Abstract
Thyroid hormone receptors (TRs) play critical roles in energy homeostasis. To understand the role of TRs in lipid homeostasis in vivo, we adopted the loss-of-function approach by creating knock-in mutant mice with targeted mutation in the TRα gene (TRα1PV mouse) or TRβ gene (TRβPV mouse). The PV mutation, identified in a patient with resistance to thyroid hormone, exhibits potent dominant-negative activity. Here we show that in contrast to TRα1PV mouse, TRβPV mice exhibited no significant reduction in WAT but had significant increases in serum free fatty acids and total triglycerides. Moreover, the liver of TRβPV mice was markedly increased (33%) with excess lipid accumulation, but the liver mass of TRα1PV mouse was decreased (23%) with paucity of lipids. These results indicate that apo-TRβ and apo-TRα1 exerted distinct abnormalities in lipid metabolism. Further biochemical analyses indicate that increased lipogenic enzyme expression, activated peroxisome proliferator-activated receptor γ (Pparγ) signaling, and decreased fatty acid β-oxidation activity contributed to the adipogenic steatosis and lipid accumulation in the liver of TRβPV mice. In contrast, the expression of lipogenic enzymes and Pparγ was decreased in the liver of TRα1PV mice. These results suggest that the regulation of genes critical for lipid metabolism by TRs in the liver is isoform dependent. These results indicate that apo-TRβ and apo-TRα1 had different effects on lipid metabolism and that both TR isoforms contribute to the pathogenesis of lipid metabolism in hypothyroidism.
Apo-TRβ and apo-TRα1 have distinct effects on lipid metabolism and that TR isoforms differentially contribute to the pathogenesis of lipid metabolism in hypothyroidism.
Thyroid hormone (T3) plays a critical role in the regulation of thermogenesis and maintenance of lipid homeostasis. T3 deficiency leads to increased body weight and cold intolerance. Excess T3 reduces plasma low-density lipoprotein cholesterol, lipoproteins, and triglycerides and leads to weight loss. The key enzymes in the lipogenic and lipolytic pathways are regulated by T3 in both the liver and adipose tissues (1,2). T3 acts via binding to thyroid hormone receptors (TRs) that are members of the nuclear receptor superfamily. TRs are ligand-dependent transcription factors encoded by two different genes, TRα and TRβ, located on human chromosomes 17 and 3, respectively. The four T3-binding TR isoforms, α1, β1, β2, and β3, are derived from the TRα and TRβ genes by alternative splicing of the primary transcripts. Each TR isoform has unique developmental and tissue-specific expression patterns (3). TRs regulate transcription by binding to the thyroid hormone response elements (TREs) in the promoter regions of T3-target genes (4). In addition to the effects of T3 and the various types of TREs, TR transcription is modulated by tissue- and development-dependent TR isoform expression (3) and by a host of corepressors and coactivators (4,5).
Studies using TR subtype knockout mice have shown that TRα1 is essential for maintaining proper thermogenesis and that TRβ is important in regulating cholesterol metabolism (6,7,8,9,10). These findings suggest tissue-dependent T3-mediated TR isoform action in the maintenance of metabolic homeostasis. In hypothyroidism, however, TRs function as aporeceptors. Studies of mice deficient in all TRs (TRα1−/− and TRβ−/− mice) have shown that they exhibit a milder overall phenotype than the debilitating symptoms of severe hypothyroidism (11), highlighting the important role of apo-TRs in the pathogenesis of hypothyroidism. Indeed, knock-in mutant mice harboring different mutations in the TRα gene exhibit abnormalities in lipid metabolism. The TRα1PV mouse that harbors a frameshift mutation in the C-terminal 16 amino acids displays a lean phenotype, partly due to the reduction in white fat mass [white adipose tissue (WAT)] (12). The TRα1R384C knock-in mutant mouse also exhibits a lean phenotype with reduction in fat mass (13). The TRα1P398H knock-in mutant mouse, interestingly, has increased body fat accumulation and elevated serum levels of leptin, glucose, and insulin (14). These results indicate that apo-TRα1 severely perturbs lipid metabolism and energy balance but in a mutation-site-dependent manner. What is not clear is whether apo-TRβ with the same mutation as the TRα1 mutant could lead to a similar or a distinct impairment in lipid metabolism.
