Abstract
The urinary concentrations of 8-isoprostane and 8-hydroxy-2’-deoxyguanosine (8-OHdG), which are biomarkers of oxidative stress, were measured in 677 Japanese people without any diseases, and their correlations with lifestyle facotrs, lifestyle-related blood biochemical parameters, and dietary intake of antioxidative vitamins were investigated. The mean urinary concentration of 8-isoprostane and 8-OHdG was 0.58 ng/mg creatinine and 8.43 ng/mg creatinine, respectively. Mean urinary 8-isoprostane was significantly different in terms of age, gender, smoking and alcohol consumption but not different in terms of body mass index (BMI) and exercise. By multiple regression analysis, urinary 8-isoprostane was significantly influenced by smoking and age. On the other hand, mean urinary 8-OHdG showed differences only by age group. Multiple regression analysis revealed that urinary 8-OHdG was significantly influenced by age, smoking, body weight, levels of high-sensitivity C-reactive protein (Hs-CRP) and low density lipoprotein-cholesterol in females, although it was significantly influenced by body weight in males. The present study shows that urinary 8-isoprostane is associated with lipid peroxidation related-lifestyles such as smoking, and urinary 8-OHdG is associated with arteriosclerosis related-factors such as Hs-CRP. Our findings suggest that 8-isoprostane and 8-OHdG appear to be prospective biomarkers for early prediction of lifestyle related-disease risk at the population level.
Keywords: 8-isoprostane, 8-OHdG, biomarkers, cross-sectional study, healthy people
Introduction
As a result of oxygen metabolic processes, cells continuously produce free radicals and reactive oxygen species (ROS) such as superoxide anion (O2−) and hydroxyl radicals (OH•) [1, 2]. These free radicals are generally neutralized by the antioxidant defense system comprising enzymes including catalase, superoxide dismutase, glutathione peroxidase and low-molecular-weight antioxidants such as β-carotene and tocopherol [3]. Oxidative stress is defined as a situation in which an increased level of ROS generation overwhelms the antioxidative defense capacity, resulting in oxidative damage to lipids, DNA and proteins [1]. Oxidative stress is suspected to contribute to the initiation and progression of many diseases and even to the normal aging process. Since ROS themselves are very reactive and have an extremely short half-life, direct determination of them in tissue or body fluids is generally impracticable [1], the measurement of biomarkers of oxidatively modified cellular constituents in biological samples as “intermediate endpoints or early-outcome predictors” of disease development provides a promising strategy in public health field [4].
F2-isoprostanes, a group of bioactive prostaglandin F2-like compounds generated by oxidatively catalyzed reaction of arachidonic acid, are considered as the reliable marker of lipid peroxidation in vivo [5]. The 8-isoprostane (8-iso-prostaglandin F2α; the major F2-isoprostane), the well known compound belonging to the F2-isoprostane class, is usually quantified in urine instead of plasma for practical use because of the short half life of plasma F2-isoprostane [5]. Elevated levels of plasma and/or urinary 8-isoprostane have been reported in several conditions such as diabetes [6, 7], alcoholic liver disease [8], and cardiovascular disease [9].
It is known that oxidative DNA damage occurs continuously in living cells. 8-Hydroxy-2'-deoxyguanosine (8-OHdG), a product of oxidatively modified DNA base guanine is the most representative product that may reflect oxidative damage induced by ROS [1]. Urinary 8-OHdG was reported in good association with diabetes mellitus [10], chronic renal failure [11], and cancer [12].
These oxidative stress biomarkers were presumed to change in the “pre-clinical stages of disease” among healthy people because of the influence of unhealthy behavior related to the lifestyles, such as smoking and alcohol drinking. However, few studies engaged in assessment of these oxidative stress biomarkers for a population who have no disease [13]. Moreover, no data are available regarding the gender differences in levels of 8-isoprostane and 8-OHdG in people who have different lifestyle habits, but have no disease at the time of health checkup.
The present study aimed to examine whether there is any difference in the levels of biomarkers of oxidative stress (8-isoprostane and 8-OHdG) among Japanese people who have different lifestyle habits in order to find the usefulness of these biomarkers as early predictors of disease risk at population level.
Materials and Methods
Subjects
Data were obtained from a worksite lifestyle intervention study in Japanese city offices in which 847 individuals were participated. For the purpose of this study, we excluded subjects who have any history of cancer, stroke, diabetes, ischaemic heart disease or asthma, and who takes any kind of medicines or supplements such as vitamins. A total of 680 subjects were selected, and then 3 persons were excluded from data analysis because their urinary concentrations of 8-isoprostane and 8-OHdG were under the limit of detection (LOD). Therefore, 677 subjects aged 20–67 years were available for final data analysis. All subjects were instructed to overnight fasting and not consume any beverage and food except plain water before the measurement. The ethics committee of Okayama University approved the study, and all subjects gave informed consent.
Health assessment
Health assessment period was from September to December, 2007. Blood samples were collected after overnight fasting for at least 10 h. Serum and plasma were preserved at 4°C for the measurement of total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), and high-sensitivity C-reactive protein (Hs-CRP). Their body composition was evaluated by using the following respective parameters such as body weight, body mass index (BMI). BMI was calculated by body weight (kg)/height (m)2 and BMI over 25 was diagnosed as obesity according to the criteria for Japanese [14]. Information on lifestyles including cigarette smoking, alcohol consumption, exercise, and dietary habit was obtained by self-reported questionnaires. The amount of alcohol consumption was defined by that one unit was considered to be equivalent to 9–12 g of ethanol [15]. The alcohol intake habit was converted into the number of units per week, and over 20 units of alcohol consumption per week represented excessive drinking. Information on dietary habit was obtained by a food frequency questionnaire [16].
Analysis of oxidative stress biomarkers
Urinary 8-isoprostane and 8-OHdG were determined in spot urine samples stored at −80°C before analysis, because Helmersson and Basu reported that urinary F2-isoprostanes isomers levels in spot urines showed no significant variation from levels measured in 24-h urine samples in the same healthy individuals by radioimmunoassay [17]. Møller and Loft indicated that the correlation coefficient of 8-OHdG measurements by enzyme-linked immunosorbent assay (ELISA) between spot and 24-h urine sample was 0.87 [18]. Urinary 8-isoprostane was analyzed by commercially available competitive enzyme immunoassay (EIA) kit (Cayman Chemical Company, Ann Arbor, MI) [19–21], and the intra-assay and inter-assay coefficients of variation (CV) were 5.4% and 11.0%, respectively. Measurement of 8-OHdG was carried out with an ELISA kit from the Japan Institute for the Control of Aging, Fukuroi, Shizuoka, Japan [22], and the intra-assay and inter-assay CV were 5.2% and 8.1%, respectively. Values for 8-isoprostane and 8-OHdG were normalized by per milligram of creatinine measured in urine (Creatinine test kit, R&D Systems, Minneapolis, MN).
