Abstract
The overexpression of murine double minute 2 (MDM2) is found in several human tumors, and increased expression of MDM2 inactivates the apoptotic and cell cycle arrest function of p53. Interleukin-16 (IL-16) is a pleiotrophic cytokine and the properties of IL-16 suggest that it involve in the pathophysiological process of chronic inflammatory diseases. In this study, we investigated the expression of MDM2 in intestinal metaplasia and gastric cancer as well as the effect of H. pylori infection and IL-16 on epithelial cell proliferation and MDM2 expression in gastric cells in vitro. The expression of MDM2 on gastric biopsies was studied immunohistochemistry. AGS cells were incubated with a combination of IL-16 and Helicobacter pylori (H. pylori). Gastric epithelial cell proliferation was studied by BrdU uptake and the expressions of MDM2 were studied by ELISA. There was no significant difference on the expression of MDM2 between with and without H. pylori infected chronic gastritis. In H. pylori infected gastric mucosa; the MDM2 expression was higher on intestinal metaplasia and gastric cancer than chronic gastritis. IL-16 administration was increased MDM2 expression and cell proliferation on AGS cells, which was decreased by H. pylori infection. In conclusion, the expression of MDM2 in long-term H. pylori infected gastric mucosa may indicate a risk for carcinogenesis. IL-16 secretion in H. pylori infected mucosa is one of the factors for gastric cancer. The expression of MDM2 on mucosa can be a mediator for gastric cancer.
Keywords: Helicobacter pylori, gastric cancer, intestinal metaplasia, MDM2
Introduction
The development of gastric carcinoma involves a multistep process from chronic gastritis to atrophy, intestinal metaplasia and dysplasia. It has been shown that atrophic gastritis and intestinal metaplasia significantly increase the risk of gastric carcinoma. The infection of Helicobacter pylori (H. pylori) induced chronic gastritis is significantly more pronounced in gastric carcinoma patients [1, 2]. H. pylori colonization alters specific host physiology, such as gastrin secretion and gastric epithelial cell cycle events. H. pylori colonization also increases apoptosis and proliferation in human gastric tissue [3–6]. H. pylori infection also induces infiltrations of neutrophils, macrophages, dentric cells, as well as B and T cells [7]. This infiltration of inflammatory cells is an important factor for mucosal change from chronic gastritis to intestinal metaplasia, and this infiltration is one of the factors for gastric carcinogenesis.
Interleukin-16 (IL-16) was initially described as a T cell chemoattractant. In vivo studies have more fully characterized IL-16 as an immunomodulatory cytokine that contributes to the regulatory process of CD4+ T cell recruitment and activation at sites of inflammation in association with asthma and several autoimmune diseases [8]. Although IL-16 can be produced by a variety of cell types, the production and timing of release of bioactive IL-16 vary depending on the cell origin. CD8+ T cells contain processed bioactive IL-16 whose release can be triggered within 1–4 h by a variety of stimuli, such as histamine and serotonin. In contrast, CD4+ T cells constitutively generate pro-IL-16 protein that is processed and secreted as bioactive IL-16 approximately 12–24 h after stimulation [9]. Lunden et al. reported that the increased numbers of CD4+ T cells in the antrum of H. pylori infected gastric mucosa, but comparable numbers of CD8+ T cells [7]. From these studies, the infiltration of inflammatory cells is important factor for gastric mucosal injury and cell cycle, but the influence of IL-16 expression on gastric mucosa by long-term infection with H. pylori, is not clear.
The p53 transcript factor functions as a tumor suppressor by inducing the expression of genes that inhibit cell growth and promote apoptosis [10]. The mdm2 gene encodes a 491 amino-acid protein that contains a binding domain for the tumor suppressor of p53 [11]. Murine double minute 2 (MDM2) was shown to bind the tumor suppressor p53 and inhibit p53-mediated transsactivation. The overexpression of MDM2 was another mechanism by which the cell could inactivate p53 in the process of transformation. The overexpression of the MDM2 gene is frequent in many human breast carcinomas, soft tissue sarcomas and other cancers, suggesting that MDM2 overexpression may be one of the common causes of oncogenesis [12].
