Abstract
Previously, we have reported that 17β-estradiol (E2) induces an increase in firing activity of primate LH-releasing hormone (LHRH) neurons. The present study investigates whether E2 alters LHRH release as well as the pattern of intracellular calcium ([Ca2+]i) oscillations and whether G protein-coupled receptor 30 (GPR30) plays a role in mediating the rapid E2 action in primate LHRH neurons. Results are summarized: 1) E2, the nuclear membrane-impermeable estrogen, estrogen-dendrimer conjugate, and the plasma membrane-impermeable estrogen, E2-BSA conjugate, all stimulated LHRH release within 10 min of exposure; 2) whereas the estrogen receptor antagonist, ICI 182,780, did not block the E2-induced LHRH release, E2 application to cells treated with pertussis toxin failed to induce LHRH release; 3) GPR30 mRNA was expressed in olfactory placode cultures, and GPR30 protein was expressed in a subset of LHRH neurons; 4) pertussis toxin treatment blocked the E2-induced increase in [Ca2+]i oscillations; 5) knockdown of GPR30 in primate LHRH neurons by transfection with small interfering RNA (siRNA) for GPR30 completely abrogated the E2-induced changes in [Ca2+]i oscillations, whereas transfection with control siRNA did not; 6) the estrogen-dendrimer conjugate-induced increase in [Ca2+]i oscillations also did not occur in LHRH neurons transfected with GPR30 siRNA; and 7) G1, a GPR30 agonist, resulted in changes in [Ca2+]i oscillations, similar to those observed with E2. Collectively, E2 induces a rapid excitatory effect on primate LHRH neurons, and this rapid action of E2 appears to be mediated, in part, through GPR30.
Estradiol induces a rapid excitatory effect in primate LHRH neurons and GPR30 plays a role in mediating the rapid excitatory action.
Since 1941 when Hans Selye (1) first described progesterone causing anesthetic action with a short latency, rapid action of steroid hormones has been reported in many types of tissues and cells (2,3). Electrophysiological studies in the late 1950s through 1980s further suggested that gonadal steroids modify neuronal activity in the hypothalamus within a minute [see reviews (4,5)]. Because rapid action of steroids can often be induced by application of a membrane-impermeable form, it is known as membrane action of steroid hormones.
Several recent studies indicate that estrogens induce a direct rapid action in LH-releasing hormone (LHRH) neurons. 17β-Estradiol (E2) hyperpolarizes guinea pig LHRH neurons (6), modifies cAMP production in GT1–7 cells (7), and stimulates intracellular calcium [Ca2+]i oscillations in mouse LHRH neurons (8). We have also shown that E2 induces a rapid action in primate LHRH neurons with or without the presence of tetrodotoxin. Using a patch clamp recording method, both E2 and E2-BSA, a plasma membrane-impermeable form of E2, increased firing activity within a few minutes (9). Similarly, with a calcium imaging method, both E2 and estrogen dendrimer conjugate [EDC (10)], a nuclear membrane-impermeable form of E2, resulted in an increase in the pulse frequency and synchronization of [Ca2+]i oscillations with a short latency in LHRH neurons (11).
The mechanism of rapid action of estrogens in LHRH neurons is still unclear. In a study in mouse LHRH neurons, Temple and colleagues (8) concluded that rapid E2 effects are mediated through estrogen receptor β (ERβ), because mouse LHRH neurons expressed ERβ, but not ERα, and the nuclear ER antagonist, ICI 182,780, suppressed the effect of E2 on [Ca2+]i oscillations. However, in primate LHRH neurons, E2 appears to cause its action through a different mechanism, because ICI 182,780 failed to block the E2-induced changes in [Ca2+]i oscillations and synchronization (11). Searching for a potential mediator of rapid E2 action, we thought that the role of G protein-coupled receptor 30 (GPR30) in primate LHRH neurons might be worthwhile to study. It has been shown that E2 rapidly activates adenylyl cyclase and the MAPKs, Erk-1 and Erk-2, through GPR30, resulting in mobilization of [Ca2+]i stores in cancer cells (12,13,14,15,16,17,18). Here, we investigated how rapid E2 action modifies the pattern of LHRH release as well as [Ca2+]i oscillations and report the possible role of GPR30 in rapid action of E2 in primate LHRH neurons. The results show that GPR30 is, in part, involved in estrogen action in primate LHRH neurons.
Results
Effects of E2, EDC, and E2-BSA on LHRH release (experiment 1)
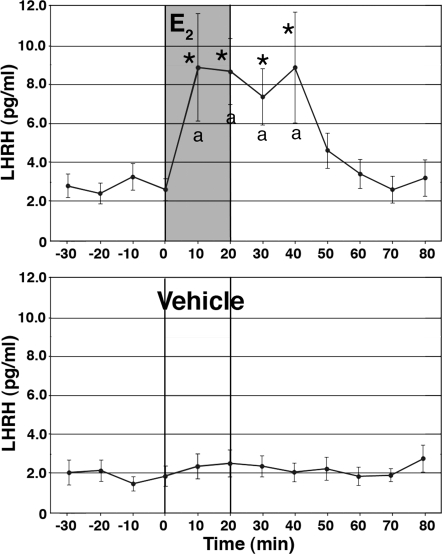
Previously, we observed that E2 induced a rapid action in primate LHRH neurons (9,11). To assess physiological consequences of the rapid E2 action, in the first series of studies we examined the effect of E2 on LHRH release. After 60 min of control perfusion, E2 at 1 nm was perfused for 20 min, whereas perifusate samples were continuously collected at 10-min intervals for an additional 60 min. E2 infusion for 20 min resulted in a significant increase in LHRH release. LHRH levels were elevated at the first sample after the initiation of infusion and continued through the entire 20-min infusion period to 20 min after termination of infusion (n = 11; Fig. 1). Vehicle did not change LHRH levels (n = 11). The E2-induced LHRH increase was significantly higher than preinfusion levels (P < 0.05) as well as from the vehicle treatment during the corresponding period (P < 0.05).
Figure 1.
E2 stimulated LHRH release in cultured primate LHRH neurons. E2 at 1 nm or vehicle was infused for 20 min whereas perifusate samples were collected at 10-min intervals. Note that E2 resulted in an increase in LHRH release within 10 min, and effects lasted for an additional 20 min after infusion was terminated. Vehicle had no effect. *, P < 0.05 vs. before E2; a, P < 0.05 vs. vehicle.