Statistical analysis
Data are presented as mean ± standard error (S.E.) unless stated elsewhere. Log-transformed data of urinary 8-isoprostane, 8-OHdG, serum TG, Hs-CRP, dietary intake of retinol and vitamin C were used in all analyses because of their skewed distributions. Unpaired t-test was used to test gender differences in health assessment data and oxidative stress biomarkers and to compare the concentrations of oxidative stress biomarkers by gender in different age groups. One-way analysis of variance (ANOVA) was used to determined differences in concentrations of oxidative stress biomarkers among groups of BMI, alcohol consumption, and exercise. Pearson’s correlation analysis was performed to examine the relation between oxidative stress biomarkers and the variables. A stepwise multiple regression analysis was performed to test the relationship between oxidative stress biomarkers and the variables that have potential association with the biomarkers. A probability value of p<0.05 was considered to be statistically significant. All analyses were performed using Statistical Package of SPSS 12.0 for Windows.
Results
Subject characteristics
The characteristics of the subjects are presented in Table 1. The proportion of male smokers was greater than that of the females, and the proportion of excessive drinkers (over the 20 units per week) in the males were greater than that of the females. Health assessment data by gender are shown in Table 2. The levels of TG, LDL-c, Hs-CRP in males were significantly higher than those in females. The males smoked more cigarettes a day and had longer smoking period than the females. For dietary antioxidant vitamin intakes, the females consumed more dietary retinol and vitamin C daily than the males.
Table 1.
Characteristics of subjects
| Variable | All |
Male |
Female |
|||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| Total | 677 (100.0) | 293 (100.0) | 384 (100.0) | |||
| Age (year) | <40 | 309 ( 45.6) | 130 ( 44.4) | 179 ( 46.6) | ||
| ≥40 | 368 ( 54.4) | 163 ( 55.6) | 205 ( 53.4) | |||
| BMI (kg/m2) | <18.5 | 67 ( 9.9) | 14 ( 4.8) | 53 ( 13.8) | ||
| 18.5–24.9 | 477 ( 70.5) | 205 ( 70.0) | 272 ( 70.8) | |||
| ≥25.0 | 133 ( 19.6) | 74 ( 25.3) | 59 ( 15.4) | |||
| Smoking | Never | 441 ( 65.1) | 119 ( 40.6) | 322 ( 83.9) | ||
| Smokers | 236 ( 34.9) | 174 ( 59.4) | 62 ( 16.1) | |||
| Alcohol drinking (units/week) | No | 222 ( 32.8) | 62 ( 21.2) | 160 ( 41.7) | ||
| <20 | 428 ( 63.2) | 209 ( 71.3) | 219 ( 57.0) | |||
| ≥20 | 27 ( 4.0) | 22 ( 7.5) | 5 ( 1.3) | |||
| Exercise (times/week) | No | 382 ( 56.4) | 128 ( 43.7) | 254 ( 66.1) | ||
| 1–5 | 261 ( 38.6) | 143 ( 48.8) | 118 ( 30.7) | |||
| 6 or over | 34 ( 5.0) | 22 ( 7.5) | 12 ( 3.1) | |||
BMI indicates body mass index.
1 unit of alcohol = 9–12 g of ethanol
Table 2.
Health parameters by gender
| Variable | All (n = 677) | Male (n = 293) | Female (n = 384) | p value |
|---|---|---|---|---|
| Age (year) | 41.4 ± 0.4 | 41.4 ± 0.6 | 41.4 ± 0.6 | 0.990 |
| Weight (kg) | 60.4 ± 0.5 | 68.5 ± 0.6 | 54.3 ± 0.5 | <0.001** |
| BMI (kg/m2) | 22.7 ± 0.1 | 23.6 ± 0.2 | 21.9 ± 0.2 | <0.001** |
| TC (mg/d1) | 203.0 ± 1.4 | 205.1 ± 2.0 | 201.3 ± 1.9 | 0.163 |
| HDL-c (mg/dl) | 63.4 ± 0.6 | 56.0 ± 0.7 | 69.0 ± 0.7 | <0.001** |
| LDL-c (mg/dl) | 124.2 ± 1.3 | 129.9 ± 1.9 | 119.9 ± 1.7 | <0.001** |
| TG (mg/dl) | 80.7 ± 0.01 | 100.3 ± 0.01 | 68.4 ± 0.01 | <0.001** |
| High-sensitivity CRP (mg/dl) | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.02 ± 0.03 | <0.001** |
| Life style | ||||
| Cigarette number a day | 6.36 ± 0.39 | 12.0 ± 0.7 | 2.1 ± 0.3 | <0.001** |
| Smoking period (year) | 6.73 ± 0.42 | 12.2 ± 0.7 | 2.5 ± 0.4 | <0.001** |
| Smoking (No./day×year) | 130.2 ± 9.4 | 255.1 ± 17.9 | 35.0 ± 5.8 | <0.001** |
| Alcohol drinking (units/week) | 3.74 ± 0.23 | 6.18 ± 0.43 | 1.88 ± 0.19 | <0.001** |
| Dietary antioxidant vitamins | ||||
| Tocopherol (mg/day) | 6.98 ± 0.09 | 6.99 ± 0.15 | 6.97 ± 0.11 | 0.937 |
| β-Carotene (µg/day) | 2793.6 ± 65.4 | 2711.4 ± 98.8 | 2856.3 ± 87.2 | 0.273 |
| Retinol (µg/day) | 421.79 ± 0.01 | 391.56 ± 0.01 | 446.48 ± 0.01 | 0.001** |
| Vitamin C (mg/day) | 58.14 ± 0.01 | 51.72 ± 0.02 | 63.58 ± 0.01 | <0.001** |
Each value represents the mean ± SE. (log-transformed data in TG, Hs-CRP, retinol and vitamin C)
**p<0.01 (by Unpaired t test)
BMI indicates body mass index;TC, total cholesterol; TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein; CRP, C-reactive protein.
Individuals identified as under-reporting their dietary antioxidant vitamins intake were excluded from a food frequency questionnaire.
Oxidative stress biomarkers by age, gender, and lifestyles
The concentrations of oxidative stress biomarkers according to the lifestyles are described in Table 3-1 and Table 3-2. The concentration of urinary 8-isoprostane in males was significantly higher than that in females (p<0.001), and subjects under 40 years had higher levels of urinary 8-isoprostane than those over 40 years. Smoking and alcohol drinking were significantly positively associated with urinary 8-isoprostane. Drinkers who consumed alcohol over 20 units per week showed the highest level of 8-isoprostane. However, BMI and exercise did not show any significant relevance to urinary 8-isoprostane.
