Abstract
GnRH regulates gonadotrope function through a complex transcriptional network that includes three members of the immediate early gene family: Egr1, Jun, and Atf3. These DNA-binding proteins act alone or in pairs to confer hormonal responsiveness to Cga, Lhb, Fshb, and Gnrhr. Herein we suggest that the transcriptional response of Jun requires a functional interaction between the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of DNA-binding proteins and β-catenin (officially CTNNB1), a coactivator of TCF/LEF. Supporting data include demonstration that GnRH increases activity of TOPflash, a TCF/LEF-dependent luciferase reporter, in LβT2 cells, a gonadotrope-derived cell line. Additional cotransfection experiments indicate that a dominant-negative form of TCF7L2 (TCFDN) that binds DNA, but not β-catenin, blocks GnRH induction of TOPflash. Overexpression of AXIN, an inhibitor of β-catenin, also reduces GnRH stimulation of TOPflash. Transduction of LβT2 cells with TCFDN adenoviruses diminishes GnRH stimulation of Jun mRNA without altering expression of Egr1 and Atf3, two other immediate early genes that confer GnRH responsiveness. Reduction of β-catenin in LβT2 cells, through stable expression of short hairpin RNA, also selectively compromises GnRH regulation of Jun expression and levels of JUN protein. Finally, overexpression of TCFDN attenuates GnRH regulation of Cga promoter activity, a known downstream target of JUN. Together, these results indicate that GnRH regulation of Jun transcription requires a functional interaction between TCF/LEF and β-catenin and that alteration of either impacts expression of JUN downstream targets such as Cga.
A functional interaction between TCF/LEF proteins and β-catenin mediates GnRH regulated expression of Jun and downstream targets of JUN in gonadotropes.
The hypothalamic neurohormone GnRH acts through a specific G-protein coupled receptor (GPCR), the GnRH receptor (GnRHR), to regulate synthesis and secretion of LH and FSH from gonadotropes in the anterior pituitary (1,2,3,4,5,6). LH and FSH are heterodimers; their synthesis requires expression of three genes, Cga, Lhb, and Fshb (1,5). Cga encodes an α-subunit common to both hormones (1,5). Lhb and Fshb encode β-subunits unique to each hormone (1,5). Once secreted, LH and FSH bind to distinct GPCRs in male and female gonads to regulate gametogenesis and steroidogenesis (7,8,9). Together, these organs and hormones comprise a hypothalamic-pituitary-gonadal axis characterized by positive and negative feedback loops essential for maintaining proper reproductive function (1,2,3,4,5,6,7,8,9).
GnRH regulates synthesis of LH and FSH primarily at the level of transcription (1,5). Upon binding of GnRH to its receptor, transduction of the transcriptional signal flows through parallel cascades of multiple kinase families that phosphorylate a number of downstream targets including several DNA-binding proteins (10,11,12,13,14,15). Activation of these signaling cascades culminates in the regulated transcription of at least 75 other genes including Cga, Lhb, Fshb, and Gnrhr, four signature genes that endow gonadotropes with full functionality (16,17,18,19).
The accumulation of mRNAs regulated by GnRH, along with their encoded proteins, occurs in three distinct waves designated as primary, secondary, or tertiary (16,17,18,19,20). Cga, Lhb, Fshb, and Gnrhr reside within the tertiary network of GnRH-responsive genes. They respond more slowly to GnRH because changes in their transcription depend on the proteins encoded by the primary and secondary response genes (20). For example, three members of the primary GnRH response network are the immediate early genes (IEGs) Egr1, Atf3, and Jun; each encodes a unique DNA-binding protein that ultimately confers GnRH responsiveness to secondary and tertiary genes. Thus, EGR1 confers GnRH responsiveness to Lhb and to Atf3 (21,22). In addition, EGR1 autoregulates transcription of its own gene (23,24). In contrast, JUN, as a component of AP1, confers GnRH responsiveness to Cga, Fshb, and Gnrhr (25,26,27,28,29,30). Moreover, JUN also positively autoregulates its own expression (31). Finally, ATF3 contributes to the GnRH responsiveness of Cga by forming a heterodimer with JUN (15).
GnRH has recently been reported to stimulate the nuclear localization of β-catenin (officially CTNNB1) and modulate the activity of TOPflash, an artificial T-cell factor (TCF)/lymphoid enhancer factor (LEF)-dependent luciferase reporter in LβT2 cells (32). These changes were associated with parallel increases in GnRH-dependent mRNAs specific for Jun, Fra1, and Myc (32). These three genes are known to be regulated in cancer and hematopoietic cells by members of the TCF/LEF family and β-catenin (33,34). In addition, recent work has also revealed instances in which the full transcriptional activity of JUN on the Jun promoter requires TCF7L2 and β-catenin (33). Thus, these collective findings suggest that GnRH regulation of Jun expression requires a functional interaction between TCF/LEF family members and β-catenin and that alteration of either would have a secondary impact on the ability of JUN to confer GnRH responsiveness to downstream tertiary gene targets. The experiments reported herein address this possibility.
Results
β-Catenin mediates GnRH stimulation of a synthetic TCF-dependent promoter
To begin to examine whether GnRH signals through β-catenin to modulate the activity of TCF/LEF proteins, we conducted transient expression experiments in LβT2 cells with TOPflash, a luciferase-containing construct that harbors a synthetic TCF/LEF-dependent promoter that contains several adjacent TCF regulatory elements (35,36). TOPflash is also widely used as a readout of β-catenin activity (35,36). To attribute possible increases in TOPflash activity to a functional interaction between TCF proteins and β-catenin, a subset of cells were transfected with expression vectors that encode a dominant-negative form of TCF7L2 (TCFDN) that contains a DNA-binding domain but lacks a β-catenin-binding domain (37,38). Controls included cells transfected with a CMV expression vector that does not encode a protein. LβT2 cells were transiently transfected and treated with either vehicle or 100 nm GnRH for 24 h.