The creation of knock-in mutant mice with an identical mutation in the TRβ (TRβPV mouse) or TRα gene (TRα1PV mouse) at the same corresponding C terminus of receptors allows us to address this important question. The PV mutation was identified in a patient with resistance to thyroid hormone (RTH). It is due to a C-insertion at codon 448 of the TRβ1 that leads to a mutant that has complete loss of T3 binding and transcription activity (15,16). The TRβPV mouse faithfully reproduces human RTH with dysregulation of the pituitary-thyroid axis (17), whereas the TRα1PV mouse has normal thyroid-pituitary functions (18). Although both the homozygous (TRβPV/PV) and heterozygous (TRβPV/+) mice are viable with no severe fertility defects, homozygous TRα1PV/PV mice die shortly after birth, and heterozygous TRα1PV/+ mice are dwarfs with reduced fertility (18). Recently, we found that the reduction in the WAT contributes to the dwarfism of TRα1PV/+ mice and that apo-TRα1 (TRα1PV) acts to repress adipogenesis of WAT by inhibition of the expression and by repression of the transcriptional activity of peroxisome proliferator-activated receptor γ (Pparγ) (12). In the present study, we found that in contrast to TRα1PV/+ mice, no abnormality in the WAT of TRβPV mice was detected. However, the liver of TRβPV mice was enlarged with excess accumulation of lipids. In contrast, the liver of the TRα1PV/+ mice was smaller than the wild-type mice with paucity in hepatic fat accumulation. Further molecular analyses indicated that the expression and activity of Pparγ was increased in the liver of TRβPV/PV mice, whereas the expression of Pparγ was repressed in the liver of TRα1PV/+ mice. Differential regulation of lipogenic genes by apo-TRβ and apo-TRα1 in accord with the lipid phenotype was also observed in the liver of these two mutant mice. These results indicated that apo-TRβ and apo-TRα1 have different effects on lipid metabolism and that both TR isoforms contribute to the pathogenesis of lipid metabolism changes in hypothyroidism.
Results
Differential effects of the apo-TR isoforms on the adipogenesis of WAT
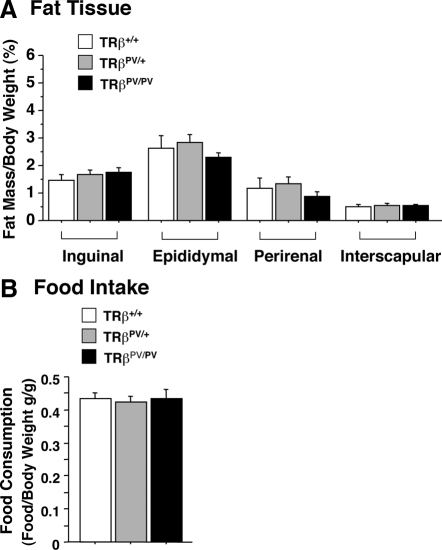
Recently we reported that the inguinal, epididymal, and perirenal fat tissues of TRα1PV/+ mice are markedly smaller than those of wild-type siblings (32–60% reduction) (12). The reduction of WAT mediated by the apo-TRα1 (TRα1PV) is not due to the increased lipolysis but is due to impaired lipogenesis of WAT (12). We therefore ascertained whether apo-TRβ (TRβPV) exerted similar effects on the adipogenesis of fat tissues in TRβPV mice. Surprisingly, no significant effects on the mass of the WAT of either the heterozygous TRβPV/+ or the homozygous TRβPV/PV mice were detected (Fig. 1A). Moreover, no effect was found on the mass of the brown adipose tissue (interscapular fat) due to the expression of TRβPV in TRβPV mice (Fig. 1A).
Figure 1.
No significant changes in the weight of fat tissues in TRβPV mice (A) and food intake (B). A, Inguinal, epididymal, perirenal, and interscapular fat tissues were weighed after dissection from mice at age 4–5 months. Ratios of fat mass vs. body weight were determined. The data are expressed as mean ± sem (n = 4–9). B, Food consumption by TRβPV mice. Food consumed by mice in 2 d was measured, and the food intake was normalized by body weight and expressed as grams per gram body weight0.75. The data are expressed as mean ± sem (n = 4–8), and the experiments were repeated three times.
We further determined the lipid-related hormones in the serum. Table 1 shows that consistent with no changes in WAT, the serum leptin levels were not significantly altered. Interestingly, free fatty acids (FFAs) and total triglycerides (TGs) were significantly elevated in TRβPV/+ mice as well as homozygous TRβPV/PV mice. Basal glucose and insulin levels, however, were not affected.
Table 1.
Lipid-related serum hormones and factors in TRβPV mice
| Mouse/genotype | Leptin (ng/ml) | FFA (μm) | TG (mg/dl) | Glucose (mg/dl) | Insulin (ng/ml) |
|---|---|---|---|---|---|
| WT | 8.359 ± 0.896 (n = 15) | 552.143 ± 39.178 (n = 14) | 90.5 ± 7.318 (n = 14) | 114.714 ± 6.929 (n = 14) | 1.165 ± 0.079 (n = 15) |
| TRβPV/+ | 8.722 ± 0.861 (n = 16) | 720.812 ± 49.134 (n = 16) | 124.75 ± 11.47 (n = 16) | 114.750 ± 5.695 (n = 16) | 1.271 ± 0.1 (n = 16) |
| TRβPV/PV | 9.258 ± 0.702 (n = 19) | 666.765 ± 20.489 (n = 17) | 127.706 ± 6.254 (n = 17) | 110.529 ± 5.952 (n = 17) | 1.251 ± 0.073 (n = 19) |
| Ratios | |||||
| TRβPV/+/WT | 1.04 (NS) | 1.31a | 1.38a | 1.00 (NS) | 1.09 (NS) |
| TRβPV/PV/WT | 1.11 (NS) | 1.21a | 1.41b | 0.96 (NS) | 1.07 (NS) |
Data are means ± sem. NS, Not significant; WT, wild type.