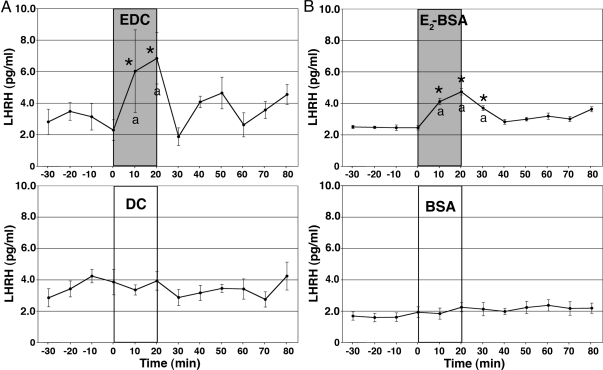
Because we previously found that the nuclear membrane-impermeable estrogen, EDC, stimulates [Ca2+]i oscillations (11), next we similarly examined the effect of EDC (1 nm) on LHRH release. Dendrimer conjugate (DC 1 nm; see Ref. 10) was used as a control. EDC resulted in a significant increase in LHRH release (n = 12; Fig. 2A). EDC-induced LHRH elevations were significantly higher than those of preinfusion levels (P < 0.05) as well as from DC treatment (n = 9) during the corresponding period (P < 0.05). Interestingly, however, the EDC-induced LHRH increase was limited to the EDC exposure period, and therefore the EDC-stimulated period was shorter than that with E2 (1 nm).
Figure 2.
Both EDC and E2-BSA stimulated LHRH release. The effects of a 20-min infusion of EDC or DC at 1 nm (A) and the effects of a 20-min infusion of E2-BSA conjugate or BSA at 1 nm (B) on LHRH release are shown. Note that EDC, but not DC, induced an increase in LHRH release within 10 min, but the effectiveness did not continue after EDC infusion. Similarly, E2-BSA, but not BSA, resulted in an elevation of LHRH release within 10 min, which lasted for an additional 10 min. *, P < 0.05 vs. before EDC; a, P < 0.05 vs. DC. *, P < 0.05 vs. before E2-BSA; a, P < 0.05 vs. BSA.
To further pursue the issue of membrane action of E2, we examined the effect of E2 17 hemisuccinate-BSA (E2-BSA, Steraloids, Newport, RI) at 1 nm concentration. E2-BSA conjugate significantly stimulated LHRH release (P < 0.05 for both preinfusion levels and BSA alone; n =10, Fig. 2B). The magnitude of LHRH response to E2-BSA was smaller than the response to E2, and the E2-BSA effect did not last as long as the response induced by E2. BSA alone did not cause any effects (n = 9; Fig. 2B).
Effects of ICI 182,780 on the E2-induced LHRH release (experiment 1)
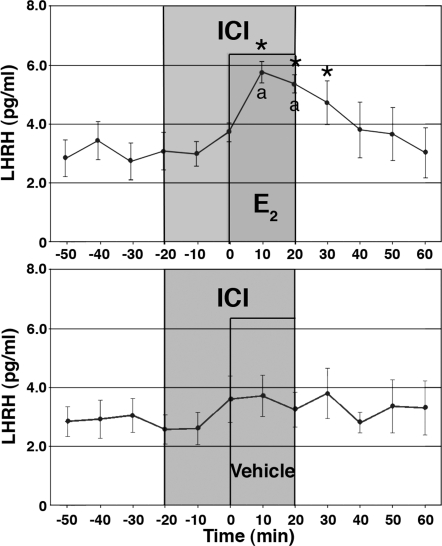
In a previous study (11) ICI 182,780 failed to block the E2-induced changes in [Ca2+]i oscillations. Thus, we also examined the effect of ICI 182,780 on the E2-induced LHRH release. Cells were exposed to ICI 182,780 (100 nm) for a 40-min period starting 20 min before the E2 exposure. As shown in Fig. 3, E2 at 1 nm (n = 8) clearly induced a significant increase in LHRH release in the presence of ICI 182,780 (for both, P < 0.05 vs. before E2 and ICI 182,780 alone). Although LHRH release in ICI 182,780 plus E2 appeared to be lower than that in E2 alone, values were not statistically different. ICI 182,780 at 100 nm alone (n = 8) did not cause a statistically significant effect.
Figure 3.
Treatment with ICI 182,780 does not block the E2-induced LHRH release. E2 at 1 nm or vehicle was infused for 20 min in the presence of ICI 182,780 at 100 nm. Note that E2 with ICI 182,780 resulted in an increase in LHRH release, whereas vehicle with ICI 182,780 did not cause any significant effect. *, P < 0.05 vs. before E2; a, P < 0.05 vs. vehicle.
The E2-induced LHRH release failed to occur in pertussis toxin (PTX)-treated LHRH neurons (experiment 1)
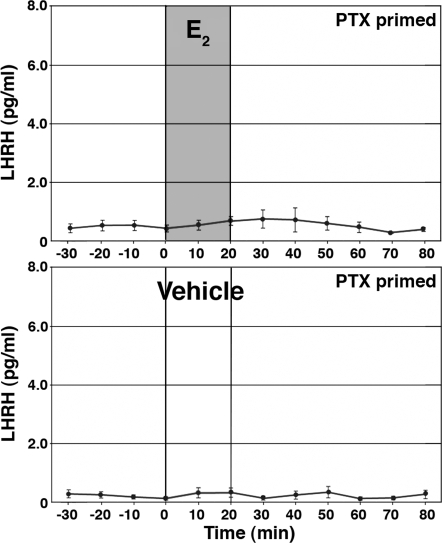
To examine the possible involvement of G protein-coupled receptors (GPCRs) mediating E2 action, we examined whether the E2-induced LHRH release could be seen in LHRH cells treated with PTX (100 ng/ml concentration), a broad-spectrum G protein blocker, for 16–20 h. The dose and exposure time were based on Filardo et al. (13). As shown in Fig. 4, E2 did not cause increases in LHRH release in cultures treated with PTX (n = 13). Vehicle infusion in PTX-treated cultures did not cause any changes (n = 13). The high KCl challenge test at the end of the experiments indicated that LHRH neurons were viable throughout the study even after PTX treatment (data not shown).
Figure 4.
Treatment with PTX blocked the E2-induced LHRH release. E2 at 1 nm or vehicle was infused for 20 min in cells treated with PTX for 16–20 h. Note that E2 failed to stimulate LHRH release.
Identification of GPR30 mRNA in the olfactory placode and hypothalamus (experiment 2)
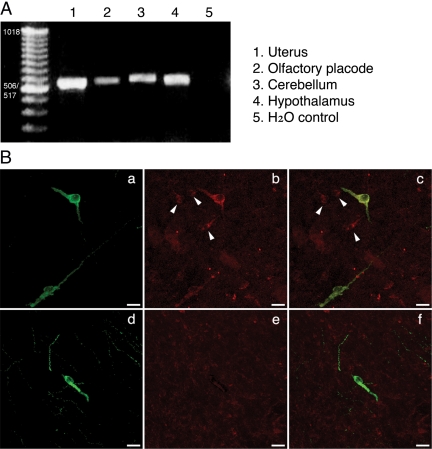
Because we speculated a possible involvement of GPR30 in the rapid E2 action, we examined the presence of GPR30 mRNA in placode tissue as well as in the hypothalamus, cerebellum, and uterus using RT-PCR with primers derived from human cDNA sequences. As shown in Fig. 5A, cDNA products identified as GPR30 were amplified by RT-PCR in all tissues examined. Subsequently, GPR30 cDNAs obtained from olfactory placode cultures were cloned and sequenced for identification of monkey GPR30 cDNAs. The results revealed that rhesus monkey cDNA sequences were similar, but not identical, to those found in humans, i.e. 96% identity at the position between bp 258 and 896. Deduced amino acid sequence of GPR30 between the rhesus monkey and human had 99.0% identity (data not shown). The cDNA and deduced amino acid sequences were identical with sequences predicted by the rhesus monkey genome program (GenBank data described XM_001084531). These results indicate that GPR30 was present in olfactory placode as well as in the brain of the rhesus monkey.