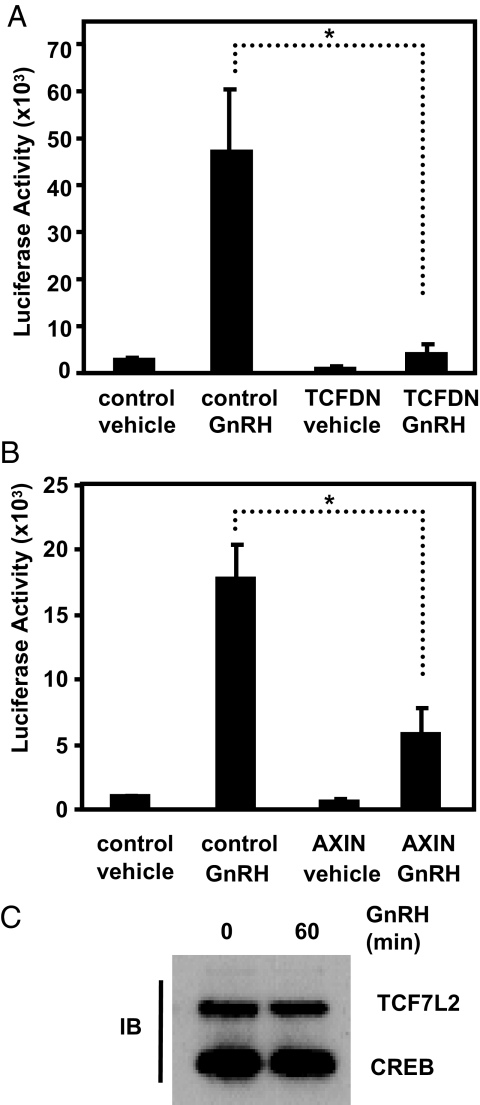
GnRH stimulated a large increase in TOPflash activity when compared with vehicle-treated controls (Fig. 1A). Overexpression of TCFDN in the absence of GnRH only marginally impacts the activity of TOPflash when compared with vehicle-treated cells. In contrast, TCFDN almost completely blocked GnRH stimulation. Gardner et al. (32) have also reported that TCFDN blocks GnRH-stimulated increases in TOPflash activity. Together, these results suggest that GnRH stimulates activity of TCF/LEF-dependent promoters and that TCF7L2 requires a β-catenin-binding domain for maximal activity.
Figure 1.
GnRH regulates TCF/LEF-dependent transcription through β-catenin and TCF/LEF. A, LβT2 cells were cotransfected with TOPflash (50 ng) and either an empty CMV expression vector (control) (100 ng) or a TCFDN-encoding expression vector (100 ng) and treated with vehicle or GnRH (100 nm) for 24 h, and resulting luciferase activity was measured. B, LβT2 cells were cotransfected with TOPflash (50 ng) and either an empty CMV expression vector (control) (100 ng) or an expression vector encoding AXIN (100 ng) and treated with vehicle or 100 nm GnRH for 24 h, and resulting luciferase activity was measured. C, Nuclear extracts were isolated from LβT2 cells treated with vehicle or 100 nm GnRH for 1 h followed by immunoblot (IB) using antibodies specific for TCF7L2 and CREB (loading control). Data shown are the means ± sem from three independent experiments performed in triplicate. *, P < 0.05.
Gardner and colleagues (32) also reported that GnRH stimulates nuclear accumulation of β-catenin in LβT2 cells. This is consistent with the notion that GnRH-stimulated increases in the amount and possibly activity of β-catenin relieve TCF/LEF-dependent promoters from repression (39). To provide additional evidence that GnRH stimulated TOPflash through β-catenin, we cotransfected LβT2 cells with TOPflash and an expression vector encoding AXIN. Overexpression of AXIN reduces the transcriptional effects of β-catenin by promoting degradation of the cofactor (40,41).
AXIN significantly reduced GnRH-stimulated increases in TOPflash activity (P < 0.05; Fig. 1B) but only marginally affected basal TOPflash activity when compared with control cells. Although it is clear that AXIN reduces GnRH-regulated increases in TOPflash activity, we are unable to verify reduced levels of β-catenin in these transient transfection experiments. This result is not surprising because LβT2 cells exhibit low transient transfection efficiency. Nevertheless, the AXIN-mediated reduction in GnRH regulation of TOPflash is consistent with the TCFDN result (Fig. 1A) and suggests GnRH regulation of a synthetic TCF-dependent promoter requires β-catenin. Immunoblot analysis of nuclear extracts from LβT2 cells indicated that levels of TCF7L2 are unchanged after treatment with GnRH (Fig. 1C). This result further supports the notion changes in β-catenin underlie changes in TCF7L2 activity.
Overexpression of TCFDN selectively attenuates GnRH stimulation of Jun expression without affecting expression of Atf3 or Egr1
GnRH induction of TOPflash suggests that the neurohormone signals through β-catenin to modulate the activity of a synthetic TCF/LEF-dependent promoter. The question remains, however, whether GnRH also regulates expression of endogenous genes in gonadotropes that are dependent on TCF/LEF and β-catenin. To address this possibility, we focused on Jun based on recent reports indicating that its expression in cancer and hematopoietic cell lines is TCF/LEF and β-catenin dependent (33,34).
To examine whether GnRH signals through TCF/LEF and β-catenin to increase expression of Jun, we transduced LβT2 cells with TCFDN-expressing adenovirus. LβT2 cells were then treated with 100 nm GnRH for 1 h, and resulting changes in gene expression were examined by real-time PCR after reverse transcription of gene products. Control cells were transduced with a GFP-expressing adenovirus.
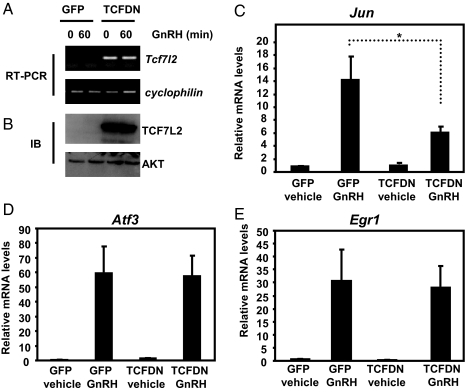
Transduced TCFDN mRNA was easily detected when compared with control cells transduced with the GFP adenovirus when examined by reverse transcription endpoint PCR (Fig. 2A). Note that the TCFDN adenovirus encodes human TCF7L2 that lacks the binding domain for β-catenin. Thus, there is no PCR signal in the GFP control because the primers used for real-time PCR are specific to human but not mouse Tcf7l2 mRNA. TCFDN-transduced LβT2 cells also exhibited high levels of TCFDN protein when examined by Western blot (Fig. 2B).