In this study, we investigated the expression of MDM2 on chronic gastritis, intestinal metaplasia and gastric cancer in H. pylori infected mucosa. In vitro study, we also investigated the effect of IL-16 administration and H. pylori infection on gastric epithelial cell proliferation and the expression of MDM2 on AGS cells.
Materials and Methods
Gastric samples
Gastric biopsies were taken through endoscopy, and classified into chronic gastritis, intestinal metaplasia and gastric cancer by histological findings. H. pylori infection was studied by taking 2 samples for culture, 2 samples for rapid urea test and 2 samples for histological findings. H. pylori infection was diagnosed if anyone of the 6 biopsies tested positive.
Expressions of MDM2 protein on gastric biopsies
The biopsies from chronic gastritis without H. pylori infection (n = 20), chronic gastritis with H. pylori infection (n = 20), intestinal metaplasia with H. pylori infection (n = 20) and gastric cancer with H. pylori infection (n = 20), were studied. The paraffin-embedded blocks were cut into 5 µm sections. Then they were mounted on slides and de-waxed in xylene and sequentially dehydrated in 100%, 80% and 70% ethanol. DNA in the specimens was degenerated by 4 M HCl for 30 min, and the intrinsic peroxidase was blocked with 0.3% hydrogen peroxide in methanol. Non-specific binding was blocked with PBS containing 3% skimmed milk (Wako, Tokyo, Japan) for 30 min. To enhance immunostaining, sections were treated with an antigen retrieval solution (10 mM citrate acid monohydrate, pH 6.0, adjusted with 2 N NaOH) and heated 3 times in a microwave oven at high power for 5 min. After the specimens were incubated with anti-MDM2 mouse antibody (Santa Cruz, CA) overnight at 4°C, they were washed with PBS, followed by incubation with biotinylated secondary anti-mouse goat antibody (Dako, Copenhagen, Denmark) diluted 20-fold for 1 h. After washing the tissues with PBS, sections were incubated with avidin-biotin complex (ABC Elite: Vector Laboratoried Inc. Burlingame, CA). Peroxides conjugates were subsequently localized using diaminobenzidine tetrahydrochloride as a chromogen. Sections were counterstained with Haematoxylin. Image Manager Software applied to a light microscope was used for computer analysis of each sample. The degree of immnopositive for MDM2 was evaluated semi-quantitatively. In random fields from representative areas of gastric biopsies, the immunoreactive cells were roughly assessed and expressed as a percentage.
Cell culture and regents
AGS cells were studied because they possess wild-type p53 [13], in contrast to KATO-III cells and other cells that have p53 deletions and/or rearrangement, which may affect the response to signals that regulate cell cycle events [14]. AGS gastric epithelial cells were grown in F-12 Ham medium (Gibco, Life-Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, MP Biomedicals, Solon, OH) and antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO). All cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cell culture experiments were carried out using 100 mm dishes (Nunc, Copenhagen, Denmark). H. pylori (NCTC 11637) were grown on Skirrow agar (Oxoid, Bashingstone, England) plate with 7% horse blood (Nippon Biotest Lab. Tokyo, Japan) in an atmosphere of 10% CO2 at 37°C. Colonies were harvested, 105 and 106 cfu/ml of H. pylori were resuspended in antibiotic-free F-12 without FBS. Experiments were performed as 104 of AGS cells were incubated with F-12 antibiotic-free media with 0.5% bovine albumin (Trace Biosciences, NZ). Using 6-well polypropylene tissue culture plates, with the combination with/without of 105, 106 cfu/ml of H. pylori and 10−10, 10−9 M of recombinant human IL-16 (Pepro Tech, Inc, Rocky hill, NJ) was added into AGS cells. Cells were harvested at 24 h incubation.