Figure 5.
A, The presence of GPR30 cDNA products were identified by RT-PCR in olfactory placode cultures, and samples were obtained from the uterus, cerebellum, and hypothalamus in female rhesus monkeys. GPR30 amplicons (∼600 bp) in olfactory placode cultures were cloned and sequenced for identification of the monkey GPR30 cDNA. H2O is control. B, Colocalization of GPR30 in LHRH neurons. Immunopositive LHRH neurons in the medial basal hypothalamus are shown in green (a and d), and immunopositive GPR30 neurons are shown in red (b). IgG does not cause any neuronal staining (e). Overlay pictures (c and f) show that LHRH neurons are double stained with GPR30 (yellow), but non-LHRH neurons (arrowheads) express only GPR30.
Colocalization of GPR30 protein in LHRH neurons (experiment 2)
To identify GPR30 proteins in LHRH neurons, sections from the monkey hypothalamus were immunostained with antibodies for LHRH and GPR30 (Fig. 5B). Analysis by confocal microscopy indicated that a subset of LHRH neurons (Fig. 5Ba) also expressed GPR30 (Fig. 5B, b and c). Interestingly, non-LHRH neurons were also immunopositive for GPR30 (Fig. 5B, b and c). Exposure to preimmune serum as a control failed to immunostain any neurons (Fig. 5B, e and f).
Effects of PTX on E2-induced changes in [Ca2+]i oscillations (experiment 3)
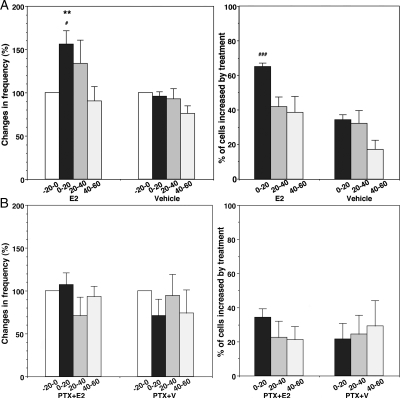
To explore the molecular mechanism of rapid E2 action, the effects of PTX on E2-induced changes in [Ca2+]i oscillations were examined. In concurrently conducted control experiments (n = 241 for E2 and n = 251 for vehicle in six cultures each) without PTX treatment, E2 (1 nm) resulted in increases in the frequency of [Ca2+]i oscillations and the percent of stimulated cells (Fig. 6A), similar to data reported previously (11). By contrast, in PTX-treated cultures, E2 (1 nm) neither stimulated the frequency of [Ca2+]i oscillations nor increased the percent of stimulated LHRH neurons (n = 227 for E2 and n = 237 for vehicle in six cultures each; Fig. 6B). The high KCl challenge test at the end of the experiments indicated that LHRH neurons were viable throughout the study regardless of the treatments.
Figure 6.
A, E2 elicited an increase in the frequency (left) and the percent of stimulated cells (right) in control cultures. In contrast (panel B), the E2-induced increase in the frequency of [Ca2+]i oscillations (left) and the percent of stimulated cells (right) failed to occur in cells pretreated with PTX. **, P < 0.01 vs. −20-0 (within treatment); #, P < 0.05 vs. vehicle; ###, P < 0.001 vs. vehicle at corresponding time (between treatments).
As seen in our previous study, E2 resulted in a more frequent synchronization of [Ca2+]i peaks among LHRH neurons (P < 0.05), whereas vehicle treatment did not induce any change in the synchronization pattern (Table 1). In contrast, E2 application in the presence of PTX failed to induce an increase in synchronization (Table 1). PTX alone did not cause any significant effects. These results suggest that a GPCR appeared to be involved in rapid E2 action.
Table 1.
Effects of treatments on the synchronization of calcium oscillations in primate LHRH neurons
| Treatments | Synchronizations in the 60-min period after initiation of treatment (n) |
|---|---|
| E2 (1 nm) | 2.17 ± 0.41a |
| Vehicle | 0.67 ± 0.33 |
| PTX + E2 (1 nm) | 1.33 ± 0.42 |
| PTX + vehicle | 1.00 ± 0.63 |
| siRNA + E2 (1 nm) | 0.83 ± 0.40 |
| siRNA + vehicle | 0.67 ± 0.21 |
| Control siRNA + E2 (1 nm) | 2.50 ± 0.62a |
| Control siRNA + vehicle | 0.67 ± 0.33 |
| siRNA + EDC (1 nm) | 0.83 ± 0.54 |
| Control siRNA + EDC (1 nm) | 2.33 ± 0.33a |
| G1 (1 nm) | 0.67 ± 0.21 |
| G1 (10 nm) | 1.50 ± 0.50 |
| Vehicleb | 1.33 ± 0.24 |
| ICI 182,780 (0.1 μm)b | 0.83 ± 0.17 |
| ICI 182,780 (1 μm) | 1.17 ± 0.40 |
Effects of GPR30 small interfering RNA (siRNA) on E2-induced changes in [Ca2+]i oscillations (experiment 3)
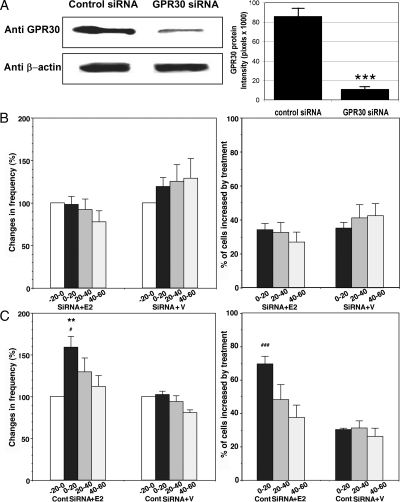
Because E2 failed to induce changes in cells treated with PTX, next we examined the effects of E2 on [Ca2+]i oscillations in primate LHRH neurons, in which GPR30 was knocked down by transfection with human siRNA for GPR30. The specificity of GPR30 siRNA in monkey placode cells was determined by Western blot analysis. The results indicated that monkey GPR30 protein expression in placode cells transfected with GPR30 siRNA was significantly lower (P < 0.001, n = 3) than that in placode cells transfected with control siRNA (Fig. 7A).
Figure 7.
