Gram-negative bacteria-producing extended-spectrum β-lactamases (ESBLs) are found to be truly multiresistant pathogens causing severe clinical problems. In our investigations, fifteen class C β-lactamases with extended substrate spectra have been reported in Gram-negative pathogens. Because of the emergence and dissemination of these enzymes, we propose that these enzymes be recognized as class C ESBLs (cESBLs), although most of the known ESBLs are class A and D β-lactamases. To decrease the selective pressure of antimicrobial drugs and minimize antimicrobial resistance, it is necessary for health-care professionals to recognize the presence of emerging cESBLs as a new and disturbing trend in antimicrobial resistance of Gram-negative pathogens. Because there is currently no drug development against cESBL-producing Gram-negative pathogens in progress and large pharmaceutical companies have largely withdrawn from research and development of new antimicrobial drugs, there is a tremendous need for the development of new β-lactams (or β-lactamase inhibitors) by focused cooperation between academia and small pharmaceutical companies, using the similar structural mechanism (a potential therapeutic target) of the extended substrate spectrum shown in most cESBLs.
The consensus view about antimicrobial resistance is that severe clinical problems arise from the emergence of antibiotic resistance in Gram-negative pathogens causing nosocomial infections, and from the lack of new antimicrobial agents to challenge the threat [1]. There are four disturbing trends (extending substrate spectra) in the increasing antimicrobial resistance of Gram-negative pathogens [1]: (i) class B β-lactamases (metallo-β-lactamases) conferring resistance to almost all β-lactam antibiotics [2]; (ii) a bifunctional aminoglycoside-modifying enzyme [3]; (iii) the evolution of a fluoroquinolone-modifying enzyme from an aminoglycoside acetyltransferase [4]; and (iv) a new plasmid-borne fluoroquinolone efflux determinant [5]. These disturbing trends indicate that options for the treatment of health-care–associated Gram-negative infections are perilously limited as the organisms expand their ability to evade existing antimicrobial agents [1],[6]. Here we wish to draw attention to a new disturbing trend (the recently emerging class C extended-spectrum β-lactamases [ESBLs]), and to the antimicrobial drug development for class C ESBLs. We suggest also that the category of ESBLs has to be expanded.
Epidemiology and Characteristics of Class C ESBLs
Generally, ESBLs are defined as β-lactamases able to hydrolyze the penicillins, cephalosporins (first-, second-, and third-generation), and monobactams (aztreonam), but not the cephamycins or carbapenems [7]. In other words, ESBLs have an extended substrate spectrum as compared with their parent types (non-ESBLs). ESBLs can also be inhibited by β-lactamase inhibitors such as clavulanic acid. Most of the known ESBLs are class A and D β-lactamases [7], but 15 class C β-lactamases with extended substrate spectra have been reported in Gram-negative pathogens isolated from clinical specimens of patients since the first description of GC1 in 1995 (Table 1). Because of the emergence and dissemination of these enzymes, we propose that these enzymes are recognized as class C ESBLs. Then class A, C, and D ESBLs would be designated aESBLs, cESBLs, and dESBLs, respectively.
Table 1. Epidemiology and characteristics of class C extended-spectrum β-lactamases (cESBLs).
