Abstract
Environmental sodium arsenite is a toxin that is associated with male infertility due to decreased and abnormal sperm production. Arsenic trioxide (ATO), another inorganic trivalent semimetal, is an effective therapy for acute promyelocytic leukemia, and there is investigation of its possible efficacy in prostate cancer. However, the mechanism of arsenic action in male urogenital tract tissues is not clear. Because the androgen receptor (AR) plays an important role in spermatogenesis and prostate cancer, we explored the possibility that trivalent arsenic regulates AR function. We found that arsenic inhibited AR transcriptional activity in prostate cancer and Sertoli cells using reporter gene assays testing several androgen response element-containing regions and by assessing native target gene expression. Arsenic inhibition of AR activity was not due to down-regulation of AR protein levels, decreased hormone binding to AR, disruption of AR nuclear translocation, or interference with AR-DNA binding in vitro. However, chromatin immunoprecipitation studies revealed that arsenic inhibited AR recruitment to an AR target gene enhancer in vivo. Consistent with a deficiency in AR-chromatin binding, arsenic disrupted AR amino and carboxyl termini interaction. Furthermore, ATO caused a significant decrease in prostate cancer cell proliferation that was more pronounced in cells expressing AR compared with cells depleted of AR. In addition, inhibition of AR activity by ATO and by the AR antagonist, bicalutamide, was additive. Thus, arsenic-induced male infertility may be due to inhibition of AR activity. Further, because AR is an important target in prostate cancer therapy, arsenic may serve as an effective therapeutic option.
Arsenic, an environmental toxin and chemotherapeutic agent, inhibits androgen receptor transcriptional activity by disrupting androgen receptor recruitment to target gene promoters in chromatin
Excessive exposure to arsenic through contaminated drinking water or occupational contact causes a range of toxic effects. Environmental arsenic is a carcinogen, and chronic exposure is associated with hepatic, cardiovascular, dermatological, and reproductive toxicity (1). Although there is an association between arsenic exposure and male infertility, the molecular mechanism is unclear. Arsenic-induced male infertility is characterized by abnormal sperm, decreased sperm counts, and decreased sperm mobility in humans as well as animal models (2,3,4).
The most common forms of inorganic arsenic compounds found in the environment are trivalent arsenite and pentavalent arsenate, which is reduced to trivalent arsenite in vivo (1,5). Circulating arsenic levels of up to approximately 0.8 μm in the blood and about 3.6 μm in the urine have been reported in chronically exposed individuals (6). In addition to being an environmental toxin, trivalent arsenic, in the form of arsenic trioxide (ATO), is also a chemotherapeutic agent for acute promyelocytic leukemia (7). The efficacy of ATO in the treatment of acute promyelocytic leukemia has stimulated investigation of ATO treatment of other leukemias and solid tumors including prostate. Previous work indicates that ATO induces apoptosis in prostate cancer cell lines at concentrations of 10 μm or greater and leads to growth inhibition at therapeutic concentrations of 2 μm or less (8,9,10).
Trivalent arsenic, including ATO and sodium arsenite, reacts with thiol-containing molecules such as cysteine and has a higher affinity for dithiols than monothiols (11,12). Because steroid receptors contain well-conserved cysteine residues in their DNA-binding domain (DBD), trivalent arsenic may interact with this region and regulate steroid receptor activity. One study has shown that arsenic inhibits glucocorticoid receptor activity by disruption of DNA binding (13). However, another study reported that arsenic-mediated inhibition of glucocorticoid receptor activity was associated with decreased hormone binding (14). Arsenic also inhibits progesterone and mineralcorticoid receptor transcriptional activity (15). Interestingly, arsenic stimulates estrogen receptor transcriptional activity by binding to the ligand-binding domain (LBD) of the receptor (16). In addition, it was reported that 2 μm arsenic inhibited androgen-induced androgen receptor (AR) transcriptional activity in hepatoma cells (15). In contrast, arsenic had no effect on androgen-induced AR activity and actually enhanced basal AR activity in prostate epithelial cells that were chronically treated with 5 μm arsenic (17). Thus, arsenic regulates many of the steroid receptors that play critical roles in disease pathogenesis including breast and prostate cancer. A variety of mechanisms have been implicated in arsenic’s actions on steroid receptors; however, little is known about how arsenic affects AR.
The AR is a member of the steroid hormone receptor family. AR has three major domains: an N-terminal domain, a DBD, and an LBD (18,19). After androgen (testosterone or its metabolite, dihydrotestosterone) binds to AR, the receptor becomes activated and interacts with DNA. AR binds to specific DNA response elements (AREs or, androgen response elements) that are associated with AR target genes (18). This interaction between the receptor and DNA allows AR to recruit coactivator proteins, such as steroid receptor coactivator (SRC)1 and transcriptional intermediary factor (TIF)2, leading to transcription of target genes (20,21).
The transactivation domains, including the activation function (AF)1 in the NH2-terminal domain and AF2 in the LBD of steroid receptors, mediate interactions with coactivators (21). AR activity depends predominately on AF1; however, AF2 plays an important role in amino and carboxyl termini (N-C) interaction (22). N-C interaction is an androgen-induced interaction between the NH2-terminal domain and the carboxyl-terminal LBD that is critical for chromatin binding capacity and for maintaining robust AR activity (22,23,24,25,26).
AR regulation of target gene expression plays an essential role in spermatogenesis (27,28). Consistent with this conclusion, the phenotype of a patient with arsenic-induced male infertility is very similar to that of a patient with an inactivating mutation in AR (29,30). Therefore, arsenic may disrupt spermatogenesis by inhibiting AR activity leading to arsenic-induced male infertility.
AR is also important in the development and progression of prostate cancer (31,32,33,34). The critical role androgens and AR play in prostate cancer is exploited in the commonly administered androgen deprivation therapy (ADT). Although the majority of patients with prostate cancer initially respond to ADT, ultimately patients relapse with an aggressive androgen-independent prostate cancer that is unresponsive to most treatments. Because AR activity is maintained in androgen-independent prostate cancer, a treatment that inhibits AR transcriptional activity is desirable (35).
Because arsenic exposure is associated with abnormal spermatogenesis and arsenic may serve as a therapeutic agent in prostate cancer, we investigated the effects of arsenic on AR function. We found that trivalent arsenic significantly inhibited AR transcriptional activity in both prostate cancer and Sertoli cells. Arsenic did not interfere with androgen binding to AR, ligand-mediated AR translocation into the nucleus, or in vitro AR-DNA binding. Nevertheless, arsenic inhibited AR N-C interaction and specific chromatin binding by AR and TIF2, which ultimately led to an inhibition of receptor activity. Consequently, trivalent arsenic inhibition of AR target gene expression may disrupt spermatogenesis, whereas, by targeting AR, arsenic may also be an effective prostate cancer therapy.