ELISA assay
AGS cells were grown on 6 well-plates (Nunc. Copenhagen, Denmark) and maintained in antibiotic-free F-12 medium without serum for 24 h prior to the experiments. The combination of IL-16 and H. pylori were added in serum-free medium, and co-cultured for 24 h. Cells were labeled with Bromodeoxyuridine (BrdU: Sigma, St. Louis, MO) for 1 h, and monolayers were washed 3 times with PBS, lysed with 1 ml of lysis buffer (50 mM NaCl, 25 mM Tris-HCl pH 8.0, 0.5% NP-40, 0.5% sodium deoxycholate and 0.02% sodium azide). Cells were scraped, and transferred to eppendolf tubes. Particulate materials were removed by centrifugation. Ten µg/ml of primary anti-BrdU antibody (Dako, Copenhagen, Denmark) and anti-MDM2 antibody (Santa Cruz, CA) were dissolved with 0.01 M phosphate buffer, 0.15 M NaCl, pH 7.2 into 10 µl, and coated each well of 96 well-plates, and incubated plates overnight at 4°C. After washing with Buffer B (0.01 M Phosphate buffer, 0.50 M NaCl, 0.1% Tween 20, pH 7.2) 3 times, 100 µl of samples (6 times) were pipetted into wells, and incubated for 2 h at room temperature. After the plates were washed with Buffer B, the plates were then blocked for 30 min with 3% skim milk (Wako, Tokyo, Japan) in buffer B. Secondary antibodies (Dako, Copenhagen, Denmark) were incubated for 1 h. Plates were further incubated with 1:10000 of alkaline phosphatase conjugated streptoavidin (Dako, Copenhagen, Denmark) for 1 h. Plates were washed with 1:20 of AmpliQ washing buffer (Dako, Copenhagen, Denmark) and the amplification reagents A and B were added to all wells. Expressions were determined by a spectrophotometer at 492 nm. The assay was followed by ampliQ detection kit (K6245, Dako, Copenhagen, Denmark). All data were expressed as % AGS cells only.
Statistical analysis
The Turney test was used to evaluate difference. A p value of <0.05 was considered significant.
Results
The expression of MDM2 on gastric mucosa
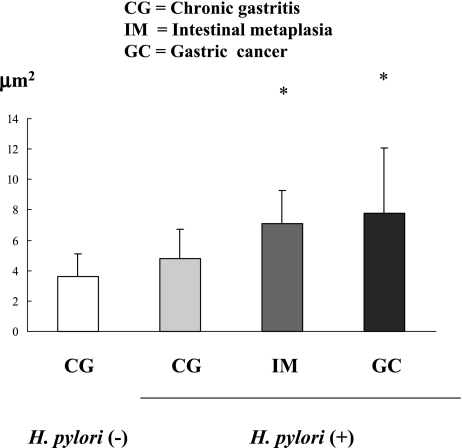
In chronic gastritis, there was not a significant difference on the expression of MDM2 protein between without H. pylori and with the infection (chronic gastritis without H. pylori infection vs. chronic gastritis with H. pylori infection: 3.60 ± 1.52 vs. 4.77 ± 1.92, p = 0.31). But in H. pylori infected gastric mucosa, the expression of MDM2 protein was increased in intestinal metaplasia and gastric cancer (chronic gastritis: 4.77 ± 1.92, intestinal metaplasia: 7.12 ± 2.16 p<0.001, gastric cancer: 7.76 ± 4.31, p<0.000) (Fig. 1, 2).
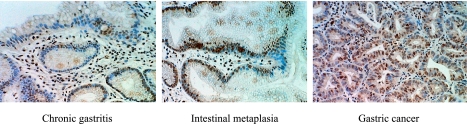
Fig. 1.
The expression of MDM2 on gastric mucosa by ABC staining. A. Chronic gastritis, B. Intestinal metaplasia, C. Gastric cancer MDM2 was stained by DAB as brown area.