P < 0.05.
P < 0.001.
That there were no changes in the mass of fat tissues but there were elevated serum FFA and TG levels prompted us to examine whether food intake was affected. We measured the food consumption and found no changes in food intake by TRβPV/+ or by homozygous TRβPV/PV mice as compared with wild-type mice (Fig. 1B). These results were consistent with no changes in the serum leptin levels (Table 1). Therefore, the elevated FFAs and TGs were not related to alteration of food intake.
Enlarged liver of TRβPV/PV mice exhibits excess lipid accumulation
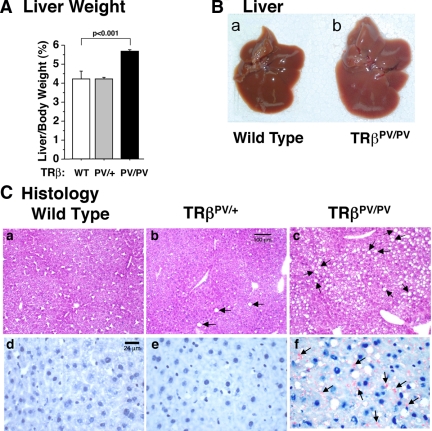
The above observations led us to ask whether there were abnormalities in the liver of TRβPV/PV mice, thereby resulting in elevated serum FFA and TG. Figure 2A shows that indeed the liver weight (percentage of ratios of liver weight vs. body weight) was significantly higher (33%) in TRβPV/PV than wild-type mice. Figure 2Bb shows an example of the enlarged liver of TRβPV/PV mice that had an apparent light grayish appearance as compared with the liver of wild-type mice (Fig. 2Ba).
Figure 2.
Enlarged fatty liver of TRβPV/PV mice. A, Comparison of liver weight of wild-type, TRβPV/+, mice, and TRβPV/PV mice aged 4–5 months. Ratios of tissue mass vs. body weight were determined. The data are expressed as mean ± sem (n = 4–9; the P value is indicated). B, Representative liver of a wild-type mouse (a) and TRβPV/PV mouse (b), showing a larger liver of TRβPV/PV mouse with grayish appearance. C, Hematoxylin- and eosin-stained (a–c) or Oil Red-O-stained (d–f) liver of a wild-type mouse (a and d), TRβPV/+ mouse (b and e), and TRβPV/PV mouse (c and f). Arrows indicate the accumulation of lipids.
Histological analysis showed that hepatocytes of TRβPV/+ mice apparently had more lipid accumulation (indicated by arrows; Fig. 2Cb) than did wild-type mice (Fig. 2Ca). Excessive lipid accumulation was clearly evident in the liver of TRβPV/PV mice (indicated by arrows; Fig. 2C, c and f).
Activation of key lipogenic genes by apo-TRβ (TRβPV) in the liver of TRβPV/PV mice
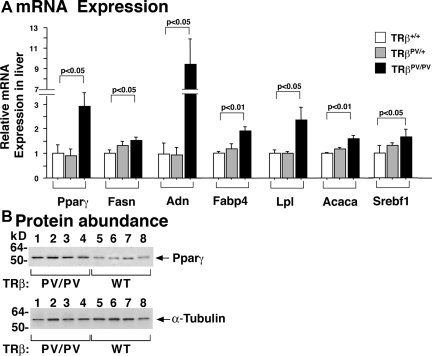
To elucidate whether altered gene expression underlies the development of fatty liver in TRβPV/PV mice, we analyzed the expression of critical genes in lipogenesis. Figure 3A shows the increased expression of Pparγ and its downstream target genes, fatty acid synthase (Fasn), adipsin (adn), ap2 (Fabp4), and lipoprotein lipase (Lpl) in the liver of TRβPV/PV mice. The expression of other lipogenic genes such as acetyl coenzyme A (CoA)-carboxylase (Acaca) and sterol regulatory element-binding transcription factor (Srebf1) was also increased. These gene expression patterns are consistent with the increased synthesis of fatty acids.
Figure 3.
Relative mRNA expression of key regulators of lipogenesis and lipolysis in the liver of TRβPV mice. A, Quantitative real time RT-PCR was used to determine the expression of key genes in lipid metabolism according to Materials and Methods. The data are expressed as mean ± sem (n = 4; the P values are indicated). B, Comparison of Pparγ protein abundance in the liver of wild-type and TRβPV/PV mice. Western blot analysis was carried out as described in Materials and Methods. The analysis used 50 μg tissue extracts. α-Tubulin was used as loading control. The lanes are marked.
Due to the critical role of Pparγ in the lipogenic pathway, we further confirmed the increased expression of Pparγ at the protein level by Western blot analysis. Figure 3B shows that consistent with the mRNA expression (Fig. 3A), the abundance of Pparγ protein was higher in the liver of TRβPV/PV mice than that of wild-type mice (compare lanes 1-4 with 5-8, Fig. 3B). The lower panel of Fig. 3B shows the corresponding loading controls. These results indicate that activation of critical lipogenic genes by TRβPV led to adipogenic steatosis in the liver of TRβPV/PV mice.
