Abstract
The therapeutic efficacy of histone deacetylase inhibitors (HDACI) is generally attributed to their ability to alter gene expression secondary to their effects on the acetylation status of transcription factors and histones. However, because HDACIs exhibit similar transcriptional effects in most cells, the molecular basis for their therapeutic selectivity toward malignant cells is largely unknown. In this study, we report that HDACI, of distinct chemotypes, quantitatively inhibit glucose transporter 1 (GLUT1)-mediated glucose transport into multiple myeloma cells through both down-regulation of GLUT1 and inhibition of hexokinase 1 (HXK1) enzymatic activity. Unexpectedly, however, this inhibition of glucose utilization is accompanied by an increase in amino acid catabolism with no increase in fatty acid oxidation. Our findings suggest that an HDACI-induced change in carbon source preference could contribute to the therapeutic efficacy of these drugs by creating a pattern of fuel utilization that is incompatible with rapid tumor growth and survival. Furthermore, these results, which implicate glucose metabolism as a target of HDACI, suggest that caution should be exercised in attributing effects of this class of drug to primary alterations in gene transcription.
The apoptotic activity of HDAC inhibitors is due in part to their ability to inhibit β-oxidation of fatty acids, glucose uptake and hexokinase activity.
Histone deactylase inhibitors (HDACI) induce differentiation and/or apoptosis in transformed cell lines yet appear to have little effect on normal cells, an observation difficult to reconcile with their observed effects on global gene transcription. Given that these agents result in a quantitative acetylation of histones, alter chromatin structure, and affect transcription of over 2% of all genes (1,2), the molecular basis of their selectivity has remained elusive. Furthermore, despite resulting in modulation of histone acetylation to a similar extent, the overall impact of HDACI treatment varies widely with cell type: in cells isolated from most hematological neoplasms, HDACI efficiently induce apoptosis, whereas cells of more solid tumor origin (breast or prostate) require much higher doses of HDACI to induce apoptosis and more often instead lead to differentiation. The ability of HDACI to facilitate differentiation and/or apoptosis in a dose- and cell type-dependent manner suggests that they may have a broad range of targets and may not function in an equivalent manner in all cells (3,4,5,6). Indeed, this is supported by the observation that cytoplasmic and nuclear factors other than histones are hyperacetylated after treatment with HDACI, including transcription factors (p53, nuclear factor-κB, signal transducer and activator of transcription 3, and CCAAT/enhancer-binding protein) as well as factors with roles outside of transcription (importin-α, tubulin, and heat-shock protein 90) (5,7,8,9,10,11,12). The challenge, therefore, is to identify among the many processes impacted by HDACI those that are involved in differentiation, apoptosis, and therapeutic efficacy in cancer.
HDACI have proven effective as treatments for non-small-cell lung cancer and tumors of gastrointestinal (larynx, colon, and rectum) and endocrine (thyroid and prostate) origins (13,14). However, hematological neoplasms have proven especially sensitive to HDACI, with meaningful responses demonstrated in T-cell lymphomas, Hodgkin’s disease, and acute myeloid and promyelocytic leukemias (15). Regardless, significant variations in responses to different HDACI in different neoplasms may reflect an absolute limitation in their therapeutic utility or the fact that the current inhibitors were optimized using non-disease-related proteins (i.e. histones). Moreover, current HDACI are relatively nonspecific, inhibiting each of 11 genetically distinct HDAC to some degree. Until recently, it was generally assumed that the therapeutic efficacy of this class of drugs related to their ability to block histone deacetylation and up-regulate transcription of some target genes. Indeed, several groups have attempted to identify the key target genes, among them p21, that mediate the cell cycle arrest and induction of apoptosis observed in several cell models. However, it is now apparent that processes other than transcription are influenced by HDACI and that targets of these agents reside in both the cytoplasm and nuclei of cells (7,8,9). Indeed, recent studies indicate that HDACI can induce both caspase-dependent and -independent apoptotic responses within the same cell (16). Thus, the mechanism of action of this class of drugs is likely more complex than originally contemplated.
Of late, significant attention has been focused on the ability of HDACI to alter cellular metabolism, an activity generally thought to be a limiting rather than a therapeutic and advantageous property of this class of compounds (17). In rodent livers and in isolated hepatocytes, for instance, the short-chain fatty acid-derived HDACI valproate (VPA) has been shown to interfere with oxidative phosphorylation, gluconeogenesis, and fatty acid oxidation, leading to accumulation of middle and short-chain carnitine esters. More specifically, VPA modulates expression and/or activity of enzymes involved in β-oxidation [acyl-coenzyme A (CoA) dehydrogenases], the tricarboxylic acid cycle (citrate synthase and succinate dehydrogenase) and the electron transport chain (cytochrome aa3), resulting in decreased mitochondrial oxygen consumption (18,19). Because of the emerging interest in metabolic enzymes as therapeutic targets in cancer, we sought to determine whether a previously unappreciated influence on cellular metabolism contributes to the ability of HDACI to induce apoptosis in malignant cells.
Given the complexity of HDACI action and the likelihood that they may not function in the same way in all cells, we elected to focus in the current study on defining the mechanism of action of HDACI in cellular models of multiple myeloma. In this hematological malignancy, glucocorticoids efficiently induce apoptosis and are generally used as frontline therapies for all stages of the disease (20). Although initially highly responsive, resistance invariably arises in treated patients. It is within the framework of this clinical problem that HDACI have emerged as potential therapies for multiple myeloma. Several HDACI efficiently induce apoptosis in myeloma cell lines, a process accompanied by induction of p21 (21,22,23). However, as with most cancers, the primary targets of HDACI in multiple myeloma remain elusive, and thus direction for the improvement of this therapeutic strategy is not obvious.
Our findings presented here demonstrate that HDACI have profound effects on cellular energy metabolism in cellular models of multiple myeloma. These effects may contribute to HDACI activities in transcription as well as induction of differentiation and apoptosis and are likely to be a major component of their efficacy in cancer therapy.