| Enzyme* | Extended Substrate Spectrum† (Parent Enzyme) | Country of Origin (Clinical Isolation) | Bacterial Species | Region (Mutation Site)‡ Causing Extended Substrate Spectrum | Reference |
| GC1 | CAZ, ATM (P99) | Japan, 1992 | E. cloacae GC1 | Ω-loop (the insertion of Ala-Val-Arg after position 210) | [8],[10] |
| SRT-1 | CAZ, CTX, CMX (SST-1) | Japan, 1985 | S. marcescens GN16694 | Ω-loop (Glu213 → Lys) | [28] |
| SMSA (SerR) | CAZ, FEP, FPI (SLS73, SerS) | France, 2000 | S. marcescens SMSA | Ω-loop (Ser220 → Tyr) | [29] |
| CHE | CTX, FEP, FPI (P99) | France, 1998 | E. cloacae CHE | R2-loop (a six-amino-acid-deletion, SKVALA at positions 289–294) | [11] |
| Ear2 | CTX, FEP (Ear1) | France, 2001 | E. aerogenes Ear2 | R2-loop (Leu293 → Pro) | [30] |
| AmpCD | CAZ, FEP, FPI, inhibitor-sensitive (AmpCR, revertant) | Japan, 1994 | E. coli HKY28 | R2-loop (a tripeptide deletion, GSD, at positions 286–288) | [31] |
| HD | CAZ, FEP, FPI (S3) | France, 2001 | S. marcescens HD | R2-loop (a four-amino-acid-deletion, MNGT, at positions 293–296) | [16] |
| EC14 | CAZ, FEP (EC1) | France, 2002–2005 | E. coli EC14 | R2-loop (Val298 → Leu) | [17] |
| EC15 | CAZ, FEP (EC1) | France, 2002–2005 | E. coli EC15 | R2-loop (His296 → Pro) | [17] |
| EC17 | CAZ, FEP (EC1) | France, 2002–2005 | E. coli EC17 | R2-loop (His296 → Pro) | [17] |
| EC19 | CAZ, FEP (EC1) | France, 2002–2005 | E. coli EC19 | R2-loop (His296 → Pro) | [17] |
| CMY-19 | CAZ, FEP, FPI (CMY-9) | Japan, 1996 | K. pneuminiae HKY327 | R2-loop (Ile292 → Ser) | [32] |
| CMY-10 | CAZ, IMP (P99) | Korea, 1999 | E. aerogenes K9911729 | R2-loop (a tripeptide deletion, PPA, at positions 303–305) | [24] |
| BER | CAZ, CTX, CRO, FEP, IMP (EC2) | France, 2006 | E. coli BER | R2-loop (the insertion of Ala-Ala after position 293) | [18] |
| MHN-7.6 | CAZ, FEP, FPI (MHN) | In vitro mutation | E. coli K12 strain MI1443 | R2-loop (Val298 → Glu) | [9] |
| AmpC1 | CAZ, FEP (P99) | In vitro mutation | E. coli JM83 | R2-loop (Leu293 → Pro) | [33] |
| Seven mutant enzymes | CAZ, FEP (CMY-2) | In vitro mutation | E. coli DH5αE | R2-loop (Val291 → Ala[Gly]; Ala292 → Pro; Leu293 → Pro; Ala294 → Glu; Leu296 → Pro; Ala298 → Val) | [34] |
| 520R | CAZ, FPI (S3) | In vitro mutation | E. coli DH5α | H-2 helix (Thr64 → Ile) | [35] |
| KL | CAZ, FEP, FPI (S4) | France, 2001 | E. coli KL | H-11 helix (Val350 → Phe) | [19] |
*: Crystallographic structures from distinct GC1 (Protein Data Bank [PDB] code 1GCE) and CMY-10 (PDB code 1ZKJ) only have been resolved. SerR is the in vitro site-directed mutant of SLS73 (SerS). All enzymes except plasmid-encoded CMY-10 and CMY-19 are chromosomal cESBLs. All enzymes except several enzymes (SerR, SerS, AmpCR, AmpC1 [in vitro Leu-293-Pro mutant of P99], seven mutants of CMY-2, MHN-7.6, and 520R) are the naturally (clinically) occurring cESBLs produced by clinical isolates. AmpCD is the only inhibitor-(tazobactam and sulbactam)sensitive cESBL.
†: CAZ, ceftazidime; CTX, cefotaxime; CMX, cefmenoxime; CRO, ceftriaxone; FEP, cefepime; FPI, cefpirome; IMP, imipenem; ATM, aztreonam. Each cESBL has extended its substrate specificity in comparison with each parent enzyme (non-cESBL).
