Abstract
Pore-forming toxins (PFTs) are commonly associated with bacterial pathogenesis. In eukaryotes, however, PFTs operate in the immune system or are deployed for attacking prey (e.g. venoms). This review focuses upon two families of globular protein PFTs: the cholesterol-dependent cytolysins (CDCs) and the membrane attack complex/perforin superfamily (MACPF). CDCs are produced by Gram-positive bacteria and lyse or permeabilize host cells or intracellular organelles during infection. In eukaryotes, MACPF proteins have both lytic and non-lytic roles and function in immunity, invasion and development. The structure and molecular mechanism of several CDCs are relatively well characterized. Pore formation involves oligomerization and assembly of soluble monomers into a ring-shaped pre-pore which undergoes conformational change to insert into membranes, forming a large amphipathic transmembrane β-barrel. In contrast, the structure and mechanism of MACPF proteins has remained obscure. Recent crystallographic studies now reveal that although MACPF and CDCs are extremely divergent at the sequence level, they share a common fold. Together with biochemical studies, these structural data suggest that lytic MACPF proteins use a CDC-like mechanism of membrane disruption, and will help understand the roles these proteins play in immunity and development.
Introduction
Pore-forming toxins (PFTs) are proteins that possess the ability to switch from a water-soluble form to a membrane-inserted pore form. Over 80 different families of PFTs have been characterized (Saier et al., 2006), these include short peptides and large globular proteins. PFTs constitute approximately one-third of all the characterized bacterial toxins (Alouf and Popoff, 2006), and are found across both Gram-positive and Gram-negative bacterial species (Parker and Feil, 2005, Aroian and van der Goot, 2007). Although PFTs have been considered traditionally as virulence factors contributing to bacterial invasion and infection, these molecules also play key roles in eukaryotes. Notable examples of eukaryotic PFTs include C9 and perforin, proteins of the immune system (Voskoboinik et al., 2006), and venoms used by sea anemones to kill prey (Nagai et al., 2002).
A remarkable aspect of PFTs is their ability to exist in both water-soluble and membrane-inserted forms. In the soluble form, the membrane spanning portions of the PFT are commonly stabilized by interaction with the core of the molecule (Tilley and Saibil, 2006). Pore formation thus often involves an extensive conformational change that permits the membrane spanning residues to insert into the lipid bilayer (Tilley and Saibil, 2006).
Pore-forming toxins are broadly classified into two groups depending upon whether membrane spanning is achieved using α-helices (α-PFTs, e.g. colicin) or β-strands (β-PFTs, e.g. perfringolysin O, PFO). While extensive structural and mechanistic information is available for pore formation by various β-PFTs, the structure of the membrane-inserted form of an α-PFT remains to be determined.
In addition to conformational mobility, another key feature of many PFTs is their ability to self-assemble into doughnut-shaped oligomers (Tilley and Saibil, 2006). Together, oligomerization and conformational change can permit formation of pores that permeabilize membranes and aid processes such as bacterial pathogenesis, for example, through the transport of toxic proteinaceous agents (Tweten, 2005; Aroian and van der Goot, 2007). Depending on the toxin, the pore diameter may vary from 1 to 50 nm (Parker and Feil, 2005; Aroian and van der Goot, 2007).
In this review we focus on the species distribution, structure and mechanism of one of the largest families of β-PFTs, the membrane attack complex/perforin/cholesterol-dependent cytolysin (MACPF/CDC) superfamily.
Cholesterol-dependent cytolysins in bacterial pathogenesis
Pathogenic Gram-positive bacteria such as Clostridium perfringens, Bacillus anthracis and Streptococcus pneumoniae produce CDCs to aid tissue or cell invasion (Table 1). The majority of characterized CDCs are secreted toxins. Exceptions include pneumolysin (PLY), which lacks an N-terminal secretion signal. In many Streptococcus pneumoniae strains, it is hypothesized that PLY is released via autolysin mediated bacterial autolysis. However, studies on the WU2 strain reveal release of PLY in the absence of autolysis, suggesting an unconventional secretion mechanism is responsible for toxin release in this strain (Balachandran et al., 2001).
Table 1.
List of current identified members of the CDC subclass.
