Abstract
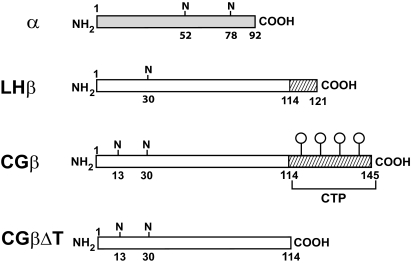
Although the LHβ- and chorionic gonadotropin-β- (CGβ) subunits share a high degree of sequence identity (>85%) in the first 114 amino acids, there is considerable sequence divergence at their carboxy ends. The CGβ-subunit terminates with a unique carboxyl-terminal extension (115–145; carboxyl-terminal peptide), which contains four O-linked oligosaccharides, whereas the LHβ-subunit bears a hydrophobic heptapeptide (115–121) at its carboxy terminus. LH is released through the regulated pathway in the pituitary, whereas CG is secreted constitutively from the placenta. We previously demonstrated in rat somatotroph-derived GH3 cells that the LH is associated primarily with a regulated routing, and although the majority of CG was released constitutively from the cells, there was a fraction that was segregated through the regulated pathway. Moreover, we showed that the LHβ heptapeptide is a determinant for the regulated secretion of LH. Given that the primary evolutionary change between LHβ and CGβ occurred at the carboxy terminus, these data suggested that the presence of the CGβ carboxyl-terminal peptide region is responsible for the constitutive secretion of CG. A CG114 mutant (CGΔT) was constructed and expressed in GH3 cells. Steady-state labeling and pulse-chase experiments demonstrated that the CGΔT entered the regulated pathway resulting in over 4-fold increase in the intracellular pool. The secretagogue, forskolin, stimulated CGΔT release over 3-fold, which was accompanied by a parallel intracellular decrease, and only marginal stimulation of CG was seen. Immunofluorescence demonstrated a unique membrane pattern of staining for CGΔT compared with dispersed cytoplasmic puncta for CG. Stimulation with forskolin caused a significant reduction in the relative fluorescence of CGΔT cells compared with a minor reduction for CG. These data show that the CGΔT analog resembles LH in its intracellular trafficking, further supporting the hypothesis that determinants at the carboxyl-terminal end of the CGβ-subunit evolved from the LHβ-subunit primarily to overcome the slow release and intracellular storage of LH resulting in rapid secretion of CG from the placenta.
Truncation of the carboxyl end CGβ reveals sorting activity analogous to LH, supporting the hypothesis that LH/CG evolution resulted in altered secretory signals.
Chorionic gonadotropin (CG) from the placenta and its pituitary counterpart, LH, comprise a family of heterodimeric glycoprotein hormones, including FSH and TSH that share a common α-subunit but differ in their hormone-specific β-subunits. Evolved from an ancestral LHβ gene, the CGβ-subunit shows greater than 85% sequence identity in the first 114 amino acids, which is responsible for their binding to a common gonadal receptor (1,2,3) (Fig. 1). One major difference between them is the carboxyl-terminal stretch with four serine O-linked carbohydrate units [carboxyl-terminal peptide (CTP)] in the CGβ-subunit (Fig. 1). In contrast, the LHβ-subunit has a hydrophobic heptapeptide that terminates at residue 121 (2,3). It was proposed that a frame-shift mutation in the LHβ/CGβ ancestral gene led to a read-through at amino acid 114, generating the CTP coding sequence (2,3).
Figure 1.
Schematic diagram of the gonadotropin subunits. These include α-subunit, LHβ-subunit (the cross-hatched area of region 114–121 of LHβ denotes the hydrophobic heptapeptide), CGβ-subunit (the cross-hatched area of region 114–145 denotes the CTP sequence with four O-linked oligosaccharides), and CGβΔT, carboxyl-terminal mutant of CGβ-subunit truncated at amino acid 114. N, Asn-linked oligosaccharide.
Previously we showed that despite the high degree of sequence similarity, the LH and CGβ-subunits differ substantially from each other in their rate of secretion and assembly with the α-subunit (4,5,6). The CGβ-subunit is secreted quantitatively as a monomer and assembles efficiently, whereas secretion and assembly of LHβ is inefficient, and the heptapeptide of LHβ contributed to this intracellular behavior (6). In terms of the secretion pathways, the major route of CG secretion from the placenta is constitutive (7), and LH exits the pituitary via the regulated pathway (8).
The members of the glycoprotein hormone family contain asparagine (N)-linked oligosaccharides, the structures of which are hormone specific. Previously we demonstrated that truncating the CGβ-subunit at residue 114 (CGβΔT) not only inhibited assembly with the α-subunit but also altered the processing of the N-linked oligosaccharides (9,10). Moreover, fusing the CTP sequence to the FSHβ-subunit promoted secretion of the FSH heterodimer compared with wild-type FSH (11). Taken together, these data suggested that the carboxyl-terminal region is involved in the folding of the CGβ-subunit.
The experiments described above were performed with transfected CHO cells, which secrete proteins only by the constitutive route, thus precluding studies of glycoprotein hormone secretion through the regulated pathway (12). To link the intracellular trafficking of the gonadotropins with their sorting, we used the GH3 cell line, which is derived from pituitary somatotrophs and contain storage vesicles responsive to secretagogues (13,14,15). We previously showed that transfected GH3 cells secrete LH through the regulated secretory pathway, and CG was secreted constitutively, although a fraction was secreted by the regulated route. Using this GH3 model, we recently demonstrated that the LHβ carboxyl-terminal heptapeptide contributes to the sorting of LH to the regulated pathway (15).