‡: Ω-loop lays from residues 189 to 226 in P99 β-lactamase. R2-loop lays from residues 289 to 307 in CMY-10 β-lactamase. The position of the N-terminal amino acid of the mature enzyme (without the respective signal peptide) is designated as position 1 of the amino acid sequence. The tripeptide deletion of AmpCD is located just before the R2-loop but causes a structural change in the R2-loop. Glu213 → Lys, the substitution of glutamic acid (Glu) by lysine (Lys) at residue 213.
The cESBLs were first defined as follows: i) extended specificity class C β-lactamase for GC1 in 1995 [8]; ii) extended-spectrum AmpC-type β-lactamase for MHN-7.6 in 1998 [9]; iii) extended-spectrum class C β-lactamase for GC1 in 1999 [10]; and iv) extended-spectrum AmpC β-lactamase (ESAC) for CHE in 2001 [11]. Class C β-lactamase was designated AmpC β-lactamase [12]. Therefore, extended-spectrum class C (AmpC) β-lactamase can be designated class C extended-spectrum β-lactamase (cESBL). Most cESBL (13 of 15 natural cESBLs produced by Gram-negative pathogens isolated from clinical specimens of patients: SMSA, CHE, Ear2, AmpCD, HD, EC14, EC15, EC17, EC19, CMY-19, BER, 520R, and KL) have extended their substrate specificity to third- and fourth-generation cephalosporins (Table 1). Some cESBLs (CMY-10 and BER) can hydrolyse carbapenems (imipenem or meropenem), which have the same substrate specificity as that of aESBLs such as GES-5 [13]. A cESBL (AmpCD) can be inhibited also by β-lactamase inhibitors (tazobactam and sulbactam) just like aESBLs and dESBLs. The hydrolytic efficiency (k cat/K m) of cESBLs for ceftazidime and cefotaxime was higher than or similar to that of SHV-38 [14] and CTX-M-15 [15], typical aESBLs. Some β-lactamase investigators [16]–[19] have tried to distinguish the difference between ESACs and cESBLs, but, except for cephamycins (cefoxitin and cefotetan), hydrolysis patterns do not differ between ESACs and cESBLs. Furthermore, ESBL-producing clinical isolates were also resistant to cephamycins by reduced outer membrane permeability [20]. In 2003, Hanson warned that if we have failed to distinguish between ESBL and plasmid-encoded class C β-lactamase (non-cESBL) producers, we would run the risk of the emergence of cESBLs [21]. Unfortunately, cESBLs have already emerged, and the phenotypic susceptibility testing to distinguish between aESBLs (or dESBLs) and emerging cESBLs is very difficult.
Treatment for cESBL-Producing Gram-Negative Pathogens
The Infectious Diseases Society of America identified six top-priority dangerous pathogens (e.g., ESBL-producing Enterobacteriaceae, Acinetobacter baumannii, Pseudomonas aeruginosa, vancomycin-resistant Enterococcus faecium, methicillin-resistant Staphylococcus aureus, and Aspergillus species) for which there are few or no drugs in late-stage development, further limiting the choice of an appropriate and safe treatment for these infections [22],[23]. Three of six dangerous pathogens are antibiotic-resistant Gram-negative bacteria. Recently, antimicrobial drugs against ESBL-producing Gram-negative pathogens accounted for about 15% (2 of 13) of all antimicrobial drugs undergoing development in phase II or later clinical studies [22]. There are no drug developments against cESBL-producing Gram-negative pathogens.
Rubinstein and Zhanel, hospital physicians, have stated that physicians are increasingly forced to use the carbapenems and fluoroquinolones (ciprofloxacin or levofloxacin) as first-line therapy for ESBL-producing Gram-negative pathogens, but the situation will become even more severe as ESBL-producing organisms increasingly become concomitantly resistant to the fluoroquinolones [6]. However, we recently found that the CMY-10 cESBL had higher imipenem-hydrolysing activity than OXA-23, a class D carbapenemase [24]. Because this extended substrate spectrum of cESBLs can threaten the management of infections by Gram-negative pathogens producing these enzymes, new antimicrobial drugs against cESBL-producing Gram-negative pathogens are urgently needed. To develop these antimicrobial drugs, it is necessary to know the operative mechanism of cESBLs to extend their substrate spectrum.