Results
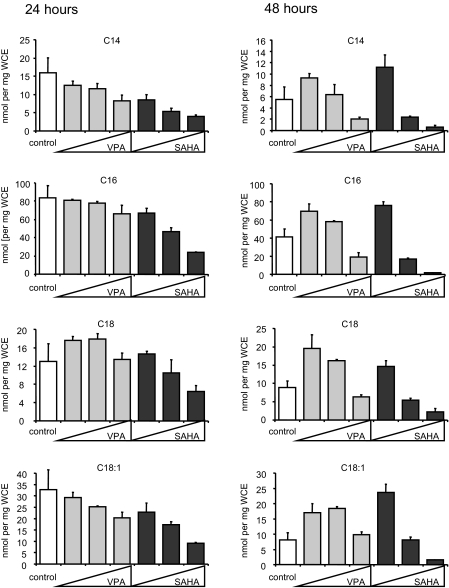
HDACI modulate cellular levels of fatty acids in myeloma cells
Whereas HDACI show great promise in the treatment of cancer and particularly of hematological malignancies, the mechanism by which they manifest their therapeutic actions is unclear. Transcriptional analyses have highlighted genes and pathways targeted by HDACI, but none of these has been definitively shown to account for the apoptotic actions of HDACI. Interestingly, the HDACI VPA has been shown to inhibit β-oxidation through sequestration of coenzyme A-SH and inhibition of acyl-carnitine dehydrogenases, leading to reduced acetyl-CoA levels and accumulation of medium- and long-chain fatty acids. Consequently, we evaluated the extent to which HDACI impact cellular metabolism in transformed cells. Initially, we asked whether HDACI would result in decreased fatty acid oxidation in transformed cells similar to that previously reported to occur in hepatocytes. To address this issue, we chose the OPM2 human multiple myeloma cell line as a model system, in part because it is known to be highly responsive to the activity of HDACI of several different chemotypes (2,22). Targeted tandem mass spectrometry was used to measure the levels of 37 acylcarnitines, ranging from C2–C20 and with varying degrees of saturation, in cells treated for 24 or 48 h with increasing concentrations of either VPA or the HDACI suberoylanilide hydroxamic acid (SAHA). Representative measurements, shown in Fig. 1, illustrate a complex relationship between HDACI treatment and fatty acid content with time- and dose-dependent changes in C14:0, C16:0, C:18:0, and C18:1 being noted. Interestingly, we observed a biphasic effect on acylcarnitine levels. More specifically, increases in acylcarnitine levels were observed after treatment of cells with low concentrations of HDACI for 48 h, as had previously been observed in hepatocytes (24). Higher concentrations resulted in decreased levels of acylcarnitines. Similar results were obtained in H929 myeloma cells treated with HDACI in the same manner (supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), demonstrating that the effects of HDACI to cause an increase in long-chain acylcarnitine species in hepatocytes can be recapitulated in cellular models of multiple myeloma. Flux through the β-oxidation pathway was analyzed by oxidation of C14-labeled oleate, and in agreement with others (24), we observed decreased metabolism of oleate after VPA treatment, indicating that the increased levels of acylcarnitines observed arises from their decreased oxidation and not from increased production (data not shown).
Figure 1.
HDACI treatment results in an altered acylcarnitine profile in multiple myeloma cells. OPM2 cells were treated with VPA (0.5, 1, or 2 mm) or SAHA (1, 2.5, or 5 μm) for 24 or 48 h. After analysis of protein concentration, lysates were deproteinated and acylcarnitines were measured by MS-MS analysis. Acylcarnitine levels were determined by normalization to lysate protein concentration. Results are representative of three independent experiments and values are calculated mean ± sd of triplicate samples.
HDACI decrease acetyl-CoA levels in cells
The effects of VPA on lipid metabolism in hepatocytes are thought to stem in part from the conversion of VPA to a CoA derivative (25). Indeed, valproyl-CoA levels rise to 300–700 μm in mitochondria in response to treatment of rat liver cells with 100 μm VPA (24,26). These data suggest the possibility that HDACI-induced changes in CoA dynamics may contribute to changes in fatty acid metabolism and possibly also to the induction of apoptosis. This general conclusion would not seem to be supported by the fact that valproyl-carnitine accounts for only 1% of the administered dose of VPA with the bulk of the drug being completely oxidized to propionyl and pentanoyl-CoA derivatives (27,28). Furthermore, treatment with SAHA, a structurally different HDACI for which a CoA derivative has not been identified, resulted in comparable changes in acylcarnitines (Fig. 1). Consequently, with a view to defining the processes regulated by HDACI that tracked with their therapeutic efficacy, we undertook a detailed comparison of the activities of both SAHA and VPA with regard to induction of apoptosis, changes in chromatin structure, and impact on acetyl-CoA metabolism.
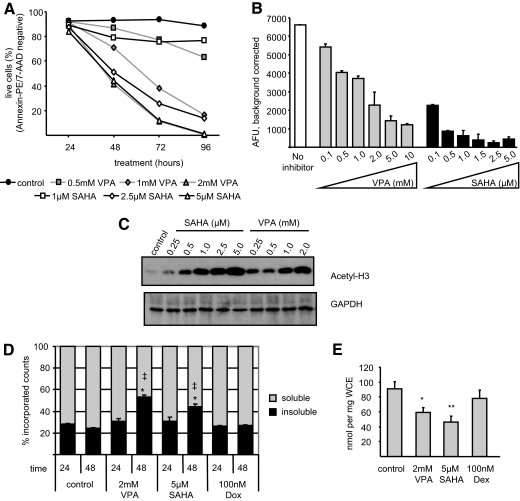
In line with previous studies (2,29), we were able to show that both VPA- and SAHA-induced apoptosis in the OPM2 cell line (Fig. 2A). A time course and dose curve analysis revealed that apoptosis at these doses was apparent at 48 h but that a maximal response was not observed until 96 h after treatment. To analyze the relative effectiveness of these HDACI in inducing apoptosis vs. their ability to inhibit deacetylation, we evaluated HDACI activity in OPM2 extracts using an in vitro deacetylation assay and found that the apoptotic activities of the selected HDACI generally correlate with their ability to inhibit HDAC (Fig. 2B). Notably, however, although SAHA is a very potent (and effective) HDACI when assayed in vitro, its relative apoptotic activity in OPM2 cells is much less than that observed for VPA (Fig. 2A). Comparison of Fig. 2, A–C, demonstrates that whereas 1 μm SAHA exhibits maximal HDAC inhibition in vitro and 1 mm VPA only 50% inhibition (Fig. 2B), the acetylation state of histones in intact cells treated with these same doses is comparable (Fig. 2C). Furthermore, these same doses induce disparate rates of apoptosis in treated cells, with VPA proving more effective (Fig. 2A). Similar observations were made in other multiple myeloma cell lines (not shown), suggesting that 1) in vitro inhibition of HDAC, although a convenient tool to assess newly developed compounds, may not provide an accurate reflection of the activity of HDACI in intact cells, and 2) the apoptotic activities of VPA may not be related solely to HDAC inhibition, leading us to further explore other processes that may be impacted by HDACI.