That the truncated analog CGβΔT exhibited altered intracellular behavior in CHO cells implied that the carboxyl terminus of the CGβ-subunit is an important locus for the intracellular routing of CG. To test this hypothesis, we examined the sorting and secretion of CG and its CGΔT truncated analog in GH3 cells. Here we show that the sorting of CGΔT resembles the trafficking of LH in that more of the truncated heterodimer is directed to the regulated pathway than wild-type CG.
Results
Steady-state labeling of CG clones
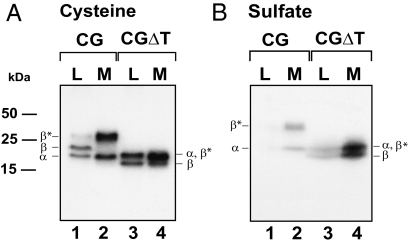
To compare the routing of CG, and its truncated mutant CGΔT, transfected GH3 cells were labeled with [35S]cysteine for 4 h (Fig. 2A), a time based on previous studies in which sufficient intracellular accumulation of these proteins is observed. Proteins were immunoprecipitated from cell lysates and media with CGβ-subunit-specific antiserum that precipitates the CGβ-subunit and the assembled α-subunit (Fig. 2A, lanes 1 and 2). The CGβ-subunit contains two N-linked oligosaccharides and four O-linked carbohydrate units that are sialylated after transit through the trans-Golgi; the addition of the O-linked oligosaccharides occurs just before secretion (5,6,13). Immunoprecipitation of lysates derived from CG-expressing cells (Fig. 2A, lane 1) precipitates the assembled α-subunit, the immature β-subunit devoid of the O-linked oligosaccharides, and the mature β-subunit (β*). In the medium, only mature β-subunits and assembled α-subunits were observed (Fig. 2A, lane 2). Although most of the CG dimer is secreted, the presence of mature β-subunit in the lysates (Fig. 2A, lane 1) reflects the fraction of CG accumulating in the regulated pool as shown previously. This contrasts with data from transfected CHO cells where no retention of mature CG dimer is seen.
Figure 2.
The synthesis and secretion of representative clones expressing either CG or CGΔT dimers after labeling for 4 h with 20 μCi/ml [35S]cysteine (A) or with 0.7 mCi/ml of [35S]sulfate (B). Media (M) and lysates (L) were immunoprecipitated with anti-CGβ serum, resolved by SDS-PAGE, and subjected to autoradiography. The migration of α- and β-subunits, and the molecular mass markers are indicated. β* represents the mature form of β-subunit. Note that α-subunit comigrates with the mature form of CGβΔT. The data shown are representative of five independent experiments.
In the case of CGΔT, the deletion of the 30-amino-acid sequence at the carboxy terminus of CGβ results in two β-forms: one that contains fully processed N-linked oligosaccharides (β*) comigrating with the α-subunit and a smaller protein (β) which, presumably, corresponds to a form bearing only one N-linked oligosaccharide (Fig. 2A, lanes 3 and 4) (10,16,17). Due to the absence of the O-linked oligosaccharides, no significant change in the migration of the CGΔT forms in the medium was seen compared with the lysate forms. In addition, removal of the CTP increased intracellular accumulation of CGΔT compared with CG dimer (Fig. 2A, lanes 1 and 3), consistent with the hypothesis that more CGΔT is entering the regulated pathway. To examine this issue further, CG and CGΔT cells were radiolabeled with [35S]sulfate (Fig. 2B). We previously demonstrated that the CG N-linked oligosaccharides are sulfated in GH3 cells (13). Because this modification occurs in the trans-Golgi and is the final step in the maturation of the oligosaccharides just before secretion (17,18,19), only those mature forms accumulating in the late stages of the secretory pathway will contain [35S]sulfate. GH3 cells expressing CG or CGΔT were labeled for 4 h with [35S]sulfate (Fig. 2B), and the lysates and media were immunoprecipitated with CGβ antiserum. The product profile was similar to that seen for the cysteine-labeled proteins. There was a 4-fold greater (4.3 ± 0.2-fold, P < 0.05) accumulation of CGΔT compared with CG (Fig. 2B, lanes 1 and 3). Moreover, more than 4-fold (4.2 ± 0.4-fold, P < 0.05) of the secreted CGΔT was sulfated compared with CG (Fig. 2B, lanes 2 and 4). This was not due to differences in the overall synthesis rates of the two cell lines, because the incorporation of [35S]cysteine in both dimers was comparable (Fig. 2A).
These data suggest that deleting the CTP not only enhances intracellular accumulation of the mutant but also results in greater incorporation of carbohydrate-linked sulfate (see Discussion).