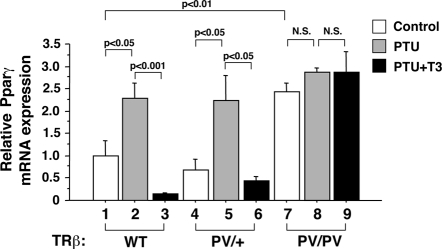
That the expression of Pparγ was activated at the mRNA and protein levels shown in Fig. 3 prompted us to ascertain whether Pparγ was a T3 target gene in the liver. We therefore rendered wild-type, TRβPV/+, and TRβPV/PV mice hypothyroid or hyperthyroid by injection of T3 into hypothyroid mice. Figure 4 shows that in wild-type mice, the expression of Pparγ mRNA was activated in the hypothyroid state (compare bar 2 with bar 1) and when T3 was administered, its mRNA expression was repressed (compare bar 3 with bar 2). In TRβPV/+ mice, the repression of Pparγ mRNA by T3 was less as compared with that in wild-type mice (compare bar 6 with bar 3). Importantly, the expression of Pparγ mRNA was constitutively activated in the liver of the TRβPV/PV mice irrespective of thyroid hormone status (bars 7–9), consistent with the dominant-negative action of TRβPV in the activation of T3-negatively regulated genes.
Figure 4.
T3 negatively regulates the expression of Pparγ in the liver. Quantitative real-time RT-PCR analysis of Pparγ mRNA expression in the liver of untreated, PTU-treated, and PTU+T3-treated TRβPV mice as described in Materials and Methods. The data are expressed as mean ± sem (n = 3–7). The P values are indicated.
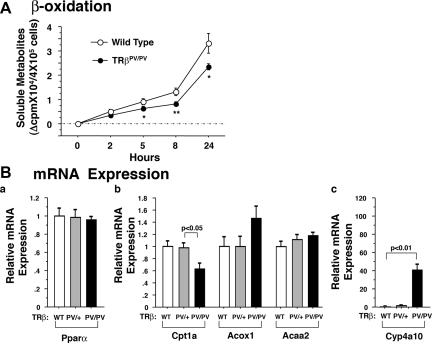
Decreased lipid β-oxidation in the liver of TRβPV/PV mice
Because decreased β-oxidation could also contribute to lipid accumulation in the liver, we examined the rates of β-oxidation in the primary hepatocytes of wild-type and TRβPV/PV mice. As shown in Fig. 5A, the β-oxidation activity in the primary hepatocytes of TRβPV/PV mice was significantly lower (24–37%) than that of wild-type mice.
Figure 5.
A, Fatty acid β-oxidation activity in TRβPV mice.In vitro β-oxidation activity in primary hepatocytes isolated from TRβPV mice as described in Materials and Methods. The data are expressed as means ± sd (n = 3–4; *, P < 0.05; **, P < 0.01). B, Relative mRNA expression of key regulators of fatty acid β-oxidation in the liver of TRβPV mice by quantitative real-time RT-PCR as described in Materials and Methods. The data are expressed as mean ± sem (n = 4–8; P values are indicated).
To identify the affected genes that mediate the decreased β-oxidation, we first examined the expression of Pparα, a known key regulator in the pathway of β-oxidation (19). Figure 5Ba shows that no changes in the expression were observed in the liver of TRβPV/PV mice. However, the expression of carnitine palmitoyl-transferase Iα (Cpt1a), a rate-controlling enzyme regulating the import of fatty acids into mitochondria, was lower (37%) in the liver of TRβPV/PV mice than in wild-type mice but not significantly affected in TRβPV/+ mice (Fig. 5Bb). In contrast, the expression of acyl-CoA oxidase (Acox1), involved in peroxisomal β-oxidation (20), was not significantly altered. No significant changes in the expression of thiolase (Acaa2) were detected (Fig. 5Bb). However, the expression of cytochrome P450 family 4 subfamily A polypeptide 10 (Cyp4a10) involved in microsomal ω-oxidation (20) was significantly increased in TRβPV/PV mice (40-fold higher than wild-type; Fig. 5Bc). These data suggest that the reduction of β-oxidation activity and the fatty liver phenotype was mainly mediated by the decreased expression of rate-determining step regulator, Cpt1a, in TRβPV/PV mice.
A distinct phenotype mediated by apo-TRα1 in the liver of TRα1PV/+ mice
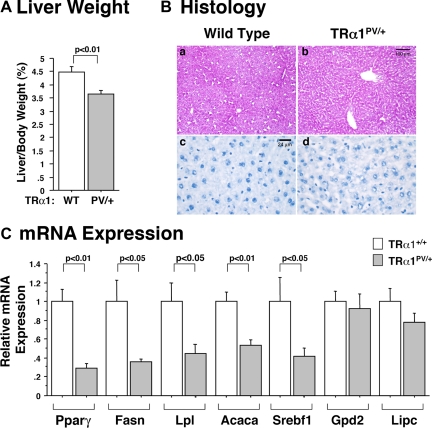
We previously reported that WAT mass is reduced as a result of impaired adipogenesis by apo-TRα1. In the present study, we found that no such defect was observed in the WAT of TRβPV mice. This differential activity mediated by apo-TR isoforms in WAT prompted us to examine whether these two apo-TR isoforms had differential effects on the liver. Interestingly, we found that in contrast to the enlarged fatty liver of TRβPV/PV mice, the liver weight of TRα1PV/+ mice was significantly decreased in TRα1PV/+ mice (23% as compared with wild-type; Fig. 6A). Histological examination indicated that the liver of TRα1PV/+ mice exhibited paucity in lipids (Fig. 6B; compare b with a and d with c). These results suggest that apo-TRβ or apo-TRα1 exerted different abnormalities in liver lipid metabolism.