Figure 2.
Treatment with HDACI results in apoptosis and decreased cellular levels of soluble acetyl-CoA. A, OPM2 cells were treated with VPA or SAHA before analysis of apoptosis using 7-AAD and annexin V PE staining followed by FACS. Values indicate the average percent remaining live (unstained) cells per treatment in triplicate samples. B, Nuclear extracts from untreated OPM2 cells were incubated with the indicated concentrations of VPA or SAHA before analysis of HDAC activity. C, OPM2 cells were incubated 24 h with SAHA or VPA before lysis and immunoblot analysis. D, OPM2 cells were incubated with [3H]acetate before addition of VPA, SAHA, or Dox and then were lysed and deproteinated, and counts were measured in soluble and insoluble fractions. *, P < 0.001 as compared with time-matched control; ‡, P < 0.01 comparing VPA and SAHA treatments. E, OPM2 cells were treated 48 h with VPA, SAHA, or Dex. Acetylcarnitine levels were measured as in Fig. 1. * and **, P < 0.05 and P < 0.01, respectively, as compared with control. Results are representative of at least three independent experiments, and values indicate mean ± sd of triplicate samples. Significance was determined by one-way ANOVA followed by Tukey comparison test.
Cellular levels of acetyl-CoA are tightly regulated. However, previous studies indicate that VPA perturbs cellular acetyl-CoA homeostasis, leading to reduced acetyl-CoA levels in rat hepatocytes and human fibroblasts (24,30). Thus, we sought to determine whether the acute administration of HDACI had any impact on acetyl-CoA metabolism in myeloma cells. Specifically, we asked whether VPA or SAHA would induce a significant shift in labeled acetyl groups from a soluble pool (i.e. acetyl-CoA) to a protein-associated fraction (presumably acetylated proteins) over time. OPM2 cells were incubated with [3H]acetate before treatment with VPA, SAHA, or doxorubicin (Dox) to label the cellular pool of acetyl-CoA. After 24 or 48 h treatment, cell extracts were prepared and treated with methanol to precipitate proteins, and the proportion of soluble vs. insoluble (protein-associated) counts was determined for each treatment. None of the treatments significantly influenced the distribution of acetate between soluble and protein-associated pools at 24 h (Fig. 2D), a time point where we observe maximal retention of acetyl groups on histones (Fig. 2C and not shown), suggesting that bulk acetylation of histones does not result in detectible depletion of the soluble acetyl-CoA pool. However, at 48 h, we observed a dramatic increase in the relative proportion of labeled acetate recovered from the insoluble vs. soluble fractions of cell extracts (Fig. 2D). Possible explanations for this finding include 1) increased incorporation of acetate derived from acetyl-CoA into proteins, 2) increased utilization of acetyl-CoA in other biological processes, or 3) an effect of HDACI to limit synthesis and replenishment of the soluble acetyl-CoA pool at the later time point. Interestingly, more robust effects are observed in VPA-treated cells, despite the fact that SAHA proved to possess the greater HDAC inhibitory potency in vitro at doses that induced similar rates of apoptosis as did VPA (Fig. 2, A and B). The specificity of this response was confirmed by demonstrating that Dox, which induces apoptosis in OPM2 cells (not shown), had no significant effect on the incorporation of acetate into proteins. Thus, it appears that acute administration of HDACI does not have a significant impact on bulk acetyl-CoA levels at a time point when we expect HDAC inhibition to be maximal but that continued administration of these drugs has a profound effect on acetyl-CoA metabolism at later time points.
We next analyzed acetyl-CoA levels in treated cells using acetylcarnitine as a surrogate measure, taking advantage of the fact that acetyl-CoA and acetylcarnitine are kept in a tight equilibrium by carnitine acetyl transferase. OPM2 cells were treated for 48 h with VPA, SAHA, or dexamethasone (Dex), all of which induce significant apoptosis in this cell line within 96 h [Fig. 2A, not shown, and previous studies (2,22)]. Using tandem mass spectrometry (MS-MS) analysis, we observed a 30% decrease in acetylcarnitine in cells treated with either VPA or SAHA (Fig. 2E). This fall in acetylcarnitine is not caused by activation of apoptosis per se, because no changes in acetylcarnitine levels were observed in cells treated with Dex. Thus, we conclude that two chemically distinct HDACI disturb acetyl-CoA equilibrium in myeloma cells, possibly contributing to their therapeutic efficacy. This intriguing observation led us to focus our continued investigation of the mechanism of action of these drugs on processes related to tumor metabolism.
HDACI inhibit glucose transporter 1 (GLUT1)-dependent glucose uptake
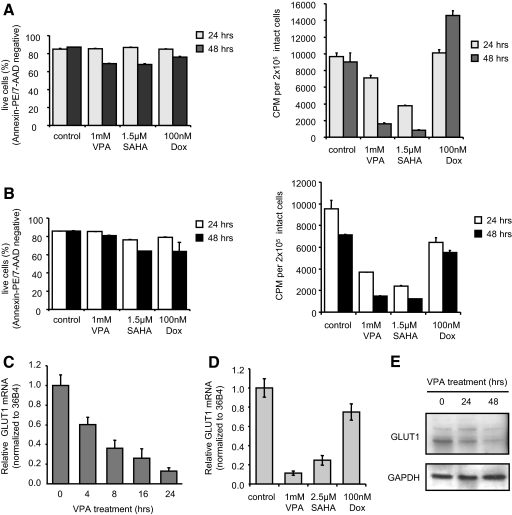
Within mammalian cells, acetyl-CoA is used for fatty acid synthesis and produced by β-oxidation of fatty acids, amino acid catabolism, and/or glycolytic flux. Given the multiple possible sources and fates of acetyl-CoA, we sought to define the mechanisms underlying HDACI-induced decreases in acetyl-CoA levels. As demonstrated in Fig. 1, at low doses and at the 48-h time point, HDACI cause a metabolic profile of accumulation of long-chain acylcarnitines that is consistent with impaired fatty acid catabolism, this constituting one mechanism for impaired replenishment of acetyl-CoA. However, both normal and neoplastic hematological cells exhibit a highly glycolytic metabolism, and a shortfall in acetyl-CoA would be expected to be replenished through increased glycolysis. Thus, we focused our studies on defining the impact of HDACI on glucose uptake. OPM2 cells were treated for 24 or 44 h with VPA, SAHA, or Dox before analysis of cellular uptake of the 3H-labeled glucose analog 2-deoxyglucose (2-DOG). We chose a dose of each compound that induced a comparable rate of apoptosis in OPM2 cells after 96 h treatment (Fig. 2A and not shown). However, at 24 and 44 h, the time points selected for analysis of glucose uptake, these compounds had minimal effects on OPM2 cell viability as determined by annexin V and 7-AAD staining (Fig. 3A, left). Interestingly, rather than increased glucose uptake as would be expected in response to a decrease in acetyl-CoA levels, we observed an HDACI-induced, time-dependent decrease in glucose uptake (5- to 10-fold at 44 h; Fig. 3A, right). No decrease in glucose uptake was noted when these cells were treated with Dox, a chemically distinct apoptotic agent. This striking effect of HDACI on glucose uptake was also observed in a second multiple myeloma cell line (H929) when assayed in the same manner (Fig. 3B).