Pulse-chase secretion kinetics
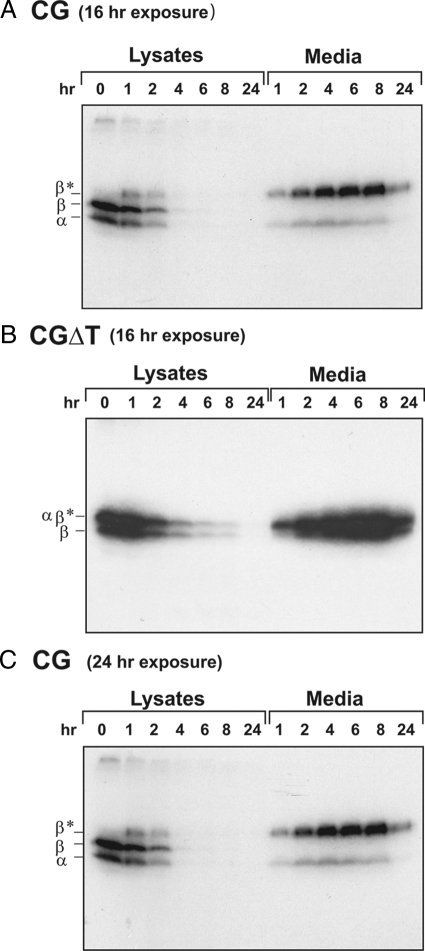
Because the enhanced intracellular accumulation implied that CGΔT is directed to the regulated pathway, we performed two additional experiments: 1) pulse-chase kinetics and 2) secretagogue-induced secretion. If more CGΔT enters the regulated pathway, we should see a greater temporal delay in its release compared with CG over several hours of chase. Transfected GH3 cells were pulse-labeled for 20 min with [35S]cysteine and chased for the indicated time periods, and CGβ was immunoprecipitated from the lysates and media (Fig. 3). At time zero of chase only, the β-subunit, without the O-linked oligosaccharides, and the coprecipitated α-subunit was detected in the lysates (Fig. 3, A and C). After 2 h of chase, the intracellular CG pool was decreased by more than 66 ± 4%. This was also reflected in the secretion kinetics where at 1 h, 50% of the CG was secreted (t½ = 50 ± 12 min; Fig. 3, A and C). Moreover, as seen above, a fraction of mature CGβ-subunit containing O-linked oligosaccharides accumulates intracellularly, indicating that after transit through the Golgi, CG is retained in the cell. At 4 h of chase, all of the intracellular pool was secreted (Fig. 3, A and C). CGΔT, by contrast, was secreted at a slower rate, and 50% of the dimer was secreted at 3 h (t½ = 2 h 45 min ± 25 min) (Fig. 3B). These data show that more CGΔT was retained in the cells compared with CG.
Figure 3.
Pulse-chase kinetics of CG (A and C) and CGΔT (B) dimers. GH3 cells were pulse-labeled with 80 μCi/ml [35S]cysteine for 20 min and then chased for the indicated times. Chase at 0 h indicates the lysate sample prepared immediately after the pulse. Lysates and media were immunoprecipitated with CGβ antiserum and subjected to SDS-PAGE followed by autoradiography. The migration of α- and β-subunits is indicated. β* represents autoradiographs for CG that exposed the mature forms of β-subunit. The data shown are representative of three independent experiments. Note that exposure was for 16 (A) or 24 h (C) for CG and 16 h for CGΔT (B).
Forskolin-treated CG and CGΔT cells
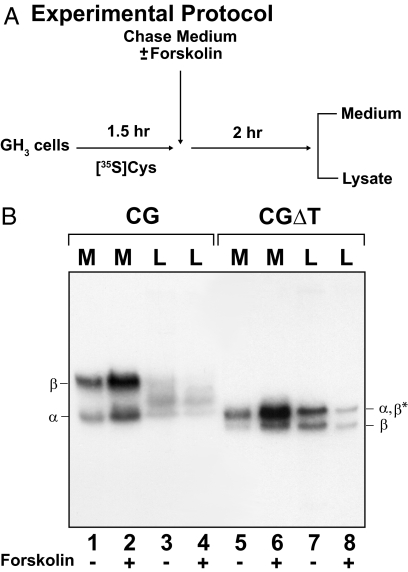
If there is increased entry of CGΔT into the regulated pathway, then its secretion should be stimulated by a secretagogue, e.g. forskolin. After the initial labeling of cells for 1.5 h to achieve maximal cellular accumulation (Fig. 4A), media and lysates were collected from one well for each dimer and served as the time zero controls (data not shown). Addition of forskolin for 2 h stimulated CGΔT release (P < 0.05) more than 3-fold (78.2 ± 4.6%) compared with unstimulated cells (26.5 ± 4.1%) (Fig. 4B, lanes 5 and 6; and Table 1). Significantly, the increased secretion was coupled to a parallel decrease of the intracellular (lysate) CGΔT pool (Fig. 4B, lanes 7 and 8, and Table 1). When the unstimulated CG-expressing cells were examined (Fig. 4B, lanes 1–4), it is clear that, compared with CGΔT, not only was the extent of accumulation lower (37.0 ± 1.6% for CG vs. 73.5 ± 4.1% for CGΔT), as shown above, but this was also true for the evoked response to forskolin (1.2-fold stimulation; Fig. 4B, lanes 1 and 2; and Table 1). That the electrophoretic migration between the intracellular and secreted subunits synthesized in either CG or CGΔT cell line was similar implies that the intracellular forms were processed to complex oligosaccharides, reflecting an accumulation of the mature proteins after transit from the late Golgi compartment. Taken together, these data show that loss of the CGβ CTP leads to a more regulated secretion of CGΔT.
Figure 4.
Secretagogue-stimulated secretion of CG and CGΔT dimers from GH3 cells. A, Experimental protocol; B, CG and CGΔT incubated in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 25 μm forskolin. All media (M) and cell lysates (L) were immunoprecipitated with CGβ antiserum. The data shown are representative of four independent experiments.
Table 1.