Fig. 2.
The Expression of MDM2 on H. pylori infected gastric mucosa in chronic gastritis (CG), intestinal metaplasia (IM) and gastric cancer (GC). There was no significant difference on CG between with and without H. pylori infection. But in H. pylori infected mucosa, the expression of MDM2 increased in IM and GC more than CG. (p<0.05)
The effect of H. pylori infection on gastric epithelial cell proliferation
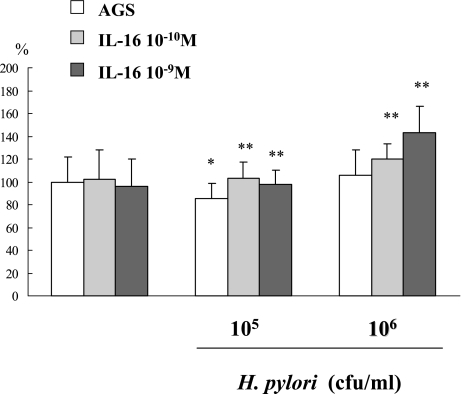
The in vitro study showed that the administration of 10−10, 10−9 M of IL-16 did not influence the BrdU uptake on AGS cells. (AGS only 100 ± 21.97%, 10−10 M IL-16 administration on AGS cells: 95.85 ± 23.90%, 10−9 M IL-16: 102.54 ± 25.77%). But the 105 cfu/ml of H. pylori incubation decreased BrdU uptake on AGS cells (AGS only: 100 ± 21.97% vs. 105 cfu/ml of H. pylori: 85.19 ± 13.57%, p<0.001), 106 cfu/ml of H. pylori infection did not decrease the BrdU uptake on AGS cells (106 cfu/ml of H. pylori: 105.78 ± 22.48%). Further 10−10, and 10−9 M of IL-16 administration increased BrdU uptake on AGS cells which was decreased by 105 cfu/ml of H. pylori infection (105 cfu/ml H. pylori: 85.19 ± 13.57% vs. 105 cfu/ml H.pylori + 10−10 M IL-16: 97.82 ± 15.24%, 105 cfu/ml H.pylori + 10−9 M IL-16: 102.80 ± 14.67%, p<0.001). Also the administration of IL-16 increased BrdU uptake on AGS cells with 106 cfu/ml of H. pylori: (106 cfu/ml H. pylori: 105.78 ± 22.48% vs. 106 cfu/ml H. pylori + 10−10 M IL-16: 143.36 ± 22.65%, 106 cfu/ml H. pylori + 10−9 M IL-16: 120.38 ± 12.62%, p<0.001) (Fig. 3).
Fig. 3.
The effect of IL-16 and H. pylori infection on BrdU uptake in AGS cells. The infection of H. pylori decreased BrdU uptake on AGS cells. But further administration of IL-16 increased BrdU uptake on AGS cells which had been decreased by H. pylori infection.
The expression of MDM2 protein levels on AGS cells
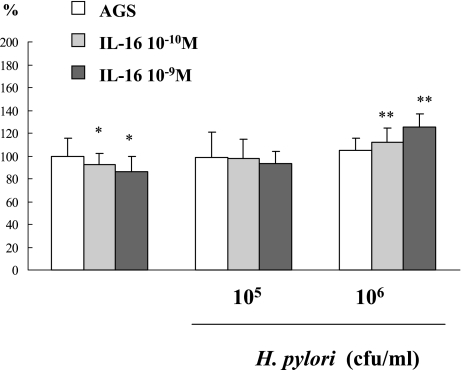
There was not a significant difference on MDM2 protein levels of AGS cells between with and without 106, 105 cfu/ml of H. pylori infection (AGS cells only: 100 ± 15.83%, 105 cfu/ml H. pylori: 98.33 ± 22.68%, 106 cfu/ml H. pylori: 104.65 ± 11.22%). Also administration of 10−10, 10−9 M of IL-16 did not have an influence on MDM2 protein levels on AGS cells (AGS cells only: 100 ± 15.83%, 10−10 M IL-16: 86.13 ± 13.38%, 10−9 M IL-16: 92.28 ± 9.64%). But on 106 cfu/ml of H. pylori incubation, the further administration of 10−10 and 10−9 M of IL-16 increased MDM2 protein levels on AGS cells infected with 106 cfu/ml of H. pylori (106 cfu/ml H. pylori: 104.65 ± 11.22%, 10−10 M IL-16 + H. pylori: 111.75 ± 12.30%, 10−9 M IL-16 + H. pylori: 125.26 ± 11.26%, p<0.05) (Fig. 4).