Antimicrobial Drug Development for cESBLs
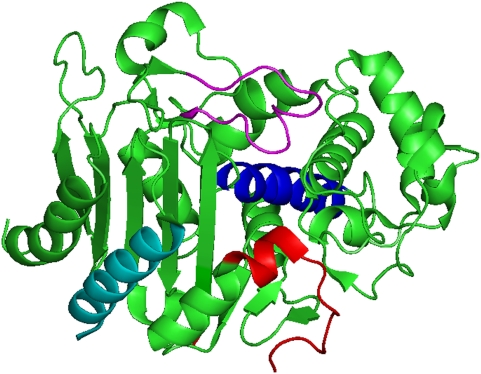
How do the cESBLs extend the substrate spectrum? The crystallographic structures can answer this question. Until now, there are two only resolved crystallographic structures of cESBLs: (i) GC1 (Protein Data Bank [PDB] code, 1GCE) [10]; and (ii) CMY-10 (PDB code, 1ZKJ) [24]. Kinetic data and the crystal structure of GC1 showed that GC1 was a natural (clinically isolated) cESBL due to the flexibility of the Ω-loop caused by the insertion of Ala-Val-Arg after position 210 [8],[10]. As shown in the Table 1, this structural characteristic of chromosomal GC1 provides insights into the molecular basis of extended substrate spectrum shown in only three cESBLs (GC1, SRT-1, and SMSA). But our kinetic data and crystal structure [24] of a plasmid-encoded cESBL (i.e., CMY-10) reveal the operative molecular strategy of most cESBLs (73%, 11 of the total 15) to extend their substrate spectrum. The region responsible for the extended substrate spectrum is the R2-loop (amino acid residues 289–307; Figure 1) [24]. Our sequence alignment of natural (clinically isolated) cESBLs shows that the R2-loop includes all regions responsible for the extended substrate spectrum in most (11 of the total 15) cESBLs: Ω-loop in three cESBLs; H-2 helix in a 520R cESBL (not natural); H-11 helix in a KL cESBL (Table 1 and Figure 1). These natural (from clinical isolates) mutations in the R2-loop can change the architecture of the active site in cESBLs, thereby affecting their hydrolysing activity. Owing to a three-amino-acid deletion (amino acid residues 303–305) in CMY-10, for example, the R2-loop in the R2 active site (i.e., the region that accommodates the R2 side-chain at C3 of the β-lactam nucleus in oxyimino-cephalosporins) displays noticeable structural alterations: the significant widening of the R2 active site. Therefore, the bulky R2 side-chain of oxyimino-cephalosporins could fit snugly into the significant widening of the R2 active site in this way. In view of no drug developments against cESBL-producing Gram-negative pathogens, new β-lactams or β-lactamase inhibitors need to be developed by the structure-based drug design (SBDD) method [25] using a similar mechanism (the significant widening of the R2 active site) of the extended substrate spectrum shown in most cESBLs. Clinically available β-lactamase inhibitors co-administered with less effective β-lactams are effective against class A β-lactamases, but show little or no activity against class C β-lactamases. Therefore, class C β-lactamases are an excellent drug target with accurate structural information [25]. Since Gram-negative pathogens producing cESBLs are increasing in emergence and spreading among organisms causing nosocomial infections (Table 1), there is an urgent need to develop an inhibitor of cESBLs or to discover new antimicrobial drugs for these cESBL-producing clinical isolates. Although large pharmaceutical companies have largely withdrawn from research and development of new antimicrobial drugs, a few academic research groups (e.g., our group, or Shoichet's laboratory [26]) and small pharmaceutical companies (e.g., Novexel [27], which has been spun out of Aventis and Anacor that has formed a worldwide strategic alliance with GlaxoSmithKline) are seeking these new β-lactamase inhibitors. The discovery of some lead compounds against CMY-10 β-lactamases by SBDD is in progress, by focused cooperation between academia and small pharmaceutical companies.