A, Transfection of cultures with human GPR30 siRNA reduced GPR30 protein expression. Immunoblot of membrane protein with antibodies against GPR30 or β-actin from control siRNA (lane 1) and GPR30 siRNA (lane 2) transfected cultures is shown on the left. Densitometry analysis, performed with ImageQuant software using results from three separate experiments, is shown on the right. Note that transfection with GPR30 siRNA, but not control siRNA, significantly (P < 0.001) reduced GPR30 expression in olfactory placode cultures. B, The E2-induced increases in the frequency of [Ca2+]i oscillations (left) and the percent of stimulated cells (right) failed to occur in cells transfected with GPR30 siRNA. C, In contrast, E2 caused changes in [Ca2+]i oscillations in cells transfected with control siRNA. **, P < 0.01 vs. −20-0 (within treatment); #, P < 0.05 vs. siRNA; ###, P < 0.001 vs. siRNA at corresponding time (between treatments).
Examination of E2 effects in GPR30 siRNA-transfected LHRH neurons (n = 237 for E2 and n = 228 for vehicle in six cultures each) indicated that neither E2 nor vehicle caused any significant changes in [Ca2+]i oscillations (Fig. 7B). In contrast, in control siRNA-transfected LHRH neurons (n =186 for E2 and n = 226 for vehicle in six cultures each), E2, but not vehicle, increased the frequency of [Ca2+]i oscillations as well as the percent of stimulated cells (Fig. 7C).
E2 clearly increased synchronization of [Ca2+]i peaks among LHRH neurons (P < 0.05) in control siRNA-transfected LHRH neurons. However, in GPR30 siRNA-transfected LHRH neurons E2 failed to induce synchronization of [Ca2+]i peaks among LHRH neurons (Table 1). Vehicle did not change any synchronization pattern in either case. Again, the high KCl challenge test at the end of the experiments indicated that LHRH neurons were viable throughout the study regardless of the treatments.
Effects of GPR30 siRNA on EDC-induced changes in [Ca2+]i oscillations (experiment 3)
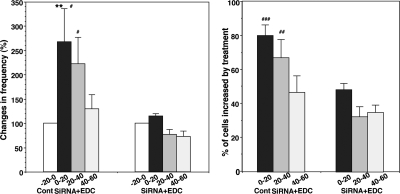
To further examine the role of GPR30, we next tested the effect of GPR30 siRNA on EDC-induced changes in [Ca2+]i oscillations. EDC failed to cause an increase in [Ca2+]i oscillations in GPR30 siRNA-transfected LHRH neurons (n = 274 in six cultures), whereas EDC clearly caused significant increases in [Ca2+]i oscillations in control siRNA-transfected LHRH neurons (n = 296 in six cultures, Fig. 8).
Figure 8.
Transfection of cells with human GPR30 siRNA abrogated EDC-induced changes in [Ca2+]i oscillations. Whereas in control siRNA-transfected cells EDC increased the frequency of [Ca2+]i oscillations (left) and the percent of stimulated cells (right), in cells transfected with siRNA for human GPR30 the EDC-induced changes in [Ca2+]i oscillations failed to occur. **, P < 0.01 vs. −20–0 (within treatment); #, P < 0.05 vs. siRNA; ##, P < 0.01 vs. siRNA; ###, P < 0.001 vs. siRNA at corresponding time (between treatments).
Similar to E2, in control siRNA-transfected LHRH neurons, EDC clearly increased synchronization of [Ca2+]i peaks among LHRH neurons (P < 0.05), whereas in GPR30 siRNA-transfected LHRH neurons the EDC-induced increase in synchronization was not observed (Table 1).
Effects of the GPR30 agonist, G1, on [Ca2+]i oscillations (experiment 3)
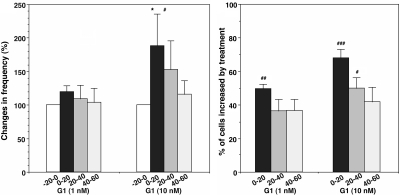
If GPR30 plays a role in rapid action of E2, G1 (a GPR30 agonist; see Ref. 19) should cause changes in [Ca2+]i oscillations similar to E2. Results indicated that G1 at 10 nm, but not 1 nm, significantly increased the frequency of [Ca2+]i oscillations (n = 239 and n = 245, for 1 and 10 nm in six cultures each, respectively), and both 10 and 1 nm concentrations of G1 increased the percent of stimulated cells (Fig. 9). Vehicle did not cause any changes (data not shown; n = 208). Although G1 at 10 nm, but not 1 nm, had a tendency to increase synchronization of [Ca2+]i peaks among LHRH neurons, it was not statistically significant (Table 1).
Figure 9.
G1 at 10 nm, but not 1 nm, caused an increase in the frequency of [Ca2+]i oscillations (left), and G1 both at 1 and 10 nm increased the percent of stimulated cells (right). *, P < 0.05 vs. −20–0 (within treatment); #, P < 0.05 vs. vehicle or G1 (1 nm); ##, P < 0.01 vs. vehicle; ###, P < 0.001 vs. vehicle or G1 (1 nm) at corresponding time (between treatments).
Effects of a high dose of ICI 182,780 on [Ca2+]i oscillations (experiment 3)
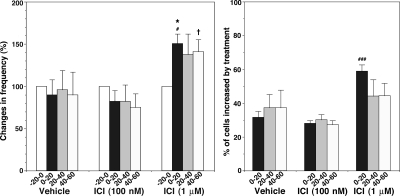
The agonistic action of ICI 182,780 at 1 μm or a higher dose for GPR30 has been reported in cancer cells (14). Thus, we examined the effect of ICI 182,780 at 1 μm on [Ca2+]i oscillations. In a previous study, we did not observe any significant changes with 0.1 μm (Ref. 11; also Fig. 10). However, ICI 182,780 at 1 μm concentration induced significant stimulatory action (P < 0.05) on the frequency of [Ca2+]i oscillations and the percent of stimulated cells (n = 225 in six cultures; Fig. 10). Synchronization of [Ca2+]i peaks among LHRH neurons was not modified by 1 μm ICI 182,780 (Table 1).
Figure 10.
ICI 182,780 at 1 μm caused an increase in the frequency of [Ca2+]i oscillations (left) and the percent of stimulated cells (right). For comparison, the data from the treatments with vehicle and 100 nm ICI 182,780, reported in a previous study (11) are included. *, P < 0.05 vs. −20-0 (within treatment); #, P < 0.05 vs. vehicle or ICI (100 nm); ###, vs. vehicle or ICI (100 nm) at corresponding time (between treatments); †, P < 0.05 vs. ICI at 100 nm, at corresponding time (between treatments).
Discussion
The results in this and previous (11) studies that ICI 182,780 neither blocked the E2-induced LHRH release nor abrogated [Ca2+]i oscillations suggest that rapid action of E2 in primate LHRH neurons is mediated by a novel ER. The present study further indicates that GPR30 appears to be, at least in part, involved in the rapid action of E2.