Figure 3.
HDACI inhibit glucose uptake and reduce GLUT1 expression before induction of apoptosis. A, Apoptosis (left) and glucose uptake (right) were analyzed in OPM2 cells treated with VPA, SAHA, or Dox. Apoptosis was analyzed as in Fig. 2A. For glucose uptake, 2 × 105 live cells (per trypan blue exclusion) were incubated 10 min with [3H]2-DOG before washing and lysis of the cells. Retained radioactivity was detected by analysis in scintillation fluid. B, Apoptosis (left) and glucose uptake (right) in similarly treated H929 cells were analyzed as described in A. C, RNA isolated from OPM2 cells treated with 2 mm VPA was reverse transcribed before analysis by RTqPCR; detected levels of GLUT1 mRNA were normalized to the similarly detected housekeeping gene 36B4 mRNA. Fold induction over control was determined by setting GLUT1 levels in untreated cells equal to 1. D, GLUT1 mRNA levels in OPM2 cells treated 24 h with VPA, SAHA, or Dox (100 nm) was analyzed as in C. E, Lysates from OPM2 cells treated with 1 mm VPA were analyzed by immunoblot for expression of GLUT1 and loading control GAPDH. Results are representative of at least three independent experiments, and values represent calculated mean ± sd of triplicate samples.
The dramatic decrease in glucose uptake led us to hypothesize that HDACI may affect the expression or activity of one or more of the GLUT proteins. Real-time quantitative PCR (RTqPCR) analysis of RNA isolated from untreated OPM2 cells indicated that GLUT1 and -8 are the most predominantly expressed glucose transporters in this cell line (not shown). Because GLUT8 is primarily associated with fructose transport, we focused our further studies on GLUT1. Importantly, treatment of OPM2 with VPA led to a decrease in GLUT1 mRNA that was apparent as early as 4 h after treatment with maximal inhibition observed at 24 h (Fig. 3C). A similar analysis of OPM2 cells revealed an 80–90% decrease at 24 h in GLUT1 mRNA in VPA- or SAHA-treated cells, whereas Dox had no significant effect on GLUT1 expression (Fig. 3D). The mRNA levels of the other GLUTs expressed in these cells were not altered significantly by HDACI treatment (not shown). Immunoblot analysis of protein extracts from cells treated in the same manner revealed a modest reduction in GLUT1 protein at 24 h, with a more pronounced decrease at 48 h (Fig. 3E). Thus, inhibition of GLUT1 mRNA expression and the subsequent decrease in the levels of its corresponding transporter protein mirror the decrease in glucose uptake observed in HDACI-treated cells. Of note, overexpression of GLUT1 has been documented in non-small-cell lung cancers, highlighting a potential correlation between expression of and reliance upon GLUT1 and clinical response to HDACI. Although some of the toxicities attributed to VPA, when used as an antiepileptic, have been attributed to reduced glucose uptake and GLUT1 mRNA expression (31), the current study advances this observation by demonstrating that these alterations in glucose metabolism are related to the HDACI inhibitor activity of VPA and SAHA. The connection between HDACI treatment and perturbation of glucose homeostasis presents a new and heretofore unappreciated activity of this class of drugs that may contribute to their therapeutic efficacy.
The acute effects of HDACI on glucose transport results from their ability to inhibit hexokinase (HXK) activity
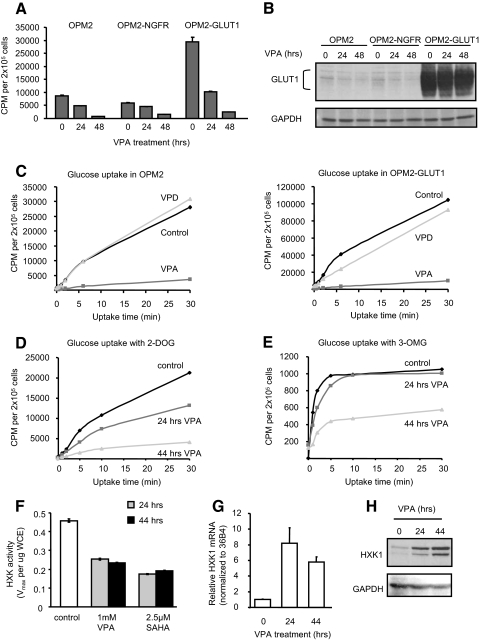
If the primary effects of HDACI on glucose transport were due to changes in GLUT1 expression manifest at the level of transcription, overexpressing this transporter using a heterologous promoter would be expected to abrogate the effects of these agents. Thus, we used a retroviral vector incorporating a rat GLUT1 cDNA with a FLAG tag inserted into an exofacial loop, allowing isolation of cells overexpressing FLAG-GLUT1 by fluorescence-activated cell sorting (FACS) (32). Glucose uptake assays comparing OPM2 parent cells with OPM2-GLUT1 cells or OPM2-nerve growth factor receptor (NGFR) (infected with an empty retroviral vector control) indicated that the vector did not affect glucose uptake, whereas overexpression of GLUT1 resulted in a 2- to 3-fold increase in glucose uptake (Fig. 4A), demonstrating that the overexpressed GLUT1 is active. Surprisingly, treatment of these OPM2 derivative cell lines with VPA for either 24 or 48 h resulted in a decrease in glucose uptake comparable in magnitude to that observed in the unmanipulated OPM2 cells (Fig. 4A). Parallel annexin V and 7-AAD analysis revealed similar rates of apoptosis in both the parental and engineered cell lines in response to HDACI (not shown). Immunoblot analysis revealed that VPA did not appreciably affect expression of FLAG-GLUT1 (Fig. 4B), and FACS analysis confirmed that levels of FLAG-GLUT1 resident on the cell surface actually increased in the presence of VPA or SAHA (supplemental Fig. 2A). Thus, overexpression of GLUT1 from a heterologous promoter was not sufficient to restore glucose uptake after HDACI treatment, suggesting that these agents may have an impact on the activity of the transporter or on other more distal steps of glucose metabolism. To examine this possibility, we analyzed glucose uptake in OPM2 (Fig. 4C, left) or OPM2-GLUT1 (Fig. 4C, right) cells over a period of 30 min. Importantly, we observed that although the Vmax was different in the two cells (consistent with overexpression of active GLUT1), the apparent Km of the transporters in these untreated cells were not different. A significant decrease in the Vmax in both cells, absent any change in the apparent Km for glucose, was observed with VPA treatment but not for its derivative valpromide, which lacks HDACI activity. Similar results were observed when the glucose transport assays were performed in cells treated for either 24 or 48 h with either VPA (Fig. 4D) or SAHA (not shown). Importantly, at no point in any of these experiments did glucose uptake in the treated cells approach that of the untreated cells. These data indicate that VPA not only slows the glucose transport rate but also actually reduces the overall capacity of the cell to transport and retain glucose.