| Species | Toxin | Toxin abbreviation | Accession code/PDB ID | Functions in disease |
|---|---|---|---|---|
| Arcanobacterium pyogenes | Pyolysin | PLO | AAC45754 | Cytotoxic for murine peritoneal macrophages and J774 cells (dose dependant) (Jost et al., 1999) |
| Bacillus anthracis | Anthrolysin O | ALO | EDT69040 | Kills human neutrophils, monocytes and macrophages (Mosser and Rest, 2006) |
| Bacillus cereus | Cereolysin | CLO | O45105 | Uncharacterized |
| Bacillus sphaericus | Sphaericolysin | BAF96950 | Damage to the ganglia of German cockroaches (Blatetela germanica) (Nishiwaki et al., 2007) | |
| Bacillus thuringiensis | Thuringiolysin | TLO | BT9727_3096* | Uncharacterized |
| Brevibacillus laterosporus | Laterosporolysin | LSL | – | Uncharacterized |
| Clostridium bifermentans | Bifermentolysin | BFL | – | Uncharacterized |
| Clostridium botulinum | Botulinolysin | BLY | – | Evidence that coronary vasconstriction is triggered by BLY causing cardiac dysfunction, leading to systemic hypertension and death in rat model (Sugimoto et al., 1997) |
| Clostridium chauvoei | Chauveolysin | CVL | – | Uncharacterized |
| Clostridium histolyticum | Histolyticolysin O | HTL | – | Uncharacterized |
| Clostridium novyi A (oedematiens) | Novyilysin | NVL | – | Uncharacterized |
| Clostridium perfringens | Perfringolysin O | PFO | P0C2E9/1PFO, 1M3I, 1M3J | Promotes dysfunctional human PMN/endothelial cell adhesion contacts and vascular leukostasis. Inhibits human PMN chemostasis and primes leukocytes for increased respiratory burst (Ellemor et al., 1999) |
| Clostridium septicum | Septicolysin O | SPL | – | Uncharacterized |
| Clostridium sordellii | Sordellilysin | SDL | – | Suggestion that severity of C. sordellii-associated disease may be related to the expression of SDL or lethal toxin (TcsL) (Voth et al., 2006) |
| Clostridium tetani | Tetanolysin | TLY | NP_782466 | Observed lysis of rabbit lysosomes in a suspension of the large granule fraction of rabbit liver (Cox et al., 1974) |
| Gardnerella vaginalis | Vaginolysin | VLY | EU522486–EU533488 | Species-specific lysis dependant on CD59. Activates p38 mitogen-activated protein kinase pathway, induces IL-8 production by human epithelial cells (Cox et al., 1974). |
| Listeria ivanovii | Ivanolysin | ILO | P31831 | Intracellular release of ILO – Does not induce IFN-γ (Kimoto et al., 2003) |
| Listeria monocytogenes | Listeriolysin O | LLO | P13128 | Intracellular release of LLO: Suppression of phagocytosis by murine macrophages. Induces the expression of IL-1α, IL-12, IFN-γ, IL-8, macrophage chemotaxis protein 1, adhesion molecules on the surface of human epithelial cells. Activates NF-κB (Kayal et al., 1999) |
| Listeria seeligeri | Seeligeriolysin O | LSO | CAA42996 | Strongly induces IL-12 but not IFN-β induces IFN-γ in naïve spleen cells. Requires Toll-like receptor 2 and 4 for signalling (Ito et al., 2005) |
| Paenibacillus alvei | Alveolysin | ALV | P23564 | Induced IL-8 expression in human polymorphonuclear leukocyte, lymphocyte, monocyte and basophil cell populations (Konig et al., 1994) |
| Streptococcus canis | Streptolysin O | SLO | Q53957 | Uncharacterized (DeWinter et al., 1999) |
| Streptococcus dysgalactiae (ssp. equisimilis) | Streptolysin O | SLO | Q54114 | Uncharacterized (Gerlach et al., 1993) |
| Streptococcus intermedius | Intermedilysin | ILY | BAA89790/1S3R | Specific for human CD59 (Giddings et al., 2004) Essential for S. intermedius infection of HepG2 cells (Sukeno et al., 2005) |
| Streptococcus pneumoniae | Pneumolysin | PLY | P0C2J9/2BK1, 2BK2 (28 Å resolution cryo EM) | Directly activates the complement cascade, induces IL-1β and TNFα expression from human monocytes. Inhibition of immunoglobulin production and proliferative response from human lymphocytes, as well as of the bactericidal activity of PMNs and monocytes (Alouf and Popoff, 2006) |
| Streptococcus pyogenes | Streptolysin O | SLO | P0C0I3 | Inhibits chemotaxis and mobility of human PMNs. Induces expression of IL-1β, IL-6 and IL-8 and release of prostaglandin E2 from human keratinocytes. Induces expression of IL-1β and TNFα expression from human monocytes (Ruiz et al., 1998) |
| Streptococcus suis | Suilysin | SLY | CAC94852 | Able to lyse epithelial cells. Possible mechanism for entry into bloodstream and brain microvascular endothelial cells leading to increased blood–brain barrier permeability and phagocytosis (Vanier et al., 2004) |
Most CDC-releasing bacteria identified to date are extracellular pathogens (of either humans or insects) that release their respective CDCs in the extracellular environment. However, at least two pathogens release their CDC (listerolysin O, LLO) inside host phagocytic cells (Listeria monocytogenes and Listeria ivanovii).
Cholesterol-dependent cytolysins perform a multitude of functions in bacterial infection. For example, these toxins disrupt plasma membranes causing cell death by necrosis (PFO) and facilitating bacterial invasion, or disrupt endosomal or phagosomal membranes to release bacteria into the interior of the cell (LLO). In addition to their pore-forming properties, many CDCs possess pro-inflammatory properties that enhance tissue damage at the site of infection (Cockeran et al., 2003). Further, certain CDCs possess the ability to kill cells through alternative mechanisms; for example, in bacterial meningitis caused by Streptococcus pneumoniae, PLY has recently been shown to form pores in the mitochrondria of neurons, activating cell death pathways (Braun et al., 2007). Table 1 summarizes the role of the known CDCs identified to date.
The structure and membrane insertion mechanism of CDCs
The first structure of a CDC family member, PFO (Rossjohn et al., 1997), revealed a flat molecule comprising a box-shaped N-terminal domain [originally annotated as three non-contiguous domains (I–III)] connected to a C-terminal Ig domain (domain 4) (Fig. 1A). An unusual feature of the N-terminal CDC domain is a central four-stranded β-sheet containing a 90° bend at its centre. Two clusters of α-helices [termed transmembrane helices (TMH) 1 and 2] are located at the base of this sheet and are suggested to be responsible for membrane penetration (Shepard et al., 1998; Shatursky et al., 1999; Fig. 1A). The first cluster of α-helices, TMH-1, is loosely sandwiched between the central β-sheet and the stalk-like β-sheet that links the N-terminal CDC domain to the C-terminal Ig domain, while the second cluster of α-helices (TMH-2) is more solvent exposed. Extensive biophysical and cryo-electron microscopy (cryo-EM) studies suggest that both clusters of α-helices unwind and adopt an amphipathic β-strand conformation in the membrane (Fig. 1B; Shepard et al., 1998; Shatursky et al., 1999; Tilley et al., 2005).
Fig. 1.