Percentage of CG and CGΔT secretion and accumulation after forskolin stimulation
| Protein | Without forskolin
|
With forskolin
|
||
|---|---|---|---|---|
| Medium | Lysate | Medium | Lysate | |
| CG (%) | 63.0 ± 1.6 | 37.0 ± 1.6 | 74.8 ± 2.1 | 25.2 ± 2.1 |
| CGΔT (%) | 26.5 ± 4.1 | 73.5 ± 4.1 | 78.2 ± 4.6a | 21.8 ± 4.6a |
[35S]Cysteine-labeled immunoprecipitated proteins from autoradiographs similar to those shown in Fig. 4B were quantitated by densitometry. To calculate the percentage of protein secretion and accumulation, the values for medium and lysate were combined and set at 100% (total synthesis). The data represent the mean values of four independent experiments ± sem.
P < 0.05 for with vs. without forskolin.
Subcellular localization of CG and CGΔT in GH3 cells
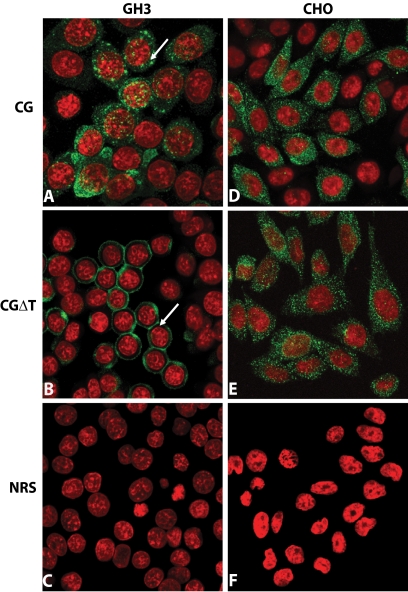
We next addressed whether the secretion patterns seen above are also associated with unique subcellular distribution of CG and CGΔT dimer. Confocal microscopy revealed that the CG cells exhibit a fluorescence pattern of randomly dispersed cytoplasmic puncta (arrow, Fig. 5A). Moreover, an unexpected cellular pattern was observed for CGΔT cells (Fig. 5B). In greater than 50% of the cells, essentially all the immunofluorescence was concentrated near the plasma membrane (arrow), giving a distinct ring appearance without significant staining around the nucleus (Fig. 5B). This staining pattern was observed for a variety of distinct clones (n = 4), expressing variable levels of CGΔT. No significant staining was seen when any of the cell lines expressing CG (not shown) or CGΔT (Fig. 5, C and F) were stained with normal rabbit serum instead of CGβ antiserum. In contrast to GH3 cell lines, there was no difference in the staining pattern for CG and CGΔT in CHO cells (Fig. 5, D–F). This indicates that the novel staining for CGΔT observed in the GH3 cells was dependent on the presence of the regulated pathway, because CHO cells lack secretory granules and release their content constitutively.
Figure 5.
Immunofluorescent localization of CG or CGΔT dimers in GH3 (A–C) and CHO (D–F) cells. The cells were immunostained with CGβ antiserum (A, B, D, and E) or normal rabbit serum (NRS; C and F represent CGΔT cells), and immunofluorescence was detected by confocal microscopy (green, positive staining with the primary antiserum; red, nuclear staining). Note the unique honeycomb pattern (arrow) of staining for CGΔT (B) vs. dispersed puncta (arrow) for CG dimer (A). There was no difference in the staining pattern for CG (D) and CGΔT (E) in CHO cells with the lattice staining pattern of CGΔT conspicuously absent in these cells. The micrographs shown are representative of eight experiments and are at ×60 magnification.
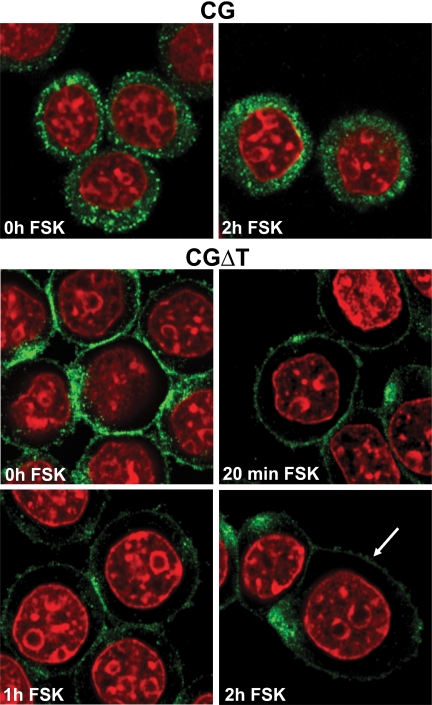
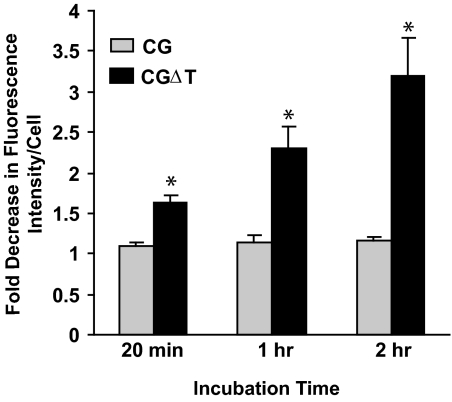
That the CGΔT accumulating in this zone of the cell is in secretagogue-releasable vesicles was demonstrated by changes in fluorescence intensity with forskolin (Figs. 6 and 7). The CG and CGΔT cells were incubated for up to 2 h with the secretagogue (Fig. 6). The relative fluorescence for the CG dimer was not significantly altered even after 2 h forskolin treatment (Figs. 6 and 7). In contrast, progressive time-dependent decrease in CGΔT fluorescence intensity with forskolin was observed (r2 = 0.87; P < 0.05), and this loss of signal was not accompanied by changes in cytoplasmic staining, implying that all of the CGΔT pool was released extracellularly (Figs. 6 and 7). It is also evident that for many CGΔT cells, clusters of puncta accumulated at a single region of the cell (see Discussion). These data show that the CGΔT heterodimer was targeted to the regulated secretory pathway in transfected GH3 cells.