Results
ATO inhibits AR transcriptional activity
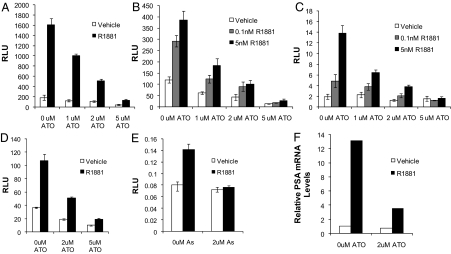
To determine the effect of arsenic on AR transcriptional activity, reporter gene assays were performed. The prostate-specific antigen (PSA) gene enhancer/promoter contains AREs and is commonly used to assess AR activity. PC3 cells are human prostate cancer cells that do not express AR. In PC3 cells transfected with a wild-type AR and treated with R1881 (an AR agonist), ATO inhibited PSA enhancer/promoter-directed luciferase activity (Fig. 1A). LNCaP and LAPC4 are androgen-dependent human prostate cancer cell lines that express AR. LNCaP cells express AR with a mutation in the LBD that can bind androgen as well as certain other steroid hormones (36). LAPC4 express a wild-type AR. Arsenic inhibited endogenous AR transcriptional activity in both LNCaP and LAPC4 cells (Fig. 1, B and C). The LNCaP-R1 cell line is an androgen-independent cell line derived from LNCaP that exhibits higher AR protein levels and increased androgen responsiveness. ATO was an effective inhibitor of AR transcriptional activity in these androgen-independent cells (data not shown).
Figure 1.
ATO inhibits AR transcriptional activity. PC3 (A and D) LNCaP (B), or LAPC4 (C) cells were transfected with an ARE (D) or PSA (ARE containing)-luciferase reporter plasmid and AR cDNA expression vector (PC3 cells only). Cells were treated with either vehicle, 0.1 or 5 nm R1881 (an AR agonist), and either 0, 1, 2, or 5 μm ATO. Luciferase activity was determined 48 h after transfection. Means of triplicate samples are plotted± sem. E, TM4 cells were transfected with an ARE-luciferase reporter plasmid and treated with vehicle or 5 nm R1881 and 0 or 2 μm sodium arsenite. Luciferase activity was determined 48 h after transfection. F, LNCaP cells were treated with vehicle or 5 nm R1881 in the presence or absence of 2 μm ATO for 48 h. RNA was extracted and then reverse transcribed to cDNA. Real-time PCR was performed using Taqman probes for PSA mRNA and 18S mRNA (control). This experiment was performed three times under similar conditions. As, Arsenic; RLU, relative luciferase units.
ATO inhibition of AR transcriptional activity was not restricted to the PSA enhancer/promoter; the same results were obtained using a simple ARE-luciferase construct (Fig. 1D) as well as the murine mammary tumor virus-luciferase construct (data not shown). Inhibition of AR activity by ATO occurred in a dose-dependent manner at 1 μm, 2 μm, and 5 μm ATO, which are clinically achievable concentrations. In addition, ATO inhibited AR activity in the absence of androgen and the presence of very low levels of androgen mimicking the conditions after ADT. The small but significant inhibition of basal AR activity by ATO may be due to low levels of residual hormone or ligand-independent activation of the receptor. Regardless, androgen-induced activity was significantly reduced by ATO in a variety of prostate cancer cell lines. Furthermore, environmental sodium arsenite completely blocked androgen-induced AR transcriptional activity in TM4 mouse Sertoli cells (Fig. 1E). Nevertheless, ATO and sodium arsenite were equally effective in inhibiting AR activity in prostate cancer cells (data not shown). Together these reporter assays show that arsenic consistently inhibited activity of wild-type as well as mutant AR in a variety of cell lines, including prostate cancer and Sertoli cells.
To confirm results of reporter gene assays, we examined androgen regulation of a native target gene (PSA) in LNCaP cells. We found that after ATO treatment PSA mRNA levels were significantly decreased, confirming our reporter gene assays (Fig. 1F). Furthermore, the inhibition of AR activity cannot be explained by cell death associated with ATO treatments because minimal cell death was observed at these concentrations and time points (data not shown).
ATO does not regulate AR protein levels
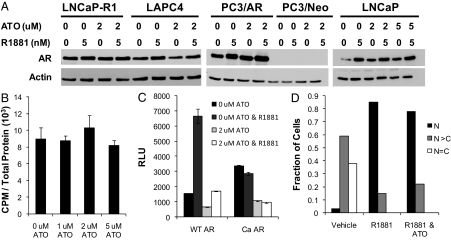
One possible mechanism by which ATO inhibits AR transcriptional activity is by down-regulation of the receptor. However, Western blot analysis showed that neither 2 μm nor 5 μm ATO in the presence or absence of androgen affected AR protein levels in LNCaP or LAPC4 cells (Fig. 2A). AR protein levels were also unchanged after ATO treatment in androgen-independent cell lines [PC3/AR, which stably express an AR cDNA (37) or in LNCaP-R1] (Fig. 2A). Whereas AR stabilization occurs as expected in the presence of androgen, ATO did not appear to influence this process (38).
Figure 2.
ATO does not affect AR levels, hormone binding, or nuclear translocation. A, LNCaP, LAPC4, LNCaP-R1, and PC3/AR cell lines were treated with either 0, 2, or 5 μm ATO and either vehicle or 5 nm R1881. AR protein levels were determined 48 h later by Western blot. PC3 cells stably transfected with an empty vector construct containing neomycin resistance (PC3/Neo) were used as a control for the PC3/AR cells. These are representative blots from four distinct experiments. B, LNCaP cells were treated with either 0, 1, 2, or 5 μm ATO for 4 h before performing radioligand binding studies. The cells were then treated for 2 h with [3H]R1881 and/or an excess of R1881. Radioligand binding studies were performed, and radioactivity was measured using a scintillation counter. Means of triplicate samples are plotted as counts per minute per total protein ± sem. C, PC3 cells were transfected with a PSA (ARE-containing)-luciferase reporter plasmid, and wild-type AR or Ca AR (AF2 LBD mutant that cannot bind hormone) cDNA expression vectors. Cells were treated with either vehicle or 5 nm R1881 and 0 or 2 μm ATO. Luciferase activity was determined 48 h after transfection. Means of triplicate samples are plotted as relative luciferase activity ± sem. D, PC3 cells were cultured on coverslips and transfected with GFP-AR. The cells were then treated with either vehicle, 1 nm R1881, or both 1 nm R1881 and 5 μm ATO for 24 h. The cells were fixed, and nuclei were stained with DAPI. GFP-AR and DAPI staining was viewed using a confocal microscope. At least 100 cells were evaluated from each treatment group and determined to have equal GFP-AR in the nucleus and cytoplasm (N = C), more GFP-AR in the nucleus than the cytoplasm (N > C), or all nuclear GFP-AR (N). The data are representative of three independent experiments. RLU, Relative luciferase units; WT, wild type.