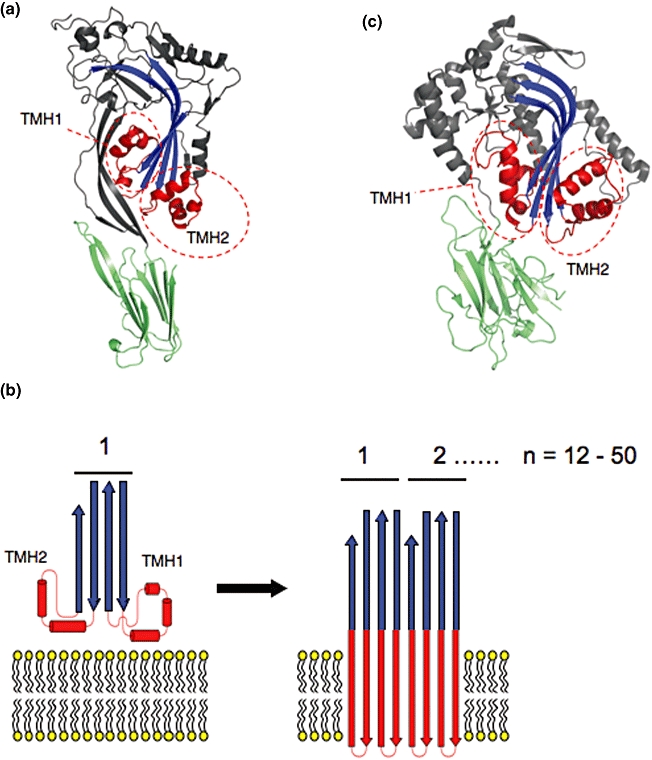
A. The structure of perfringolysin O [PDB identifier: 1PFO (Rossjohn et al., 1997)]. The central β-sheet that contains a 90° bend is in blue. The two transmembrane regions TMH1 and TMH2 are in red and are labelled. The C-terminal Ig domain is in pale green. B. Schematic showing the molecular mechanism of CDC membrane insertion. The two clusters of α-helices (red cylinders) unwind and insert into the membrane as β-sheets. C. X-ray crystal structure of Plu-MACPF [PDB identifier 2QP2 (Rosado et al., 2007)]. Colouring is as for Fig. 1A, with the central β-sheet in blue and the two clusters of α-helices corresponding to TMH1 and TMH2 labelled. The location of the binding site for CD59 on C8α and C9 is at the TMH2 region.
Initial interaction with the membrane, oligomerization and conformational change
The current model for CDC mechanism comprises an initial recognition event at the cell membrane via the C-terminal Ig domain. This is followed by lateral diffusion and association with other CDC molecules to form a pre-pore oligomer (Soltani et al., 2007). Cryo-EM and biophysical studies suggest that oligomerization occurs via edge strand hydrogen bonding between the central four-stranded β-sheet of each monomer, i.e. the fourth β-strand of one monomer interacts with the first β-strand of the next (Ramachandran et al., 2005; Tilley et al., 2005; Tweten, 2005). Following formation of the pre-pore, a triggering event transmitted from the Ig domain to the N-terminal domain permits conformational change and insertion of the transmembrane regions into the membrane. The result of this concerted activity is a giant β-barrel lined pore spanning the membrane. The precise nature of the conformational change remains unclear; however, cryo-EM studies reveal that the N-terminal domain undergoes a significant ‘collapse’ that brings TMH-1 and TMH-2 in close proximity to the membrane (Tilley et al., 2005).
Initially, it was proposed that cholesterol functions as a general receptor for CDCs (hence the family name). For PFO, biochemical data reveal that cholesterol may function as both a receptor and a trigger for the transition from the pre-pore to the pore conversion. However, recent studies using cholesterol-depleted human red blood cells have revealed that initial membrane interaction and pre-pore assembly of streptolysin O (SLO) and intermedilysin (ILY) does not require the presence of cholesterol (Giddings et al., 2003; Soltani et al., 2007). For ILY, it has been shown that CD59 functions as a glycoprotein receptor that recruits the CDC to the membrane surface and that cholesterol is instead required to trigger the conformational transition of the pre-pore to the pore form (Giddings et al., 2004). Further experiments on ILY demonstrated that a highly conserved region of the Ig domain, the undecapeptide, is not responsible for binding cholesterol-rich membranes. Instead, it was demonstrated that loops adjacent to the undecapeptide loop in ILY, called L1–L3, were responsible for membrane interactions (Soltani et al., 2007).
Structural studies on CDCs have been hampered by their conformational mobility as well as the tendency of these molecules to form oligomers of varying size. Thus much remains to be understood in regards to the mechanism of pore formation by CDCs. Most importantly, the molecular details of the interaction between CDCs and membrane receptors and a precise picture (at atomic resolution) of the conformational change in the family need to be resolved.
Membrane attack complex/perforin-like proteins
In comparison to bacteria, PFTs from eukaryotes are relatively understudied (Smyth and Trapani, 1998; Trapani, 1998). One of the largest mammalian family of PFTs is the MACPF superfamily; so named because of a domain common to proteins of the mammalian membrane attack complex (MAC) and perforin (PF) (Tschopp et al., 1986). These molecules perform crucial roles in the defence against bacterial and viral infection as well as in tumour surveillance (Smyth et al., 2000; Voskoboinik et al., 2006).
Initially identified in the late 19th Century as a lytic factor in blood, the terminal components of complement (C5b, C6, C7, C8α-β-γ and C9) assemble on the surface of Gram-negative bacteria and protozoan pathogens such as Leishmania major to form a large multi-protein complex called the MAC. Complement-associated bacterial cell lysis is optimally achieved in the presence of lysozyme, which hydrolyzes components of the bacterial cell wall (Martinez and Carroll, 1980). However, even in the absence of lysozyme complement pore formation can result in cell death through alternative non-lytic pathways (Martinez and Carroll, 1980). Consistent with these data, deficiency of MAC components results in an increased susceptibility to infection by Gram-negative bacteria such as Neisseria gonorrhoeae.