Previously, we have shown that E2 rapidly increases firing activity and the frequency of [Ca2+]i oscillations in LHRH neurons within 1 min (9,11). Because both increases in firing activity and [Ca2+]i oscillations are excitatory signals of LHRH neurons, in the present study we examined whether the LHRH release pattern was modified by E2 treatment. The results indicated that E2 stimulates LHRH release within 10 min. This finding is in line with the basic neurophysiological property showing that excitation of LHRH neurons by E2 results in neurosecretion. This rapid excitatory E2 action on LHRH release is not limited to primate LHRH neurons. In cultured mouse LHRH neurons as well, E2 causes excitatory action, because E2 stimulates [Ca2+]i oscillations and their synchronizations (8) and [Ca2+]i oscillations reflect firing activity of neurons (20). These authors did not examine the effect of E2 on LHRH release. Moreover, collateral studies with GT1–7 cells in our laboratory show that E2 induces not only an increase in the frequency of [Ca2+]i oscillations, but also LHRH release with a short latency (Noel and Terasawa, unpublished observation). Thus, it is clear that E2 can cause a rapid excitation in LHRH neurons leading to LHRH neurosecretion in vitro.
One might say that the findings of the present study are inconsistent with a well-established concept that E2 suppresses release of LHRH (and LH) in vivo with a latency of 1–2 h, whereas E2 stimulates release of LHRH (and LH) in vivo with a latency of anywhere from 12 to 48 h (depending on species) after its administration [(21,22); also see Refs. 23 and 24]. Although whether rapid action of E2 on LHRH release occurs in the hypothalamus in vivo is unknown, there are several differences between the observations in this study and in vivo results. First, in cultured LHRH neurons, E2 causes LHRH release with a significantly shorter time domain (within minutes rather than hours). Second, the excitatory E2 action in cultured primate LHRH neurons is mediated through extranuclear ER, whereas E2 action in in vivo studies is mediated by nuclear ER. In fact, this and previous studies have shown that ICI 182,780, a classical ER blocker, failed to block not only the E2-induced changes in [Ca2+]i oscillations, but also LHRH release. Moreover, EDC, a nuclear membrane-impermeable estrogen, caused effects similar to those of E2. Third, the effect of E2 reported in this study is direct action to LHRH neurons, not mediated by other interneurons. Previously we reported that our cultures contain no glia and almost no other neurons (25,26), and excitatory effects of E2 on LHRH neurons were also induced in the presence of tetrodotoxin (9,11). In contrast, in in vivo studies E2 action through ERα is likely mediated by interneurons or glia, and not direct to LHRH neurons. Mouse LHRH neurons contain only ERβ, not ERα (27), whereas interneurons, such as kisspeptin, neuropeptide Y, opioid, glutamate, and γ-aminobutyric acid neurons, as well as glia, contain ERα (24). Studies in transgenic mice further suggest that the positive feedback effect of E2 requires ERα, which interacts with an estrogen-response element, whereas the negative feedback action of E2 is mediated, in part, by an estrogen-response element -independent ERα signaling mechanism (28,29,30). Presently, whether primate LHRH neurons express ERα is unknown, although the presence of ERβ in primate LHRH neurons has been reported (31). Nonetheless, in primates as well, interneurons and glia may play a significant role in E2 feedback mechanisms in vivo, because interneurons and glia express ERα and ERβ (24).
The results in the present study indicate that rapid E2 action appears to be mediated, in part, by GPR30. First, E2 did not cause changes in either [Ca2+]i oscillations or LHRH release when LHRH neurons were treated with PTX, suggesting that the effects of E2 are mediated by a PTX-sensitive pathway, as seen in cancer cells (13). Second, in GPR30 siRNA-transfected LHRH neurons, E2 failed to induce changes in [Ca2+]i oscillations, whereas it caused an effect in LHRH neurons transfected with control siRNA. Similarly, EDC was not effective in inducing changes in [Ca2+]i oscillations in GPR30 siRNA-transfected LHRH neurons but was effective in control siRNA-transfected LHRH neurons. Finally, G1, a selective agonist for GPR30, at 10 nm resulted in an increase in the frequency of [Ca2+]i oscillations and percent of stimulated cells. Because it has been reported that ICI 182,780 at doses higher than 1 μm can be an agonist for GPR30 (14), we also examined the effect of 1 μm ICI 182,780. To our surprise, 1 μm, but not 100 nm, induced increases in the frequency of [Ca2+]i oscillations and percent of stimulated cells. However, unlike E2 and EDC, neither G1 at 10 nm nor ICI 182,780 at 1 μm caused significant changes in the synchronization frequency of [Ca2+]i oscillations. The physiological significance of [Ca2+]i synchronization is unclear at this time. Nonetheless, effects caused by G1 at 10 nm and ICI 182,780 at 1 μm are not identical to the actions of E2 and EDC, indicating that multiple mechanisms appear to be involved in the rapid E2 action in primate LHRH neurons.
The dose-dependent action of ICI 182,780, is quite intriguing. In our previous study (11) ICI 182,780 at 100 nm, a dose that should sufficiently antagonize the effect of 1 nm E2 through ERα/ERß receptors (32), failed to elicit changes in [Ca2+]i oscillations, and in the present study ICI 182,780 at 100 nm did not cause a significant change in LHRH release (Fig. 3). In contrast, without E2, ICI 182,780 at 1 μm, but not 100 nm, stimulated [Ca2+]i oscillations (Fig. 10). Although in the present study we did not examine the effect of 1 μm ICI 182,780 on LHRH release, these data are interpreted to mean that whereas the failure of ICI 182,780 at 100 nm to compete with E2 is due to the absence of ERα/ERß receptor blockade, ICI 182,780 at a higher dose (1 μm) appears to cause an agonistic action to GPR30. A similar finding has been reported in cancer cells (14).
It is possible that GPR30 in LHRH neurons is associated with ERα or ERβ, because it has been proposed that GPCRs may mediate E2 action in coupling with membrane ERα (see Ref. 33). For example, in hippocampal neurons E2 modifies signaling of the GPCR metabolic glutamate receptors, together with ERα and ERβ (34,35). However, membrane receptors can mediate E2 action independent of ERα and ERβ, the nonsteroidal ligand STX, which mimics E2 action in the brain (36), causes an effect similar to E2 in hypothalamic neurons in wild-type mice as well as in ERα and ERβ knockout mice (37). Two questions are currently under investigation: 1) whether GPR30 involved in rapid E2 action in primate LHRH neurons requires an interaction with ERα or ERβ; and 2) whether E2 action in primate LHRH neurons is mediated by two independent mechanisms, i.e. GPR30 and ERα/ERβ.
A significant number of publications support the hypothesis that rapid action of E2 is mediated by GPR30 (see reviews in Refs. 17 and 18). Activation of GPR30 by E2 in cancer cells results in transactivation of adenylyl cyclase and epidermal growth factor receptor, resulting in an intracellular cAMP increase and increases in phospholipase C, phosphatidyl-inositol 3 kinases and MAPKs, such as Erk-1/-2 (13,14). Phospholipase C activation leads to [Ca2+]i mobilization. There are a couple of publications against this hypothesis (38,39). In the present study we did not examine whether E2 activates epidermal growth factor receptor or which signal transduction pathways are involved in E2 action. Therefore, the question of whether intracellular signal transduction mechanisms specific to the rapid action of E2, similar to those described in cancer cells (16,17,18), occur in primate LHRH neurons remains to be determined.