Figure 1. Ribbon diagram of crystallographic structure of CMY-10 (a cESBL).
The image was rendered with PyMOL, available on the Internet (http://sourceforge.net/projects/pymol). The R2-loop is represented as red, while the Ω-loop, H-2 helix, and H-11 helix are depicted in violet, blue, and cyan, respectively. The R1 active site (central upper region) is surrounded by the Ω-loop and the R2 active site (central lower region) by the R2-loop and H-11 helix. The nucleophile (Ser65), attacking the carbonyl carbon of β-lactam ring, is present in the H-2 helix.
Conclusion
Since the emergence and dissemination of fifteen class C extended-spectrum β-lactamases (ESBLs) produced by Gram-negative pathogens isolated from clinical specimens of patients, the category of ESBLs has broadened to include class C β-lactamases with extended substrate spectrum. We propose that these enzymes be recognized as class C ESBLs (cESBLs). Phenotypic susceptibility testing to distinguish the difference between organisms producing general ESBLs (e.g., aESBLs or dESBLs) or emerging cESBLs is very challenging. The difficulty in type identification of ESBLs hinders hospital infection control and the ability of the physician to prescribe the most appropriate antibiotic, thus increasing the selective pressure and generating antibiotic resistance. It is necessary for health-care professionals to recognize the presence of emerging cESBLs as a new and disturbing trend in antimicrobial resistance of Gram-negative pathogens. Furthermore, there is currently no drug development in progress against cESBL-producing Gram-negative pathogens. Therefore, there is a tremendous need for the development of new β-lactams or β-lactamase inhibitors by the structure-based drug-design method using the similar structural mechanism (the significant widening of the R2 active site) of the extended substrate spectrum shown in most cESBLs.
Accession Number
The Protein Data Bank (PDB, http://www.rcsb.org/pdb/) accession code for the protein discussed in this paper is CMY-10 (1ZKJ, [24]).
Acknowledgments
We would like to thank Drs. Won-Keun Lee, Il Kwon Bae, and Marianne Manchester for critical reading of the manuscript and valuable comments. We also thank our colleagues, both past and present, at the Center for Antibiotic Resistance, Myongji University, for sharing their insights into the concepts presented here.
Footnotes
The authors have declared that no competing interests exist.
The work carried out on a class C ESBL was funded by the National Institute of Health of KCDC in the Republic of Korea, a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST), the Driving Force Project for the Next Generation of Gyeonggi Provincial Government in the Republic of Korea, the Korea Research Foundation (KRF-2008-313-C00790), and the Marine & Extreme Genome Research Center Program of the Ministry of Land, Transport, and Maritime Affairs in the Republic of Korea. The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the paper; or the decision to submit it for publication.