C6, C7, C8α, C8β and C9 all contain a common region called the MACPF domain. Upon binding of C7 to the C5bC6 complex, forming the C5b-7 complex, there is interaction with the surface of the bacteria via C7 through an as yet uncharacterized mechanism. Recruitment of the C8 complex (which comprises the MACPF components C8α and C8β together with the lipocalin C8γ) is followed by membrane insertion of the C8α component (Muller-Eberhard, 1986). Finally, the C5b-8 complex recruits and permits pore assembly by the final component C9 (Bhakdi and Tranum-Jensen, 1978). EM studies also revealed that C9 undergoes conformational change from a ellipsoid to an elongated torus (DiScipio and Berlin, 1999).
Biochemical studies on C8α and C9 have revealed that the MACPF domain is required for membrane insertion and pore formation respectively (Steckel et al., 1983). Importantly, all host cells express the MAC inhibitor CD59 that inhibits the membrane inserting activity of C8α and C9, preventing inadvertent lysis of host cells. Deficiency of CD59 can result in an overactivity of complement, uncontrolled host cell lysis and development of paroxysmal nocturnal haemoglobinuria (Walport, 2001).
In 1984, perforin was characterized as a lytic PFT produced by natural killer cells and cytotoxic T lymphocytes (Henkart et al., 1984; Podack and Konigsberg, 1984). Perforin is stored in cytoplasmic secretory granules and is released on contact to kill virus-infected or transformed cells (Voskoboinik et al., 2006). Perforin itself is able to lyse and kill cells by necrosis; however, it also permits delivery of pro-apoptopic proteases (granzymes) into the target cell (Shiver et al., 1992, Bolitho et al., 2007). Two competing models for granyzme delivery by perforin have been proposed: diffusion of the granzyme through a plasma membrane perforin channel, versus coendocytosis of perforin and granzyme with subsequent disruption of an endosome membrane by perforin to release the granzyme. However, the precise molecular mechanism remains to be understood.
Congenital perforin deficiency results in the commonly fatal immunoregulatory disease of infants, familial haemophagocytic lymphohistiocytosis (FHL) (Voskoboinik et al., 2004; 2005). Affected individuals suffer from massive accumulation of CD8+ T cells in organs and a cytokine-storm mediated immunoproliferative disorder that commonly results in severe tissue damage. Currently, the only effective treatment for severe recurrent FHL is a bone marrow transplant (Jabado et al., 1997). Overactivity of perforin also results in disease; for example, perforin is critical for the destruction of insulin-producing pancreatic β-islet cells in the NOD mouse model of Type I diabetes (Kagi et al., 1997).
MACPF proteins are eukaryote CDCs: implications for function and dysfunction
In the absence of structural information, and based upon bioinformatic studies, it was originally proposed that C9 and perforin insert into membranes using two predicted amphipathic α-helices that map to the most conserved region of the MACPF domain (residues 292–333 of the human C9 sequence; Peitsch et al., 1990). Therefore, it was postulated that C9 and perforin belonged to the α-PFT class of toxins. Several recently determined structures challenge this hypothesis. The X-ray crystal structure of Plu-MACPF (a MACPF domain containing protein from Photorhabdus luminescens; Rosado et al., 2007; Fig. 1C), the MACPF domain of C8α (Hadders et al., 2007) and the MACPF domain of C8α in complex with C8γ (Slade et al., 2008) reveal that the MACPF domain is homologous to the N-terminal portion (domains I–III) of CDCs (Fig. 1C). In particular, conservation of a complex core fold, including the membrane spanning clusters of helices (TMH1-2 or CH1-2) suggests strongly that MACPF proteins and CDCs form pores using a analogous mechanism (Fig. 2). Interestingly, it appears that highly conserved MACPF signature sequences (Ponting, 1999) map to regions that may be crucial for controlling conformational change and unfurling of the membrane spanning regions for the MACPF subclass of the MACPF/CDC family of proteins.
Fig. 2.
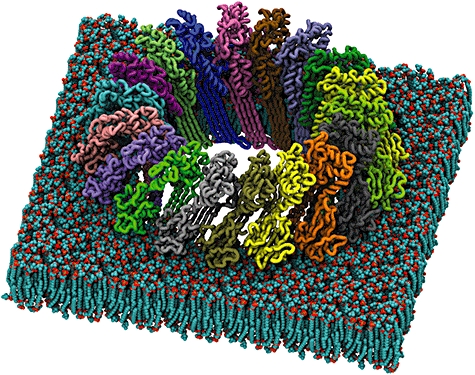
Model of the pore form of a MACPF proteins in a lipid bilayer (using the Plu-MACPF structure as a template, PDB ID: 2QP2).
The structural data provide a framework upon which we can start to understand disease-linked variants of perforin (Rosado et al., 2007). In a previous study, the rather puzzling observation was made that many disease-linked variants of perforin map to two regions that are most poorly conserved across the superfamily (Voskoboinik et al., 2006). The structural data reveal that these two regions comprise the putative perforin transmembrane sequences. These data present an exception to the rule that functional regions of proteins generally contain the highest degree of sequence conservation. A possible reason for this apparent paradox is that membrane spanning regions depend on physiochemical properties (hydrophobicity), rather than absolute sequence. Furthermore, it is important to note that not all MACPF proteins insert into membranes (e.g. C6) and thus do not require amphipathic sequences in TMH1-2/CH1-2. Indeed, many members of the family may play important non-lytic functions that are discussed at the end of this review.
The structural data also provide an explanation for the control of C8α and C9 by the host cell factor CD59. The CD59 binding site on both proteins maps to the second putative transmembrane sequence TMH-2/CH-2 (Fig. 1C). Thus, it is suggested that CD59 controls MAC function by directly interfering with the assembly of the transmembrane pore (see Fig. 1C).