Fig. 4.
The effect of IL-16 and H. pylori infection on MDM2 expression in AGS cells. The infection of H. pylori decreased MDM2 expression on AGS cells, but further administration of IL-16 increased MDM2 expression on AGS cells which had been decreased by H. pylori infection.
Discussion
Infection with H. pylori is a causality of chronic gastritis, gastroduodenal ulceration, and gastric cancer. Intestinal-type gastric carcinogenesis occurs at a later age and progresses through well-defined histological steps initiated by the transition from normal mucosa to chronic superficial gastritis, which then leads to atrophic gastritis and intestinal metaplasia and finally to dysplasia and adenocarcinona [15]. Correa reported that populations at high risk of developing gastric cancer had a higher prevalence of atrophic gastritis than populations at low risk of developing gastric cancer [16]. Gastric epithelial mucosal integrity is controlled by the balance of cell loss and renewal. But epithelial cell hyperproliferation is a factor for gastric carcinogenesis. H. pylori infection increased epithelial cell proliferation in gastric mucosa, and for stimulated gastric cell proliferation by H. pylori, the infiltration with inflammatory cells is important factor.
The human homologue of the mdm2 gene is frequently overexpressed in many human cancers, suggesting that MDM2 overexpression may be one of the common causes of oncogenesis [12, 17]. Amplification of the mdm2 gene enhances tumorigeneic potential of murine cells. Although human cancer cells frequently overexpress MDM2, and experimental evidences suggest that in normal cells, MDM2 overexpression causes G1 arrest, suggesting that MDM2 overexpression requires genetic alterations. The biochemical activities of MDM2 in cancer cells have been studied intensely because of its association with the tumor suppressor p53 and its implication in human cancer. Cancer cells that overexpress MDM2 may evade these checkpoint regulatory pathways by selecting mutated cells [18].
In this study, we demonstrated that on H. pylori infected mucosa, the expression of MDM2 was increased in intestinal metaplasia and gastric cancer. But in chronic gastritis, there was no significant difference on the MDM2 expression between with and without H. pylori infection. These results indicate that the expression of MDM2 is the trigger for the stimulation and occurrence of gastric cancer, but H. pylori infection only did not influence MDM2 expression. Kodama et al. reported that in chronic gastritis, H. pylori infection increased MDM2 protein levels more than non-infected tissues [19]. The difference between their study and ours may be the method of diagnosis for chronic gastritis. They diagnosed chronic gastritis endoscopically, but in this study, we diagnosed chronic gastritis by histological findings. In our study, there was not a significant difference on MDM2 protein levels between with and without H. pylori infected chronic gastritis, but the expression of MDM2 seemed to increase. Previous studies demonstrated that the expression of MDM2 increased in gastric cancer [20, 21]. From our study, the expression of MDM2 protein was increased in not only gastric cancer, but also in intestinal metaplasia than chronic gastritis. The histological change of intestinal metaplasia is important for the change to gastric cancer, and the stimulation of MDM2 protein may be one of the factors for the transition to gastric cancer.