Table 3-1.
Characteristics of subjects by urinary 8-isoprostane (ng/mg creatinine)
| Variable | All (n = 677) |
Male (n = 293) |
Female (n = 384) |
|||
|---|---|---|---|---|---|---|
| mean ± SE | p | mean ± SE | p | mean ± SE | p | |
| Total | 0.58 ± 0.01 | — | 0.68 ± 0.02 | — | 0.52 ± 0.02 | <0.001**$ |
| Age (year) | ||||||
| <40 | 0.63 ± 0.02 | 0.044* | 0.74 ± 0.03 | 0.101 | 0.56 ± 0.03 | 0.147 |
| ≥40 | 0.55 ± 0.02 | 0.64 ± 0.02 | 0.49 ± 0.03 | |||
| BMI (kg/m2) | ||||||
| <18.5 | 0.56 ± 0.04 | 0.646 | 0.80 ± 0.09 | 0.712 | 0.51 ± 0.05 | 0.875 |
| 18.5–24.9 | 0.58 ± 0.02 | 0.68 ± 0.02 | 0.52 ± 0.02 | |||
| ≥25.0 | 0.62 ± 0.03 | 0.68 ± 0.03 | 0.55 ± 0.05 | |||
| Smoking | ||||||
| Never | 0.51 ± 0.02 | <0.001** | 0.55 ± 0.03 | <0.001** | 0.50 ± 0.02 | 0.032* |
| Smokers | 0.75 ± 0.02 | 0.79 ± 0.02 | 0.64 ± 0.04 | |||
| Alcohol drinking (units/week) | ||||||
| No | 0.51 ± 0.03 | 0.004** | 0.67 ± 0.03 | 0.958 | 0.46 ± 0.03 | 0.018* |
| <20 | 0.62 ± 0.02 | 0.69 ± 0.02 | 0.56 ± 0.02 | |||
| ≥20 | 0.74 ± 0.09 | 0.69 ± 0.11 | 0.99 ± 0.04 | |||
| Exercise (times/week) | ||||||
| No | 0.57 ± 0.02 | 0.056 | 0.71 ± 0.03 | 0.343 | 0.50 ± 0.02 | 0.057 |
| 1-5 | 0.63 ± 0.02 | 0.68 ± 0.02 | 0.58 ± 0.03 | |||
| 6 or over | 0.47 ± 0.08 | 0.56 ± 0.07 | 0.34 ± 0.19 | |||
Table 3-2.
Characteristics of subjects by urinary 8-OHdG (ng/mg creatinine)
| Variable | All (n = 677) |
Male (n = 293) |
Female (n = 384) |
|||
|---|---|---|---|---|---|---|
| mean ± SE | p | mean ± SE | p | mean ± SE | p | |
| Total | 8.43 ± 0.01 | — | 8.18 ± 0.01 | — | 8.63 ± 0.01 | 0.086$ |
| Age (year) | ||||||
| <40 | 7.91 ± 0.01 | <0.001** | 7.76 ± 0.01 | 0.021* | 8.01 ± 0.01 | 0.003** |
| ≥40 | 8.90 ± 0.01 | 8.53 ± 0.01 | 9.20 ± 0.01 | |||
| BMI (kg/m2) | ||||||
| <18.5 | 8.63 ± 0.02 | 0.135 | 9.68 ± 0.05 | 0.107 | 8.37 ± 0.03 | 0.285 |
| 18.5–24.9 | 8.56 ± 0.01 | 8.21 ± 0.01 | 8.83 ± 0.01 | |||
| ≥25.0 | 7.90 ± 0.02 | 7.83 ± 0.02 | 7.98 ± 0.03 | |||
| Smoking | ||||||
| Never | 8.35 ± 0.01 | 0.377 | 7.98 ± 0.01 | 0.330 | 8.48 ± 0.01 | 0.109 |
| Smokers | 8.59 ± 0.01 | 8.31 ± 0.01 | 9.41 ± 0.02 | |||
| Alcohol drinking (units/week) | ||||||
| No | 8.49 ± 0.01 | 0.778 | 7.91 ± 0.02 | 0.448 | 8.73 ± 0.01 | 0.852 |
| <20 | 8.43 ± 0.01 | 8.31 ± 0.01 | 8.54 ± 0.01 | |||
| ≥20 | 7.99 ± 0.03 | 7.72 ± 0.03 | 9.29 ± 0.09 | |||
| Exercise (times/week) | ||||||
| No | 8.59 ± 0.01 | 0.229 | 8.16 ± 0.01 | 0.912 | 8.82 ± 0.01 | 0.059 |
| 1-5 | 8.15 ± 0.01 | 8.23 ± 0.01 | 8.05 ± 0.02 | |||
| 6 or over | 8.82 ± 0.03 | 7.96 ± 0.03 | 10.63 ± 0.04 | |||
*p<0.05, **p<0.01 (gender, age, and smoking by Unpaired t test; BMI, alcohol drinking, and exercise by ANOVA)
$p value by Unpaired t test to test gender differences
log-transformed data in urinary 8-isoprostane and 8-OHdG
The concentration of urinary 8-OHdG had significant relevance to age in both males and females. The subjects over 40 years had higher concentration of 8-OHdG than those under 40 years. In addition, BMI, smoking, alcohol drinking and exercise did not show significant relevance to urinary 8-OHdG.
Table 4 shows oxidative stress biomarkers by gender and age groups. Between females and males, the concentrations of urinary 8-isoprostane were significantly different in the younger groups of 20–29 and 30–39 years, however, the levels of urinary 8-OHdG were significantly different in the older groups of 50–59 and 60–69 years. Interestingly, the peak urinary 8-OHdG level in males was in the group of 50–59 years, whereas that was in the group of 60–69 years in females.
Table 4.
Oxidative stress biomarkers according to gender and age groups
| Age groups | (n) |
8-isoprostane (ng/mg creatinine) |
8-OHdG (ng/mg creatinine) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (years) | All | Male | Female | Male | Female | p | Male | Female | p |
| 20–29 | (102) | (43) | (59) | 0.78 ± 0.04 | 0.52 ± 0.05 | 0.011* | 7.59 ± 0.02 | 7.97 ± 0.02 | 0.546 |
| 30–39 | (207) | (87) | (120) | 0.72 ± 0.03 | 0.57 ± 0.03 | 0.035* | 7.85 ± 0.02 | 8.04 ± 0.02 | 0.667 |
| 40–49 | (201) | (97) | (104) | 0.66 ± 0.04 | 0.53 ± 0.03 | 0.056 | 8.19 ± 0.01 | 7.71 ± 0.02 | 0.316 |
| 50–59 | (141) | (56) | (85) | 0.61 ± 0.04 | 0.46 ± 0.04 | 0.055 | 9.35 ± 0.02 | 10.84 ± 0.02 | 0.027* |
| 60–69 | (26) | (10) | (16) | 0.68 ± 0.05 | 0.39 ± 0.12 | 0.139 | 7.50 ± 0.05 | 12.20 ± 0.02 | <0.001** |
*p<0.05, **p<0.01
Each value represents the mean ± SE.
p values were analyzed by Unpaired t test between male and female.