Controversy exists as to the subcellular localization of GPR30. Filardo and collaborators (13,14) have shown the presence of GPR30 in the plasma membrane, whereas Prossnitz and collaborators (40) have shown the presence of GPR30 in the endoplasmic reticulum, Golgi apparatus, and nuclear membrane. Another group reports that subcellular localization of GPR30 was dependent on the cell type, such that GPR30 was in the membrane of hippocampal neurons, whereas in cancer cells, GPR30 was present in the endoplasmic reticulum (41). Newer studies, however, report that GPR30 was found in the endoplasmic reticulum and Golgi apparatus in hippocampal neurons (42,43). However, Filardo and colleagues (44) have recently suggested that GPR30 was located at the cell surface and, upon E2 stimulation, GPR30 was sequestered from cell surface resulting in codistribution with clathrin-coated vesicles. Internalization of seven-transmembrane receptors commonly occurs as a consequence of receptor endocytosis after ligand binding (see Ref. 45). Although subcellular localization of GPR30 in LHRH neurons remains to be investigated, we believe that E2 in primate LHRH neurons appears to signal from the plasma membrane based on the following reasons. First, in this and our previous (11) studies, EDC caused stimulatory action in LHRH neurons within 10 min. Second, the data indicate that plasma membrane-impermeable E2-BSA also stimulated LHRH release, similar to EDC. Interestingly however, the LHRH responses to both EDC and E2-BSA were not as large as that exposed to E2, suggesting that multiple mechanisms may be present for the rapid action of E2.
Recently, Madak-Erdogan et al. (46) reported that gene stimulation by EDC was mediated by ERα, rather than GPR30, similar to gene stimulation by E2, because EDC significantly reduced gene stimulation in ERα siRNA knockdown cancer cells, whereas EDC stimulated gene expression in cells in the absence of GPR30 (i.e. siRNA knock-down cells). Moreover, in the same study G1 did not cause any gene stimulation, in contrast to G1 effects in this study. A rapid action of EDC may differ from EDC action causing gene transcription, because in the present study EDC caused its effect within 10 min, whereas in that study cells were exposed EDC for 2 h. In fact, it has been shown that EDC induces ERK phosphorylation within 2 min in cancer cells (10). Therefore, EDC, like E2, may cause its action through two different mechanisms, such as GPR30 and ERα/ERβ-mediated mechanisms. Further investigation with signal transduction experiments in LHRH neurons is needed for this speculation.
In conclusion, in the present study we found that E2 induces a rapid excitatory effect on primate LHRH neurons, and this rapid action of E2 appears to be mediated through GPR30. Perhaps, for LHRH neurons and other neurons, a mechanism of rapid excitatory action through GPR30 provides a tool for efficient neuronal function, such as the release of neuropeptides and neurotransmitters at their terminals. Nonetheless, the role of GPR30 in mediating E2 action is probably partial, because other receptors appear to be involved in cumulative action of E2 in LHRH neurons. This speculation is based on our observations that the effect of the nuclear membrane-impermeable estrogen, EDC, on LHRH release was shorter than that of E2, and the effect of the GPR30 agonist, G1, on [Ca2+]i oscillations was not as effective as E2. Although this speculation may contradict with seemingly complete elimination of the E2-induced changes in [Ca2+]i oscillations in GPR30 siRNA-transfected cells, we will not be able to solve this discrepancy until we have an answer to the question of whether two different extranuclear receptor-mediated mechanisms are operated independently or cooperatively.
Materials and Methods
Animals
Rhesus monkey embryos (Macaca mulatta) from time-mated pregnancies were delivered by cesarean section under isoflurane anesthesia. A total of 18 fetuses at embryonic d 35 (E35) to E37 were used in this study. All experimental procedures were carried out in accordance with the standards outlined in Principles for Use of Animals and Guide for the Care and Use of Laboratory Animals. The protocol used in the studies was approved by the Animal Care and Use Committee of the University of Wisconsin-Madison.
Tissue culture
Culture methods for LHRH neurons derived from the olfactory placode region have been described previously (25,47). Briefly, the olfactory placode region and ventral migratory pathway of LHRH neurons (terminal nerve region) were dissected out and divided into small (<0.5 mm3) pieces. For calcium imaging experiments, two to three pieces were plated on 25-mm round collagen-coated glass coverslips, and for perifusion experiments four to six pieces were plated on a 25-mm round collagen-coated plastic coverslip (Nalge NUNC International, Rochester, NY), producing 16–24 cultures each. Cultures were grown in growth medium (Medium 199, Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT), 0.6% glucose, and 50 μg/ml gentamycin (Sigma) under 1.5% CO2 at 37 C for at least 2 wk before experiments. On the third to fourth day in culture, cells on coverslips were exposed to the antimitotic agent 5-fluoro-5-deoxyuridine 30–40 μm for 3 d. Medium was replaced every 1–3 d. All experiments were conducted at 2–4 wk of culture.
Measurement of changes in intracellular calcium
[Ca2+]i levels were assessed by the method described previously (26,48) with some modifications. Cultured cells were loaded with 18 μm fura-2 AM (Teflab, Austin, TX) and 6 μl of a mixture of pluronic F-127 (BASF Pharma/Knoll AG, Parsippany, NY) for 30 min at 37 C under 1.5% CO2. The coverslip was then placed in a Dvorak-Stotler recording chamber. Fluorescence imaging of the dye-loaded cells was achieved with an inverted microscope. A culture was viewed through a ×20 objective lens, and a 750- × 750-μm recording field that contained the appearance of LHRH neurons was selected for data capture. Cultures were continuously perifused at a speed of 50 μl/min with serum free medium at pH 7.4, under 95% O2 and 5% CO2 at room temperature (22–25 C).
[Ca2+]i was monitored as a function of the ratio of the 510-nm fura-2 emission excited by illuminations at 340 and 380 nm with a Lambda DG-4 light source and filter exchanger (Sutter Instruments, Novato, CA). Fura-2 fluorescence was recorded at 10-sec intervals with a charge-coupled device camera (Photometrics, Tucson, AZ) and Metafluor imaging software (Molecular Devices Corp., Downingtown, PA). Images were acquired under low-light conditions, increasing the sensitivity of the camera while maintaining low levels of background noise. The ratio of the fluorescence intensities (ΔF/F0) from the 340- and 380-nm excitation wavelength-evoked images was used to calculate the free [Ca2+]i levels and saved as a text file with Excel for later analysis.
LHRH neurons for calcium imaging in cultures were easily identifiable based on their morphology, size, unique appearance, and migratory pattern: They appeared ovoid in shape with a large (≥10 μm) soma and large somatic processes, which form neuronal bundles. However, because our cultures contain numerous epithelial cells, fibroblasts, and other types of unidentified cells and occasionally a small number of non-LHRH neurons (25,26), it was important to confirm whether recorded cells were actually LHRH neurons. To test the viability of cells all cultures were routinely challenged with 56 mm KCl at the end of experiments. After the completion of each [Ca2+]i imaging experiment, a photomicrograph of the viewing area was taken, and the reference grid number of the coverslip was recorded for later identification of LHRH neurons with immunostaining (see below), and confirmation was made that recorded cells were LHRH neurons in each case.