References
- 1.Chopra I, Schofield C, Everett M, O'Neill A, Miller K, et al. Treatment of health-care-associated infections caused by Gram-negative bacteria: A consensus statement. Lancet Infect Dis. 2008;8:133–139. doi: 10.1016/S1473-3099(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JM, Bonomo RA. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: Beta-lactams in peril! Curr Opin Microbiol. 2005;8:518–524. doi: 10.1016/j.mib.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Kim C, Hesek D, Zajicek J, Vakulenko SB, Mobashery S. Characterization of the bifunctional aminoglycoside-modifying enzyme ANT(3″)-Ii/AAC(6′)-IId from Serratia marcescens. Biochemistry. 2006;45:8368–8377. doi: 10.1021/bi060723g. [DOI] [PubMed] [Google Scholar]
- 4.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, et al. Fluoroquinolone-modifying enzyme: A new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 5.Yamane K, Wachino J, Suzuki S, Kimura K, Shibata N, et al. Novel plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 2007;51:3354–3360. doi: 10.1128/AAC.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley JS, Guidos R, Baragona S, Bartlett JG, Rubinstein E, et al. Anti-infective research and development—Problems, challenges, and solutions. Lancet Infect Dis. 2007;7:68–78. doi: 10.1016/S1473-3099(06)70689-2. [DOI] [PubMed] [Google Scholar]
- 7.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nukaga M, Haruta S, Tanimoto K, Kogure K, Taniguchi K, et al. Molecular evolution of a class C β-lactamase extending its substrate specificity. J Biol Chem. 1995;270:5729–5735. doi: 10.1074/jbc.270.11.5729. [DOI] [PubMed] [Google Scholar]
- 9.Morosini MI, Negri MC, Shoichet B, Baquero MR, Baquero F, et al. An extended-spectrum AmpC-type β-lactamase obtained by in vitro antibiotic selection. FEMS Microbiol Lett. 1998;165:85–90. doi: 10.1111/j.1574-6968.1998.tb13131.x. [DOI] [PubMed] [Google Scholar]
- 10.Crichlow GV, Kuzin AP, Nukaga M, Mayama K, Sawai T, et al. Structure of the extended-spectrum class C β-lactamase of Enterobacter cloacae GC1, a natural mutant with a tandem tripeptide insertion. Biochemistry. 1999;38:10256–10261. doi: 10.1021/bi9908787. [DOI] [PubMed] [Google Scholar]
- 11.Barnaud G, Labia R, Raskine L, Sanson-Le Pors MJ, Philippon A, et al. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol Lett. 2001;195:185–190. doi: 10.1111/j.1574-6968.2001.tb10519.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomson KS, Moland ES. Version 2000: The new β-lactamases of Gram-negative bacteria at the dawn of the new millennium. Microbes Infect. 2000;2:1225–1235. doi: 10.1016/s1286-4579(00)01276-4. [DOI] [PubMed] [Google Scholar]
- 13.Bae IK, Lee YN, Jeong SH, Hong SG, Lee JH, et al. Genetic and biochemical characterization of GES-5, an extended-spectrum class A β-lactamase from Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2007;58:465–468. doi: 10.1016/j.diagmicrobio.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Poirel L, Héritier C, Podglajen I, Sougakoff W, Gutmann L, et al. Emergence in Klebsiella pneumoniae of a chromosome-encoded SHV β-lactamase that compromises the efficacy of imipenem. Antimicrob Agents Chemother. 2003;47:755–758. doi: 10.1128/AAC.47.2.755-758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirel L, Gniadkowski M, Nordman P. Biochemical analysis of ceftazidime-hydrolysing extended-spectrum β-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J Antimicrob Chemother. 2002;50:1031–1034. doi: 10.1093/jac/dkf240. [DOI] [PubMed] [Google Scholar]
- 16.Mammeri H, Poirel L, Bemer P, Drugeon H, Nordmann P. Resistance to cefepime and cefpirome due to a 4-amino-acid deletion in the chromosome-encoded AmpC β-lactamase of a Serratia marcescens clinical isolate. Antimicrob Agents Chemother. 2004;48:716–720. doi: 10.1128/AAC.48.3.716-720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammeri H, Poirel L, Fortineau N, Nordmann P. Naturally occurring extended-spectrum cephalosporinases in Escherichia coli. Antimicrob Agents Chemother. 2006;50:2573–2576. doi: 10.1128/AAC.01633-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammeri H, Poirel L, Nordmann P. Extension of the hydrolysis spectrum of AmpC β-lactamase of Escherichia coli due to amino acid insertion in the H-10 helix. J Antimicrob Chemother. 2007;60:490–494. doi: 10.1093/jac/dkm227. [DOI] [PubMed] [Google Scholar]