AR retains androgen binding capacity in the presence of ATO
To determine whether ATO inhibition of AR activity is a result of decreased hormone binding to AR, a whole-cell ligand binding assay was performed. Specific binding of R1881 to AR in the presence and absence of ATO (0–5 μm) was determined in PC3/AR and LNCaP cells. These studies revealed that ATO did not interfere with androgen binding to wild-type or mutant AR (Fig. 2B).
The AR AF2 transactivation domain is not essential for ATO-mediated inhibition of AR activity
The AF2 transactivation domain in the LBD is important for coregulator recruitment as well as AR N-C interaction. Deletion of the AF2 domain results in a constitutively active receptor that cannot bind hormone. To examine the possibility that ATO interferes with AF2 domain function, reporter gene assays were conducted in PC3 cells transfected with a constitutively active (Ca) AR lacking the AF2 domain. As expected, Ca AR stimulated luciferase production in the absence of hormone. Nevertheless, the activity of Ca AR was inhibited by ATO treatment (Fig. 2C). Therefore, AF2 domain function was not essential for ATO inhibition of AR transcriptional activity. In addition, these results are consistent with the finding that arsenic did not inhibit hormone binding to AR.
Androgen-mediated AR nuclear translocation after ATO treatment
After hormone binding, AR dissociates from its chaperone proteins and translocates into the nucleus where it can bind to chromatin and initiate target gene transcription. To determine whether ATO interferes with this process, we used a green fluorescent protein (GFP)-AR fusion protein to examine AR subcellular localization. We first verified by reporter gene assay that ATO inhibited the transcriptional activity of GFP-AR fusion protein similar to wild-type AR (data not shown). We also showed that, as expected, treatment with androgen resulted in nuclear localization of GFP-AR compared with vehicle control. Treatment with a combination of ATO and androgen yielded similar results: nuclear localization of GFP-AR. We scored these images, and the majority of the cells exhibited mostly nuclear GFP-AR after androgen treatment or a combination of ATO and androgen (Fig. 2D). Thus, ATO did not interrupt androgen-mediated AR nuclear translocation.
AR binds to an ARE-containing oligonucleotide in the presence of ATO
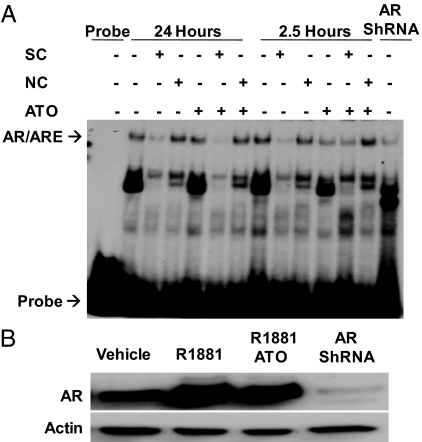
Because ATO binds to dithiol groups, the zinc fingers in the DBD of AR, which contain multiple cysteine residues, could be a target (12,39). Furthermore, a previous study showed that trivalent arsenic inhibits glucocorticoid receptor activity by preventing the receptor from binding to DNA (13). To explore the possibility that ATO inhibits AR binding to DNA, we used EMSA. A 32P-labeled consensus ARE probe was incubated in the presence of nuclear extracts from LNCaP cells. The cells were treated for 24 or 2.5 h with either vehicle or androgen in the presence or absence of ATO to explore the possibility that ATO affects AR-DNA binding in a time-dependent manner. The following controls were performed to assist in identification of the AR/ARE complex: use of nuclear extracts from LNCaP cells infected with short hairpin RNA (shRNA) lentivirus against AR, a specific competitor, and a nonspecific competitor. AR bound to the ARE probe in the presence and absence of ATO after androgen treatment at both 24 and 2.5 h (Fig. 3A). Furthermore, Western blot analysis revealed similar nuclear AR protein levels in the presence and absence of ATO in androgen-treated extracts as well as a decrease in AR levels in shRNA-infected cells (Fig. 3B).
Figure 3.
AR binding to DNA in vitro is not affected by ATO. LNCaP cells were treated with either vehicle or 5 nm R1881 in the presence or absence of 5 μm ATO for 2.5 or 24 h. Nuclear extracts were obtained and used for EMSA. A, Nuclear extracts were incubated with [32]P-labeled ARE probe, an AR antibody, and/or a specific (SC)/nonspecific (NC) competitor. All of these mixtures were then separated on a nondenaturing polyacrylamide gel. Radioactivity was measured using autoradiography. LNCaP cells infected with shRNA lentivirus against AR were used as a control. This is representative of three distinct experiments performed under similar conditions. B, Western blot analysis of AR protein levels from the nuclear extracts.
ATO interferes with AR and TIF2 recruitment to a target gene promoter in vivo
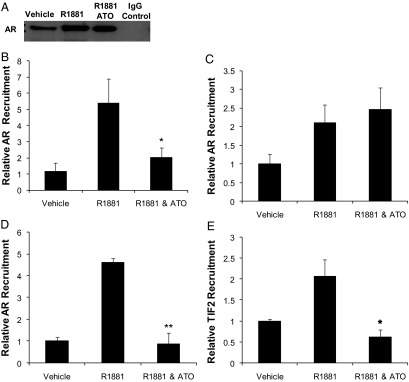
The ability of AR to bind to a DNA probe in vitro is distinct from AR binding to chromatin in vivo. Therefore, we used chromatin immunoprecipitation to determine whether ATO affects AR recruitment to the PSA proximal promoter. LNCaP cells were treated for 24 or 2.5 h with vehicle or androgen in the presence or absence of ATO. We found that there was increased recruitment of AR to the PSA promoter after androgen treatment at both 24 and 2.5 h, as expected (Fig. 4, B and C). Interestingly, 24 h but not 2.5 h ATO treatment inhibited androgen-mediated AR recruitment to the PSA promoter (Fig. 4, B and C). ATO treatment for 24 h blocked androgen-mediated AR recruitment to the PSA distal enhancer as well (Fig. 4D). ATO treatment for 24 h may be required to produce secondary effects leading to disruption of AR-chromatin interaction. Western blots of the immunoprecipitation samples revealed that there were similar amounts of cross-linked AR immunoprecipitated in the androgen compared with the androgen and ATO-treated cells (Fig. 4A). This result indicates that the differences in AR recruitment to specific AR-regulated target gene promoters were not due to differences in levels of immunoprecipitated AR. In addition, 24 h ATO treatment inhibited androgen-mediated recruitment of the AR coactivator, TIF2, to the PSA distal enhancer (Fig. 4E).