Figure 2.
GnRH-stimulated increases in Jun expression are reduced in TCFDN-expressing LβT2 cells. LβT2 cells were transduced with either GFP- or TCFDN-expressing adenoviruses (1 × 1011 vpm) and subsequently treated with either vehicle or 100 nm GnRH for 1 h. A, Representative endpoint RT-PCR experiment using primers that span either the DNA-binding domain of TCF7L2 or cyclophilin B. B, Whole-cell extracts were isolated from GFP- and TCFDN-transduced LβT2 cells and subjected to Western blot analysis using antibodies specific for TCF7L2. Quantitative real-time PCR was used to determine relative levels of Jun (C), Atf3 (D), or Egr1 (E) expression. Levels of cyclophilin expression were used to normalize samples. The expression levels of each gene are expressed relative to the amount of expression in vehicle-treated GFP-expressing LβT2 cells. Data shown are the means ± sem from three distinct experiments in which each sample was assayed in triplicate. *, P < 0.05.
As expected, GnRH dramatically stimulated expression of Jun mRNA when compared with the GFP control group (Fig. 2C). In contrast, transduced TCFDN significantly dampened the stimulatory effect of GnRH on Jun expression (P < 0.05; Fig. 2C). Moreover, transduced TCFDN had no significant effect in the absence of GnRH, suggesting that TCF7L2 contributes to the hormonal responsiveness of Jun transcription.
As noted earlier, GnRH also stimulates a rapid increase in the accumulation of Atf3 and Egr1 mRNA (16,17,18,19). In contrast to the results reported for Jun, transduced TCFDN had no impact on GnRH stimulation of Atf3 or Egr1 mRNA accumulation (Fig. 2, D and E). Collectively, these results suggest that GnRH regulation of Jun expression is unique in its requirement for a functional form of TCF7L2 when compared with the other two IEGs.
Targeted reduction of endogenous β-catenin compromises GnRH-regulated expression of Jun
The finding that TCFDN disrupts GnRH stimulated increases in Jun expression implies that GnRH signals through β-catenin to increase Jun expression. To examine this possibility, we stably transfected LβT2 cells with recombinant lentivirus that expresses short hairpin RNA (shRNA) specific for β-catenin. LβT2 cells that stably express nonsilencing shRNA were also generated and served as controls. These new lines of LβT2 cells were treated with either vehicle or 100 nm GnRH for 1 h, and gene expression was assayed using real-time PCR after reverse transcription of gene products.
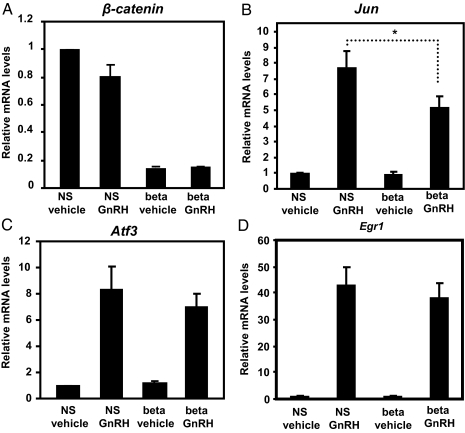
Stable expression of shRNA specific for β-catenin significantly reduced levels of its mRNA independently of GnRH when compared with the nonsilencing shRNA groups (Fig. 3A). As expected, in the absence of GnRH, levels of Jun mRNA were low and not significantly impacted by shRNA specific for β-catenin (Fig. 3B). In contrast, reduced levels of β-catenin mRNA correlated with an attenuated response of Jun mRNA to GnRH (P < 0.05; Fig. 3B). A concurrent reduction in Jun and β-catenin expression is consistent with observed reduction in TOPflash activity in the presence of AXIN (Fig. 1B). Together, both experimental paradigms suggest that GnRH regulation of TCF-dependent promoters, both natural and synthetic, requires β-catenin. Similar to the experiments with TCFDN, GnRH-stimulated increases in Egr1 and Atf3 were unaffected by the reduced levels of β-catenin mRNA (Fig. 3, C and D). Together, these results suggest that β-catenin mediates GnRH transcriptional responsiveness of Jun but not that of Egr1 or Atf3.
Figure 3.
Endogenous β-catenin is required for maximal expression of Jun in GnRH-treated cells. LβT2 cells that stably express either nonsilencing shRNA (NS) or shRNA to β-catenin (beta) were treated with either vehicle or 100 nm GnRH for 1 h. Quantitative real-time PCR was used to determine relative levels of β-catenin (A), Jun (B), Atf3 (C), or Egr1 (D) expression. Levels of cyclophilin expression were used to normalize samples. The expression levels of each gene are expressed relative to the amount of expression in vehicle-treated cells that express nonsilencing shRNA. For A, data shown are the mean ± sem; n = 3. For B–D, data shown are the means ± sem; n = 6. *, P < 0.05.
JUN protein levels are reduced in TCFDN-expressing and β-catenin-deficient cells
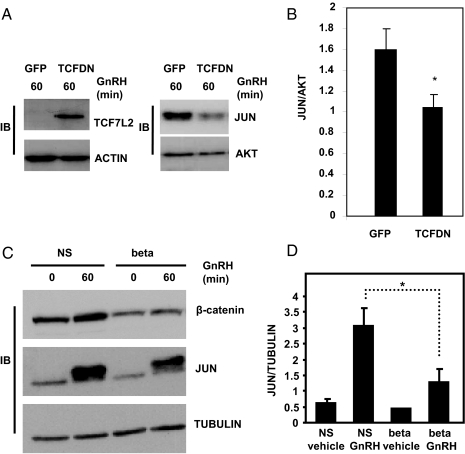
To ensure that changes in the mRNAs for Tcf7l2, β-catenin, and Jun correlated with changes in their cognate proteins, we performed immunoblot analysis of whole-cell extracts from LβT2 cells transduced with TCFDN adenovirus or from stably transfected cell lines that express either the nonsilencing or β-catenin shRNAs. Levels of JUN protein were significantly lower in cells that were transduced with TCFDN and then exposed to GnRH (100 nm, 60 min) when compared with their control counterparts (P < 0.05; Fig. 4, A and B), consistent with the notion that maximal stimulation of JUN requires TCF7L2 with an intact β-catenin-binding domain.
