strus M., Heczko P.B., Kurnyta M., Biedron R., Chmielarczyk A. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int. J. Exp. Pathol. 2007;88:155–164. doi: 10.1111/j.1365-2613.2007.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vrese M., Schrezenmeir J. Probiotics, Prebiotics, and Synbiotics. Adv. Biochem. Eng. Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]