Figure 4.
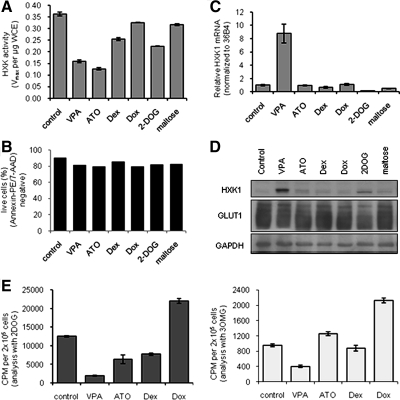
HDACI influence glucose uptake at the level of GLUT1 expression and HXK activity. A, OPM2 cells uninfected or stably infected with empty retrovirus (OPM2-NGFR) or retrovirus expressing FLAG-GLUT1 (OPM2-GLUT1) were treated with 1 mm VPA before analysis of glucose uptake as in Fig. 3A. B, Lysates of treated cells in A were analyzed by immunoblotting for GLUT1. C, Parent OPM2 (left) or OPM2-GLUT1 (right) cells were treated 48 h with 1 mm VPA or valpromide before analysis of glucose uptake rate. Treated cells were incubated with [3H]2-DOG for 0–30 min before stopping glucose uptake with phloretin. Retained radioactivity was detected as in Fig. 3A. D and E, OPM2 cells were treated with 1 mm VPA before analysis of glucose uptake rate as in C using either [3H]2-DOG (D) or [3H]3-OMG (E). F, HXK activity present in lysates of OPM2 cells treated with VPA or SAHA was analyzed and normalized to protein input. G, OPM2 cells were treated with 1 mm VPA before isolation of RNA. RTqPCR analysis of HXK1 expression was performed as in Fig. 2C. H, HXK1 and GAPDH expression in lysates of OPM2 cells treated with 1 mm VPA was analyzed by immunoblotting. Results are representative of at least three independent experiments and values represent mean ± sd of triplicate samples.
Because 2-DOG, the substrate used for our glucose uptake studies, does not participate in glycolysis downstream of HXK, we reasoned that HDACI must target either GLUT1 and/or HXK activity. To dissect the relative contributions of glucose transport and phosphorylation to the observed effects of HDACI, we compared glucose uptake in OPM2 cells treated for 24 or 44 h with VPA using either 2-DOG or a second glucose analog, 3-O-methylglucose (3-OMG), which cannot be phosphorylated by HXK and thus more directly reports on the activity of GLUT1 absent involvement of HXK. Analysis with 2-DOG indicated a time-dependent decrease in the rate of glucose uptake, with 2- and 5-fold decreases observed at 24 and 44 h, respectively (Fig. 4D). A parallel analysis of the same treated cells using 3-OMG revealed only a slight delay in uptake at 24 h but a 2-fold decrease in uptake after 44 h VPA treatment (Fig. 4E). These data indicate that VPA has both an acute and a chronic effect on glucose uptake in cells. The acute effect is manifest primarily at the level of HXK, as evidenced by reduced 2-DOG uptake at 24 h with a corresponding minimal change in uptake of 3-OMG, whereas chronic treatment has a more profound effect on glucose transport per se and likely reflects the down-regulation of the GLUT1 transporter observed at 44 h (Fig. 4E).
Analysis of HXK activity in lysates of OPM2 cells treated 24 or 44 h with VPA or SAHA revealed a 50% decrease in the Vmax of HXK activity at 24 h that was maintained through 44 h (Fig. 4F). RTqPCR analysis indicated that HXK1 was the most abundantly expressed HXK in OPM2 cells, with a barely detectable level of HXK3 and no detection of HXK2 or HXK4 (not shown). Further RTqPCR analysis of RNA from OPM2 cells treated 24 or 44 h with VPA revealed an 8- to 10-fold induction of HXK1 mRNA expression by VPA (Fig. 4G), suggesting that decreased glucose uptake and HXK activity is accompanied by the adaptive response of HXK induction. The observed increase in HXK1 mRNA translated into increased protein expression as shown by immunoblot analysis of lysates of OPM2 cells treated 24 or 44 h with VPA or SAHA (Fig. 4H and not shown). Thus, surprisingly, HDACI are regulating HXK1 not at the level of transcription or expression but at the level of enzymatic activity. These data indicate that HDACI affect glucose homeostasis by inhibiting HXK activity and down-regulating GLUT1 expression.
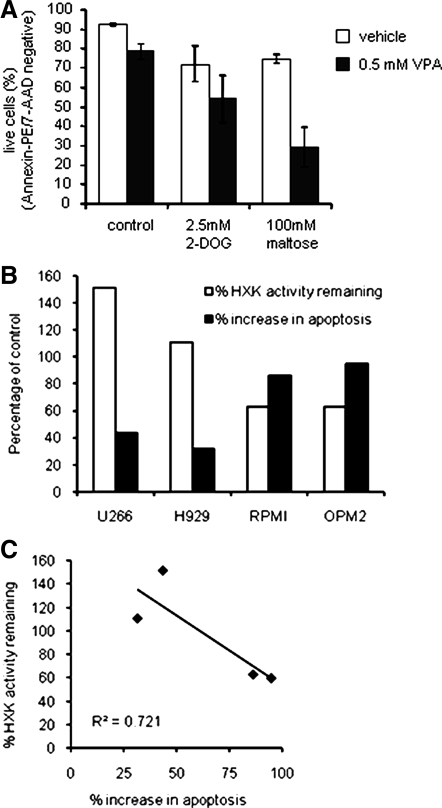
To investigate the contribution of the inhibition of glycolysis to the induction of apoptosis by HDACI, OPM2 cells were treated with a low dose of VPA, itself causing only a 10% reduction in viability, together with the glycolytic inhibitor 2-DOG, which upon phosphorylation acts as a HXK inhibitor during extended treatment, or maltose, which binds reversibly to the exofacial binding site of glucose transporters but cannot be transported. 2-DOG alone increased apoptosis by approximately 20% (Fig. 5A), and in cells cotreated with 2-DOG and VPA, we observed an additive effect between the two treatments, suggesting that HXK inhibition 1) contributes to induction of apoptosis by VPA and 2) poses a common mechanism by which VPA and 2-DOG induce apoptosis. Maltose, through inhibition of glucose transport, also itself increased apoptosis by 20%, but in the presence of VPA, we observed a cooperative induction of apoptosis, suggesting synergy between HXK and glucose transport inhibition (Fig. 5A). These data strongly suggest that the inhibition of HXK by HDACI is their primary means of influencing glycolysis and contributes to their apoptotic activity.