Figure 4.
JUN protein levels are reduced in β-catenin-deficient and TCFDN-expressing cells. A, Whole-cell extracts isolated from LβT2 cells transduced with either GFP- or TCFDN-expressing adenoviruses (5 × 1010 vpm) and treated with 100 nm GnRH for 1 h, were subject to immunoblot analysis using antibodies specific for TCF7L2, JUN, actin (loading control), and AKT (loading control). B, Arbitrary densitometric units of JUN divided by arbitrary densitometric units of AKT. Data shown in B are the means ± sem from three distinct experiments. *, P < 0.05. C, Whole-cell extracts were isolated from LβT2 cells stably expressing nonsilencing shRNA (NS) or shRNA to β-catenin (beta) and subjected to immunoblot analysis using antibodies specific for β-catenin, JUN, and tubulin (loading control). D, Arbitrary densitometric units of JUN divided by arbitrary densitometric units of tubulin. Data shown in D are the means ± sem from three distinct experiments. *, P < 0.05.
With respect to β-catenin protein, GnRH (100 nm, 60 min) stimulated accumulation of the coactivator in the stable cell line that expresses the nonsilencing shRNA (Fig. 4C). Although levels of β-catenin protein were lower and refractory to stimulation by GnRH in the stable cell lines that express shRNA specific for the coactivator (Fig. 4C), the silencing of β-catenin protein was not as robust relative to its cognate mRNA (Fig. 3A). This may be attributed to a requirement of β-catenin for growth (37), which would favor the selection and propagation of clones with moderate reductions in β-catenin protein vs. clones with dramatic reductions in the coactivator. Additionally, β-catenin is also a required component of the cadherin cell adhesion complex (42,43), and alterations in cell morphology have been demonstrated downstream of GnRH (43,44), providing another role for the coactivator in gonadotropes. Nevertheless, the concurrent reduction of β-catenin and JUN protein (P < 0.05; Fig. 4, C and D) is consistent with the possibility that GnRH signals through TCF7L2 and β-catenin to regulate transcription of Jun, an event that leads to subsequent accumulation of JUN protein.
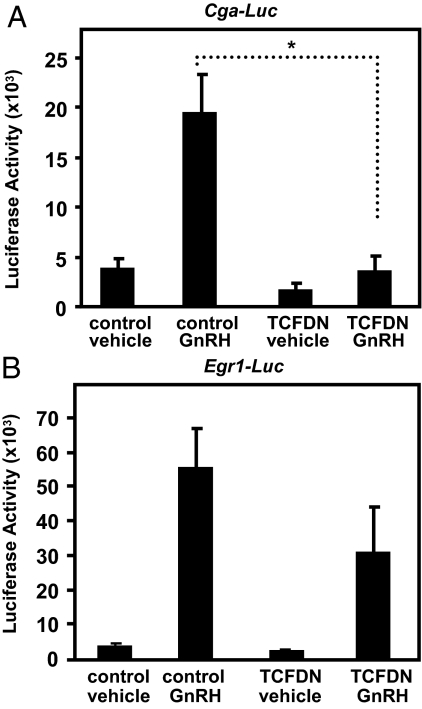
Transduced TCFDN comprises activity of a JUN-responsive promoter in LβT2 cells
Prior reports indicate that full activity of the human CGA requires binding of JUN to tandem cAMP response elements (15,27). Because GnRH-stimulated JUN confers hormonal responsiveness to Cga in LβT2 cells, then TCFDN-dependent reductions in JUN should correlate with attenuated response of a CGA luciferase reporter after a GnRH challenge. To examine this possibility, LβT2 cells were transiently cotransfected with a CGA luciferase reporter and an expression vector encoding TCFDN. After transient transfection, LβT2 cells were treated with GnRH (100 nm) for 24 h and then assayed for luciferase activity.
TCFDN had a marginal effect (∼2-fold reduction) on CGA promoter activity in vehicle-treated LβT2 cells (Fig. 5A). In contrast, TCFDN significantly reduced GnRH stimulated increases in CGA promoter activity by over 4-fold (P < 0.01; Fig. 5A). Because GnRH stimulation of Egr1 occurs independently of TCF/LEF (Fig. 2), we also examined whether a murine Egr1 luciferase reporter (45) is refractory to TCFDN. As expected, GnRH increased the activity of the Egr1 promoter reporter (Fig. 5B). Although TCFDN appeared to reduce GnRH-stimulated increases in Egr1 promoter activity, the effect was not statistically significant (Fig. 5B). Together, these data indicate a strong correlation between TCFDN-induced reductions in Jun expression (Fig. 2) and JUN protein (Fig. 4) and reduced activity of a JUN-regulated promoter reporter in GnRH-treated LβT2 cells. These data also suggest that the requirement for TCF/LEF is specific for Jun but not for the other two IEGs that confer GnRH responsiveness to downstream gene targets.
Figure 5.
CGA transcription is compromised by TCFDN. A, LβT2 cells were cotransfected with a human CGA-Luc promoter reporter (50 ng) and either an empty CMV expression vector (control) (100 ng) or a TCFDN expression vector (100 ng) and then treated with vehicle or 100 nm GnRH for 24 h. B, LβT2 cells were cotransfected with an Egr1-Luc promoter reporter (50 ng) and either an empty CMV expression vector (control) (100 ng) or a TCFDN expression vector (100 ng) and then treated with vehicle or GnRH for 24 h. Data shown are the means ± sem from three independent experiments performed in triplicate. *, P < 0.05.
Discussion
Prior reports have revealed that GnRH regulates transcription of a large network of genes (16,17,18,19). Genes within this regulated network have been categorized as primary, secondary, or tertiary genes based on how rapidly levels of their mRNAs change in response to a GnRH stimulus (16,17,18,19). Primary genes that encode DNA-binding proteins play a vital role in the GnRH network because, as DNA binding proteins, they confer hormonal responsiveness to secondary and tertiary genes that contain the appropriate regulatory elements. The findings presented herein have provided greater insight into how GnRH coordinates activation across this transcriptional network. Specifically, our data indicate maximal levels of Jun expression in response to GnRH requires a functional interaction between TCF7L2 and β-catenin. Moreover, alterations in the activity of TCF7L2 or levels of β-catenin have a secondary impact of the GnRH responsiveness of JUN-dependent downstream promoters such as Cga. Finally, the requirement for TCF7L2 and β-catenin is selective for Jun because neither the transcription factor nor coactivator are required for GnRH regulated expression of Egr1 or Atf3, two other members of the primary network of neurohormone-responsive genes.