Figure 6.
A, Comparison of liver weight of TRα1PV mice with wild-type siblings. Mice aged 4–5 months were used in the analysis. Ratios of liver mass vs. body weight were determined. B, Hematoxylin- and eosin-stained (a and b) or Oil Red-O-stained (c and d) liver of wild-type mouse (a and c) and TRα1PV/+ mouse (b and d). The liver of TRα1PV/+ mouse exhibits lipid paucity. C, Relative mRNA expression of key regulators of lipogenesis and lipolysis in the liver of TRα1PV mice by quantitative real-time RT-PCR as described in Materials and Methods. The data are expressed as mean ± sem (n = 4–5; P values are indicated).
To identify altered gene expression that led to lipid paucity in the liver of TRα1PV/+ mice, we examined the key genes involved in lipid synthesis. As shown in Fig. 6C, the expression of key genes involved in the lipogenesis such as Pparγ, Fasn, Lpl, Acaca, and Srebf1 was reduced, ranging from 75–20%, whereas the expression of lipolytic enzymes Gpd2 and Lipc was not changed. These results indicate that the decrease in lipids in the liver of TRα1PV/+ mice was mainly due to the reduction in lipogenesis. The differential gene expression profiles of key genes in the lipid metabolism between TRα1PV/+ mice and TRβPV/PV mice indicate that apo-TRα1 and apo-TRβ acted to lead to different defects in liver. These results suggest that TRα1 and TRβ could play distinct roles in hepatic lipid metabolism.
Discussion
Although the effects of T3 on energy homeostasis and lipid metabolism have long been recognized (21), the molecular mechanisms by which T3 regulates these processes have only recently begun to be understood. Studies of genetically engineered mice lacking individual TR isoforms or all TRs indicate that TR isoforms mediate different metabolic processes. TRα1 is a major regulator of body temperature (7,9,10), and TRβ plays a key role in regulating cholesterol pathways (6). Investigations using mice lacking all TRs revealed that the deleterious effects of total TR deficiency are less severe than congenital hypothyroidism, thus highlighting the critical roles of apo-TRs in the pathogenesis of hypothyroidism. The creation of several TRα1 knock-in mutant mice led to the understanding that apo-TRα1 could derail metabolic homeostasis, causing loss of WAT and hypermetabolism (12,13) and visceral adiposity (14). However, it was less clear whether an apo-TRβ that has the same mutation site as the apo-TRα1 could lead to the same abnormalities in lipid metabolism. The availability of the TRβPV knock-in mouse that harbors the same mutation as the TRα1PV knock-in mouse presented the opportunity to address this question. It is interesting that the two apo-TR isoforms exerted distinct effects in two major target organs involved in lipid homeostasis. The WAT mass was reduced in TRα1PV/+ mice (12) whereas no loss of WAT in TRβPV/PV mice was discovered in the present study. The liver was enlarged with excess lipid accumulation in TRβPV/PV mice, but the liver was smaller with a paucity of lipids in TRα1PV/+ mice. These results suggest apo-TR isoforms mediate distinct defects that could underlie the pathogenesis of hypothyroidism.
The distinct abnormalities in the liver of TRα1PV/+ and TRβPV mice have allowed us to ascertain whether these two apo-TR isoforms differentially regulate the key lipid metabolic genes in the same target tissues. Indeed, we found that consistent with the corresponding lipid phenotype in the liver, a panel of lipogenic genes was activated by TRβPV but repressed by TRα1PV. At present, it is not clear how these two apo-receptors that have the same mutation site at the corresponding C-terminal region could differentially regulate the same target genes in the liver. TRβPV and TRα1PV differ mainly in the A/B domain that is involved in the interaction with receptor coregulatory proteins to affect transcriptional activity (22). It is possible that the different length and sequences in the A/B domain of TRβPV and TRα1PV could lead to the differential recruitment of different receptor interacting proteins at the amino-terminal region, thereby differently affecting the regulation of the same target genes. Alternatively, the different A/B domain could indirectly affect the recruitment of different corepresssors at the corepressor binding surface that involved helix 1, helix 11, and helix 12 (23), thus leading to differential transcriptional responses. The elucidation of these possibilities will require future studies.