To know the mechanisms for the different expression of MDM2, we studied the expression of MDM2 and gastric epithelial cell proliferation on the H. pylori infected cells in vitro. In this study, we noticed the effect of IL-16 on H. pylori infected gastric epithelial cells. Keates et al. demonstrated that IL-16 protein levels were increased in Crohn’s diseases, and anti-IL-16 antibody reduced TNBS-induces injury [22]. IL-16 was originally isolated as lymphocyte chemoattractant factor, and IL-16 is a proinflammatory cytokine that induces migration of CD4+ T lymphocytes as well as activated CD4-bearing monocytes/macrophages and eosinophils. IL-16 is produced predominately by activated CD8+ T lymphocytes and to a lesser extent by CD4+ T lymphocytes and eosinophils [23]. In humans, H. pylori infection results in active chronic gastritis with continuous infiltration of neutrophils, B cells and T cells. Lundgren et al. reported increased numbers of CD4+ T cells in the antrum of H. pylori-positive individuals [7]. Stimulated infiltration of these inflammatory cells by H. pylori infection is known as an important factor for gastric carcinogenesis, and the effect of IL-16 may be one of the important factors for chronic inflammatory diseases including chronic gastritis by long-term infection with H. pylori.
In this study, we demonstrated that H. pylori incubation decreased BrdU uptake on AGS cells, but further administration of IL-16 increased cell proliferation, which was decreased by H. pylori infection. On the other hand, Joe et al. demonstrated H. pylori increased tritiated thymidine incorporation on KATO III cells [24]. The reasons of the differentiation between these studies were the difference of cell lines and concentration of H. pylori administration. In most H. pylori infected patients, the bacteria infected in childhood and persisted into adulthood. This long-term infection with H. pylori causes stimulation of inflammatory cells including monocytes, neutrophils. Especially, the IL-8 is one of the important cytokines for H. pylori infected gastric mucosa for the occurrence of gastrointestinal ulceration and gastric cancer. We investigated the expression of IL-16 in gastric mucosa, and there was no significant difference in the expression of IL-16 on gastric mucosa (data not shown). But the administration of IL-16 increased BrdU uptake on AGS cells which was decreased by H. pylori infection. In vivo studies, we reported that H. pylori infection increased cell proliferation on mice gastric mucosa [25, 26]. One of the reasons for the difference between our in vivo and in vitro study was the presence of inflammatory cell infiltration. In gastric mucosa, infiltration of inflammatory cells and expression of cytokines by H. pylori infection, stimulate cell proliferation on gastric mucosa, and the proliferation is increased by not only H. pylori infection directly. The expression of IL-16 is one of the important factors for stimulation of cell growth in H. pylori infected gastric mucosa. One of the reasons for stimulation of cell growth is, stimulation of oxidative stress by H. pylori infection. Inflammation provides several sources of oxidants including leukocytes, cytokines. The infiltration of this reactive oxygen damaged gastric mucosa, and continual damage by long-term infection of H. pylori leads to proteome changes related to cell proliferation and carcinogenesis.
In our study, the expression of MDM2 protein was decreased by H. pylori infection, but further administration of IL-16 increased MDM2 protein which had been decreased by H. pylori. From these results, H. pylori infection only is not a trigger for the expression of MDM2, but infiltration of inflammatory cells or the expressions of cytokines including IL-16 are important factors for the expression of MDM2. Ohmiya et al. reported that MDM2 SNP309, a single-mucleotide polymorphism in the MDM2 promoter can impact tumorgenesis especially in the atrophic gastritis. SNP309 elevated the risk for carcinoma in the stomach after establishment of severe atrophic gastritis [27]. The presence of SNP309 in the MDM2 gene leads to higher expression of MDM2 protein. From their study, SNP309 can impact tumorigenesis especially in atrophic gastritis. And also SNP309 elevated the risk of carcinoma in the stomach after establishment of atrophic gastritis. The expression of MDM2 and SNP309 can be markers for gastric cancer in atrophic gastritis, but severe inflammatory changes in gastric mucosa are necessary for these expressions.