Figure 6.
Immunofluorescence of CG- and CGΔT-expressing cells in GH3 cells after incubation with 25 μm forskolin (FSK) up to 2 h. The corresponding controls (no primary antiserum) performed concurrently with this experiment are similar to Fig. 5C. There was greater loss of fluorescence signal of CGΔT compared with CG dimer after incubation with forskolin (arrow). The micrographs shown are representative of the staining pattern seen in four independent experiments at ×100 magnification.
Figure 7.
Fluorescent intensity of CG and CGΔT after incubation without or with forskolin. Results are the mean ± sem (n = 4). *, P < 0.05 for CG vs. CGΔT. Fold decrease in fluorescence intensity per cell for CGΔT cells was correlated with the incubation time (r2 = 0.87; P < 0.05).
Discussion
Based on extensive sequence identity, it has been suggested that the CGβ gene evolved from an ancestral LHβ gene (2,15,20). A single-base-pair deletion at codon 114 generated an mRNA encoding the CGβ-subunit composed of 145 amino acids compared with the 121 amino acids of the LHβ-subunit, resulting in two proteins with different COOH termini (2,20). Thus, the apparent major evolutionary change from LHβ to the CGβ-subunit was the appearance of the CTP. The seven amino acids at the carboxy terminus of LHβ are very hydrophobic (2,15,21); the CGβ carboxy end is hydrophilic, containing several serine residues, four of which are O-glycosylated (1). Here we show that truncating the carboxy terminus of the CGβ-subunit to 114 amino acids shifts the secretion of this CG analog from a primarily constitutive route to a more regulated pathway. This was demonstrated in GH3 cells by the following: 1) greater intracellular accumulation of the CGΔT mutant, 2) slower release of CGΔT compared with CG, and 3) increase in the forskolin-releasable pool. Thus, despite the otherwise identical amino acid sequence between CG and CGΔT, loss of the CTP leads to a molecular signature more resembling LH than CG.
When comparing the biosynthesis of LH and CG, three important differences emerge: 1) the pathways of secretion, 2) polarity of their release, and 3) posttranslational processing of the N-linked oligosaccharides. The N-linked carbohydrates in CG terminate with Gal-sialic acid, whereas in LH, N-acetyl galactosamine-sulfate serves as the terminal oligosaccharide (18). LH is packaged into storage granules (8,22,23,24) and released exocytotically by GnRH via the basolateral-like surface near the capillaries of pituitary gonadotroph cells (21,22,25). In contrast, CG is secreted by an apical route through the villous and directly into the intervillous space created by the implanted placenta (26,27,28).
Although the regulated release of CGΔT is analogous to LH, the determinants accounting for this behavior are not clear. The LHβ- and CGβ-subunits share 85% amino acid identity, and aside from the carboxyl-terminal differences, the LHβ contains one N-linked oligosaccharide at position 30, whereas CGβ contains two N-linked units at sites 13 and 30 (Fig. 1). We cannot exclude the possibility that there is some signaling generated between these determinants and the CTP that overrides the regulated function. When the LHβ heptapeptide is deleted from the LHβ-subunit, the resulting LHΔT dimer displays a more constitutive phenotype comparable to that seen for CG (15). Thus, the data suggest that multiple distal secretory signals in the LH and CGβ-subunits interact with their corresponding carboxy termini. In this regard, it is known that the CTP sequence interacts with other domains in the CGβ-subunit and ultimately participates in the overall folding of the subunit (10,29).
It is curious that when the GH3 cells were labeled with [35S]sulfate, the extent of labeling of CGΔT was over 4-fold greater than CG. Although this suggests that the CTP masks a determinant for sulfate recognition, we cannot exclude the possibility that the posttranslational changes are due to induced changes in the folding of CGΔT as discussed above. The latter is consistent with previous studies demonstrating the altered processing of CGΔT Asn-linked oligosaccharides in CHO cells compared with CG (9,10).
Immunofluorescence and confocal microscopy revealed a unique pattern of staining for CGΔT compared with CG dimer. Almost the entire staining was located in the region of the plasma membrane and densely packed there, and little of the punctate staining around the nucleus and the Golgi was observed. In addition, zones of clustered puncta were observed in CGΔT cells. The nature of such aggregates is not clear, but the protein may be accumulating in an intermediate endosomal-Golgi compartment, not all of which is secretagogue releasable. In contrast, CG dimer cells showed random dispersed cytoplasmic puncta, similar to those observed for LH dimer in GH3 cells (15). In the case of CGΔT, the characteristic honeycomb pattern was reduced after forskolin stimulation. This is a major distinction between CGΔT and LH/CG. Because little perinuclear staining was observed, the data suggest that CGΔT segregates into distinct secretion vesicles. That CGΔT may sort to a unique granule-like structure compared with LH is consistent with numerous observations that the type of regulated cargo synthesized in transfected cell lines with regulated secretion pathways influences granule biogenesis (30,31,32,33). Even within the same cell, different secretory proteins can sort from one another into distinct secretion vesicles (34). Alternatively, the staining of CGΔT dimer reflects regulated sorting of the dimer, because for CG dimer, it is expected that there will be staining dispersed in the cell given its constitutive behavior. In the case of LH, the staining observed throughout the cytoplasm (15) is indicative of the inefficient assembly of the LHβ-subunit. Although, like CGΔT, the regulated LH dimer pool may be concentrated at the periphery, unassembled LHβ-subunit accumulating in the ER and Golgi will score positive with the CGβ antiserum, which recognizes both LH dimer and free β-subunit. Thus, the dispersed staining of the LHβ-subunit obscures the distinct peripheral signal of LH dimer as seen for CGΔT. Similarly in transfected GH3 cells expressing only the CGβΔT-subunit (data not shown), less of it appears in the periphery compared with the CGΔT dimer. Because deletion of the CTP partially inhibits assembly (29), it is apparent that more CGβΔT accumulates in the perinuclear region and less is observed in the region of the plasma membrane.