Log-transformed data in urinary 8-isoprostane and 8-OHdG
Relationship between oxidative stress biomarkers and health examination variables
The results of Pearson’s correlation analysis between oxidative stress biomarkers and health assessment data are presented in Table 5-1 and Table 5-2. Urinary 8-isoprostane in all subjects was significantly positively correlated with body weight, smoking, and alcohol consumption, and negatively correlated with age, dietary intake of retinol and vitamin C. In males, urinary 8-isoprostane was significantly negatively correlated with age and dietary intakes of β-carotene, retinol and vitamin C, although in females there were no significantly correlations with these parameters. However, urinary 8-isoprostane in females was significantly positively correlated with alcohol consumption.
Table 5-1.
Pearson’s correlation of urinary 8-isoprostane with each parameter
| Variable | All (n = 677) |
Male (n = 293) |
Female (n = 384) |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (year) | −0.098 | 0.011* | −0.115 | 0.049* | −0.090 | 0.079 |
| Weight (kg) | 0.130 | 0.001** | 0.025 | 0.675 | 0.053 | 0.304 |
| BMI (kg/m2) | 0.068 | 0.076 | −0.006 | 0.912 | 0.051 | 0.319 |
| TC (mg/dl) | −0.048 | 0.214 | −0.090 | 0.126 | 0.030 | 0.559 |
| HDL-c (mg/dl) | −0.050 | 0.189 | −0.033 | 0.575 | 0.063 | 0.219 |
| LDL-c (mg/dl) | −0.049 | 0.204 | −0.088 | 0.134 | −0.069 | 0.176 |
| TG (mg/dl) | 0.057 | 0.140 | −0.030 | 0.603 | 0.022 | 0.662 |
| Hs-CRP (mg/dl) | 0.020 | 0.605 | 0.031 | 0.591 | −0.040 | 0.436 |
| urinary 8-OHdG (ng/mg Cre) | −0.066 | 0.086 | −0.099 | 0.090 | −0.036 | 0.485 |
| Life style | ||||||
| Smoking (No./day × year) | 0.242 | <0.001** | 0.267 | <0.001** | 0.133 | 0.009** |
| Alcohol drinking (units/week) | 0.121 | 0.002** | 0.046 | 0.436 | 0.104 | 0.041* |
| Dietary antioxidant vitamins | ||||||
| Tocopherol (mg/day) | −0.005 | 0.906 | −0.056 | 0.341 | 0.032 | 0.529 |
| β-Carotene (µg/day) | −0.051 | 0.189 | −0.149 | 0.011* | 0.023 | 0.658 |
| Retinol (µg/day) | −0.100 | 0.010** | −0.120 | 0.040* | −0.056 | 0.275 |
| Vitamin C (mg/day) | −0.092 | 0.017* | −0.124 | 0.034* | −0.030 | 0.561 |
Table 5-2.
Pearson’s correlation of urinary 8-OHdG with each parameter
| Variable | All (n = 677) |
Male (n = 293) |
Female (n = 384) |
|||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age (year) | 0.205 | <0.001** | 0.129 | 0.028* | 0.248 | <0.001** |
| Weight (kg) | −0.142 | <0.001** | −0.139 | 0.017* | −0.125 | 0.014* |
| BMI (kg/m2) | −0.099 | 0.010** | −0.126 | 0.031* | −0.068 | 0.186 |
| TC (mg/dl) | 0.112 | 0.004** | −0.044 | 0.452 | 0.076 | 0.136 |
| HDL-c (mg/dl) | 0.074 | 0.053 | 0.061 | 0.302 | 0.047 | 0.353 |
| LDL-c (mg/dl) | 0.105 | 0.006** | −0.007 | 0.905 | 0.186 | <0.001** |
| TG (mg/dl) | −0.014 | 0.707 | −0.075 | 0.201 | 0.068 | 0.186 |
| Hs-CRP (mg/dl) | 0.070 | 0.067 | −0.031 | 0.595 | 0.140 | 0.006** |
| Life style | ||||||
| Smoking (No./day × year) | 0.040 | 0.296 | 0.052 | 0.380 | 0.157 | 0.002** |
| Alcohol drinking (units/week) | 0.007 | 0.849 | −0.006 | 0.914 | 0.084 | 0.099 |
| Dietary antioxidant vitamins | ||||||
| Tocopherol (mg/day) | −0.057 | 0.136 | −0.102 | 0.083 | −0.031 | 0.551 |
| β-Carotene (µg/day) | −0.003 | 0.931 | −0.074 | 0.205 | 0.032 | 0.528 |
| Retinol (µg/day) | −0.029 | 0.453 | −0.084 | 0.152 | −0.012 | 0.811 |
| Vitamin C (mg/day) | −0.021 | 0.593 | −0.080 | 0.171 | −0.005 | 0.921 |
*p<0.05, **p<0.01
Log-transformed data in 8-isoprostane, 8-OHdG, TG, Hs-CRP, retinol and vitamin C.
BMI indicates body mass index; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein; TG, triglycerides; Hs-CRP, high-sensitivity C-reactive protein; Cre, creatinine.
Urinary 8-OHdG in all subjects was significantly positively correlated with age, TC and LDL-c, and negatively correlated with body weight and BMI. In both males and females, significant correlations were shown between urinary 8-OHdG and age or body weight. In females, there were significantly positive correlations between urinary 8-OHdG and LDL-c, Hs-CRP, and smoking. However, no significant correlation between urinary 8-OHdG and dietary intakes of antioxidant vitamins were observed.
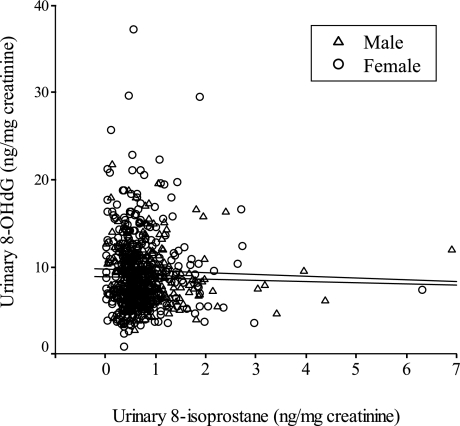
As shown in Fig. 1 and Table 5-1, we did not find any significant association between urinary 8-isoprostane and 8-OHdG (r = −0.066, p = 0.086).