Historically, β-catenin has been viewed as a member of the WNT canonical signaling cascade that plays important roles during development (46,47,48,49,50). For instance, Wnt4 is important for the growth of the embryonic pituitary (49,50), whereas Wnt5 regulates the shape of the endocrine gland during development (47). TCF7L2 seems to restrict the growth of the pituitary, because embryonic pituitary glands of Tcf7l2 null mice are enlarged and exhibit excessive proliferation (48). β-Catenin, by binding to the DNA-binding protein paired like homeobox 1 (PROP1), induces the expression of Pit1, resulting in the differentiation of pituitary precursor cells into somatotropes, lactotropes, and thyrotropes (51). Levels of Lhb gene expression were also reduced in embryonic pituitary glands devoid of β-catenin (51). In addition to roles in WNT signaling and development, β-catenin is also an important cofactor in the cadherin cell adhesion complex (42). Recently, it has been shown that GnRH induces changes in gonadotrope cell morphology by altering the actin cytoskeleton (44), suggesting concurrent changes in the cadherin complex. Although it is clear that Wnt and β-catenin are important for development and cell adhesions, recent data (32), including our own, indicate that GnRH has hijacked components of the WNT signal transduction cascade to regulate transcription in differentiated gonadotropes.
Although we have focused on GnRH, FSH, and prostaglandin E2 (PGE2) have also been shown to signal through β-catenin to regulate transcription (52,53,54). For instance, FSH-stimulated increases in aromatase expression in ovarian granulosa cells are mediated by β-catenin (52). PGE2, by binding to its heterotrimeric GPCR on colon cancer cells, stimulates the accumulation of β-catenin and increases TCF/LEF transcriptional activity (53,54). GnRH is thought to primarily signal through activation of Gq/11 (55). In contrast, FSH and PGE2 both signal through activation of Gs (7,53), suggesting that the role of β-catenin in mediating responsiveness to hormones that bind to GPCRs is not restricted to one type of G protein.
Glycogen synthase kinase-β (GSK3β) may serve as an important signaling intermediate that links GnRH to increases in β-catenin levels or activity (39,56). WNT inhibition of GSK3β activity prevents the phosphorylation of residues in the N terminus of β-catenin, resulting in the accumulation of the coactivator (39,56). Recently, Gardner and colleagues reported that GnRH stimulates the rapid phosphorylation of GSK3β on serine 9, leading to inhibition of the kinase (32). We have also observed a strong correlation between GnRH-stimulated increases in GSK3β phosphorylation and increased activity of β-catenin in LβT2 cells (unpublished data, n = 3). In colon cancer cells, PGE2 inhibits GSK3β activity via phosphorylation of serine 9, an event that contributes to increases in TOPflash activity (53,54). In addition to serine 9, recent work has revealed that p38 MAPK can phosphorylate and inactivate GSK3β through serine 389 (57). This inactivation stimulated the accumulation of β-catenin in mouse embryonic fibroblasts and embryonic stem cells (57). This result may be relevant to GnRH regulation of β-catenin because the neurohormone signals through p38 MAPK (13). Together these reports suggest that inhibition of GSK3β activity may serve as a broad mechanism for hormones to modulate β-catenin downstream of a GPCR. Further studies will be required to reveal the signaling cascade that links GnRH to induced phosphorylation of GSK3β and concomitant changes in the levels and activity of β-catenin.
Thus far, we have emphasized the selective requirement for TCF7L2 and β-catenin in GnRH-regulated expression of Jun along with their indirect impact on JUN downstream targets such as Cga. It is important to note, however, that β-catenin plays another distinct role in the network of GnRH-responsive genes. Recently, we reported that β-catenin can also act independently of GnRH to support the permissive role of the nuclear receptor steroidogenic factor 1 (SF1) (officially NR5A1) in allowing Lhb to respond to GnRH-stimulated rises in EGR1 (58). Moreover, SF1 is required for expression of Cga, Fshb, and Gnrhr. For example, mice that lack SF1 in gonadotropes are hypogonadal and infertile due to insufficient synthesis and secretion of LH and FSH that in turn are a consequence of attenuated expression of Gnrhr, Fshb, and Lhb (59,60). This suggests that β-catenin may act independently of GnRH to support the permissive role of SF1 in allowing all four gonadotrope signature genes to respond to GnRH. This enabling role of β-catenin may also explain why FSH-stimulated increases in aromatase gene expression in granulosa cells requires a functional interaction between SF1 and β-catenin (52). Interestingly, FSH also stimulates increases in Jun expression, suggesting that β-catenin may play both permissive and hormone-responsive roles in granulosa cells as it does in gonadotropes (61). Finally, there is a growing number of reports indicating that transcriptional activity of a number of nuclear receptors, including SF1, require a functional interaction with β-catenin (62). Thus, the permissive requirement for β-catenin may be extended generally to members of the nuclear receptor family.
A prior report has revealed that a splice variant of Tcf7l2 originally identified in embryonic pituitary may influence whether β-catenin interacts with TCF/LEF family members or SF1 (38,63). This Tcf7l2 isoform (also known as TCF-4N) lacks a DNA-binding domain but retains an N-terminal β-catenin interaction domain (38,63). TCF-4N was found to inhibit β-catenin-regulated increases in TOPflash activity (38). Conversely, TCF-4N increased synergistic interactions between β-catenin and SF1 on the inhibin-α promoter (38). Although it is tempting to suggest that TCF-4N would favor Lhb gene expression and inhibit Jun expression in gonadotropes, our immunoblot experiments suggest that LβT2 cells express only full-length TCF7L2 (data not shown).
In summary, this report indicates that GnRH signals through β-catenin and TCF7L2 to increase expression of Jun. Although the requirement for β-catenin and TCF7L2 appears to be limited to one of the three IEGs that confer hormonal responsiveness to secondary and tertiary genes of the GnRH network, the potential impact is significant because three of the four signature genes (Gnrhr, Fshb, and Cga) respond to GnRH-mediated changes in JUN (25,26,27,28,29,30). Consequently, blockade of any point along the signaling route that links GnRH to β-catenin is likely to wreak havoc with gonadotrope function.