- 19.Mammeri H, Nazic H, Naas T, Poirel L, Leotard S, et al. AmpC β-lactamase in an Escherichia coli clinical isolate confers resistance to expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 2004;48:4050–4053. doi: 10.1128/AAC.48.10.4050-4053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson KS. Controversies about extended-spectrum and AmpC β-lactamases. Emerg Infect Dis. 2001;7:333–336. doi: 10.3201/eid0702.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson ND. AmpC β-lactamases: What do we need to know for the future? J Antimicrob Chemother. 2003;52:2–4. doi: 10.1093/jac/dkg284. [DOI] [PubMed] [Google Scholar]
- 22.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, et al. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 23.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Jung HI, An YJ, Lee JH, Kim SJ, et al. Structural basis for the extended substrate spectrum of CMY-10, a plasmid-encoded class C β-lactamase. Mol Microbiol. 2006;60:907–916. doi: 10.1111/j.1365-2958.2006.05146.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AC. The process of structure-based drug design. Chem Biol. 2002;10:787–797. doi: 10.1016/j.chembiol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Powers RA, Morandi F, Shoichet BK. Structure-based discovery of a novel, noncovalent inhibitor of AmpC β-lactamase. Structure. 2002;10:1013–1023. doi: 10.1016/s0969-2126(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 27.Fox JL. The business of developing antibacterials. Nat Biotechnol. 2006;24:1521–1528. doi: 10.1038/nbt1206-1521. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura N, Minami S, Mitsuhashi S. Sequences of homologous β-lactamases from clinical isolates of Serratia marcescens with different substrate specificities. Antimicrob Agents Chemother. 1998;42:176–179. doi: 10.1128/aac.42.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidri N, Barnaud G, Decre D, Cerceau C, Lalande V, et al. Resistance to ceftazidime is associated with a S220Y substitution in the omega loop of the AmpC β-lactamase of a Serratia marcescens clinical isolate. J Antimicrob Chemother. 2005;55:496–499. doi: 10.1093/jac/dki025. [DOI] [PubMed] [Google Scholar]
- 30.Barnaud G, Benzerara Y, Gravisse J, Raskine L, Sanson-Le Pors MJ, et al. Selection during cefepime treatment of a new cephalosporinase variant with extended-spectrum resistance to cefepime in an Enterobacter aerogenes clinical isolate. Antimicrob Agents Chemother. 2004;48:1040–1042. doi: 10.1128/AAC.48.3.1040-1042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doi Y, Wachino J, Ishiguro M, Kurokawa H, Yamane K, et al. Inhibitor-sensitive AmpC β-lactamase variant produced by an Escherichia coli clinical isolate resistant to oxyiminocephalosporins and cephamycins. Antimicrob Agents Chemother. 2004;48:2652–2658. doi: 10.1128/AAC.48.7.2652-2658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wachino J, Kurokawa H, Suzuki S, Yamane K, Shibata N, et al. Horizontal transfer of blaCMY-bearing plasmids among clinical Escherichia coli and Klebsiella pneumoniae isolates and emergence of cefepime-hydrolyzing CMY-19. Antimicrob Agents Chemother. 2006;50:534–541. doi: 10.1128/AAC.50.2.534-541.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakulenko SB, Golemi D, Geryk B, Suvorov M, Knox JR, et al. Mutational replacement of Leu-293 in the class C Enterobacter cloacae P99 β-lactamase confers increased MIC of cefepime. Antimicrob Agents Chemother. 2002;46:1966–1970. doi: 10.1128/AAC.46.6.1966-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlow M, Hall BG. Experimental prediction of the evolution of cefepime resistance from the CMY-2 AmpC β-lactamase. Genetics. 2003;164:23–29. doi: 10.1093/genetics/164.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raimondi A, Sisto F, Nikaido H. Mutation in Serratia marcescens AmpC β-lactamase producing high-level resistance to ceftazidime and cefpirome. Antimicrob Agents Chemother. 2001;45:2331–2339. doi: 10.1128/AAC.45.8.2331-2339.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]