Fig. 1.
Pearson’s correlation between urinary 8-isoprostane and 8-OHdG in Japanese healthy people. No significant correlations were found in both males (r = −0.099, p = 0.090, n = 293), and females (r = −0.036, p = 0.485, n = 384).
Multiple regression analysis for oxidative stress biomarkers
The result of multiple regression analysis for urinary 8-isoprostane is showed in Table 6-1. The model for males demonstrated that age and smoking were important influential factors of urinary 8-isoprostane (12.0%) after adjustment for BMI, body weight, alcohol consumption, and dietary intakes of vitamins. On the other hand, the model for females revealed that smoking and age were important influential factors of 8-isoprostane (2.4%). That is to say, the more they smoked, the higher the concentrations of urinary 8-isoprostane were. Moreover, the level of urinary 8-isoprostane was decreased with aging in both males and females.
Table 6-1.
Multiple regression analysis for urinary 8-isoprostane
| Subjects | Explanatory variable | β | p value | Adjusted R2 |
|---|---|---|---|---|
| Malea) | 0.120 | |||
| Age | −0.252 | <0.001 | ||
| Smoking | 0.363 | <0.001 | ||
| Femaleb) | 0.024 | |||
| Smoking | 0.146 | 0.004 | ||
| Age | −0.108 | 0.035 |
The result of multiple regression analysis for urinary 8-OHdG is described in Table 6-2. The model for males indicated body weight was important influential factor of urinary 8-OHdG (1.6%), and the model for females revealed that age, body weight, smoking, Hs-CRP and LDL-c were important influential factors of 8-OHdG, which explained 12.7% of the variation of urinary 8-OHdG. That is to say, aging was the most influence factor for urinary 8-OHdG. Leanness and smoking also had influence on urinary 8-OHdG. Moreover, the more they smoked, the higher the level of Hs-CRP, and the higher the concentrations of urinary 8-OHdG were.
Table 6-2.
Multiple regression analysis for urinary 8-OHdG
| Subjects | Explanatory variable | β | p value | Adjusted R2 |
|---|---|---|---|---|
| Malec) | 0.016 | |||
| Weight | −0.139 | 0.017 | ||
| Femaled) | 0.127 | |||
| Age | 0.168 | 0.002 | ||
| Weight | −0.250 | <0.001 | ||
| Hs-CRP | 0.209 | <0.001 | ||
| Smoking | 0.102 | 0.036 | ||
| LDL-c | 0.109 | 0.050 |
β indicates standardized partial regression coefficient.
Log-transformed data in 8-isoprostane, 8-OHdG, TG, Hs-CRP, retinol and vitamin C.
Variables included in the model, for a): weight, BMI, TC, HDL-c, LDL-c, TG, Hs-CRP, alcohol, exercise, tocopherol, β-carotene, retinol, vitamin C; for b): weight, BMI, TC, HDL-c, LDL-c, TG, Hs-CRP, alcohol, exercise, tocopherol, β-carotene, retinol, vitamin C; for c): age, BMI, TC, HDL-c, LDL-c, TG, Hs-CRP, smoking, alcohol, exercise, tocopherol, β-carotene, retinol, vitamin C; for d): BMI, TC, HDL-c, TG, alcohol, exercise, tocopherol, β-carotene, retinol, vitamin C.
BMI indicates body mass index; TC, total cholesterol; TG, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein; Hs-CRP, high-sensitivity C-reactive protein.
Discussion
The present study investigated the association of oxidative stress biomarkers 8-isoprostane and 8-OHdG with lifestyles in Japanese people. 8-Isoprostane and 8-OHdG are markers of oxidative damage of DNA and membrane lipid. There were few studies that evaluated the association of these oxidative stress-related biomarkers with lifestyles or lifestyle-related blood biochemical parameters in healthy people.
The present results showed that mean urinary 8-isoprostane levels in males were high in comparison with that in females and there was a negative association between urinary 8-isoprostane and age. Gender difference in urinary 8-isoprostane was supported by several methods such as EIA [23], radioimmunoassay [24], and gas chromatography-mass spectrometry (GC/MS) [25]. The association of urinary 8-isoprostane with age was not fully examined in the published literatures. A few reports showed no agreement on the association of urinary 8-isoprostane with age [26, 27]. It is unknown why urinary 8-isoprostane was higher among people under 40 years, however, negative association of urinary 8-isoprostane with age in our study was coincided with the report by Frisard et al. [27].
Positive association of urinary 8-isoprostane with smoking was demonstrated by many researchers [28–30]. In the long-term smokers, oxidative stress and inflammation are involved in the development of atherosclerosis and chronic obstructive pulmonary disease (COPD) [2]. ROS production in circulating phagocytes, oxidized proteins, lipid peroxidation products such as malonaldehyde and F2-isoprostane, and reduced levels of antioxidants such as vitamin C, β-carotene and glutathione were detected in serum of smokers [31]. Our result that the level of urinary 8-isoprostane was negatively correlated with dietary antioxidant vitamins might support the possible consumption of antioxidant vitamins in serum by smoking. Therefore, a significant increase in 8-isoprostane in smokers and an association of 8-isoprotane with smoking habits were not surprising results.
We did not observe gender difference in mean urinary 8-OHdG levels, however, in the groups of 50–59 and 60–69 years, urinary 8-OHdG in females was significantly high compared with those in males and in female group below 40 years. In published literatures, no consensus has been reached on the association of urinary 8-OHdG with aging [13, 32]. A lower urinary 8-OHdG level in women than in men was reported by Loft et al. [33], whereas several findings suggested that the level of urinary 8-OHdG in females was higher than those in males [34]. In female groups of 50–59 and 60–69 years of the present study, high urinary 8-OHdG levels may be contributed by postmenopausal state. Nakano et al. reported that at menopausal age, urinary 8-OHdG was elevated by increased body iron status [35]. In addition, during menopause, a decreased production of oestrogen may also be involved in the elevation of urinary 8-OHdG. Much attention has been focused on the antioxidant property of oestrogen related to the suppression of cholesterol synthesis in atherosclerotic patients [13]. Therefore, oestrogen levels and body iron status should be kept in mind while discussing the gender difference in urinary 8-OHdG.
In both sexes, urinary 8-OHdG was negatively associated with body weight, which can be supported by reports that showed an association of weight loss with 8-OHdG levels regarding to a cancer risk of low BMI [36] and a negative association of BMI with urinary 8-OHdG [37, 38]. However, increased levels of urinary 8-OHdG in obese children was observed [39]. Therefore, more studies are needed to fully understand the relationship between 8-OHdG and body weight in healthy people.