Materials and Methods
Chemicals and GnRH
GnRH, Triton X-100, Igepal CA-300, sodium deoxycholate, and Nonidet P-40 were purchased from Sigma Chemical Co. (St. Louis, MO); sodium dodecyl sulfate (SDS) was from Bio-Rad Laboratories (Hercules, CA); glycine was from J. T. Baker (Phillipsburg, NJ); and TRIzol and Lipofectamine were purchased from Invitrogen (Carlsbad, CA).
DNA constructs
The −1500/+45-bp human CGA promoter-luciferase promoter has been described previously (27). The −1381/+79 mouse Egr1-luciferase promoter was kindly provided by Dr. Larry Jameson (Northwestern University, Chicago, IL) (45). The TOPflash luciferase reporter vector was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). TCFDN was cloned into pcDNA3 and was kindly provided by Dr. Frank McCormick (University of California School of Medicine, San Francisco, CA) (37). The murine Axin1 in a pcS2_MT expression vector was generously provided by Dr. Frank Costantini (Columbia University, New York, NY) (40).
Transient transfections
LβT2 cells maintained at 37 C with 5% CO2 in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) (complete media) (Invitrogen) were plated into a 24-well plate (250,000 cells per well). Twenty-four hours later, LβT2 cells were washed with PBS (Sigma), and a transfection cocktail containing DNA constructs, DMEM, and Lipofectamine (1.6 μl/well) was added in accordance with the manufacturer’s protocol. Transfection medium was removed after an overnight incubation, and cells were treated with vehicle or 100 nm GnRH diluted in DMEM supplemented with 1% P/S for 24 h. Luciferase activity was examined using the dual luciferase reporter assay kit (Promega Corp., Madison, WI). The amounts of DNA constructs used are in the figure legends.
shRNA-expressing LβT2 cells and GnRH treatment
Expression Arrest GIPZ lentiviral shRNAmir vectors (nonsilencing and β-catenin) were purchased from Open Biosystems (Huntsville, AL). Lentiviral packaging was done in TLA-HEK293T cells in accordance with the Trans-Lentiviral GIPZ packaging system. Five hours after transfection, the packaging cocktail was aspirated and replaced with complete medium. Seventy-two hours after transfection, HEK293T cells were lysed and lentiviral containing supernatants were isolated after centrifugation (3000 rpm for 20 min).
LβT2 cells in six-well plates (2.5 × 106 per well) were transduced with lentivirus diluted in 2 ml DMEM containing 8 μg/ml Sequabrene (Sigma). Six hours later, an equal amount of DMEM supplemented with 20% FBS was added to each well. The following day, the transfection cocktail was removed and replaced with complete medium. Puromycin (350 ng/ml) diluted in complete medium was used to select for shRNA-expressing cells. Cells were then plated and maintained in complete medium in 96-well plates to cultivate individual clones. Levels of β-catenin expression in shRNA-expressing cells were examined by quantitative real-time (QRT)-PCR (Fig. 3A) and by Western blot (Fig. 4A).
For GnRH treatment, serum-starved (overnight) shRNA-expressing cells in six-well plates (80% confluent) were treated with either vehicle (PBS) or GnRH for 60 min. Treatments were removed, and 1 ml TRIzol was added.
Adenoviral transduction of LβT2 cells and GnRH treatment
TCFDN was excised from pcDNA3 with EcoR1 and BamH1 and subcloned into EcoR1 and BrglII sites of the pDC316(io) shuttle vector, and TCFDN adenoviruses were constructed using the AdMax system (Microbix Biosystems, Toronto, Ontario, Canada). LβT2 cells (1 × 106 per well, 80% confluent) in six-well plates were transduced with adenoviruses diluted in 1 ml DMEM supplemented with 2% FBS and 1% P/S. Twelve to 14 h after transduction, adenoviral-containing medium was removed and new medium (DMEM containing 2% FBS and 1% P/S) was added. At 48 h after transduction, an overnight serum starve was initiated, and approximately 16 h later, transduced cells were treated with vehicle or GnRH (100 nm) for 60 min. Treatments were then removed, and 1 ml TRIzol was added to each well. Viral particles per milliliter (vpm) was determined by measuring sample OD at 260 nm and the relationship of 1012 vpm per OD. The amount of adenovirus used (denoted in the figure legends) was based on levels of TCFDN expression and functional outcome.
RNA isolation and cDNA synthesis
Total RNA was isolated from TRIzol in accordance with the manufacturer’s protocol. All RNA work was done with diethylpyrocarbonate-treated water. Before cDNA synthesis, total RNA (0.5 μg) was incubated with 1 μl deoxyribonuclease (deoxyribonuclease I, ribonuclease free; Roche Diagnostics, Indianapolis, IN), 2 μl 5× First Strand Buffer (Invitrogen) in a total volume of 10 μl for 15 min at room temperature. One microliter EDTA (25 mm) was then added to each reaction and incubated at 70 C for 15 min. The reaction was then placed on ice for 1 min, and 4 μl qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) was added per sample. Twenty-microliter cDNA reactions were then carried out after the qScript cDNA SuperMix protocol. Samples were then diluted 1:10 in diethylpyrocarbonate-treated water and stored at −70 C.
QRT-PCR
Relative mRNA levels were determined using QRT-PCR and the 7000 ABI Prism sequence detection software system (Applied Biosystems, Foster City, CA). Primer Express 2.0 software (Applied Biosystems) was used to design intron spanning primers (except Jun, which lacks apparent introns). Primer sets were optimized using a range of primer concentrations, and 300 nm was optimal for Atf3, Jun, and cyclophilin B (officially Ppib), whereas 150 nm was optimal for β-catenin and Egr1 primer sets. Primer efficiency was then determined over a range of cDNA dilutions (1:1, 1:10, 1:100, and 1:1000). The amplification efficiency of the QRT reaction for each gene was between 93 and 105%, and therefore relative levels of expression were calculated using the comparative method (ΔΔCT). Cyclophilin B expression was used to normalize samples. Samples were assayed in triplicate.