On the basis of in vitro studies, four possible mechanisms were proposed to account for the dominant-negative activity of TRβ mutants: 1) formation of inactive dimers between mutant and wild-type TRs (w-TRs), 2) competition between mutant and w-TRs for binding to TREs, 3) competition for limited amounts of auxiliary proteins such as retinoid X recetors (RXRs), and 4) stable association of TRβ mutants with corepressors, resulting in repression of T3 target genes (24,25). However, only after mouse models of human RTH became available was it possible to determine which of these possibilities operates in vivo. Using the mouse model of RTH that we had created (TRβPV mice) (17), we determined how TRβPV could affect the gene regulatory activity of w-TRs on T3-target genes in the liver (26). We found that in liver nuclear extracts of TRβPV/+ mice, TRβPV forms not only TRE-bound homodimers (inactive TRβPV/TRβPV homodimers) but also TRE-bound heterodimers with w-TRβ, w-TRα1, or RXR. In TRβPV/PV mice, in addition to TRβPV/TRβPV homodimers, the lack of w-TRβ facilitates the formation of TRE-bound TRβPV/w-TRα1 and TRβPV/RXR heterodimers. Therefore, in vivo, TRβPV competes with w-TRβ or w-TRα1 for binding to TRE and for heterodimerization with RXRs (25). Such competition leads to repression of the positively T3-regulated target genes, S14, malic enzyme, and type 1 deiodinase, in the liver of TRβPV mice. In the pituitary, such competition leads to the activation of the negatively T3-regulated gene, such as the TSH-β gene, because TRβ is the major TR isoform in the pituitary (17). In the present study, we found that Pparγ was a T3-negatively regulated gene (Fig. 4). Its expression was constitutively activated in the liver of the TRβPV/PV mice irrespective of thyroid hormone status, consistent with the dominant-negative action of TRβPV in the activation of the T3-negatively regulated gene.
TRβ is known to be the major TR isoform in the liver, and many TR-mediated T3 effects are believed to act via TRβ in this target tissue (26). Therefore, it was reasonable to expect that only the mutation of the major TRβ isoform would lead to observable phenotypic abnormalities. However, the present study shows that mutation of TRα1, which is a less abundant TR isoform in the liver, led to the repression of PPARγ and manifestation of abnormality in lipid metabolism in TRα1PV/+ mice. As described above, the dominant-negative activity of TRβ is stronger in tissues where TRβ is the predominantly expressed isoform (22,26). It would seem reasonable to expect that abnormal gene regulatory activity of the less abundantly expressed TRα1PV would be compensated by the predominantly expressed w-TRβ in the liver. However, the findings that mutation of a less abundant TR (TRα1PV) in the liver could manifest distinct abnormalities in lipid metabolism suggest that TRα1PV not only could act via dominant-negative activity by interference with the transcription of w-TRs but also could possibly act via a gain of function. Still, at present, how TRα1PV could act via this mode of action in the liver is not clear.
The deleterious effects of apo-TRα1 on the liver are not limited to TRα1PV. The TRα1R384C mouse exhibits reduced content of glycogen in the liver (13). However, male TRα1P398H mice exhibit hepatic steatosis (14,27), indicating contrasting effects of TRα1PV and TRα1P398H on liver lipid metabolism. These particular contrasting effects were also observed in WAT in that the former led to a lean phenotype with impaired adipogenesis in WAT (12), whereas the latter led to visceral adiposity (14). The underlying mechanisms to account for the contrasting abnormalities of lipid metabolism between TRα1PV and TRα1P398H mice are not clear. It is possible that the structures of TRα1PV and TRα1P398H differ, resulting in the recruitment of different corepressors to differentially affect their in vivo functions. If apo-TRα1 were to act via gains of function, it could also be possible that the different TRα1 mutants could assume different intrinsic biological activities due to their different structures. Investigation of these possibilities awaits future studies.
Recent studies have indicated that thyroid hormone could also act via nongenomic action through a plasma membrane receptor (28). The plasma membrane receptor is located on integrin αvβ3 at the arginine-glycine-aspartic acid (RGD) recognition site important to binding by the integrin of extracellular matrix proteins (29,30). Interestingly, snake venom-derived RGD-containing disintegrin was found to inhibit adipogenesis of primary cultured fibroblastic preadipocytes (31). These cell-based studies further expanded the complexity of understanding the regulation of adipogenesis by thyroid hormone.
Obesity and disorders of lipid metabolism are major health issues. The findings that the apo-TR isoforms act differentially in a target-tissue-dependent manner could help direct the design and development of T3 analogs to treat these disorders. One could envision if fatty liver were detected in patients with hypothyroidism, it would be more beneficial to treat with a TRβ-specific analog such as GC-1 without a major undesirable effect in other organs such as the heart (32). As additional advances are made in better understanding the actions of TR isoforms in lipid metabolism, novel T3 analogs for improved treatment strategies would certainly be forthcoming.
Materials and Methods
Animals
This animal study was carried out according to the protocol approved by the National Cancer Institute Animal Care and Use Committee. Genotyping of TRβPV and TRα1PV mice was performed using specific primers (17,18). Wild-type littermates were used for the comparison of phenotypes. To determine the effect of T3 on target gene expression in vivo, wild-type male mice (age 4–5 months) were divided into three groups (n = 4–5) and treated with or without propylthiourea (PTU) and injected with or without T3 as described before (12). The hypothyroid or hyperthyroid state of mice was confirmed by the determination of serum thyroid hormone levels.