The relationship between smoking and urinary 8-OHdG is complicated. Several reports showed that an association of urinary 8-OHdG with smoking was related to oxidative damage in lung tissue due to various chemicals in cigarette smoke [33]. However, negative correlations between passive smoking and urinary 8-OHdG have previously been reported [40]. Although the reason for this discrepancy is unknown, the grade of the cigarette filter or differences in the quality of cigarettes among countries might be a factor.
LDL-c has been known as a risk factor for atherosclerosis [41], and Hs-CRP, which involves in inflammation, is also accepted as a risk factor for cardiovascular events in association with atherosclerosis [42], although it is not clear whether plasma Hs-CRP reflects the arterial inflammation because Hs-CRP is generated in the liver. Increased immunoreactivity for 8-OHdG was observed in atherosclerosis plaque of the carotid artery wall [43] and common intima-media thickness is a sign of atherosclerosis [44]. So far, few studies have provided evidence that urinary 8-OHdG increased in atherosclerotic patients. Our results that showed a positive association of urinary 8-OHdG with Hs-CRP and LDL-c may suggest a potential relationship between urinary 8-OHdG and atherosclerosis. Moreover, age, smoking, Hs-CRP, and LDL-c, indicated as influential factors of urinary 8-OHdG by multiple regression analysis, are known as coronary risk factors [41]. Therefore, urinary 8-OHdG may be a useful biomarker for early prediction of atherosclerosis risk.
Our results did not show a significantly correlation between urinary 8-isoprostane and 8-OHdG among all subjects (n = 677). In the previous studies, some researchers reported the elevated levels of 8-isoprostane and 8-OHdG in the diabetics, although these studies have not shown the association between 8-isoprostane and 8-OHdG [45, 46]. A study by England et al. suggested there was no correlation between any products of oxidative DNA damage including 8-hydroxyguanine and F2-isoprostanes levels among healthy volunteers who were non-smokers (n = 53) [47]. Moreover, Harman et al. reported a significant correlation between urinary 8-iso-prostaglandin F2α (IsopF2α) and urinary 8-OHdG or 5-hydroxymethyl-2'-deoxyuridine (5-OHmU) among 234 healthy subjects including 138 smokers, but they did not observe such a correlation among never-smokers [48]. Their results showed that 5-OHmU was more strongly correlated with urinary IsopF2α than 8-OHdG. Whereas in our study, in which the subjects included 35% smokers, there was no correlation between urinary 8-isoprostane and 8-OHdG, and there was also no correlation between these 2 markers among non-smokers (r = −0.069, p = 0.146, n = 441). Therefore, the relation between isoprostane and 8-OHdG might not be so strong. Further investigation is needed to confirm these results.
As for the measurement of 8-isoprostane, several researchers pointed out that mass spectrometric methods have high sensitivity and specificity compared with immunoasays [49]. However, at present, no more suitable methods instead of immunoassays are available for the large scaled cross-sectional study. Since many clinical researchers have employed EIA Kit for the measurement of 8-isoprostane [19–21], we used the most popular commercially available competitive EIA kit of Cayman for the detection of urinary 8-isoprostane. The concentrations of urinary 8-isoprostane by mass spectrometric methods in human controls were reported in the range of 0.16–1.88 (ng/mg creatinine) [50–52]. Therefore, our results, in which the mean urinary 8-isoprostane was 0.58 (ng/mg creatinine), did not stray from the values by mass spectrometric methods.
At present, there are two accepted methods for measuring urinary 8-OHdG: high performance liquid chromatography with electrochemical detection (HPLC-ECD) method [53] and ELISA [22, 54]. Urinary 8-OHdG data by these two methods showed a good correlation (r = 0.833; p<0.0001) [55]. For unknown reasons, 10% of the urine samples showed more than 4 times increase in 8-OHdG value by ELISA in comparison with HPLC-ECD method. There are two commercial kits for quantifying 8-OHdG using a monoclonal antibody N45.1 from the Japan Institute for the Control of Aging (Fukuroi, Shizuoka) and another monoclonal antibody (clone 1F7) from Trevigen (Gaithersburg, MD). Chiou et al. found a good correlation between the two kits with a correlation coefficient of 0.9 [12].
Although the present findings are significant, several limitations of the study should be noted. First, causal relationships could not be determined because this study was a cross-sectional study. Second, some reporting bias may have been introduced because the information on lifestyle habits like smoking, drinking, and dietary intakes was obtained via self-reported questionnaires. Third, since there is no validated method for measurement of isoprostane at population level, present results should be interpreted with caution. Further studies are needed to examine the analytical methods of 8-isoprostane in comparison with the “gold standard” mass spectrometric methods and to confirm the urinary level of 8-isoprostane in healthy subjects with increased sample size.
In conclusion, this study shows that urinary 8-isoprostane is associated with lipid peroxidation related-lifestyles such as smoking, and urinary 8-OHdG is associated with arteriosclerosis related-factors such as higher levels of Hs-CRP and LDL-c, body weight, and smoking. The present findings suggest that 8-isoprostane and 8-OHdG appear to be prospective biomarkers for early prediction of lifestyle related-disease risk at the population level.
Acknowledgments
This work was supported in part by funding from the Junpukai and in part from Health Science Center Foundation. We gratefully acknowledge the technical contribution from K. Takemoto, A. Minoura, S. Hamanishi, U. Akazawa, and A. Ohashi.