We conclude, the expression of MDM2 on gastric mucosa may be one of the factors for gastric carcinogenesis by long-term H. pylori infection, because MDM2 expresses on gastric mucosa in the early phase of gastric cancer. Also, the infiltration of IL-16 by H. pylori infection is a factor for stimulation of MDM2 expression on H. pylori infected gastric mucosa. The expression of IL-16 on H. pylori infected gastric mucosa can be a trigger for stimulation of epithelial cell proliferation on H. pylori infected gastric mucosa.
References
- 1.Stolte M., Meining A. Helicobacter pylori gastritis of the gastric carcinoma phenotype: Is histology capable of identifying high-risk gastritis? . J. Gastroenterol. 2000;35, Suppl 12:98–101. [PubMed] [Google Scholar]
- 2.Haruma K., Kamada T., Ito M., Kitadai Y., Yoshihara M., Sumii K., Kajiyama G. Helicobacter pylori infection is a major risk factor for gastric carcinoma in young patients. Scand. J. Gastroenterol. 2000;35:255–259. doi: 10.1080/003655200750024100. [DOI] [PubMed] [Google Scholar]
- 3.Moss S.F., Calam J., Agarwal B., Wang S., Holt P.R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannick E.E., Bravo L.E., Zarama G., Realpe J.L., Zhang X.J., Ruiz B., Fontham E.T., Mera R., Miller M.J., Correa P. Inducible nitric oxide synthase, nytrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 5.Peek R.M., Moss S.F., Tham K.T., Pérez-Pérez G.I., Wang S., Miller G.G., Atherton J.C., Holt P.R., Blaser M.J. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 6.Jones N.L., Shannon P.T., Cutz E., Yeger H., Sherman P.M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 1997;151:1675–1703. [PMC free article] [PubMed] [Google Scholar]
- 7.Lundgren A., Trollmo C., Edebo A., Svennerholm A.M., Lundin B.S. Helicobacter pylori-specific CD4+ T cells home to and accumulate in the human Helicobacter pylori-induced gastric mucosa. Infect. Immun. 2005;73:5612–5619. doi: 10.1128/IAI.73.9.5612-5619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruikshank W.W., Kornfeld H., Center D.M. Interleukin-16. J. Leukoc. Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 9.Glass W.G., Sarisky R.T., Vecchio A.M. Not-so-sweet sixteen: the role of IL-16 in infectious and immune-mediated inflammatory disease. J. Interferon Cytokine Res. 2006;26:511–520. doi: 10.1089/jir.2006.26.511. [DOI] [PubMed] [Google Scholar]
- 10.Vousden K.H. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 11.Saji S., Okumura N., Eguch H., Nakashima S., Suzuki A., Toi M., Nozawa Y., Saji S., Hayashi S. MDM2 enhances the finctionfunction of estrogen receptor alpha in human breast cancer cells. Biochem. Abiophys. Res. Comm. 2001;281:259–265. doi: 10.1006/bbrc.2001.4339. [DOI] [PubMed] [Google Scholar]
- 12.Iwakuma T., Lozano G. MDM2, an introduction. Mol. Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 13.Chen G., Sordillo E.M., Ramey W.G., Reidy J., Holt P.R., Krajewski S., Reed J.C., Blaser M.J., Moss S.F. Apotosis in gastric epithelial cells induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem. Biophys. Res. Commun. 1997;239:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 14.Bates S., Vousden K.H. p53 in signaling checkpoint arrest or apoptosis. Curr. Opin. Genet. Dev. 1996;6:12–18. doi: 10.1016/s0959-437x(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 15.Fox J.G., Wang T.C., Rogers A.B., Poutahidis T., Ge Z., Taylor N., Dangler C.A., Israel D.A., Krishna U., Gaus K., Peek R.M. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterol. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 16.Correa P. In: Chronic atrophic gastritis as a precusor of cancer. in Precancerous lesions of the gastrointestinal tract. Sherlock P., Morson B., Barbara L., Veronesi U., editors. Raven Press, New York; New York, USA.: 1983. pp. 145–153. [Google Scholar]