It is clear that the evolutionary site surrounding amino acid residue 114 is the basis for the differential polarity of secretion and sorting described above for LH and CG. Previously, we demonstrated in polarized MDCK cells that the CTP sequence is the signal driving CG apically into the maternal blood (16,35) and that deleting the CTP reversed its polarity to the basolateral surface in vitro. During peak CG synthesis, the placenta primarily employs a pathway of rapid unregulated secretion instead of maintaining a low basal level with pulsatile secretagogue-stimulated surges of the hormone. This is supported by the largely constitutive secretion of the CG dimer and the more regulated release of CGΔT from GH3 cells shown here. Thus, the determinants at the carboxyl end of the CGβ-subunit apparently evolved from the LHβ-subunit primarily to overcome the slow release and intracellular storage of pituitary LH resulting in a rapid secretion of CG from the placenta.
Materials and Methods
Cell culture and transfection
GH3 cells were a gift from Dr. Dennis Shields (Albert Einstein College of Medicine, New York, NY). The cells were grown (no more than 35 passages) at 37 C in Ham’s F-12 medium (Mediatech Inc., Herndon, VA) supplemented with 12.5% horse serum (Invitrogen Corp., Carlsbad, CA), 2.5% fetal bovine serum (Harlan Bioproducts for Science, Inc., Indianapolis, IN), 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified 5% CO2 incubator. To obtain clones expressing α-, CGβ-, or CGβΔT-subunits (Fig. 1) and corresponding dimers, semiconfluent cells grown in six-well plates were transfected with 4 μg plasmid DNA using Lipofectamine 2000 (Invitrogen) and processed after 48 h following the manufacturer’s instructions (15). Stable clones were selected approximately 16 d later with 0.25 mg/ml G418 (Research Product International, Mt. Prospect, IL). Single colonies were isolated and subsequently screened by immunoprecipitating proteins from the media and lysates of metabolically labeled cells (see below). Clones maintained in culture in the presence of 0.125 mg/ml G418 were used for the experiments described below. The pM2 HA vector is a PSV2 neo derivative, which contains the ampicillin resistance and the neomycin resistance gene, and driven by the Harvey murine sarcoma virus long terminal repeat (5,7,36).
Metabolic labeling and immunoprecipitation
For continuous labeling experiments, dimer cells were plated into six-well dishes and grown approximately 4 d to near confluency. Cells were labeled for 4 h with 20 μCi/ml [35S]cysteine (specific activity > 1000 Ci/mmol; MP Biochemicals Inc., Irvine, CA) in Ham’s F-12 medium without cysteine and G418 but supplemented with 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics. Labeling with inorganic sulfate was performed for 4 h in Ham’s F-12 sulfate-free medium with 0.7 mCi/ml carrier-free sulfate (Na2[35S]O4; MP Biomedicals) supplemented with 7.5% dialyzed fetal bovine serum, glutamine, and antibiotics (13,15).
All labeled media and cell lysates were treated with iodoacetamide and phenylmethane sulfonyl fluoride to inhibit inadvertent disulfide bond formation and proteolytic activity, respectfully. After centrifugation to remove cell debris, samples were precleared with 7.5 μl/ml normal rabbit serum (Sigma Chemical Co., St. Louis, MO) and Pansorbin (EMD BioSciences Inc., La Jolla, CA). The supernatants (2 ml) were divided into equal aliquots, and proteins were immunoprecipitated for 2 h at room temperature with α or CGβ rabbit polyclonal antisera and Pansorbin (5). Equal volumes of samples were separated by SDS-PAGE (15% acrylamide). The gels were soaked in 1 m sodium salicylate for 15 min, dried, and exposed to x-ray film (15).
Storage and release assays
To analyze gonadotropin storage and secretion behavior, cells expressing CG or CGΔT dimers were preincubated for 1.5 h with cysteine-free medium (Ham’s F-12) followed by a 20-min pulse-label in this medium containing 80 μCi/ml [35S]cysteine. At the end of the pulse, the medium was aspirated and cells were washed twice with prewarmed chase medium comprised of Ham’s F-12, 1 mm unlabeled l-cysteine (Sigma), 7.5% dialyzed fetal bovine serum, glutamine, antibiotics and then incubated in this medium for up to 24 h. All labeled media and cell lysates were immunoprecipitated with CGβ antiserum and analyzed by SDS-PAGE.