Abbreviations
- ROS
reactive oxygen species
- O2−
superoxide anion
- OH·
hydroxyl radicals
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- LOD
limit of detection
- TC
total cholesterol
- TG
triglycerides
- HDL-c
high-density lipoprotein-cholesterol
- LDL-c
low-density lipoprotein-cholesterol
- Hs-CRP
high-sensitivity C-reactive protein
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- EIA
enzyme immunoassay
- CV
coefficients of variation
- SE
standard error
- ANOVA
one-way analysis of variance
- GC/MS
gas chromatography-mass spectrometry
- COPD
chronic obstructive pulmonary disease
- IsopF2α
8-iso-prostaglandin F2α
- 5-OHmU
5-hydroxymethyl-2'-deoxyuridine
- HPLC-ECD
high performance liquid chromatography with electrochemical detection
References
- 1.Halliwell B., Gutteridge J.M.C. In: Oxidative stress, in Free Radicals in Biology and Medicine 3rd ed. Halliwell B., Gutteridge J.M.C., editors. Oxford University Press; New York: 1999. pp. 246–350. [Google Scholar]
- 2.Halliwell B., Gutteridge J.M.C. In: Reactive species can be poisonous, in Free Radicals in Biology and Medicine 4th ed. Halliwell B., Gutteridge J.M.C., editors. Oxford University Press; New York: 2007. pp. 440–487. [Google Scholar]
- 3.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 4.Bonassi S., Au W.W. Biomarkers in molecular epidemiology studies for health risk prediction. Mutat. Res. 2002;511:73–86. doi: 10.1016/s1383-5742(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 5.Morrow J.D., Hill K.E., Burk R.F., Nammour T.M., Badr K.F., Roberts L.J. 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davì G., Ciabattoni G., Consoli A., Mezzetti A., Falco A., Santarone S., Pennese E., Vitacolonna E., Bucciarelli T., Costantini F., Capani F., Patrono C. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 7.Gopaul N.K., Anggård E.E., Mallet A.I., Betteridge D.J., Wolff S.P., Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS. Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 8.Hill D.B., Awad J.A. Increased urinary F2-isoprostane excretion in alcoholic liver disease. Free Radic. Biol. Med. 1999;26:656–660. doi: 10.1016/s0891-5849(98)00250-0. [DOI] [PubMed] [Google Scholar]
- 9.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic. Res. 2000;33:S85–97. [PubMed] [Google Scholar]
- 10.Kanauchi M., Nishioka H., Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron. 2002;91:327–329. doi: 10.1159/000058412. [DOI] [PubMed] [Google Scholar]
- 11.Akagi S., Nagake Y., Kasahara J., Sarai A., Kihara T., Morimoto H., Yano A., Nakao K., Nanba K., Ichikawa H., Makino H. Significance of 8-hydroxy-2'-deoxyguanosine levels in patients with chronic renal failure. Nephrology. (Carlton) 2003;8:192–195. doi: 10.1046/j.1440-1797.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiou C.C., Chang P.Y., Chan E.C., Wu T.L., Tsao K.C., Wu J.T. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin. Chim. Acta. 2003;334:87–94. doi: 10.1016/s0009-8981(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 13.Kimura S., Yamauchi H., Hibino Y., Iwamoto M., Sera K., Ogino K. Evaluation of urinary 8-hydroxydeoxyguanine in healthy Japanese people. Basic Clin. Pharmacol. Toxicol. 2006;98:496–502. doi: 10.1111/j.1742-7843.2006.pto_217.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y., Inoue S., Ikeda Y., Sakata T., Saito Y., Sato Y., Shirai K., Oono M., Miyazaki S., Tokunaga K., Fukagawa K., Nakamura T. A new criteria of obesity. J. Jpn. Soc. Study Obesity. 2000;6:18–28. (in Japanese) [Google Scholar]
- 15.Hiro H., Shima S. Availability of the Alcohol Use Disorders Identification Test (AUDIT) for a complete health examination in Japan. Nihon Arukoru Yakubutsu Igakkai Zasshi. 1996;31:437–450. (in Japanese) [PubMed] [Google Scholar]
- 16.Takahashi K., Yoshimura Y., Kaimoto T., Kunii D., Komatsu T., Yamamoto S. Validation of a Food Frequency Questionnaire based on food groups for estimating individual nutrient intake. Jpn. J. Nutr. 2001;59:221–232. (in Japanese) [Google Scholar]
- 17.Helmersson J., Basu S. F2-isoprostane excretion rate and diurnal variation in human urine. Prostaglandins. Leukot. Essent. Fatty Acids. 1999;61:203–205. doi: 10.1054/plef.1999.0091. [DOI] [PubMed] [Google Scholar]
- 18.Møller P., Loft S. Dietary antioxidants and beneficial effect on oxidatively damaged DNA. Free Radic. Biol. Med. 2006;41:388–415. doi: 10.1016/j.freeradbiomed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Michoulas A., Tong V., Teng X.W., Chang T.K., Abbott F.S., Farrell K. Oxidative stress in children receiving valproic acid. J. Pediatr. 2006;149:692–696. doi: 10.1016/j.jpeds.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Shibata H., Nabika T., Moriyama H., Masuda J., Kobayashi S. Correlation of NO metabolites and 8-iso-prostaglandin F2a with periventricular hyperintensity severity. Arterioscler. Thromb. Vasc. Biol. 2004;24:1659–1663. doi: 10.1161/01.ATV.0000137415.67349.3c. [DOI] [PubMed] [Google Scholar]
- 21.Devries M.C., Hamadeh M.J., Glover A.W., Raha S., Samjoo I.A., Tarnopolsky M.A. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic. Biol. Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Saito S., Yamauchi H., Hasui Y., Kurashige J., Ochi H., Yoshida K. Quantitative determination of urinary 8-hydroxydeoxyguanosine (8-OH-dg) by using ELISA. Res. Commun. Mol. Pathol. Pharmacol. 2000;107:39–44. [PubMed] [Google Scholar]
- 23.Martino F., Loffredo L., Carnevale R., Sanguigni V., Martino E., Catasca E., Zanoni C., Pignatelli P., Violi F. Oxidative stress is associated with arterial dysfunction and enhanced intima-media thickness in children with hypercholesterolemia: the potential role of nicotinamide-adenine dinucleotide phosphate oxidase. Pediatrics. 2008;122:e648–655. doi: 10.1542/peds.2008-0735. [DOI] [PubMed] [Google Scholar]
- 24.Patrignani P., Panara M.R., Tacconelli S., Seta F., Bucciarelli T., Ciabattoni G., Alessandrini P., Mezzetti A., Santini G., Sciulli M.G., Cipollone F., Davì G., Gallina P., Bon G.B., Patrono C. Effects of vitamin E supplementation on F(2)-isoprostane and thromboxane biosynthesis in healthy cigarette smokers. Circulation. 2000;102:539–545. doi: 10.1161/01.cir.102.5.539. [DOI] [PubMed] [Google Scholar]
- 25.Ward N.C., Hodgson J.M., Puddey I.B., Mori T.A., Beilin L.J., Croft K.D. Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic. Biol. Med. 2004;36:226–232. doi: 10.1016/j.freeradbiomed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Cracowski J.L., Baguet J.P., Ormezzano O., Bessard J., Stanke-Labesque F., Bessard G., Mallion J.M. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertension. 2003;41:286–288. doi: 10.1161/01.hyp.0000050963.16405.e6. [DOI] [PubMed] [Google Scholar]