- 17.Meltzer P.S. MDM2 and p53:a question of balance. J. Natl. Cancer Inst. 1994;86:1265–1266. doi: 10.1093/jnci/86.17.1265. [DOI] [PubMed] [Google Scholar]
- 18.Deb S.P. Cell cycle regulatory functions of the human oncoprotein MDM2. Mol. Cancer Res. 2003;1:1009–1016. [PubMed] [Google Scholar]
- 19.Kodama M., Fujioka T., Murakami K., Okimoto T., Sato R., Watanabe K., Nasu M. Eradication of Helicobacter pylori reduced the immunohistochemical detection of p53 and MDM2 in gastric mucosa. J. Gastroenterol. Hepatol. 2005;20:941–946. doi: 10.1111/j.1440-1746.2005.03880.x. [DOI] [PubMed] [Google Scholar]
- 20.Günther T., Schneider-Stock R., Häckel C., Kasper H.U., Pross M., Hackelsberger A., Lippert H., Roessner A. Mdm2 gene amplification in gastric cancer correlation with expression of Mdm2 protein and p53 alterations. Mod. Pathol. 2000;13:621–626. doi: 10.1038/modpathol.3880107. [DOI] [PubMed] [Google Scholar]
- 21.Tanière P., Martel-Planche G., Maurici D., Lombard-Bohas C., Scoazec J., Montesano R., Berger F., Hainaut P. Molecular and clinical differences between adenocarcinomas of the esophagus and of the gastric cardia. Am. J. Pathol. 2001;158:33–40. doi: 10.1016/S0002-9440(10)63941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keates A.C., Castagliuolo I., Cruickshank W.W., Qiu B., Arseneau K.O., Brazer W., Kelly C.P. Interleukin-16 is up-regulated in Crohn’s disease and participates in TNBS colitis in mice. Gastroenterol. 2000;119:972–982. doi: 10.1053/gast.2000.18164. [DOI] [PubMed] [Google Scholar]
- 23.Enarsson K., Lundin B.S., Johnsson E. Brezicka., Quiding-Jarbrink M. CD4+CD25high regulatory T cells reduce T cell transendothelial migration in cancer patients. Eur. J. Immunol. 2007;37:282–291. doi: 10.1002/eji.200636183. [DOI] [PubMed] [Google Scholar]
- 24.Joe T., Kataoka H., Tanida S., Watanabe K., Ohshima T., Sasaki M., Nakao H., Ohhara H., Higashiyama S., Ito M. Helicobacter pylori-stimulated interleukin-8 (IL-8) promotes cell proliferation through transactivation of epidermal growth factor receptor (EGFR) by disintegrin and metalloproteinase (ADAM) activation. Dig. Dis. Sci. 2005;50:2081–2089. doi: 10.1007/s10620-005-3011-0. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Nakajima N., Ito Y., Iwasaki A., Arakawa Y., Kuwayama H. Gastric epithelial cell proliferation and apoptosis in Helcobacter pylori-infected mice. Aliment Pharmacol. Ther. 2000;14, Suppl 1:68–73. doi: 10.1046/j.1365-2036.2000.014s1068.x. [DOI] [PubMed] [Google Scholar]
- 26.Akai Y., Nakajima N., Ito Y., Matsui T., Iwasaki A., Arakawa Y. Green Tea Polyphenols Reduce Gastric Epithelial Cell Proliferation and Apoptosis Stimulated by Helicobacter pylori Infection. J. Clin. Biochem. Nutr. 2007;40:108–115. doi: 10.3164/jcbn.40.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohmiya N., Taguchi A., Mabuchi N., Itoh A., Hirooka Y., Niwa Y., Goto H. MDM2 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. J. Clin. Oncol. 2006;24:4434–4440. doi: 10.1200/JCO.2005.04.1459. [DOI] [PubMed] [Google Scholar]