For each QRT-PCR, a master mix containing 12.5 μl Platinum SYBR Green qPCR SuperMix-UDG, 0.5 μl ROX reference dye (Invitrogen), and primers were added to 5 μl cDNA. The final volume of a QRT-PCR was 25 μl. QRT-PCR conditions were 50 C for 2 min, 95 C for 10 min and then 40 cycles of 95 C for 15 sec and 60 C for 1 min. Primer sequences for real-time PCR include cJun (forward 5′-AGTTCTTGTGCCCCAAGAACG-3′ and reverse 5′-AAGCGTGTTCTGGCTATGCAG-3′), Atf3 (forward 5′-TTACCGTCAACAACAGACCCCT-3′ and reverse 5′-CGCCTCCTTTTCCTCTCATCTT-3′), Egr1 (forward 5′-GAACCCCTTTTCAGCCTAGTTCA-3′ and reverse 5′-AGGATGAAGAGGTCGGAGGATT-3′), cyclophilin B (forward5′-CAAAGACACCAATGGCTCACAG-3′ and reverse 5′-CCACATCCATGCCCTCTAGAAC-3′), and β-catenin (forward 5′-CGCAAGAGCAAGTAGCTGATATTG and reverse 5′-CGGACCCTCTGAGCCCTAGT-3′).
Western blot
Protein extracts were prepared one of three ways. Nuclear extracts were prepared as previously described (58,64), or whole-cell lysates were collected using the following two methods. Cells were rinsed twice in cold PBS and lysed in lysis buffer containing 50 mm Tris (pH 7.5), 150 mm NaCl, 10 mm NaF, 1 mm EDTA, 1 mm EGTA, 0.5% deoxycholate, and 1% Triton X-100. Lysis buffer was supplemented with the Mini Protease Inhibitor cocktail (Roche). Cell homogenates were then cleared by centrifugation at 16,000 × g for 10 min and supernatants analyzed. For samples that were lysed in TRIzol, after RNA isolation, the protein was extracted by the modified protocol provided for Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH). Extracted proteins were solubilized in 5 m urea, 1% SDS, and 25 mm dithiothreitol and heated at 50 C. Protein concentrations were determined by Coomassie Plus protein assay (Pierce, Rockford, IL) using BSA as a standard. Protein samples were separated on 12% polyacrylamide SDS gels. Proteins were then transferred to a polyvinylidene difluoride membrane (Bio-Rad) in Towbin buffer. Membranes were blocked in 5% nonfat milk in Tris-buffered (pH 8.0) saline containing 0.05% Tween 20 (TBST). Membranes were incubated for either 1 h at room temperature or overnight at 4 C using primary antibodies for β-catenin (BD Transduction Laboratories, Lexington, KY), JUN, AKT (Cell Signaling Technology, Beverly, MA), TCF7L2, total CREB (Upstate), tubulin (Abcam, Cambridge, MA), or actin (Sigma) diluted in TBST containing 5% nonfat milk. Membranes were rinsed three times with TBST and then incubated in horseradish peroxidase-conjugated secondary antibody (Pierce) for 1 h at room temperature. The membrane was rinsed three times with TBST, and antigen-antibody complexes were detected by chemiluminescence (Immobilon horseradish peroxidase; Millipore, Billerica, MA).
Statistics
Levels of luciferase activity, gene expression, and arbitrary densitometric units were analyzed by one-way ANOVA, and differences between groups were determined with the Tukey post hoc test. Levels of arbitrary densitometric units in Fig. 4B were analyzed by a two-tailed Student’s t test.
Acknowledgments
We thank Anthony Zeleznik for help in constructing the adenoviral vectors. Additionally, we thank Derek Pouchnik and the facilities at the Washington State University Bioinformatics Core Laboratories for assistance with real-time PCR and Dr. Maria Herndon for critical evaluation of this manuscript.
Footnotes
This work was supported by National Institutes of Health Grant R01 HD055776 (to J.H.N.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: FBS, Fetal bovine serum; GPCR, G protein-coupled receptor; GSK3β, glycogen synthase kinase-β; IEG, immediate early gene; LEF, lymphoid enhancer factor; PGE2, prostaglandin E2; P/S, penicillin-streptomycin; QRT, quantitative real-time; SDS, sodium dodecyl sulfate; SF1, steroidogenic factor 1; shRNA, short hairpin RNA; TCF, T-cell factor; TCFDN, dominant-negative form of TCF7L2.
References
- Gharib SD, Wierman ME, Shupnik MA, Chin WW 1990 Molecular biology of the pituitary gonadotropins. Endocr Rev 11:177–199 [DOI] [PubMed] [Google Scholar]
- Wierman ME, Gharib SD, Chin WW 1988 The structure and regulation of the pituitary gonadotrophin subunit genes. Baillieres Clin Endocrinol Metab 2:869–889 [DOI] [PubMed] [Google Scholar]
- Crowley Jr WF, Filicori M, Spratt DI, Santoro NF 1985 The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41:473–531 [DOI] [PubMed] [Google Scholar]
- Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP 1991 Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res 47:155–187 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Quirk CC, Nilson JH 2004 Multiple and overlapping combinatorial codes orchestrate hormonal responsiveness and dictate cell-specific expression of the genes encoding luteinizing hormone. Endocr Rev 25:521–542 [DOI] [PubMed] [Google Scholar]
- Ferris HA, Shupnik MA 2006 Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod 74:993–998 [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E 1997 The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18:739–773 [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL 2002 The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Ascoli M 1993 The lutropin/choriogonadotropin receptor. 4 years later. Endocr Rev 14:324–347 [DOI] [PubMed] [Google Scholar]
- Naor Z, Benard O, Seger R 2000 Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab 11:91–99 [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Roberson MS 2000 Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. J Biol Chem 275:14182–14189 [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Zhang T, Fewtrell C, Roberson MS 1999 Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem 274:29796–29804 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Zhang T, Li HL, Mulvaney JM 1999 Activation of the p38 mitogen-activated protein kinase pathway by gonadotropin-releasing hormone. Endocrinology 140:1310–1318 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA 1995 A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol 15:3531–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS 2005 Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol 19:2624–2638 [DOI] [PubMed] [Google Scholar]
- Ruf F, Fink MY, Sealfon SC 2003 Structure of the GnRH receptor-stimulated signaling network: insights from genomics. Front Neuroendocrinol 24:181–199 [DOI] [PubMed] [Google Scholar]
- Ruf F, Sealfon SC 2004 Genomics view of gonadotrope signaling circuits. Trends Endocrinol Metab 15:331–338 [DOI] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC 2001 Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem 276:47195–47201 [DOI] [PubMed] [Google Scholar]
- Yuen T, Wurmbach E, Ebersole BJ, Ruf F, Pfeffer RL, Sealfon SC 2002 Coupling of GnRH concentration and the GnRH receptor-activated gene program. Mol Endocrinol 16:1145–1153 [DOI] [PubMed] [Google Scholar]
- Salisbury TB, Binder AK, Nilson JH 2008 Welcoming β-catenin to the gonadotropin-releasing hormone transcriptional network in gonadotropes. Mol Endocrinol 22:1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SI, Dexheimer V, Nishida E, Kitajima S, Thiel G 2008 Expression of the transcriptional repressor ATF3 in gonadotrophs is regulated by Egr-1, CREB and ATF2 following GnRH receptor stimulation. Endocrinology 149:6311–6325 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J 1999 Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol 19:2567–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson ED, Yu J, Mustelin T 2005 Co-factors p300 and CBP catch Egr1 in their network. Prostate 63:407–410 [DOI] [PubMed] [Google Scholar]