Figure 4.
ATO interferes with AR and TIF2 recruitment to chromatin. LNCaP cells were treated with vehicle, 5 nm R1881, or 5 nm R1881 and 5 μm ATO after which chromatin immunoprecipitation was performed. A, After immunoprecipitation with AR (PG21) antibody, a fraction of each sample was immunoblotted for AR (N-20). After either 24 h (B) or 2.5 h (C) treatment, AR recruitment to the PSA proximal promoter was determined by real-time PCR. AR (D) and TIF2 (E) recruitment to the PSA distal enhancer after 24 h treatment was examined using real-time PCR. These are the averages of at least three independent experiments plotted as means ± sem. P values were determined by a comparison of androgen treatment alone vs. a combination of androgen and ATO. *, P < 0.05; **, P < 0.01.
ATO disrupts androgen-mediated AR N-C interaction
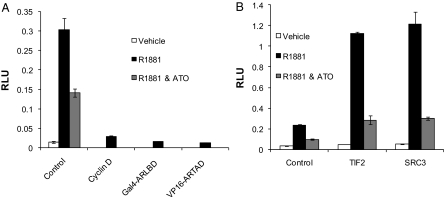
Li et al. (24) demonstrated that disruption of AR N-C interaction did not affect AR binding to an ARE oligonucleotide in vitro but blocked AR binding to AREs in chromatin. To determine whether the differential effects of ATO on AR binding to DNA in vitro compared with chromatin in vivo are due to disruption of AR N-C interaction, we performed mammalian two-hybrid assays. This system allows for the specific assessment of the interaction between the amino and carboxyl termini of AR. PC3 cells were transfected with the Gal4-LUC reporter, Gal4-ARLBD (the carboxyl terminus of AR, amino acids 614–919, fused to the Gal4 DBD), and/or VP16-ARTAD [transcriptional activation domain (TAD) of VP16 fused to AR amino acids 1-565]. As expected, transfection with either the VP16-ARTAD or Gal4-ARLBD alone in the presence of androgen failed to induce luciferase activity (Fig. 5A). However, transfection with both constructs resulted in a significant increase in luciferase activity after androgen treatment indicative of AR N-C interaction (Fig. 5A). The combination of ATO and 24 h androgen treatment resulted in a decrease in luciferase activity compared with androgen treatment alone (Fig. 5A). This result reveals that ATO interfered with AR N-C interaction.
Figure 5.
ATO disrupts androgen-mediated AR N-C interaction. PC3 cells were transfected with Gal4-LUC reporter and (A) control: Gal4-ARLBD + VP16-ARTAD + empty vector (EV); cyclin D: Gal4-ARLBD + VP16-ARTAD + cyclin D; Gal4-ARLBD: Gal4-ARLBD + EV; VP16-ARTAD: VP16-ARTAD + EV (B) Gal4-ARLBD + VP16-ARTAD + either EV Control or TIF2 or SRC3. All cells were treated with vehicle, 1 nm R1881, or 1 nm R1881 and 5 μm ATO for 24 h. Luciferase activity was determined 48 h after transfection. Means of triplicate samples are plotted ± sem. RLU, Relative luciferase units.
It has been shown that TIF2 and SRC3, known AR coactivators, increase N-C interaction possibly by binding to the amino and carboxyl termini of AR (22,40). We observed this same effect (Fig. 5B). Interestingly, coactivator-stimulated N-C interaction was also inhibited by ATO treatment, indicating that ATO may prevent coactivator interaction with the N-terminal domain and/or the LBD of AR (Fig. 5B).
ATO inhibits prostate cancer cell proliferation
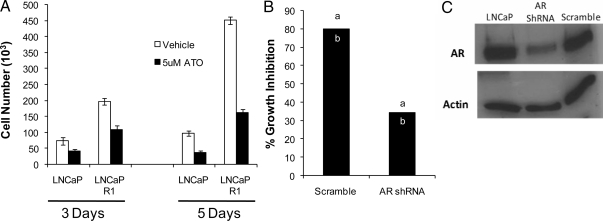
In order for ATO to be a possible therapeutic option in prostate cancer, it should inhibit malignant processes such as cell growth. We found that ATO significantly inhibited cell proliferation in both LNCaP as well as the androgen-independent derivative LNCaP-R1 cells (Fig. 6A). ATO inhibited proliferation to a similar extent in LNCaP and LNCaP-R1 cells. To assess the role of ATO-mediated inhibition of AR activity on ATO’s antiproliferative effects, we generated an LNCaP AR knockdown cell line (Fig. 6C). Depletion of AR resulted in an approximate 3-fold decrease in cell growth compared with control cells, as previously reported (41). Nevertheless, after ATO treatment, there was a greater decrease in proliferation of LNCaP cells (∼80% inhibition) compared with LNCaP AR knockdown cells (∼35% inhibition) (Fig. 6B). Thus, inhibition of AR transcriptional activity by ATO is in part mediating its antiproliferative effects.
Figure 6.
ATO inhibits prostate cancer cell proliferation. A, Viable LNCaP and LNCaP-R1 cells were counted after treatment for 3 d and 5 d ± 5 μm ATO in media supplemented with 5% charcoal-stripped serum. Experiments were performed in triplicate. The data represent mean cell number ± sem. B, Viable LNCaP scramble control and LNCaP AR knockdown cells were counted after 5 d treatment ± 5 μm ATO. Two experiments were performed in triplicate and are plotted as percent inhibition in cell proliferation compared with vehicle control. a and b indicate the average values for each experiment. C, Western blot analysis of AR levels from LNCaP, AR shRNA-infected LNCaP, and scramble control infected LNCaP cells.
Inhibition of AR transcriptional activity by ATO and bicalutamide is additive
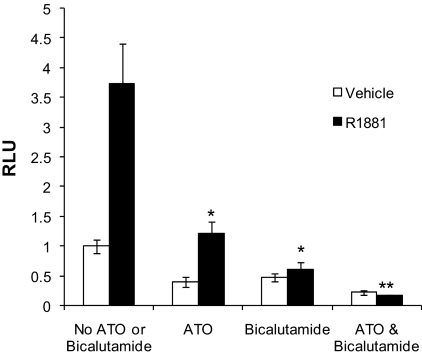
Bicalutamide is an AR antagonist often used in androgen deprivation therapy. We examined AR activity after individual and combined ATO and bicalutamide treatment. These PSA reporter studies revealed that, in the presence or absence of androgen, the greatest inhibition of AR transcriptional activity occurred after treatment with a combination of ATO and bicalutamide (Fig. 7). Thus, ATO did not compromise bicalutamide-mediated inhibition of AR activity, and, in fact, the combined treatment was most effective.