- 27.Frisard M.I., Broussard A., Davies S.S., Roberts L.J. 2nd., Rood J., de Jonge L., Fang X., Jazwinski S.M., Deutsch W.A., Ravussin E., For the Louisiana Healthy Aging Study Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62:752–759. doi: 10.1093/gerona/62.7.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow J.D., Frei B., Longmire A.W., Gaziano J.M., Lynch S.M., Shyr Y., Strauss W.E., Oates J.A., Roberts L.J. 2nd. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 29.Reilly M., Delanty N., Lawson J.A., FitzGerald G.A. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Gutteridge J.M., Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 31.Reilly M., Delanty N., Lawson J.A., FitzGerald G.A. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Hofer T., Karlsson H.L., Möller L. DNA oxidative damage and strand breaks in young healthy individuals: a gender difference and the role of life style factors. Free Radic. Res. 2006;40:707–714. doi: 10.1080/10715760500525807. [DOI] [PubMed] [Google Scholar]
- 33.Loft S., Vistisen K., Ewertz M., Tjønneland A., Overvad K., Poulsen H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 34.Wu L.L., Chiou C.C., Chang P.Y., Wu J.T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Nakano M., Kawanishi Y., Kamohara S., Uchida Y., Shiota M., Inatomi Y., Komori T., Miyazawa K., Gondo K., Yamasawa I. Oxidative DNA damage (8-hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy people. Free Radic. Biol. Med. 2003;35:826–832. doi: 10.1016/s0891-5849(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 36.Mizoue T., Kasai H., Kubo T., Tokunaga S. Leanness, smoking, and enhanced oxidative DNA damage. Cancer Epidemiol. Biomarkers Prev. 2006;15:582–585. doi: 10.1158/1055-9965.EPI-05-0658. [DOI] [PubMed] [Google Scholar]
- 37.Mizoue T., Tokunaga S., Kasai H., Kawai K., Sato M., Kubo T. Body mass index and oxidative DNA damage: a longitudinal study. Cancer Sci. 2007;98:1254–1258. doi: 10.1111/j.1349-7006.2007.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichiba M., Yamada S., Ishii K., Gonda K., Murai R., Shimomura T., Saeki T., Kanbe T., Tanabe Y., Yoshida Y., Tsuchiya H., Hoshikawa Y., Kurimasa A., Kishimoto Y., Kawasaki H., Shiota G. Significance of urinary excretion of 8-hydroxy-2'-deoxyguanosine in healthy subjects and liver disease patients. Hepatogastroenterology. 2007;54:1736–1740. [PubMed] [Google Scholar]
- 39.Sebekova K., Somoza V., Jarcuskova M., Heidland A., Podracka L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int. J. Pediatr. Obes. 2008;21:1–7. doi: 10.1080/17477160802248039. [DOI] [PubMed] [Google Scholar]
- 40.Smith C.J., Fischer T.H., Heavner D.L., Rumple M.A., Bowman D.L., Brown B.G., Morton M.J., Doolittle D.J. Urinary thromboxane, prostacyclin, cortisol, and 8-hydroxy-2'-deoxyguanosine in nonsmokers exposed and not exposed to environmental tobacco smoke. Toxicol. Sci. 2001;59:316–323. doi: 10.1093/toxsci/59.2.316. [DOI] [PubMed] [Google Scholar]
- 41.Homma Y. Predictors of atherosclerosis. J. Atheroscler. Thromb. 2004;11:265–270. doi: 10.5551/jat.11.265. [DOI] [PubMed] [Google Scholar]
- 42.Ridker P.M. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am. J. Cardiol. 2003;92:17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 43.Martinet W., Knaapen M.W., De Meyer G.R., Herman A.G., Kockx M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa T., Sasahara T., Kiritoshi S., Sonoda K., Senokuchi T., Matsuo T., Kukidome D., Wake N., Matsumura T., Miyamura N., Sakakida M., Kishikawa H., Araki E. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi S., Suzuki G., Kusunoki Y., Yamane K., Egusa G., Kohno N. Increasing of oxidative stress from mitochondria in type 2 diabetic patients. Diabetes Metab. Res. Rev. 2004;20:399–404. doi: 10.1002/dmrr.469. [DOI] [PubMed] [Google Scholar]
- 46.Kanabrocki E.L., Murray D., Hermida R.C., Scott G.S., Bremner W.F., Ryan M.D., Ayala D.E., Third J.L., Shirazi P., Nemchausky B.A., Hooper D.C. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol. Int. 2002;19:423–439. doi: 10.1081/cbi-120002914. [DOI] [PubMed] [Google Scholar]
- 47.England T., Beatty E., Rehman A., Nourooz-Zadeh J., Pereira P., O’Reilly J., Wiseman H., Geissler C., Halliwell B. The steady-state levels of oxidative DNA damage and of lipid peroxidation (F2-isoprostanes) are not correlated in healthy human subjects. Free Radic. Res. 2000;32:355–362. doi: 10.1080/10715760000300351. [DOI] [PubMed] [Google Scholar]
- 48.Harman S.M., Liang L., Tsitouras P.D., Gucciardo F., Heward C.B., Reaven P.D., Ping W., Ahmed A., Cutler R.G. Urinary excretion of three nucleic acid oxidation adducts and isoprostane F(2)alpha measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free Radic. Biol. Med. 2003;35:1301–1309. doi: 10.1016/j.freeradbiomed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Morrow J.D. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 50.Davì G., Guagnano M.T., Ciabattoni G., Basili S., Falco A., Marinopiccoli M., Nutini M., Sensi S., Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 51.Barden A., Zilkens R.R., Croft K., Mori T., Burke V., Beilin L.J., Puddey I.B. A reduction in alcohol consumption is associated with reduced plasma F2-isoprostanes and urinary 20-HETE excretion in men. Free Radic. Biol. Med. 2007;42:1730–1735. doi: 10.1016/j.freeradbiomed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Liang Y., Wei P., Duke R.W., Reaven P.D., Harman S.M., Cutler R.G., Heward C.B. Quantification of 8-iso-prostaglandin-F(2alpha) and 2,3-dinor-8-iso-prostaglandin-F(2alpha) in human urine using liquid chromatography-tandem mass spectrometry. Free Radic. Biol. Med. 2003;34:409–418. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 53.Frenkel K., Zhong Z.J., Wei H.C., Karkoszka J., Patel U., Rashid K., Georgescu M., Solomon J.J. Quantitative high-performance liquid chromatography analysis of DNA oxidized in vitro and in vivo. Anal. Biochem. 1991;196:126–136. doi: 10.1016/0003-2697(91)90128-g. [DOI] [PubMed] [Google Scholar]
- 54.Yin B., Whyatt R.M., Perera F.P., Randall M.C., Cooper T.B., Santella R.M. Determination of 8-hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic. Biol. Med. 1995;18:1023–1032. doi: 10.1016/0891-5849(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 55.Shimoi K., Kasai H., Yokota N., Toyokuni S., Kinae N. Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2'-deoxyguanosine in human urine. Cancer Epidemiol. Biomarkers Prev. 2002;11:767–770. [PubMed] [Google Scholar]