Figure 5.
HDACI cooperates with other glycolytic inhibitors to induce apoptosis. A, OPM2 cells were treated with vehicle, 2-DOG, or maltose for 72 h in the presence or absence of VPA. Apoptosis was analyzed as in Fig. 2A. Data represent the average of the percentage of live (unstained) cells detected in three independent experiments ± sd. B, Myeloma cell lines (H929, U266, RPMI, OPM2, and KMS11) were treated 44 h (HXK activity) or 96 h (apoptosis) with or without 1 mm VPA before analysis of HXK activity and apoptosis as in Figs. 4F and 2A, respectively. Data are calculated as percent change relative to the untreated control and are representative of at least three independent experiments. C, Data from B are plotted to reveal a correlation between induction of apoptosis and inhibition of HXK activity.
We and others have observed that myeloma cell lines exhibit differing levels of sensitivity to HDACI despite the fact that these drugs achieve similar levels of histone acetylation in treated lysates. Thus we asked whether differential inhibition of HXK correlated with the ability of HDACI to induce apoptosis in these cells. The results of this analysis, shown in Fig. 5, B and C, appear to suggest that HXK1 is indeed a primary target of HDACI in these cellular models of multiple myeloma. These data provide strong support for our hypothesis that HDACI regulation of glycolysis contributes to their therapeutic efficacy. Interestingly, we also demonstrated that HDACI similarly affected glucose uptake in NB4 acute promyelocytic leukemia, suggesting that this pathway may be a common target of this class of drugs in hematological malignancies (supplemental Fig. 3).
HDACI-mediated inhibition of glucose uptake is accompanied by a compensatory increase in amino acid oxidation
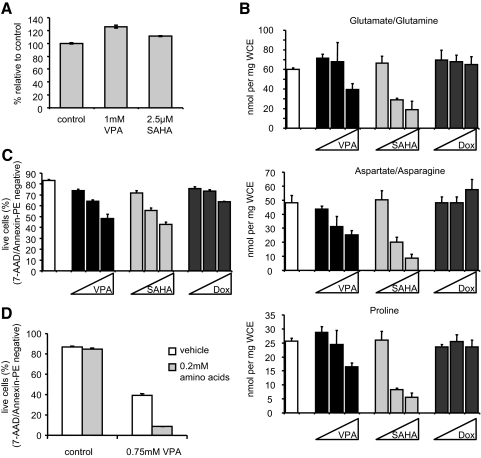
Cancer cells in general are known to rely heavily on glycolysis for cellular energy (33). Because HDACI treatment causes a strong suppression of glycolysis, we asked whether the loss of this energy source would translate to a similar reduction in cellular levels of ATP. OPM2 cells were treated with vehicle, VPA, or SAHA for 44 h, a time at which glucose transport is maximally decreased, and cell viability was analyzed by trypan blue exclusion. Equal numbers of intact cells were subjected to a chemiluminescent assay that uses luciferase activity to determine relative levels of ATP in the cell lysates. Although a 5-fold reduction in glucose uptake was observed in response to VPA or SAHA treatment (not shown), we observed no decrease in ATP content in a parallel analysis of the treated cells (Fig. 6A). These data suggest that an alternative metabolic program has been used to maintain cellular levels of ATP.
Figure 6.
Reduction of glucose uptake by HDACI does not reduce ATP levels but instead results in metabolism of amino acids. A, OPM2 cells were treated 44 h with VPA or SAHA before determining the number of remaining intact cells by trypan blue staining. An equal number of live cells was included in a chemiluminescent assay using luciferase activity to determine the relative levels of ATP in each sample. The data are expressed as mean percent ± sd relative to control where control equals 100% and are representative of three independent experiments. B, OPM2 cells were treated 48 h with VPA (0.5, 1, or 2 mm), SAHA (1, 2.5 or 5 μm), or Dox (25, 50, or 100 nm). Amino acid levels present in whole-cell extracts were determined using MS-MS analysis of deproteinated lysates and normalized to protein concentrations in the original lysates. C, Before lysis, apoptosis in cells analyzed in A was measured as in Fig. 2A. D, OPM2 cells were treated 96 h with vehicle or VPA in the presence or absence of supplemental nonessential amino acids. Apoptosis was analyzed as in Fig. 2A. Results are representative of at least three independent experiments, and graphed values represent calculated mean ± sd of triplicate samples.
Ordinarily, inhibition of glycolysis results in induction of fatty acid and/or amino acid catabolism to produce acetyl-CoA and cellular energy. The inability of HDACI-treated cells to restore acetyl-CoA levels to that observed in untreated cells (Fig. 2E) suggested that the energy demands of the cell were not being met. Thus, we next measured levels of each of 15 amino acids in OPM2 cells treated with VPA or SAHA to examine whether amino acids/proteins were being used as a fuel source, with a parallel experiment performed in the presence of Dox as a control. MS-MS analysis revealed little change in the 15 amino acids surveyed after 24 h, but a significant decrease in the concentrations of most amino acids occurred after 48 h of HDACI treatment, with the most dramatic decreases in glutamate/glutamine, aspartate/asparagine, and proline (Fig. 6B) as well as leucine/isoleucine, valine, and glycine (not shown). Similar results were obtained in a comparable analysis of H929 cells (supplemental Fig. 1B). Whereas changes in glucose transport and fatty acid metabolism were observed after 12 and 24 h, it was clear from these experiments that amino acid utilization was a late response that mirrored the onset of apoptosis (Fig. 6C). In contrast, these wholesale decreases in amino acid content did not occur when cells were treated with Dox, despite similar induction of apoptosis (Fig. 6, B and C). Thus, we extended our studies to explore the possible relationship between amino acid utilization and apoptosis.