Figure 7.
ATO and bicalutamide treatment effectively inhibit AR transcriptional activity. LNCaP cells were transfected with a PSA (ARE containing)-luciferase reporter plasmid. Cells were treated with either vehicle or 1 nm R1881 in the presence or absence of 20 μm Casodex. The cells were also treated with either 0 or 2 μm ATO. Luciferase activity was determined 48 h after transfection and treatment using a luminometer. Means of triplicate samples are plotted ± sem. P values were determined by comparison of androgen treatment vs. androgen plus ATO or androgen plus bicalutamide, or all three treatments. *, P < 0.05; **, P < 0.01. RLU, Relative luciferase units.
Discussion
Our findings demonstrate that the mechanism by which arsenic inhibits AR activity is distinct from that of other steroid receptors (13,14,16). Arsenic did not prevent hormone binding or AR binding to an ARE-containing oligonucleotide probe in vitro. Furthermore, arsenic did not disrupt androgen-mediated AR nuclear translocation or change AR protein levels. However, arsenic disrupted AR recruitment to the PSA promoter and enhancer in vivo. This decrease in AR-chromatin interaction coincided with, and may result from, arsenic interference with androgen-induced AR N-C interaction. Targeting AR N-C interaction by an environmental toxin /therapeutic agent is novel and has ramifications for arsenic-induced male infertility as well as prostate cancer therapeutics.
Interestingly, whereas arsenic had no effect on AR binding to an ARE probe in vitro, arsenic disrupted AR binding to a native AR target gene promoter in vivo. One possible mechanism for this differential effect is that arsenic interferes with AR N-C interaction. In the presence of androgen, the FxxLF sequence in the N terminus of AR binds with high affinity to the AF2 domain, termed N-C interaction, and is important for AR transcriptional activity (23). Li et al. (24) found that interruption of N-C interaction prevented AR from binding to chromatin in vivo but not to naked DNA in vitro. Consistent with these findings, we show that arsenic inhibited androgen-induced AR N-C interaction and AR binding to chromatin but not AR binding to an ARE-containing oligonucleotide. Arsenic also inhibited coactivator enhancement of androgen-mediated AR N-C interaction. Consequently, arsenic disrupted not only interaction of the amino and carboxyl termini of AR but also coactivator interaction with either one or both of the AR termini. It is more likely that ATO disrupts the AF1-containing amino terminus than the AF2-containing carboxyl terminus of AR based on our work with Ca AR, which lacks the AF2 domain. Ca AR cannot undergo N-C interaction or hormone binding but is still inhibited by arsenic. This result implicates the N-terminal domain containing the AF1 region as the more important target of arsenic action.
It is unlikely that arsenic binds directly to the AR amino or carboxyl terminus to interfere with N-C interaction, given that arsenic did not disrupt hormone or DNA binding and required prolonged treatment. Because arsenic interrupted AR recruitment to the PSA promoter after 24 h but not 2.5 h treatment, arsenic may act on AR indirectly. Arsenic may inhibit N-C interaction by indirectly modulating an AR coregulator or disrupting AR phosphorylation or other posttranslational modifications. Arsenic may alter the kinetics of AR recruitment to AR target gene promoters. It may increase the dissociation rate of AR from chromatin.
One possibility is that arsenic prevents recruitment of coactivators that enhance AR N-C interaction. p160 coactivators, such as TIF2, have been shown to enhance AR N-C interaction by binding to both the amino-terminal and carboxyl-terminal domains (22,40). We found that arsenic inhibited TIF2 recruitment to an AR target gene promoter in vivo. Arsenic also inhibited p160 coactivator stimulation of AR N-C interaction. Therefore, arsenic may indirectly modulate AR activity by inhibiting TIF2 function and perhaps other p160 coactivators as well. However, because arsenic interfered with AR binding to chromatin, the decrease in TIF2 recruitment may be secondary to decreased AR recruitment.
In addition to inhibiting p160 coactivator recruitment to AR transcriptional complexes, arsenic could also increase recruitment of corepressors that interfere with AR N-C interaction (22,23). The corepressor-silencing mediator for retinoid and thyroid hormone receptors (SMRT) interacts with the LBD of AR and inhibits N-C interaction as well as competes with p160 coactivators (42). Cell cycle proteins, cyclin D1 and tumor suppressor p53, can also prevent AR N-C interaction (43,44).
Arsenic may also modulate posttranslational modification of AR, including phosphorylation and acetylation, although the effect of these modifications on AR function is not completely understood. Currently, most of the AR posttranslational modifications affect AR nuclear-cytoplasmic shuttling and have not been shown to modulate AR N-C interaction (45). Nevertheless, AR N-C interaction plays an important role in AR transactivation, and there may be posttranslational modifications of AR that affect N-C interaction.
Arsenic affects multiple signaling pathways in a cell type-dependent manner. An arsenic-regulated pathway that is particularly relevant in prostate cancer and androgen signaling is MAPK (45,46). However, arsenic activates MAPK in prostate cancer cells (data not shown), which is more consistent with enhancement, not inhibition, of AR activity (47,48,49,50). Thus, arsenic regulation of MAPK may not explain arsenic’s inhibitory effects on AR.
Arsenic inhibition of AR activity has significant implications in the realm of male infertility and prostate cancer therapy. Because AR transcriptional activity is essential for spermatogenesis, inhibition of AR activity by sodium arsenite may be involved in the pathogenesis of arsenic-induced male infertility (2,3,27,28). We found that sodium arsenite inhibited AR transcriptional activity in mouse Sertoli cells. Sodium arsenite and ATO have the same molecular mechanism of action; therefore, sodium arsenite most likely regulates AR activity through the same mechanism as ATO. Furthermore, ATO inhibited AR activity as well as cell proliferation in androgen-dependent and -independent prostate cancer cells. Because ATO’s antiproliferative effects were mediated, in part, by its ability to inhibit AR activity, ATO may be most effective in the treatment of prostate cancer where AR signaling is intact. Current models suggest that AR activity is maintained or enhanced in recurrent prostate cancer (32).