The role of C-terminal domains in MACPF function
All CDCs characterized to date contain a C-terminal Ig domain that is critical for interacting with lipid or protein cofactors. Further, this domain is of key importance for triggering conformational change in the N-terminal lytic CDC domain [domains I–III (Polekhina et al., 2005)]. Bioinformatic studies reveal that MACPF proteins are also found in concert with one or more C-terminal domains. However, rather than the common Ig domain found in CDCs, a wider variety of C-terminal domain folds are represented in the MACPF branch of the family. For example, perforin contains an EGF-like domain followed by a C2 domain; C8α contains an EGF-like followed by a thrombospondin type 1 domain; mammalian-derived C9 contains an EGF-like domain and Plu-MACPF contains a β-prism domain (Rosado et al., 2007). The structure of Plu-MACPF (which appears non-lytic, but binds to membranes) reveals that the β-prism domain is similarly located to the Ig domain of the CDCs. Studies on perforin have revealed that the C2 domain is responsible for initial interaction with the membrane (Voskoboinik et al., 2006). Thus for perforin, we propose that C-terminal domain may perform a similar role to the Ig domains of CDCs by interacting with lipids or protein receptors and triggering conformational change and membrane insertion (Fig. 2). However, it is clear that the C-terminal domains of other MACPF proteins perform roles distinct from membrane interaction. For example, the C-terminal domains of C8α are not essential for formation of a functional MAC (Scibek et al., 2002; Slade et al., 2006).
The broader MACPF proteins family in defence and attack
The development of powerful informatic tools such as PSI-BLAST now permits the identification of a large number (> 500) of MACPF proteins. Predictably, many of these proteins appear to be involved in immune defence or attack (Table 2). Notably, in plants the MACPF protein constitutively activated cell death-1 (CAD-1) is important for defence against bacterial infection. Interestingly, CAD-1 knockouts result in an overactivity of the plant immune response (Morita-Yamamuro et al., 2005).
Table 2.
List of current identified members of the MACPF subclass.
| MACPF subclasses | Common names | Description of expression pattern and function |
|---|---|---|
| The following subclasses contain proteins with demonstrated lytic activity: | ||
| C9-like | C6, C7, C8α, C8β, C9 | Vertebrate membrane attack complex (MAC) |
| Roles within the MAC: | ||
| C9 – membrane insertion, pore formation and lytic activity | ||
| C8α, C7 – role in anchoring the MAC to the target membrane | ||
| C6 and C8β– no detected ability to insert into membranes (Muller-Eberhard, 1986) | ||
| Perforin-like | perforin | Released from granules within natural killer and cytotoxic T lymphocytes to lyse targeted cells in the immune response (Voskoboinik et al., 2006). |
| Sea anemone toxins | PsTX-60A | Haemolytic toxin released from the sea anenome nemocysts to kill prey. |
| PsTX-60B | Species include Phyllodiscus semoni and Actineria villosa (Oshiro et al., 2004). | |
| AvTX-60A | ||
| The following proteins have not been demonstrated to have lytic activity: | ||
| Apextrin | apextrin | Located in secretory vesicles in sea-urchin eggs, Apextrin becomes localized to the apical extracellular matrix upon fertilization of the cells in the blastula (Haag et al., 1999) |
| Upregulation upon bacterial infection in amphioxus (Huang et al., 2007) | ||
| Astrotactin | Astrotactin-1 Astrotactin-2 | Astrotactin-1 required for neuronal cells migration along glial fibres, possibly neuronal adhesion molecules (Zheng et al., 1996) |
| Chlamydia proteins | – | Hypothetical proteins of Chlamydia trachomatis, Chlamydophila pneumoniae and C. muridium (Ponting, 1999) |
| Cyano-bacteria | – | Hypothetical protein of Trichodesmium erythraeum (cyanobacteria) |
| DBCCR/BRINP | DBCCR1 (BRINP1), DBCCR1-like protein 1 (BRINP3), DBCCR1-like protein 2 (BRINP2) | DBCCR-1, deleted in bladder cancer candidate region-1 gene, tumour suppressor gene commonly deleted in bladder cancer. Overexpression of DBCCR-1 suppresses tumour cell growth. Involved in neuronal development (Motomiya et al., 2007) Also referred to as FAM5 family of proteins. |
| EPCS50 | EPCS50 | EPCS50 expressed in the trophoblast upon implantation of the murine embryo (Hemberger et al., 2000) |
| Fungal proteins | SpoC1-C1C | Expressed during maturation of the conidia (specialized organ for asexual reproduction) of Emericella nidulans, mRNA levels drop upon germination (Stephens et al., 1999) |
| Malarial proteins | SPECT2 and MAOP | SPECT2 and MAOP are essential for parasite invasion into the human liver (Ishino et al., 2004) and the mosquito host (Kadota et al., 2004) respectively |
| MPS | MPS, MPG | Macrophage Proliferation-specific Gene-1 detected in differentiated macrophages (Spilsbury et al., 1995) |
| Plant proteins | CAD1 | Arabidopsis thaliana CAD1 involved in plant immune response (Morita-Yamamuro et al., 2005) |
| Plu-MACPF | Plu-MACPF | Hypothetical protein from the bacteria, Photorhabdus luminescens (Rosado et al., 2007) |
| Tsl | Tsl | Torso-like protein (Tsl) from Drosophila melanogaster, is hypothesized to activate the receptor, Torso, via the protein Trunk (Stevens et al., 1990) |
Several organisms use MACPF proteins as weapons of attack. For example, sea anemone venom contains haemolytic MACPF proteins and the malaria parasite uses two MACPF proteins to invade the mosquito midgut and to breach the liver sinusoidal membrane (Ishino et al., 2004; Kadota et al., 2004).