Oxidative metabolism of amino acids contributes to apoptosis induced by HDACI
The data presented thus far suggest that the induction of metabolic stress in cells by HDACI makes a previously unrecognized contribution to their apoptotic activity. Furthermore, if apoptosis occurred secondarily to the depletion of readily utilizable fuel sources, supplementation with an appropriate carbon source might reduce or delay the apoptotic effects of HDACI. Given the effects of HDACI on GLUT1 and HXK1 activity, it was not surprising that glucose supplementation was without effect (not shown). Furthermore, because HDACI generated an acylcarnitine profile consistent with inhibition of fatty acid oxidation, it was also expected that oleate supplementation would fail to block the apoptotic response, and this indeed was found to be the case (not shown). Because GLUT8 was expressed in these cells, we also unsuccessfully attempted to rescue apoptosis using fructose (not shown). Finally, we examined whether supplementation with amino acids would impact cell viability. For this experiment, OPM2 cells were treated with 0.75 mm VPA, which achieved approximately 50% apoptosis at 96 h, in the absence or presence of a cocktail of nonessential amino acids (Fig. 6D). The surprising result was that rather than reducing the apoptotic activity of VPA, the amino acid treatment dramatically increased the efficacy of this HDACI, suggesting the possibility that a product of amino acid catabolism was contributing to HDACI-activated apoptosis. Taken as a whole, our data show that both inhibitors of glycolysis and added amino acids enhance HDACI-induced apoptosis, suggesting that HDACI-mediated killing of multiple myeloma cells occurs in part via forcing these cells to increase oxidation of fuels other than glucose, most notably amino acids. Amino acid treatment alone did not affect cell viability (Fig. 6D) or HXK activity or expression (data not shown). Somewhat unexpectedly, the glycolytic inhibitors 2-DOG and maltose did not cooperate with amino acid treatment to increase apoptosis (supplemental Fig. 4A). Likewise, we saw no changes in acylcarnitine and/or amino acid levels in cells treated with 2-DOG or maltose (supplemental Fig. 4, B and C), possibly because these inhibitors block metabolism at only a single point (glycolysis) as opposed to the multiple pathways targeted by HDACI. Similar rates of apoptosis were observed with the doses selected, demonstrating that altered metabolic profile is not directly linked to the induction of apoptosis (supplemental Fig. 4D).
Altered metabolic profile tracks with HDACI treatment and not with induction of apoptosis
Several agents have been shown to induce apoptosis in myeloma cells, leading us to ask whether the observed alterations in metabolism were unique to HDACI or a feature common to agents that induced apoptosis in myeloma cells. To address this issue, HXK activity was analyzed in OPM2 cells treated with VPA, arsenic trioxide (ATO), Dex, or Dox, with the metabolic inhibitors 2-DOG and maltose used for comparison. Doses were selected that induced similar rates of apoptosis (Fig. 7B). As expected, VPA and 2-DOG reduced HXK activity, whereas Dox and maltose were without effect. Interestingly, both ATO and Dex (albeit to a lesser degree) inhibited HXK activity (Fig. 7A). Induction of HXK1 mRNA and protein expression, however, proved to be unique to HDACI (Fig. 7, C and D). Further analysis revealed that the level of inhibition of glucose uptake (as assessed using labeled 2-DOG), observed in cells treated with VPA, ATO, Dex, and Dox correlated well with their ability to inhibit HXK1 activity (Fig. 7E, left). However, VPA alone was shown to inhibit glucose uptake when assessed using 3-OMG, a likely reflection of the ability of HDACI to down-regulate GLUT1 expression (Fig. 7E, right). Similarly, we observed that the changes in fatty acid and amino acid metabolism, as well as cooperation with amino acid treatment to induce apoptosis, were unique to HDACI and not common to other agents that induce apoptosis (ATO, Dex, and Dox).
Figure 7.
HDACI regulation of metabolism is unique among chemotherapeutics. OPM2 cells were treated 44 h with VPA (1 mm), ATO (0.75 μm), Dex (100 nm), Dox (100 nm), 2-DOG (2.5 mm), or maltose (100 mm) before analysis of apoptosis (B) as in Fig. 2A, HXK activity (A) and HXK 1 mRNA levels (C), and HXK1, GLUT1, and GAPDH protein (D) expression as in Fig. 4. E, OPM2 cells were treated as in A–D, with the exception of ATO (1 μm), before analysis of glucose uptake using 2-DOG (left) and 3-OMG (right) glucose analogs as in Fig. 4D. Results are representative of at least three independent experiments, and graphed values represent calculated mean ± sd of triplicate samples.
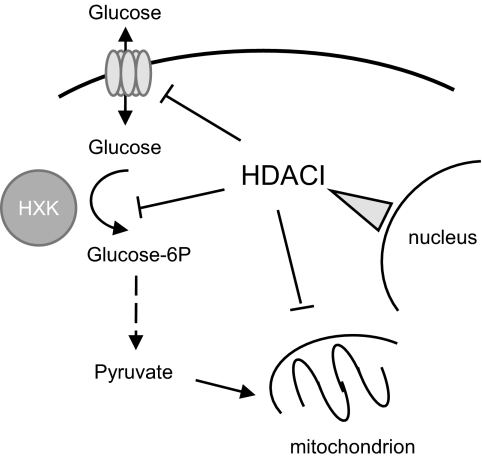
We conclude from the results presented here that it is the combined ability of the HDACI to inhibit glucose uptake (GLUT1) and glucose utilization (HXK1), together with their previously established impact on fatty acid β-oxidation, that contributes to their ability to reprogram cellular metabolism and impact cell viability. A model describing the possible interrelationship between HDACI and these activities is shown in Fig. 8.
Figure 8.
Mechanisms of action of HDACI. In addition to regulating transcription, HDACI can modulate metabolism through inhibition of mitochondrial β-oxidation of fatty acids as well as inhibition of glycolysis at the levels of transport (GLUT1) and retention (HXK).
Discussion
The widespread use of HDACI in analysis of transcriptional activation has enabled a specific evaluation of the role of HDAC and corepressors in control of transcription and downstream cellular processes. However, recent studies demonstrate that the effects of HDACI extend beyond modulation of chromatin and transcription, with targets of acetylation present in multiple cellular compartments. Work presented here adds to our understanding of HDACI function by showing that in addition to modulating transcription and activating apoptotic signaling cascades, drugs of this class can also impact cellular metabolism in malignant cells, an activity that may contribute to their therapeutic efficacy.