Our results also suggest that ATO may be useful in conjunction with bicalutamide, an AR antagonist, in prostate cancer therapy. The doses of ATO used in these experiments are clinically effective concentrations that do not induce significant toxicity (10). In addition, ATO inhibited AR activity in the presence of very low androgens. This is clinically relevant because low tissue levels of androgen may persist after ADT (51,52). Bicalutamide and ATO together optimally inhibited AR activity and perhaps may prevent progression to androgen independence. We observed an additive effect on AR activity using a combination of ATO and bicalutamide. This additive effect may reflect the fact that ATO and bicalutamide influence AR activity through different mechanisms.
Our studies reveal that trivalent arsenic inhibits AR transcriptional activity by disrupting AR recruitment to AR target gene promoters in vivo. This effect may play a significant role in arsenic-induced male infertility. In addition, ATO-mediated inhibition of AR activity represents a compelling new prostate cancer therapeutic opportunity either alone or in combination with ADT.
Materials and Methods
Cell culture and chemical reagents
The human prostate cancer cell lines LNCaP.FGC (ATCC catalog no. CRL 1740; batch F-11701) and PC-3 (ATCC catalog no. CRL 1435; batch F-11154) were obtained from American Type Culture Collection (Manassas, VA). LAPC-4 cells were generously provided by Dr. Charles Sawyers (Sloan Kettering Memorial Center, New York, NY). TM4 cells were generously provided by Dr. Jeffrey J. Lysiak (University of Virginia, Charlottesville, VA). LNCaP AR knockdown cells were derived from LNCaP cells infected with an AR shRNA construct and selected using 10 μg/ml blasticidin S. A LNCaP control cell line was created by infection with a scramble shRNA construct followed by selection. Cell culture media (RPMI-1640 and DMEM) were obtained from Life Technologies, Inc. (Gaithersburg, MD). Fetal bovine serum (FBS) was obtained from Hyclone Laboratories, Inc. (Logan, UT). LNCaP and PC-3 cell lines were cultured in RPMI supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine (Life Technologies, Inc.) and 10% FBS. The LAPC-4 cell line was cultured in Isocove’s media (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% FBS, 10 nm dihydrotestosterone, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. ATO and sodium arsenite were purchased from Sigma (St. Louis, MO), and R1881 was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA).
Plasmids
The PSA luciferase (PSA-Luc) reporter plasmid (kindly provided by Dr. Carlos Perez-Stable, University of Miami, Miami, FL) consists of the PSA promoter and 5′ flanking region, which contain both the distal (−5325 to −4023) and proximal (−542 to +12) ARE-containing enhancer regions but lacks the intervening sequences (52). The ARE luciferase (ARE-Luc) reporter plasmid and ARΔAF2 mutant (CaAR) were provided by Dr. Zafar Nawaz (University of Miami). The following DNA constructs were generously provided: GFP-AR, Gal4-ARLBD, VP16-ARTAD, Gal4-LUC, pRC/CMV-cyclin D1 (Dr. Karen Knudsen, Thomas Jefferson University, Philadelphia, PA); SRC3, TIF2 (Dr. Carolyn Smith, Baylor College of Medicine, Houston, TX), AR shRNA, scramble shRNA (Dr. Paul Rennie, University of British Columbia, Vancouver, British Columbia, Canada).
Reporter gene assays and transfections
All transfections were carried out using the cationic lipid reagent Lipofectamine (Invitrogen Life Technologies) according to the manufacturer’s instructions. For luciferase assays, cells were plated at a density of 2 × 106 cells in 60-mm dishes 16–20 h before transfection. Immediately before transfection, media were replaced with unsupplemented DMEM. For luciferase assays, cells were transfected with 5 μg reporter PSA luc or ARE luc, 250 ng CMVhAR, pARS (caAR), or no AR (LNCaP cells). After a 4- to 5-h incubation with DNA/lipid complexes, cells were refed with RPMI supplemented with 2% charcoal-dextran-stripped serum and treated with vehicle, 0.1 nm, or 5 nm R1881, and/or 20 μm bicalutamide in the absence or presence of 1, 2, or 5 μm ATO. Cells were harvested 48 h after transfection, lysed, and assessed for luciferase activity using the Promega luciferase assay kit (Promega Corp., Madison, WI).
RNA extraction and real-time RT-PCR
Total RNA was harvested using the trizol method according to the manufacturer’s protocol (Invitrogen Life Technologies). Total RNA (500 ng) was reverse transcribed using cDNA archive kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using ABI Prism 7700. Taqman probes from Applied Biosystems for 18S and PSA were used. cDNA (100 ng) was used for PSA PCR and 1 ng cDNA was used for 18S. The comparative threshold cycle method was used to determine the relative expression level of PSA mRNA.
Western blot analysis
Cells were treated for 48h with vehicle or 5 nM R1881 and 0, 2, or 5 μm ATO. Cells were grown to approximately 80% confluence in 60-mm plates and than harvested. Total protein (40 μg) from each sample was resolved on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline [20 mm Tris base (pH 7.5) and 50 mm NaCl and 2.5 mm EDTA] containing 0.1% Tween 20 (TBS-T), and then probed with either anti-AR (N-20; 1:200) or anti-actin (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) primary antibodies diluted in 5% nonfat dry milk in Tris-buffered saline-Tween 20. After washing in Tris-buffered saline-Tween 20, membranes were incubated with their appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) and developed using an enhanced chemiluminescence detection system (Amersham Biosciences, Arlington Heights, IL) according to the instructions of the manufacturer.
Radioligand binding assay
A whole-cell monolayer binding assay was used to assess specific R1881 binding to AR. Cells were grown to approximately 80% confluence in 100-mm plates. Twenty-four hours before the ligand-binding assays, the cells were refed with RPMI supplemented with 2% charcoal-dextran-stripped serum. Four hours before the ligand-binding assay, cells were treated with 0, 1, 2, or 5 μm ATO; 2 h before the ligand-binding assays, cells were treated with 0.1 nm [3H]R1881 in the absence or presence of 100 nm unlabeled (cold) R1881. The cells were collected, and radioactivity was measured using a scintillation counter as previously described (37). The cells treated with [3H]R1881 alone were used to determine total binding, and cells treated with [3H]R1881 and cold R1881 were used to determine nonspecific binding. Specific R1881 binding was calculated by subtraction of nonspecific from total binding. Means of triplicate samples were plotted as counts per minute/total protein ± sem.
GFP-AR and confocal microscopy
One day after plating on coverslips, PC3 cells were transfected with 2 μg GFP-AR and refed with serum-free RPMI. Twenty-four hours after transfection, cells were treated for 24 h with vehicle or 1 nm R1881 in the absence or presence of 5 μm ATO. The cells were fixed in 4% formaldehyde in PBS for 10 min. Next, the cells were permeablized in PBS containing 1% BSA-0.1% Triton X-100 for 5 min. The cells were washed with PBS, and the coverslips were mounted on glass slides in ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen). The images were then collected using confocal microscopy (Carl Zeiss, Thornwood, NY).