To examine forskolin-stimulated release of dimers, three wells of CG and CGΔT were labeled with 25 μCi/ml [35S]cysteine for 1.5 h. Medium and lysate from one well were collected and served as the time zero controls for subsequent experiments. Cells in the other wells were washed in chase medium, and then one well received chase medium with forskolin (25 μm final concentration) for 2 h (Fig. 4A). The remaining well was incubated without secretagogue (13,15). Labeled proteins secreted into the medium and remaining in cells at the end of an experiment were immunoprecipitated with CGβ-subunit-specific antiserum and analyzed by gel electrophoresis and fluorography as described above.
Immunofluorescence and confocal microscopy
GH3 cells expressing CG or CGΔT were grown on Fisherbrand Superfrost-Plus microscopy slides (Fisher Scientific, Pittsburgh, PA) in petri dishes as described above. The cells were fixed with 4% paraformaldehyde for 20 min at room temperature, washed in PBS, and permeabilized with 0.2% Tween 20 for 10 min (15). Cells were then incubated in 20% normal goat serum (Vector Laboratories, Burlingame, CA) for 1 h to block nonspecific binding and washed three times for 10 min in 2% BSA (Sigma) in PBS. Cells were incubated at room temperature with CGβ antiserum (1:250 dilution) for 30 min, washed, and stained with goat antirabbit IgG conjugated to Alexa Fluor 488 (1:250 dilution; Invitrogen) for 20 min. After three washes in 2% BSA/PBS, and once in PBS, nuclei were counterstained with TOPRO-iodide (Invitrogen) for 15 min. After several washes with PBS, the cells were mounted in VectaShield mounting medium (Vector). Staining patterns were analyzed using an Olympus FV-500 confocal microscope with a z-interval of 0.5–1 μm using ×60 and ×100 objectives.
To estimate the effect of forskolin stimulation on the pattern and intensity of staining, GH3 cells expressing CG or CGΔT were incubated for 20 min or 1 or 2 h in fresh medium with or without 25 μm forskolin. Cells were fixed for indirect immunofluorescence staining and examined using confocal microscopy. Processing of images was performed using the Metamorph image software package (Molecular Devices Corp., Downingtown, PA). The relative fluorescence intensity per cell of CG and CGΔT cells (three to five fields per slide; 200–300 cells) incubated with or without forskolin was measured with the NIH ImageJ program. The experiment was repeated four times.
Analysis of data
The labeled bands from autoradiography were scanned using a GS-710 calibrated Imaging Densitometer, and the intensity of bands was quantified using Quantity One software (Bio-Rad Laboratories, Inc.). Equal exposure times for the fluorograms were used when comparing the results of protein synthesis and secretion. The secretion t½ for dimers corresponds to the time when 50% of the labeled dimer accumulated in the medium. To calculate the percentage of protein secretion (medium) and accumulation (lysate), the values for medium and lysate were first combined and set at 100% (total synthesis). The relative fluorescence intensity of untreated or forskolin-treated cells is expressed as fold decrease in fluorescence intensity per cell. To evaluate the time course of forskolin exposure and the fluorescence intensity, regression analysis was used. Each experiment was repeated three to eight times and analyzed using a paired t test, and the results are expressed as the mean ± sem, with P < 0.05 considered significantly different.
Acknowledgments
We thank Dr. Christopher Pearl for helpful discussion during the course of the study. We also thank Drs. David Sheff and Rick Sifers for their comments regarding the manuscript. We are grateful to Dennis Oakley for his help with confocal microscopy and to Laura Kyro and Sharon Thomas for preparation for the manuscript.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant DR065155 and NIH Neuroscience Blueprint Core Grant NS057105 to Washington University and the Bakewell Family Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: CG, Chorionic gonadotropin; CTP, carboxyl-terminal peptide.
References
- Birken S, Canfield RE 1977 Isolation and amino acid sequence of COOH-terminal fragments from the β-subunit of human choriogonadotropin. J Biol Chem 252:5386–5392 [PubMed] [Google Scholar]
- Talmadge K, Vamvakopoulos NC, Fiddes JC 1984 Evolution of the genes for the β-subunits of human chorionic gonadotropin and luteinizing hormone. Nature 307:37–40 [DOI] [PubMed] [Google Scholar]
- Fiddes JC, Goodman HM 1980 The cDNA for the β-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3′-untranslated region. Nature 286:684–687 [DOI] [PubMed] [Google Scholar]
- Corless CL, Matzuk MM, Ramabhadran TV, Krichevsky A, Boime I 1987 Gonadotropin β-subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. J Cell Biol 104:1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Kornmeier CM, Whitfield GK, Kourides IA, Boime I 1988 The glycoprotein α-subunit is critical for secretion and stability of the human thyrotropin β-subunit. Mol Endocrinol 2:95–100 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Spangler MM, Camel M, Suganuma N, Boime I 1989 Mutagenesis and chimeric genes define determinants in the β-subunits of human chorionic gonadotropin and lutropin for secretion and assembly. J Cell Biol 109:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyan M, Boime I 1997 Secretion of chorionic gonadotropin from human trophoblasts. Placenta 18:237–241 [DOI] [PubMed] [Google Scholar]