A variety of pathogenic bacteria produce MACPF proteins; indeed Plu-MACPF from the insect pathogenic enterobacteria P. luminescens proved useful for structural studies. It remains to be understood whether these bacterial MACPF domain-containing proteins have pore-forming functions in pathogenesis.
MACPF proteins in development
Interestingly, several MACPF proteins have been identified that may play roles in development rather than immune defence or attack. In most of these cases it is not yet clear whether the MACPF protein has a lytic or non-lytic function.
In insects, Torso-like protein (Tsl) is maternally secreted at the anterior and posterior poles of the oocyte. Through an as yet uncharacterized mechanism, Tsl secretion results in Trunk-mediated activation of the Torso receptor tyrosine kinase. Torso-signalling results in development of anterior and posterior structures. Accordingly, Tsl knockouts are embryonically lethal (Stevens et al., 1990).
The sea urchin protein, apextrin, was initially identified in secretory vesicles within eggs (Haag et al., 1999). Closely related molecules have been subsequently been identified in several other sea urchin species, Cnidaria [hydrazoans (Hydra magnipapillata) and corals (Acropora millepora)] (Miller et al., 2007). Haag and colleagues initially postulated that the role of apextrin was in cell adhesion in developing embryos (Haag et al., 1999). However, more recent experiments in amphioxus (lancelet) suggest that apextrin may also play a role in immune defence against bacterial infection (Huang et al., 2007).
In mammals the large (over 1000 amino acids) proteins astrotactin-1 and -2 play important roles in neural development. Astrotactin 1 is hypothesized to be a neuronal adhesion molecule (Zheng et al., 1996) and precursor neuronal cells in the cerebella cortex of humans and mice produce astrotactin-1 in order to migrate along glial fibres. Targeted disruption of astrotactin-1 gene in mice resulted in mice with cerebella that were 10% smaller and reduction in the ability of granule cells to migrate (Adams et al., 2002). Similarly human deleted in bladder cancer candidate region-1 (DBCCR-1)/BMP/RA inducible neural-specific protein-1 (BRINP-1) is also thought to play a role in neural development (Kawano et al., 2004). Other data also suggest this latter molecule is a tumour suppressor that may modulate the cell cycle. Finally, studies on the mammalian MACPF protein EPCS50, reveal that this protein is produced in the developing trophoblast. It is suggested that this molecule may be involved in trophoblast invasion of the uterine lining (Hemberger et al., 2000).
Concluding statements
Recent structural studies have permitted the unification of CDC and MACPF proteins as a single superfamily and thus suggest that MACPF proteins and CDCs share a common mechanism of oligomerization and pore formation. However, important distinctions between the families remain to be understood. Most notably, while all CDCs appear to function as lytic toxins, the same cannot be said for MACPF proteins. Molecules such as C6 and C8β appear non-lytic and perhaps function as scaffolds and/or regulators of lytic molecules such as C9.
Interestingly, several members of the MACPF family appear to perform novel roles in development. While intriguing, a role for proteins of the same family in immunity or development is not unprecedented. For example, members of the Toll-like receptor family play well-defined roles in fly development and mammalian immunity.
Many unanswered questions remain about the MACPF/CDC family. In particular, the absence of a high-resolution structure of either a MACPF or a CDC in the pore form precludes an understanding of the fine details of the conformational re-arrangements that these remarkable proteins undergo. Future structural and biochemical studies will no doubt start to shed light on these processes as well as addressing the role of lytic or non-lytic MACPF proteins in development.
Acknowledgments
M.A.D. is supported by an National Health and Medical Council (NHMRC) of Australia Peter Doherty Fellowship, J.C.W. by an NHMRC Principal Research Fellowship, A.M.B. by an NHMRC Senior Research Fellowship, I.V. is supported by a NHMRC R. Douglas Wright Fellowship, J.A.T. is supported by a NHMRC Senior Principal Research Fellowship. We thank the NHMRC and the Australian Research Council for project and Program Grant support.
References
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. doi: 10.1242/dev.129.4.965. [DOI] [PubMed] [Google Scholar]
- Alouf JE, Popoff MR. The Comprehensive Sourcebook of Bacterial Protein Toxins Amsterdam. Boston, MA: Elsevier; 2006. p. xxiii. [Google Scholar]
- Aroian R, van der Goot FG. Pore-forming toxins and cellular non-immune defenses (CNIDs) Curr Opin Microbiol. 2007;10:57–61. doi: 10.1016/j.mib.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Balachandran P, Hollingshead SK, Paton JC, Briles DE. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J Bacteriol. 2001;183:3108–3116. doi: 10.1128/JB.183.10.3108-3116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci USA. 1978;75:5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19:339–347. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Braun JS, Hoffmann O, Schickhaus M, Freyer D, Dagand E, Bermpohl D, et al. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun. 2007;75:4245–4254. doi: 10.1128/IAI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockeran R, Anderson R, Feldman C. Pneumolysin in the immunopathogenesis and treatment of pneumococcal disease. Expert Rev Anti Infect Ther. 2003;1:231–239. doi: 10.1586/14787210.1.2.231. [DOI] [PubMed] [Google Scholar]
- Cox CB, Hardegree C, Fornwald R. Effect of tetanolysin on platelets and lysosomes. Infect Immun. 1974;9:696–701. doi: 10.1128/iai.9.4.696-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWinter LM, Low DE, Prescott JF. Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet Microbiol. 1999;70:95–110. doi: 10.1016/s0378-1135(99)00128-5. [DOI] [PubMed] [Google Scholar]
- DiScipio RG, Berlin C. The architectural transition of human complement component C9 to poly(C9) Mol Immunol. 1999;36:575–585. doi: 10.1016/s0161-5890(99)00073-5. [DOI] [PubMed] [Google Scholar]