EMSA
LNCaP cells were treated with vehicle or 5 nm R1881 in the absence or presence of 5 μm ATO for 2.5 or 24 h. The nuclear extracts were obtained using the Active Motif nuclear extraction kit. For the binding reaction, 5 μg of the nuclear extracts were incubated with 2 μg polydeoxyinosinic deoxycytidylic acid, and approximately 20,000 cpm 32P-end-labeled DNA fragment containing the ARE consensus sequence in DNA binding buffer for 30 min at room temperature as previously described (53). The nuclear extracts were also incubated in the absence or presence of a specific or nonspecific competitor in 100-fold excess. The reaction products were run on a 5% nondenaturing polyacrylamide gel, and autoradiography was performed.
Chromatin immunoprecipitation
LNCaP cells were grown in phenol red-free RPMI with 2% charcoal-dextran-stripped serum for 3 d to 80–90% confluence before the treatment with vehicle or 5 nm R1881 in the absence or presence of 5 μm ATO for 2.5 or 24 h. Chromatin was then cross-linked with 1% formaldehyde for 10 min at room temperature. The cross-linked chromatin was then sonicated, diluted, and immunoprecipitated with AR (PG21) antibody (Upstate Biotechnology, Inc., Lake Placid, NY), TIF2 (M-343) antibody (Santa Cruz Biotechnology), or normal rabbit IgG control at 4 C overnight. Protein A agarose beads with salmon sperm DNA were added and then washed with a low-salt buffer, followed by a high-salt buffer, then LiCl buffer, and finally Tris-EDTA buffer. The protein-DNA complexes were eluted and the cross-links reversed. The DNA fragments were purified with the QIAGEN PCR purification kit (QIAGEN, Chatsworth, CA). The fragments were then analyzed by real-time PCR. Real-time PCR was performed using an icycler iQ PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) with iQ SyberGreen supermix (Bio-Rad). The primers for the PSA distal enhancer were as follows: forward, 5′-CCAAATCTTGTAGGGTGAC-3′; and reverse, 5′-CAATATGTTCCTCCAGAGTAG-3′. The primers for the PSA proximal promoter were as follows: forward, 5′-TCTGCCTTTGTCCCCTAGAT-3′; and reverse, 5′-AACCTTCATT CCCCAGGACT-3′.
Mammalian two-hybrid
PC3 cells were plated at a density of 2 × 106 cells in 60-mm dishes 16–20 h before transfection. Immediately before transfection, media were replaced with unsupplemented DMEM. Cells were transfected with 0.75 μg of Gal4-LUC reporter, VP16-ARTAD, and Gal4-ARLBD as well as empty vector or 1.5 μg of coregulators (cyclin D1, SRC3, TIF2). Each plate was transfected with an equivalent amount of DNA. After a 4- to 5-h incubation with DNA/lipid complexes, cells were refed with phenol red-free RPMI supplemented with 2% charcoal-dextran-stripped serum and treated with or without 5 μm ATO. Cells were treated 24 h after transfection with either vehicle or 5 nm R1881; 24 h after R1881 treatment, cells were harvested, lysed, and assessed for luciferase activity using the Promega luciferase assay kit.
Cell proliferation assay
Cells were plated at an initial density of 40,000 per well in six-well dishes. The following day, cells were treated with media supplemented with 5% charcoal-stripped serum ± 5 μm ATO. After the appropriate treatment period, cells were trypsinized and viable cells were counted using a hemocytometer. Experiments were performed in triplicate.
Acknowledgments
We thank Drs. Carolyn Smith, Jeffrey J. Lysiak, Carlos Perez-Stable, Karen Knudsen, Zafar Nawaz, Charles Sawyers, and Paul Rennie for generously providing reagents. We also thank Zafar Nawaz and Sarath Dhananjayan for their invaluable advice on ChIP as well as Carol Maiorino, Yassin Flores, and Shuyun Rao for their assistance.
Footnotes
This work was supported by Grant F30 ES014989-01 from the National Institute of Environmental Health Sciences (to A.E.R.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: ADT, Androgen deprivation therapy; AF, activation function; AR, androgen receptor; ARE, androgen response element; ATO, arsenic trioxide; Ca, constitutively active; DAPI, 4,6-diamidino-2-phenylindole; DBD, DNA-binding domain; GFP, green fluorescent protein; LBD, ligand-binding domain; PSA, prostate-specific antigen; N-C, amino and carboxyl-termini; shRNA, short hairpin RNA; SRC, steroid receptor coactivator; TAD, transcriptional activation domain; TIF, transcriptional intermediary factor.