Determination of serum hormones and glucose
Serum levels of total T4 and T3 were determined by using a GammaCoat T4 or T3 RIA kit (DiaSorin, Stillwater, MN) according to the manufacturer’s instructions. Serum glucose (nonfasting) was determined by using the method of glucose oxidation (Accu-Chek glucose monitor; Roche Diagnostics Co., Indianapolis, IN). TGs and FFAs were measured using assay kits (catalog no. TR22421 from Thermo Trace Ltd., Melbourne, Australia, and catalog no. 1383175 from Roche Diagnostics GmbH, Mannheim, Germany, respectively) according to the manufacturer’s instructions. Serum adiponectin, insulin, and leptin were measured by RIAs (catalog nos. MADP-60HK, SRI-13K, and ML-82K, respectively; Linco Research, Inc., St. Charles, MO).
Quantitative real-time RT-PCR
RNA from fat tissues was extracted using an RNeasy lipid tissue mini kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Liver RNA was prepared by using Trizol reagent (Invitrogen, Carlsbad, CA). The determination of mRNA by real-time RT-PCR was carried out as described previously (33) by using total fat RNA (100 ng) or total liver RNA (200 ng). The primers for mouse Pparα and sterol regulatory element-binding transcription factor 1 (Srebf1) are mPparα-F, CGTACGGCAATGGCTTTATC; mPparα-R, AATCCCCTCCTGCAACTTCT; mSrebf1-F, CTGGCTGAGGCGGGATGAAGAG; and mSrebf1-R, TACCGTGAGCTACCTGGACTGA.
The primer sequences for mouse lipoprotein lipase (Lpl), Pparγ adipsin (Adn), aP2 (Fabp4), acetyl CoA carboxylase (Acaca), fatty acid synthase (Fasn), α-glycerol phosphate dehydrogenase (Gpd2), hepatic lipase (Lipc), acyl-CoA oxidase (Acox1), carnitine palmitoyl-transferase Iα (Cpt1a), cytochrome P450 family 4 subfamily A polypeptide 10 (Cyp4a10), thiolase (Acaa2), and the 18S rRNA (internal controls) were described previously (12,14,33).
Western blot analysis
The liver and fat tissues were dissected and cut into small pieces. Cytosolic extract was prepared by using a NE-PER kit (Pierce Chemical Co., Rockford, IL; catalog no. 78833) according to the manufacturer’s protocols. The protein concentration was determined by the Bradford method (Pierce) with BSA (Pierce) as the standard. Western blot analysis was carried out as described previously (34). For the detection of Pparγ, cellular fractions (25 μg) were separated by SDS-PAGE. The primary antibody used in the Western blot analysis was monoclonal antibody (1:100 dilution; catalog no. sc-7273; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For the control of protein loading, α-tubulin was used (monoclonal anti-α-tubulin antibody clone DM1A; 1:6000 dilution; Sigma Chemical Co., Inc., St. Louis, MO).
Isolation of primary mouse hepatocytes and in vitro β-oxidation analysis
Hepatocytes were isolated by a two-step collagenase perfusion of the liver as described previously (35). In vitro β-oxidation activity was determined as previously described with modification (36). Briefly, 400,000 hepatocytes were seeded into six-well plates. After overnight incubation, 1 ml fresh medium containing 12 mCi [9,10(n)-3H]palmitic acid/BSA was added to cells. After the given incubation period at 37 C, the medium was removed and centrifuged for 5 min. Next, 0.9 ml supernatant was mixed with 100 ml 10% BSA/PBS and then mixed with 200 ml 70% perchloric acid. Supernatant-containing water-soluble β-oxidation products were counted after vortex and centrifuge.
Acknowledgments
We thank Dr. Valentina Factor for her assistance in the preparation of mouse primary hepatocytes.
Footnotes
The present research was supported by the Intramural Research Program of Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 8, 2009
Abbreviations: CoA, Coenzyme A; FFA, free fatty acids; Pparγ, peroxisome proliferator-activated receptor γ; PTU, propylthiourea; RTH, resistance to thyroid hormone; RXR, retinoid X receptor; TG, triglyceride; TR, thyroid hormone receptor; TRE, thyroid hormone response element; WAT, white adipose tissue; w-TR, wild-type TR.