Perifusion experiments
Perifusion experiments were conducted using Sykes-Moore chambers as described previously (47). Briefly, 2–4 wk after the initiation of culture, LHRH neurons on two coverslips were placed face-to-face, separated by a rubber O-ring, forming a chamber with a volume of 200 μl. Before the initiation of sample collection, cultured cells were then perifused with a modified Krebs-Ringer phosphate buffer (25) with 0.05% BSA and 0.1% glucose (pH = 7.4) under 95% O2 and 5% CO2at 37 C for at least 1 h. Perifusates were collected at 30 μl/min in 10-min fractions for up to 6 h using the ACUSYST (Endotronics, Minneapolis, MN) perifusion system. To test the viability of cells, all cultures were challenged with 56 mm KCl at the end of experiments. All samples were stored at −80 C for later LHRH RIA. After the perifusion experiment, cells were fixed with 2% paraformaldehyde (pH = 7.6) and immunostained for LHRH and neuron-specific enolase (see below).
RT-PCR analysis, cloning, and sequencing
To determine whether the rhesus monkey brain and olfactory placode region contained GPR30 mRNAs, total RNA from the hypothalamus and cerebellum of one adult rhesus monkey brain and 20 olfactory placode cultures from six fetuses were isolated using RNA STAT-60 (Tel-Test, Friendswood, TX) following the manufacturer’s protocol. Briefly, the tissue was homogenized (1 ml of RNA STAT-60 per 50–100 mg of tissue), chloroform was added (0.2 ml/ml homogenate), and the mixture was spun. To precipitate RNA, isopropanol was added (0.5 ml) to the aqueous layer. The RNA pellet was washed (75% ethanol), air dried, and resuspended (diethylpyrocarbonate-treated water). Individual RNA samples were then analyzed for the presence of GPR30 mRNAs using RT-PCR.
For reverse transcription (RT), 1 μg of each sample was used. First-strand cDNA was synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. After RT, 3 μl aliquots of each reaction were used in PCR. PCR primers designed to detect GPR30 mRNA were based on human cDNA sequences (see below) and were synthesized by the University of Wisconsin Biotechnology Center. Each RT aliquot used for PCR was combined with 2.5 mm deoxynucleotide triphosphates (Amersham Biosciences, Princeton, NJ), 1× PCR Buffer II with MgCl2, 1.25U AmpliTaq (Applied Biosystems, Branchburg, NJ), and 12.5 pmol of either GPR30 or glyceraldehyde-3-phosphate dehydrogenase primers in a final volume of 50 μl. LHRH and glyceraldehyde-3-phosphate dehydrogenase primers were used as PCR-positive controls. Negative controls for PCR used aliquots of water mixed with each PCR/primer cocktail. PCR primer sequences and GenBank accession numbers referenced for their design are as follows: Upstream primer (5′-ATGGATGTGACTTCCCAAGC-3′) and downstream primer (5′-AACCTCACATCCGACTGCTC-3′) were derived from the alignment of human GPR30 mRNA sequences (accession no. AF027956). Upstream and downstream primers start at positions 1 and 1112 within the coding region of these mRNAs. PCR amplification conditions consisted of one cycle at 94 C for 1 min, 55 C for 30 sec, 72 C for 2 min; 40 cycles at 94 C for 30 sec, 55 C for 30 sec, 72 C for 2 min; and a 72 C incubation for 10 min.
After PCR amplification, 20-μl aliquots of each reaction were run on a 1% agarose TBE gel with ethidium bromide. Target PCR products were isolated and purified with the Geneclean II kit (Bio101, La Jolla, CA), and sequenced on an AB1 3700 DNA analyzer (University of Wisconsin Biotechnology Center) using the BigDye Terminator mix (PE Applied Biosystems, Foster City, CA).
Transfections
After 2 wk of cell plating, cultured LHRH neurons were transferred to serum free medium without phenol red (DMEM/F-12; Sigma, St. Louis, MO) and transiently transfected with human GPR30 siRNA or nonspecific presynthesized control siRNA using Fugene HD Transfection Reagent (Roche Diagnostics, Indianapolis, IN) at 37 C, following the manufacturer’s instruction to interfere with GPR30 expression. Sequences of GPR30 siRNA provided by Dharmacon (Lafayette, CO) were 5′-CUUCAGCGAAUCUCACUCCUU-3′ for sense and 5′-UUGAAGUCGCUUAGAGUGAGGAA-3′ for antisense. Experiments were conducted 48–72 h later.
Protein isolation and Western blot analyses
Olfactory placode cells were homogenized in lysis buffer (1% Triton X-100, 100 mm NaCl, 50 mm HEPES, 10 mm EDTA, 2 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 10 mm NaF, 4 mm Na4P2O7, 5 μg/ml leupeptin, 5 μg/ml aprotinin) at a ratio of 1 g tissue per 5 ml buffer. Cellular debris was cleared by centrifugation (12,000 × g, 15 min, 4 C). The protein concentration of the supernatant was determined using a Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) with BSA as the standard.
Proteins were denatured in Laemmli, separated on 10% SDS-PAGE gels, and transferred to Immobilon-P polyvinylidene fluoride membranes (Sigma). Blots were blocked in 5% nonfat milk/TBST (Tris-buffered saline, 0.1% Tween 20) at room temperature for 1 h, incubated with either an affinity purified polyclonal human GPR30 antibody raised in a rabbit (1:200 dilution, described in Ref. 12) or actin (1:1000 dilution) in 2% nonfat milk/TBST overnight at 4 C, rinsed three times for 10 min each with TBST at room temperature, incubated with goat antirabbit IgG horseradish peroxidase conjugate (1:5000 dilution of 1 mg/ml stock; Jackson ImmunoResearch Laboratories, West Grove, PA), and then rinsed three times for 10 min each with TBST. Immunoreactive proteins were detected using enhanced chemiluminescence (ECL Plus Western Blotting Detection Reagents; Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s recommendations. Bands were imaged using a Storm model 840 PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA). Equal loading was assessed by reprobing the same blot with an antibody against the β-actin. Protein expression was quantitated using ImageQuant software (Molecular Dynamics, Inc.) and standardized for loading control.
Immunocytochemistry
To determine the colocalization of GPR30 in LHRH neurons, a serial section of the hypothalamus was double immunostained with an affinity-purified polyclonal human GPR30 antibody [1:400 (13)] and labeled with Texas Red and then exposed to the monoclonal antibody HU4H (gift from Dr. Henryk Urbanski, Oregon National Primate Research Center, 1:1000) and labeled with fluorescein isothiocyanate, as described previously (49). Immunostained images were obtained using a confocal microscope (Leica, model TCS SP2 AOBS; Leica Corp., Deerfield, IL) at the Wisconsin National Primate Research Center, University of Wisconsin-Madison. FITC and Texas Red were excited by an argon (488 nm) and helium-neon laser (594 nm), respectively, and a z-series of optical sections from immunostained LHRH neurons were collected at 0.5-μm intervals and images were reconstructed using NIH Image.