- Yu J, de BI, Liang H, Adamson ED 2004 Coactivating factors p300 and CBP are transcriptionally crossregulated by Egr1 in prostate cells, leading to divergent responses. Mol Cell 15:83–94 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, White BR, Burns AT, Cherrington BD, Otis AM, Clay CM 2003 c-Jun N-terminal kinase activation of activator protein-1 underlies homologous regulation of the gonadotropin-releasing hormone receptor gene in αT3-1 cells. Endocrinology 144:839–849 [DOI] [PubMed] [Google Scholar]
- White BR, Duval DL, Mulvaney JM, Roberson MS, Clay CM 1999 Homologous regulation of the gonadotropin-releasing hormone receptor gene is partially mediated by protein kinase C activation of an activator protein-1 element. Mol Endocrinol 13:566–577 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Schultz K, Nilson JH 1996 The cAMP response elements of the α-subunit gene bind similar proteins in trophoblasts and gonadotropes but have distinct functional sequence requirements. J Biol Chem 271:31650–31656 [DOI] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KH, Chin WW, Kaiser UB 2004 Essential role of the homeodomain for pituitary homeobox 1 activation of mouse gonadotropin-releasing hormone receptor gene expression through interactions with c-Jun and DNA. Mol Cell Biol 24:6127–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Xu S, Xu J, Spiryda LB, Park JS, Jeong KH, McGee EA, Kaiser UB 2002 Direct binding of AP-1 (Fos/Jun) proteins to a SMAD binding element facilitates both gonadotropin-releasing hormone (GnRH)- and activin-mediated transcriptional activation of the mouse GnRH receptor gene. J Biol Chem 277:37469–37478 [DOI] [PubMed] [Google Scholar]
- Angel P, Hattori K, Smeal T, Karin M 1988 The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 55:875–885 [DOI] [PubMed] [Google Scholar]
- Gardner S, Maudsley S, Millar RP, Pawson AJ 2007 Nuclear Stabilization of β-catenin and inactivation of glycogen synthase kinase-3β by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol 21:3028–3038 [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A 2005 Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437:281–285 [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C 1999 Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA 96:1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H 1997 Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787 [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K 1999 The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature 399:798–802 [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F 1999 β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426 [DOI] [PubMed] [Google Scholar]
- Kennell JA, O'Leary EE, Gummow BM, Hammer GD, MacDougald OA 2003 T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with β-catenin to coactivate C/EBPα and steroidogenic factor 1 transcription factors. Mol Cell Biol 23:5366–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R 2006 Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433 [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry III WL, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F 1997 The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181–192 [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho Eh, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F 1999 Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145:741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ 2008 Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Pawson AJ, Millar RP, Maudsley S 2004 Cytoskeletal reorganization dependence of signaling by the gonadotropin-releasing hormone receptor. J Biol Chem 279:1980–1993 [DOI] [PubMed] [Google Scholar]
- Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM 2007 Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology 148:1736–1744 [DOI] [PubMed] [Google Scholar]
- Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL 2002 GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol 16:221–233 [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Torres M 1997 WNTs modulate cell fate and behavior during vertebrate development. Trends Genet 13:157–162 [DOI] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA 2004 WNT5A signaling affects pituitary gland shape. Mech Dev 121:183–194 [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Davis SW, Camper SA 2007 TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol 311:396–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA 2008 WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn 237:1006–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG 2002 Identification of a Wnt/Dvl/β-catenin → Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111:673–685 [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG 2006 Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell 125:593–605 [DOI] [PubMed] [Google Scholar]
- Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH 2006 Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci USA 103:12435–12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS 2005 Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310:1504–1510 [DOI] [PubMed] [Google Scholar]
- Shao J, Jung C, Liu C, Sheng H 2005 Prostaglandin E2 stimulates the β-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem 280:26565–26572 [DOI] [PubMed] [Google Scholar]
- Shacham S, Harris D, Ben-Shlomo H, Cohen I, Bonfil D, Przedecki F, Lewy H, Ashkenazi IE, Seger R, Naor Z 2001 Mechanism of GnRH receptor signaling on gonadotropin release and gene expression in pituitary gonadotrophs. Vitam Horm 63:63–90 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Kishida S, Yamamoto H 2006 Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med 38:1–10 [DOI] [PubMed] [Google Scholar]
- Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M 2008 Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science 320:667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury TB, Binder AK, Grammer JC, Nilson JH 2007 Maximal activity of the luteinizing hormone β-subunit gene requires β-catenin. Mol Endocrinol 21:963–971 [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL 2001 Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 128:147–154 [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL 2001 Hypomorphic phenotype in mice with pituitary-specific knockout of steroidogenic factor 1. Genesis 30:65–69 [DOI] [PubMed] [Google Scholar]
- Sharma SC, Richards JS 2000 Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC 2005 Interaction of nuclear receptors with the Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26:898–915 [DOI] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons Jr RH, MacDougald OA, Camper SA 2001 Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome 12:843–851 [DOI] [PubMed] [Google Scholar]
- Gummow BM, Winnay JN, Hammer GD 2003 Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin α gene. J Biol Chem 278:26572–26579 [DOI] [PubMed] [Google Scholar]