- Conn PM, McArdle CA, Andrews WV, Huckle WR 1987 The molecular basis of gonadotropin-releasing hormone (GnRH) action in the pituitary gonadotrope. Biol Reprod 36:17–35 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Hsueh AJ, Lapolt P, Tsafriri A, Keene JL, Boime I 1990 The biological role of the carboxyl-terminal extension of human chorionic gonadotropin β-subunit. Endocrinology 126:376–383 [DOI] [PubMed] [Google Scholar]
- Muyan M, Boime I 1998 The carboxyl-terminal region is a determinant for the intracellular behavior of the chorionic gonadotropin β-subunit: effects on the processing of the Asn-linked oligosaccharides. Mol Endocrinol 12:766–772 [DOI] [PubMed] [Google Scholar]
- Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I 1992 Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin β-subunit to the follitropin β-subunit. Proc Natl Acad Sci USA 89:4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Kelly RB 1987 Constitutive and regulated secretion of proteins. Annu Rev Cell Biol 3:243–293 [DOI] [PubMed] [Google Scholar]
- Bielinska M, Rzymkiewicz DM, Boime I 1994 Human luteinizing hormone and chorionic gonadotropin are targeted to a regulated secretory pathway in GH3 cells. Mol Endocrinol 8:919–928 [DOI] [PubMed] [Google Scholar]
- Muyan M, Rzymkiewicz DM, Boime I 1994 Secretion of lutropin and follitropin from transfected GH3 cells: evidence for separate secretory pathways. Mol Endocrinol 8:1789–1797 [DOI] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Pearl CA, Comstock A, Boime I 2008 A carboxyl-terminal sequence in the lutropin β-subunit contributes to the sorting of lutropin to the regulated pathway. J Biol Chem 283:11485–11492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Krieger M, Corless CL, Boime I 1987 Effects of preventing O-glycosylation on the secretion of human chorionic gonadotropin in Chinese hamster ovary cells. Proc Natl Acad Sci USA 84:6354–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Boime I 1988 Site-specific mutagenesis defines the intracellular role of the asparagine-linked oligosaccharides of chorionic gonadotropin β-subunit. J Biol Chem 263:17106–17111 [PubMed] [Google Scholar]
- Green ED, Boime I, Baenziger JU 1986 Differential processing of Asn-linked oligosaccharides on pituitary glycoprotein hormones: implications for biologic function. Mol Cell Biochem 72:81–100 [DOI] [PubMed] [Google Scholar]
- Green ED, Gruenebaum J, Bielinska M, Baenziger JU, Boime I 1984 Sulfation of lutropin oligosaccharides with a cell-free system. Proc Natl Acad Sci USA 81:5320–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maston GA, Ruvolo M 2002 Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol 19:320–335 [DOI] [PubMed] [Google Scholar]
- Keutmann HT, Williams RM, Ryan RJ 1979 Structure of human luteinizing hormone β-subunit: evidence for a related carboxyl-terminal sequence among certain peptide hormones. Biochem Biophys Res Commun 90:842–848 [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Childs GV 1988 Differential storage and release of luteinizing hormone and follicle-releasing hormone from individual gonadotropes separated by centrifugal elutriation. Endocrinology 122:1282–1290 [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR 2003 The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Suppl 61:463–476 [PubMed] [Google Scholar]
- Thomas SG, Clarke IJ 1997 The positive feedback action of estrogen mobilizes LH-containing, but not FSH-containing secretory granules in ovine gonadotropes. Endocrinology 138:1347–1350 [DOI] [PubMed] [Google Scholar]
- Currie RJ, McNeilly AS 1995 Mobilization of LH secretory granules in gonadotrophs in relation to gene expression, synthesis and secretion of LH during the preovulatory phase of the sheep oestrous cycle. J Endocrinol 147:259–270 [DOI] [PubMed] [Google Scholar]
- Boyd JD, Hamilton WJ 1970 The human placenta. Cambridge, UK: W Heffer and Sons; 157–174 [Google Scholar]
- Wynn RM 1972 Cytotrophoblastic specializations: an ultrastructural study of the human placenta. Am J Obstet Gynecol 114:339–355 [DOI] [PubMed] [Google Scholar]
- Thiede HA, Choate JW 1963 Chorionic gonadotropin localization in the human placenta by immunofluorescent staining. II. Demonstration of hCG in the trophoblast and amnion epithelium of immature and mature placentas. Obstet Gynecol 22:433–443 [PubMed] [Google Scholar]
- Muyan M, Furuhashi M, Sugahara T, Boime I 1996 The carboxy-terminal region of the β-subunits of luteinizing hormone and chorionic gonadotropin differentially influence secretion and assembly of the heterodimers. Mol Endocrinol 10:1678–1687 [DOI] [PubMed] [Google Scholar]
- Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M 2004 Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem 279:20242–20249 [DOI] [PubMed] [Google Scholar]
- Kim T, Gondre-Lewis MC, Arnaoutova I, Loh YP 2006 Dense-core secretory granule biogenesis. Physiology (Bethesda) 21:124–133 [DOI] [PubMed] [Google Scholar]
- Kim T, Loh YP 2006 Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol Biol Cell 17:789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP, Kim T, Rodriguez YM, Cawley NX 2004 Secretory granule biogenesis and neuropeptide sorting to the regulated secretory pathway in neuroendocrine cells. J Mol Neurosci 22:63–71 [DOI] [PubMed] [Google Scholar]
- Sobota JA, Ferraro F, Back N, Eipper BA, Mains RE 2006 Not all secretory granules are created equal: partitioning of soluble content proteins. Mol Biol Cell 17:5038–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Boime I 2004 Luteinizing hormone and follicle-stimulating hormone exhibit different secretion patterns from cultured Madin-Darby canine kidney cells. Biol Reprod 70:649–655 [DOI] [PubMed] [Google Scholar]
- Sachais BS, Snider RM, Lowe 3rd JA, Krause JE 1993 Molecular basis for the species selectivity of the substance P antagonist CP-96,345. J Biol Chem 268:2319–2323 [PubMed] [Google Scholar]