- Ellemor DM, Baird RN, Awad MM, Boyd RL, Rood JI, Emmins JJ. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect Immun. 1999;67:4902–4907. doi: 10.1128/iai.67.9.4902-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach D, Kohler W, Gunther E, Mann K. Purification and characterization of streptolysin O secreted by Streptococcus equisimilis (group C) Infect Immun. 1993;61:2727–2731. doi: 10.1128/iai.61.6.2727-2731.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings KS, Johnson AE, Tweten RK. Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci USA. 2003;100:11315–11320. doi: 10.1073/pnas.2033520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;11:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- Haag ES, Sly BJ, Andrews ME, Raff RA. Apextrin, a novel extracellular protein associated with larval ectoderm evolution in Heliocidaris erythrogramma. Dev Biol. 1999;211:77–87. doi: 10.1006/dbio.1999.9283. [DOI] [PubMed] [Google Scholar]
- Hadders MA, Beringer DX, Gros P. Structure of C8alpha-MACPF reveals mechanism of membrane attack in complement immune defense. Science. 2007;317:1552–1554. doi: 10.1126/science.1147103. [DOI] [PubMed] [Google Scholar]
- Hemberger M, Himmelbauer H, Ruschmann J, Zeitz C, Fundele R. cDNA subtraction cloning reveals novel genes whose temporal and spatial expression indicates association with trophoblast invasion. Dev Biol. 2000;222:158–169. doi: 10.1006/dbio.2000.9705. [DOI] [PubMed] [Google Scholar]
- Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified ctyoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Liu H, Han Y, Fan L, Zhang Q, Liu J, et al. Profile of acute immune response in Chinese amphioxus upon Staphylococcus aureus and Vibrio parahaemolyticus infection. Dev Comp Immunol. 2007;31:1013–1023. doi: 10.1016/j.dci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kawamura I, Kohda C, Tsuchiya K, Nomura T, Mitsuyama M. Seeligeriolysin O, a protein toxin of Listeria seeligeri, stimulates macrophage cytokine production via Toll-like receptors in a profile different from that induced by other bacterial ligands. Int Immunol. 2005;17:1597–1606. doi: 10.1093/intimm/dxh341. [DOI] [PubMed] [Google Scholar]
- Jabado N, de Graeff-Meeder ER, Cavazzana-Calvo M, Haddad E, Le Deist F, Benkerrou M, et al. Treatment of familial hemophagocytic lymphohistiocytosis with bone marrow transplantation from HLA genetically nonidentical donors. Blood. 1997;90:4743–4748. [PubMed] [Google Scholar]
- Jost BH, Songer JG, Billington SJ. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infect Immun. 1999;67:1723–1728. doi: 10.1128/iai.67.4.1723-1728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci USA. 2004;101:16310–16315. doi: 10.1073/pnas.0406187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Odermatt B, Seiler P, Zinkernagel RM, Mak TW, Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med. 1997;186:989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Nakatani T, Mori T, Ueno S, Fukaya M, Abe A, et al. Identification and characterization of novel developmentally regulated neural-specific proteins, BRINP family. Brain Res Mol Brain Res. 2004;125:60–75. doi: 10.1016/j.molbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31:1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Kawamura I, Kohda C, Nomura T, Tsuchiya K, Ito Y, et al. Differences in gamma interferon production induced by listeriolysin O and ivanolysin O result in different levels of protective immunity in mice infected with Listeria monocytogenes and Listeria ivanovii. Infect Immun. 2003;71:2447–2454. doi: 10.1128/IAI.71.5.2447-2454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B, Koller M, Prevost G, Piemont Y, Alouf JE, Schreiner A, Konig W. Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): generation of interleukin-8. Infect Immun. 1994;62:4831–4837. doi: 10.1128/iai.62.11.4831-4837.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RJ, Carroll SF. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980;28:735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K, Funayama N, et al. The innate immune repertoire in cnidaria – ancestral complexity and stochastic gene loss. Genome Biol. 2007;8:R59. doi: 10.1186/gb-2007-8-4-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita-Yamamuro C, Tsutsui T, Sato M, Yoshioka H, Tamaoki M, Ogawa D, et al. The Arabidopsis gene CAD1 controls programmed cell death in the plant immune system and encodes a protein containing a MACPF domain. Plant Cell Physiol. 2005;46:902–912. doi: 10.1093/pcp/pci095. [DOI] [PubMed] [Google Scholar]
- Mosser EM, Rest RF. The Bacillus anthracis cholesterol-dependent cytolysin, anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 2006;6:56. doi: 10.1186/1471-2180-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomiya M, Kobayashi M, Iwasaki N, Minami A, Matsuoka I. Activity-dependent regulation of BRINP family genes. Biochem Biophys Res Commun. 2007;352:623–629. doi: 10.1016/j.bbrc.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Nagai H, Oshiro N, Takuwa-Kuroda K, Iwanaga S, Nozaki M, Nakajima T. Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski. Biochem Biophys Res Commun. 2002;294:760–763. doi: 10.1016/S0006-291X(02)00547-8. [DOI] [PubMed] [Google Scholar]