References
- Diamant S, Gorin E, Shafrir E 1972 Enzyme activities related to fatty-acid synthesis in liver and adipose tissue of rats treated with triiodothyronine. Eur J Biochem 26:553–559 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP 1991 Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest 87:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR 2003 Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol 213:1–11 [DOI] [PubMed] [Google Scholar]
- Cheng SY 2000 Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord 1:9–18 [DOI] [PubMed] [Google Scholar]
- O'Malley BW 2003 Sequentiality and processivity of nuclear receptor coregulators in regulation of target gene expression. Nuclear Receptor Signal 1:e010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Salto C, Forrest D, Angelin B, Vennstrom B 2002 Requirement for thyroid hormone receptor β in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol 16:1767–1777 [DOI] [PubMed] [Google Scholar]
- Johansson C, Gothe S, Forrest D, Vennstrom B, Thoren P 1999 Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-β or both α1 and β. Am J Physiol 276:H2006–2012 [DOI] [PubMed] [Google Scholar]
- Marrif H, Schifman A, Stepanyan Z, Gillis MA, Calderone A, Weiss RE, Samarut J, Silva JE 2005 Temperature homeostasis in transgenic mice lacking thyroid hormone receptor-α gene products. Endocrinology 146:2872–2884 [DOI] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA 2001 Thyroid hormone-sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest 108:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B 1998 Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennstrom B, Forrest D 1999 Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Araki O, Furuya F, Kato Y, Cheng SY 2007 Impaired adipogenesis caused by a mutated thyroid hormone α1 receptor. Mol Cell Biol 27:2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Alkemade A, Mittag J, Nordstrom K, Katz A, Rozell B, Westerblad H, Arner A, Vennstrom B 2007 Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J 26:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Schultz JJ, Brent GA 2003 A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem 278:38913–38920 [DOI] [PubMed] [Google Scholar]
- Meier CA, Dickstein BM, Ashizawa K, McClaskey JH, Muchmore P, Ransom SC, Menke JB, Hao EH, Usala SJ, Bercu BB 1992 Variable transcriptional activity and ligand binding of mutant β-1 3,5,3′-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol 6:248–258 [DOI] [PubMed] [Google Scholar]
- Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD 1991 Characterization of seven novel mutations of the c-erbAβ gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest 88:2123–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S 2000 Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S 2001 A targeted dominant negative mutation of the thyroid hormone α1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett L, Galli A, Crabb D 2000 The role of hepatic peroxisome proliferator-activated receptors (PPARs) in health and disease. Liver 20:191–199 [DOI] [PubMed] [Google Scholar]
- Pegorier JP, Le May C, Girard J 2004 Control of gene expression by fatty acids. J Nutr 134:2444S–2449S [DOI] [PubMed] [Google Scholar]
- Mariash CN 2003 Thyroid hormone and the adipocyte. J Clin Endocrinol Metab 88:5603–5604 [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK 2006 Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428 [DOI] [PubMed] [Google Scholar]
- Marimuthu A, Feng W, Tagami T, Nguyen H, Jameson JL, Fletterick RJ, Baxter JD, West BL 2002 TR surfaces and conformations required to bind nuclear receptor corepressor. Mol Endocrinol 16:271–286 [DOI] [PubMed] [Google Scholar]
- Yen PM, Cheng SY 2003 Germline and somatic thyroid hormone receptor mutations in man. J Endocrinol Invest 26:780–787 [DOI] [PubMed] [Google Scholar]
- Yen PM, Ikeda M, Brubaker JH, Forgione M, Sugawara A, Chin WW 1994 Roles of v-erbA homodimers and heterodimers in mediating dominant negative activity by v-erbA. J Biol Chem 269:903–909 [PubMed] [Google Scholar]
- Zhang XY, Kaneshige M, Kamiya Y, Kaneshige K, McPhie P, Cheng SY 2002 Differential expression of thyroid hormone receptor isoforms dictates the dominant negative activity of mutant β-receptor. Mol Endocrinol 16:2077–2092 [DOI] [PubMed] [Google Scholar]
- Liu YY, Heymann RS, Moatamed F, Schultz JJ, Sobel D, Brent GA 2007 A mutant thyroid hormone receptor-α antagonizes peroxisome proliferator-activated receptor-α signaling in vivo and impairs fatty acid oxidation. Endocrinology 148:1206–1217 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Leonard JL, Davis FB 2008 Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29:211–218 [DOI] [PubMed] [Google Scholar]
- Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ 2005 Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146:2864–2871 [DOI] [PubMed] [Google Scholar]
- Cody V, Davis PJ, Davis FB 2007 Molecular modeling of the thyroid hormone interactions with αvβ3 integrin. Steroids 72:165–170 [DOI] [PubMed] [Google Scholar]
- Lin YT, Tang CH, Chuang WJ, Wang SM, Huang TF, Fu WM 2005 Inhibition of adipogenesis by RGD-dependent disintegrin. Biochem Pharmacol 70:1469–1478 [DOI] [PubMed] [Google Scholar]
- Baxter JD, Webb P, Grover G, Scanlan TS 2004 Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metab 15:154–157 [DOI] [PubMed] [Google Scholar]
- Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC, Cheng SY 2003 Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis 24:1467–1479 [DOI] [PubMed] [Google Scholar]
- Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY 2005 An unliganded thyroid hormone β receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol 25:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY, Factor VM, Thorgeirsson SS 1996 Reduced growth capacity of hepatocytes from c-myc and c-myc/TGF-α transgenic mice in primary culture. Biochem Biophys Res Commun 222:64–70 [DOI] [PubMed] [Google Scholar]
- Linden D, William-Olsson L, Rhedin M, Asztely AK, Clapham JC, Schreyer S 2004 Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J Lipid Res 45:1279–1288 [DOI] [PubMed] [Google Scholar]