References
- Hughes MF 2002 Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16 [DOI] [PubMed] [Google Scholar]
- Pant N, Murthy RC, Srivastava SP 2004 Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol 23:399–403 [DOI] [PubMed] [Google Scholar]
- Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM 2003 Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 5:27–31 [PubMed] [Google Scholar]
- Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Thompson C, Ladich ER 2002 Pathology related to chronic arsenic exposure. Environ Health Perspect 110:883–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S 2001 The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol 176:127–144 [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waalkes MP 2008 Inorganic arsenic and human prostate cancer. Environ Health Perspect 116:158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Jr WH, Schipper HM, Lee JS, Singer J, Waxman S 2002 Mechanisms of action of arsenic trioxide. Cancer Res 62:3893–3903 [PubMed] [Google Scholar]
- Lu M, Xia L, Luo D, Waxman S, Jing Y 2004 Dual effects of glutathione-S-transferase pi on As2O3 action in prostate cancer cells: enhancement of growth inhibition and inhibition of apoptosis. Oncogene 23:3945–3952 [DOI] [PubMed] [Google Scholar]
- Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A 2001 Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res 61:5432–5440 [PubMed] [Google Scholar]
- Murgo AJ 2001 Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute cooperative research and development studies. Oncologist 6:22–28 [DOI] [PubMed] [Google Scholar]
- Scott N, Hatlelid KM, MacKenzie NE, Carter DE 1993 Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol 6:102–106 [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ 1993 Transfer of arsenite from glutathione to dithiols: a model of interaction. Chem Res Toxicol 6:598–602 [DOI] [PubMed] [Google Scholar]
- Kaltreider RC, Davis AM, Lariviere JP, Hamilton JW 2001 Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ Health Perspect 109:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Miyashita Y, Simons Jr SS 1990 Structurally based, selective interaction of arsenite with steroid receptors. J Biol Chem 265:16039–16042 [PubMed] [Google Scholar]
- Bodwell JE, Gosse JA, Nomikos AP, Hamilton JW 2006 Arsenic disruption of steroid receptor gene activation: complex dose-response effects are shared by several steroid receptors. Chem Res Toxicol 19:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica A, Pentecost E, Martin MB 2000 Effects of arsenite on estrogen receptor-α expression and activity in MCF-7 breast cancer cells. Endocrinology 141:3595–3602 [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Webber MM, Waalkes MP 2005 Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environ Health Perspect 113:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann EP 2002 Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- Janne OA, Palvimo JJ, Kallio P, Mehto M 1993 Androgen receptor and mechanism of androgen action. Ann Med 25:83–89 [DOI] [PubMed] [Google Scholar]
- Heemers HV, Tindall DJ 2007 Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- He B, Lee LW, Minges JT, Wilson EM 2002 Dependence of selective gene activation on the androgen receptor NH2- and COOH-terminal interaction. J Biol Chem 277:25631–25639 [DOI] [PubMed] [Google Scholar]
- He B, Wilson EM 2002 The NH(2)-terminal and carboxyl-terminal interaction in the human androgen receptor. Mol Genet Metab 75:293–298 [DOI] [PubMed] [Google Scholar]
- Li J, Fu J, Toumazou C, Yoon HG, Wong J 2006 A role of the amino-terminal (N) and carboxyl-terminal (C) interaction in binding of androgen receptor to chromatin. Mol Endocrinol 20:776–785 [DOI] [PubMed] [Google Scholar]
- Langley E, Kemppainen JA, Wilson EM 1998 Intermolecular NH2-/carboxyl-terminal interactions in androgen receptor dimerization revealed by mutations that cause androgen insensitivity. J Biol Chem 273:92–101 [DOI] [PubMed] [Google Scholar]
- Langley E, Zhou ZX, Wilson EM 1995 Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem 270:29983–29990 [DOI] [PubMed] [Google Scholar]
- Isomaa V, Parvinen M, Janne OA, Bardin CW Nuclear androgen receptors in different stages of the seminiferous epithelial cycle and the interstitial tissue of rat testis. Endocrinology 116:132–137 [DOI] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA 1994 Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology 134:2307–2316 [DOI] [PubMed] [Google Scholar]
- Brinkmann AO, Jenster G, Ris-Stalpers C, van der Korput JA, Bruggenwirth HT, Boehmer AL, Trapman J 1995 Androgen receptor mutations. J Steroid Biochem Mol Biol 53:443–448 [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein KL 2005 Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. J Cell Biochem 95:657–669 [DOI] [PubMed] [Google Scholar]
- Debes JD, Tindall DJ 2002 The role of androgens and the androgen receptor in prostate cancer. Cancer Lett 187:1–7 [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Leav I, Kwan PW, Bubley GJ, Balk SP 2001 Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc Natl Acad Sci USA 98:10823–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin ME, Balk SP 2004 Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 91:483–490 [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL 2004 Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E 1992 The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol 41:665–669 [DOI] [PubMed] [Google Scholar]
- Dai JL, Maiorino CA, Gkonos PJ, Burnstein KL 1996 Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids 61:531–539 [DOI] [PubMed] [Google Scholar]
- Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM 1995 Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol 9:208–218 [DOI] [PubMed] [Google Scholar]
- Freedman LP 1999 Strategies for transcriptional activation by steroid/nuclear receptors. J Cell Biochem (Suppl 32–33):103–109 [DOI] [PubMed] [Google Scholar]
- Shen HC, Buchanan G, Butler LM, Prescott J, Henderson M, Tilley WD, Coetzee GA 2005 GRIP1 mediates the interaction between the amino- and carboxyl-termini of the androgen receptor. Biol Chem 386:69–74 [DOI] [PubMed] [Google Scholar]
- Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS 2006 Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res 66:10613–10620 [DOI] [PubMed] [Google Scholar]
- Liao G, Chen LY, Zhang A, Godavarthy A, Xia F, Ghosh JC, Li H, Chen JD 2003 Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J Biol Chem 278:5052–5061 [DOI] [PubMed] [Google Scholar]
- Shenk JL, Fisher CJ, Chen SY, Zhou XF, Tillman K, Shemshedini L 2005 p53 Represses androgen-induced transactivation of prostate-specific antigen by disrupting hAR amino- to carboxyl-terminal interaction. J Biol Chem 276:38472–38479 [DOI] [PubMed] [Google Scholar]
- Burd CJ, Petre CE, Moghadam H, Wilson EM, Knudsen KE 2005 Cyclin D1 binding to the androgen receptor (AR) NH2-terminal domain inhibits activation function 2 association and reveals dual roles for AR corepression. Mol Endocrinol 19:607–620 [DOI] [PubMed] [Google Scholar]
- Ponguta LA, Gregory CW, French FS, Wilson EM 2008 Site specific androgen receptor serine phosphorylation linked to epidermal growth factor dependent growth of castration-recurrent prostate cancer. J Biol Chem 283:20989–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AM, Pramanik R, Nicholson LJ, Dew TK, Martin FL, Muir GH, Morris JD 2007 Ras-MEK-ERK signaling cascade regulates androgen receptor element-inducible gene transcription and DNA synthesis in prostate cancer cells. Int J Cancer 121:520–527 [DOI] [PubMed] [Google Scholar]
- Hong SH, Privalsky ML 2000 The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol Cell Biol 20:6612–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma WY, Li J, Goranson A, Dong Z 1999 Requirement of erk, but not JNK, for arsenite-induced cell transformation. J Biol Chem 274:14595–14601 [DOI] [PubMed] [Google Scholar]
- Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ 2001 Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem 276:22177–22182 [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T, Hanioka N, Yoshida H, Jinno H, Ando M 2003 Arsenite and arsenate activate extracellular signal-regulated kinases 1/2 by an epidermal growth factor receptor-mediated pathway in normal human keratinocytes. Br J Dermatol 149:1116–1127 [DOI] [PubMed] [Google Scholar]
- Mohler JL, Gregory CW, Ford III OH, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS 2004 The androgen axis in recurrent prostate cancer. Clin Cancer Res 10:440–448 [DOI] [PubMed] [Google Scholar]
- Heracek J, Hampl R, Hill M, Starka L, Sachova J, Kuncova J, Eis V, Urban M, Mandys V 2007 Tissue and serum levels of principal androgens in benign prostatic hyperplasia and prostate cancer. Steroids 72:375–380 [DOI] [PubMed] [Google Scholar]
- Grad JM, Dai JL, Wu S, Burnstein KL 1999 Multiple androgen response elements and a myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol 13:1896–1911 [DOI] [PubMed] [Google Scholar]