To identify whether the recorded cells were LHRH neurons, cultures were stained using standard immunocytochemical procedures with an antisera cocktail, GF-6 and LR-1 [gifts from Dr. N. M. Sherwood (University of British Columbia, Victoria, Canada; 1:9000 dilution) and Dr. R. A. Benoit (University of Montreal, Montreal, Canada; 1:15,000), respectively] for 40–42 h, VECTASTAIN ABC peroxidase system (VECTOR Laboratories, Burlingame, CA), and 3.3′-diaminobenzidine as the chromagen. LHRH-immunopositive neurons were identified by matching the photomicrograph and digitized fluorescence images (48,50). Neurons that were not LHRH immunopositive were excluded from analysis. Other cells, such as fibroblasts and epithelial cells, as well as cells yet to be identified, were defined as nonneural cells and also excluded from analysis.
LHRH assay
LHRH concentrations in media samples were measured in 200 μl aliquots (single) with RIA using antiserum R-1245 or R42 (a gift from Dr. T. Nett, Colorado State University, Fort Collins, CO) as described previously (47,50). Synthetic LHRH (Richelieu Laboratory, Inc., Montreal, Canada) was used for both the trace and the reference standard. The standard curve was constructed using the culture medium as a part of the assay buffer. The sensitivity of the assay at 95% binding was 0.05 pg/tube. Intra- and interassay coefficients of variation were 8.3% and 11.4%, respectively.
Data analysis
Effects of treatments (E2 vs. vehicle, EDC vs. DC, E2-BSA vs. BSA, E2 effects in the presence of ICI 182,780, and E2 effects in the presence of PTX) were examined using two-way ANOVA repeated measure followed by Dunnett multiple comparison test.
Peaks in [Ca2+]i oscillations were detected using Pulsar algorithm (51), as described previously (11). After the detection of [Ca2+]i peaks, the number of [Ca2+]i peaks, the amplitude of [Ca2+]i peaks, and the interpeak interval during 20-min blocks (−20 to 0, 0–20, 20–40, and 40–60 min after the treatment) were calculated in individual LHRH neurons from the Pulsar results. Subsequently, mean values for these parameters of all LHRH neurons (15–60 cells per culture) in each culture were calculated. Finally, for statistical comparison overall mean (±sem) for each treatment group was calculated from the mean value of each culture. For the purpose of graphic presentation the frequency (numbers per 20-min period) of [Ca2+]i peaks was normalized by designating the mean value at −20 to 0 min as 100%. All statistical analyses were conducted with raw data.
Synchronization of [Ca2+]i peaks among multiple numbers of cells in a culture was examined as previously described (11). Briefly, using our Pulsar algorithm (51), the average rate of [Ca2+]i peaks during the entire recording period for a single culture was calculated. Second, the rate of [Ca2+]i peaks occurring during 50-sec periods were plotted. A synchronization was considered to have occurred if the mean rate during the two successive 50-sec periods was greater than the mean + 3 sd, based on our previous observations (26,48). The total number of synchronizations occurring during a 60-min period after the initiation of treatment (i.e. 10 min during and 50 min after) was expressed as the synchronization frequency.
The effects of treatments (E2 in the presence or absence of PTX, E2 or EDC in GPR30 siRNA and control siRNA-transfected cells, G1, and ICI 182,780) on the frequency, amplitude, interpeak interval, and proportion of the cell number with increased [Ca2+]i oscillations during the 20-min block, were compared with those in respective controls using two-way ANOVA repeated measure, followed by Dunnett multiple comparison test. The effects of treatments on the synchronization frequency during the 60 min after the initiation of a treatment were compared with that in respective controls in the same 60-min period using t test (unpaired, two tailed). All groups consisted of six cultures per group. Data are presented as means ± sem. Statistical significance was established at P < 0.05.
Acknowledgments
We thank Drs. John and Benita Katzenellenbogen (University of Illinois, Urbana, IL) for the gift of EDC and control dendrimer. We also thank Samuel Frost for his technical assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01HD15433 and R01HD11355 (to E.T.), R01CA119165 (to E.J.F.), and T32 HD41921 (to S.D.N.) and was possible to perform by NIH supports (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: [Ca2+]i, Intracellular calcium; DC, dendrimer conjugate; E2 , 17β-estradiol; EDC, estrogen dendrimer conjugate; ER, estrogen receptor; GPR30, G protein-coupled receptor 30; LHRH, LH-releasing hormone; PTX, pertussis toxin; RT, reverse transcriptase; siRNA, small interfering RNA; TBST, Tris-buffered saline, 0.1% Tween 20.
References
- Selye H 1941 The anesthetic effect of steroid hormones. Proc Soc Exp Biol Med 46:116–121 [Google Scholar]
- Watson CS, Campbell CH, Gametchu B 1999 Membrane oestrogen receptors on rat pituitary tumour cells: immuno-identification and responses to oestradiol and xenoestrogens. Exp Physiol 84:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M 2000 Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev 52:513–556 [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 2005 Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol 26:65–84 [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW 2008 Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ 1995 Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS 2006 Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 20:491–502 [DOI] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E 2008 Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 149:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ 1997 Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45:607–617 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton Jr AR 2000 Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P 2005 GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–367 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ 2008 Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Constantin S, Wray S 2008 Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase A-dependent phosphorylation. Endocrinology 149:3500–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongthammakun S, Terasawa E 1993 Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- Mizuno M, Terasawa E 2005 Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta). J Neuroendocrinol 17:238–245 [DOI] [PubMed] [Google Scholar]
- Knobil E 1980 The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 36:53–88 [DOI] [PubMed] [Google Scholar]
- Herbison AE 2006 Physiology of the GnRH neuronal network. In: Neill JD, ed. Knobil and Neill’s physiology of reproduction. San Diego: Academic Press; 1415–1482 [Google Scholar]
- Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P 1993 A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- Richter TA, Keen KL, Terasawa E 2002 Synchronization of Ca2+ oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol 88:1559–1567 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM 2008 Classical estrogen receptor α signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 149:5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z 2007 Gonadotropin-releasing hormone neurons express estrogen receptor-β. J Clin Endocrinol Metab 92:2827–2830 [DOI] [PubMed] [Google Scholar]
- Hermenegildo C, Cano A 2000 Pure anti-oestrogens. Hum Reprod Update 6:237–243 [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER 2007 Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG 2005 Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG 2007 Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27:9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ 2003 Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ 2006 A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH 2008 G protein coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y 2006 G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ 2007 Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M 2008 Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett 441:94–99 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ 2002 Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Prog Neurobiol 66:61–79 [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Mogi K, Claude P 1999 Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Schanhofer WK, Keen KL, Luchansky L 1999 Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG 2005 Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- Gearing M, Terasawa E 1988 Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW 1982 Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]