- Nishiwaki H, Nakashima K, Ishida C, Kawamura T, Matsuda K. Cloning, functional characterization, and mode of action of a novel insecticidal pore-forming toxin, sphaericolysin, produced by Bacillus sphaericus. Appl Environ Microbiol. 2007;73:3404–3411. doi: 10.1128/AEM.00021-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro N, Kobayashi C, Iwanaga S, Nozaki M, Namikoshi M, Spring J, Nagai H. A new membrane-attack complex/perforin (MACPF) domain lethal toxin from the nematocyst venom of the Okinawan sea anemone Actineria villosa. Toxicon. 2004;43:225–228. doi: 10.1016/j.toxicon.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Peitsch MC, Amiguet P, Guy R, Brunner J, Maizel JV, Tschopp J. Localization and molecular modelling of the membrane-inserted domain of the ninth component of human complement and perforin. Mol Immunol. 1990;27:589–602. doi: 10.1016/0161-5890(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Podack ER, Konigsberg PJ. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984;160:695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polekhina G, Giddings KS, Tweten RK, Parker MW. Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc Natl Acad Sci USA. 2005;102:600–605. doi: 10.1073/pnas.0403229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP. Chlamydial homologues of the MACPF (MAC/perforin) domain. Curr Biol. 1999;9:R911–R913. doi: 10.1016/s0960-9822(00)80102-5. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Tweten RK, Johnson AE. The domains of a cholesterol-dependent cytolysin undergo a major FRET-detected rearrangement during pore formation. Proc Natl Acad Sci USA. 2005;102:7139–7144. doi: 10.1073/pnas.0500556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado CJ, Buckle AM, Law RH, Butcher RE, Kan WT, Bird CH, et al. A common fold mediates vertebrate defense and bacterial attack. Science. 2007;317:1548–1551. doi: 10.1126/science.1144706. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Ruiz N, Wang B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol. 1998;27:337–346. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- Saier MH, Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibek JJ, Plumb ME, Sodetz JM. Binding of human complement C8 to C9: role of the N-terminal modules in the C8 alpha subunit. Biochemistry. 2002;10:14546–14551. doi: 10.1021/bi026641j. [DOI] [PubMed] [Google Scholar]
- Shatursky O, Heuck AP, Shepard LA, Rossjohn J, Parker MW, Johnson AE, Tweten RK. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- Shepard LA, Heuck AP, Hamman BD, Rossjohn J, Parker MW, Ryan KR, et al. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37:14563–14574. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Su L, Henkart PA. Cytotoxicity with the target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71:315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Slade DJ, Chiswell B, Sodetz JM. Functional studies of the MACPF domain of human complement protein C8alpha reveal sites for simultaneous binding of C8beta, C8gamma, and C9. Biochemistry. 2006;25:5290–5296. doi: 10.1021/bi0601860. [DOI] [PubMed] [Google Scholar]
- Slade DJ, Lovelace LL, Chruszcz M, Minor W, Lebioda L, Sodetz JM. Crystal structure of the MACPF domain of human complement protein C8 alpha in complex with the C8 gamma subunit. J Mol Biol. 2008;379:331–342. doi: 10.1016/j.jmb.2008.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Trapani JA. The relative role of lymphocyte granule exocytosis versus death receptor-mediated cytotoxicity in viral pathophysiology. J Virol. 1998;72:1–9. doi: 10.1128/jvi.72.1.1-9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani CE, Hotze EM, Johnson AE, Tweten RK. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc Natl Acad Sci USA. 2007;104:20226–20231. doi: 10.1073/pnas.0708104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilsbury K, O'Mara MA, Wu WM, Rowe PB, Symonds G, Takayama Y. Isolation of a novel macrophage-specific gene by differential cDNA analysis. Blood. 1995;85:1620–1629. [PubMed] [Google Scholar]
- Steckel EW, Welbaum BE, Sodetz JM. Evidence of direct insertion of terminal complement proteins into cell membrane bilayers during cytolysis. Labeling by a photosensitive membrane probe reveals a major role for the eighth and ninth components. J Biol Chem. 1983;10:4318–4324. [PubMed] [Google Scholar]
- Stephens KE, Miller KY, Miller BL. Functional analysis of DNA sequences required for conidium-specific expression of the SpoC1-C1C gene of Aspergillus nidulans. Fungal Genet Biol. 1999;27:231–242. doi: 10.1006/fgbi.1999.1145. [DOI] [PubMed] [Google Scholar]
- Stevens LM, Frohnhofer HG, Klingler M, Nusslein-Volhard C. Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature. 1990;346:660–663. doi: 10.1038/346660a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Haque A, Horiguchi Y, Matsuda M. Botulinolysin, a thiol-activated hemolysin produced by Clostridium botulinum, inhibits endothelium-dependent relaxation of rat aortic ring. Toxicon. 1997;35:1011–1023. doi: 10.1016/s0041-0101(97)00002-0. [DOI] [PubMed] [Google Scholar]
- Sukeno A, Nagamune H, Whiley RA, Jafar SI, Aduse-Opoku J, Ohkura K, et al. Intermedilysin is essential for the invasion of hepatoma HepG2 cells by Streptococcus intermedius. Microbiol Immunol. 2005;49:681–694. doi: 10.1111/j.1348-0421.2005.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Tilley SJ, Saibil HR. The mechanism of pore formation by bacterial toxins. Curr Opin Struct Biol. 2006;16:230–236. doi: 10.1016/j.sbi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Trapani JA. Dual mechanisms of apoptosis induction by cytotoxic lymphocytes. Int Rev Cytol. 1998;182:111–192. doi: 10.1016/s0074-7696(08)62169-5. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322:831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier G, Segura M, Friedl P, Lacouture S, Gottschalk M. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect Immun. 2004;72:1441–1449. doi: 10.1128/IAI.72.3.1441-1449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik I, Thia MC, De Bono A, Browne K, Cretney E, Jackson JT, et al. The functional basis for hemophagocytic lymphohistiocytosis in a patient with co-inherited missense mutations in the perforin (PFN1) gene. J Exp Med. 2004;200:811–816. doi: 10.1084/jem.20040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik I, Thia MC, Trapani JA. A functional analysis of the putative polymorphisms A91V and N252S and 22 missense perforin mutations associated with familial hemophagocytic lymphohistiocytosis. Blood. 2005;105:4700–4706. doi: 10.1182/blood-2004-12-4935. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- Voth DE, Martinez OV, Ballard JD. Variations in lethal toxin and cholesterol-dependent cytolysin production correspond to differences in cytotoxicity among strains of Clostridium sordellii. FEMS Microbiol Lett. 2006;259:295–302. doi: 10.1111/j.1574-6968.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]