One challenge in the development of new cancer therapeutics is the identification of processes that are either preferentially represented in transformed cells or which play a more important role in malignant vs. normal cells. Although this objective has been met on several levels through the targeting of specific antigens on the cell surface or by interfering with growth factor signaling, little progress has been made until recently in exploiting cellular energy metabolism. With a few exceptions, transformed cells rely more heavily on glycolytic than on oxidative metabolism to generate the ATP and NADH/NADPH required for cellular growth. It was initially considered that this bias could be explained by the relatively low oxygen tension in tumors and the necessity to use anaerobic metabolism of glucose to pyruvate to meet the cellular energy demands. However, as first suggested by Warburg over 80 yr ago, even in the face of a readily available oxygen supply, glycolysis is the preferred mechanism of producing ATP in transformed cells. One hypothesis is that oxidative metabolism leads to the generation of reactive oxygen species (ROS) and in doing so increases the chances for DNA mutations that may negatively impact tumor growth and/or cell survival. Indeed, it has been shown that hypoxia-inducible factor (HIF-1), which is activated during aerobic glycolysis in the absence of hypoxia (34), actively suppresses mitochondrial function and in doing so may help to protect the cell from ROS-induced DNA damage. Recent studies indicate that cancer cells possess the ability to up-regulate oxidative phosphorylation upon inhibition of glycolysis, but in these studies, the increased mitochondrial activity was insufficient to sustain cellular ATP levels (33). Despite this plasticity, we have made the unexpected observation in the current study that HDACI have a dramatic effect on glucose uptake into multiple myeloma cells through their ability to both down-regulate the expression of the GLUT1 glucose transporter and through their inhibitory actions on HXK1. In doing so, these agents disrupt glycolysis and, secondary to a depletion of cellular acetyl-CoA, facilitate a dramatic switch in carbon utilization from glucose and fatty acids to oxidative metabolism of amino acids. These data suggest that the therapeutic efficacy of HDACI as anticancer agents in some situations may reside, at least in part, with their ability to affect metabolism.
As clinical exposure to HDACI has increased, it has become clear that the hematological malignancies are more responsive than are solid tumors (15). There may be many reasons to explain this selectivity, one being their reliance upon glycolysis. Indeed, in addition to these two myeloma cell lines, we also observe similar suppression of glucose uptake by HDACI in NB4 acute promyelocytic leukemia cells (supplemental Fig. 3). The range of VPA inhibition of HXK activity observed in a panel of myeloma cell lines, and their corresponding observed rates of apoptosis, highlights a potential reason for differing sensitivity among cancers to HDACI. The efficacy of HDACI, therefore, either in vitro or in patients, likely represents the sum of the different effects of HDACI on transcription and metabolism. Of note, the effects of HDACI on glucose metabolism occur at concentrations that are readily achievable clinically (35,36).
It is possible that in cells where either HXK3 or another GLUT are used, i.e. GLUT3, that the efficacy of the HDACI may be limited. In this regard, it is interesting that in cultured breast cancer cells, which express a low level of HXK3 and several different GLUT whose expression is not subject to regulation by HDACI (data not shown), we did not observe either HXK inhibition or induction of HXK1 (not shown). Neither did we see the same alterations in acylcarnitine and amino acid profiles in these cells (not shown). It is important to point out that although cancer cells preferentially use glycolysis that this is not a predictor of response to HDACI, because at therapeutically relevant doses, these agents do not significantly 1) induce apoptosis in A549 lung cancer cells or 2) inhibit HXK activity in either A549 cells or LNCaP prostate cancer cells, and no obvious change in fatty-acid or amino acid metabolism was noted in these cell models (supplemental Fig. 5 and not shown). Given these findings, it will be interesting to determine whether expression of GLUT1 and concomitant lack of expression of the other GLUT tracks with response to HDACI, a study that is currently amenable to clinical investigation.
Although we observe dramatic changes in cellular metabolism in response to HDACI, and we believe that this has important therapeutic consequences, it is clear that there are additional processes that are targeted by this class of drugs that contribute to their therapeutic efficacy. Notable in this regard was the inability to block HDACI-induced apoptosis in these cells by supplementation with glucose, fructose, oleate, or amino acids, suggesting that HDACI-mediated apoptosis was not necessarily associated with nutrient deprivation. Indeed, one of the most dramatic results observed was that amino acid supplementation dramatically increased the efficacy (and potency) of HDACI, suggesting that it was some aspect of amino acid catabolism, rather than lack of amino acids per se, that was linked to the apoptotic response. Although the mechanism for this interesting result is currently unknown, it is worth noting that in some patients with an underlying genetic defect in the urea cycle, VPA can induce hyperammonemic encephalophathy without altered liver function (37,38). This raises the interesting possibility that localized increases in ammonia production, secondary to increased amino acid catabolism, may underlie some of the therapeutic effects of the HDACI.
Although we have established a clear link between HDACI exposure, decreases in glucose uptake, and ultimately amino acid catabolism, key questions remain that, when answered, will further enhance our understanding of this class of drugs. Although GLUT1 mRNA expression is clearly regulated by HDACI, the mechanism by which this occurs is unclear. Previous studies indicate that GLUT1 is primarily regulated at the mRNA level, which would account for the ensuing down-regulation of GLUT1 protein we observed. GLUT1 mRNA stability is modulated by AU-rich sequence binding proteins such as Hu antigen R—also ELAV (embryonic lethal, abnormal vision, drosophila)-like 1, whose activity was recently shown to be sensitive to ROS (39). HDACI are known to potently induce ROS (40), which may account for the connection between HDACI and GLUT1 mRNA regulation. The role of ROS induction in HDACI induction of apoptosis is unclear; however, ameliorating ROS production with the ROS scavenger ascorbate elicited no change in induction of apoptosis by HDACI (not shown), and thus its role in GLUT1 regulation is uncertain. Alternatively, the aberrant induction of HIF-1 during aerobic glycolysis may contribute to GLUT1 expression and its regulation by HDACI; GLUT1 mRNA expression is induced by HIF-1, which is also inhibited by HDACI (34,41,42).
One finding of particular note was that HDACI exerted an inhibitory effect on HXK at the level of enzymatic activity rather than transcription and/or expression. Such a level of regulation disagrees with current dogma regarding HDACI action that has been built upon several studies illustrating direct regulation of gene expression by HDACI. Although perplexing, this novel mechanism of regulation of metabolism implies an as yet unappreciated level of possible regulation by HDACI. The activity of other proteins, such as p53, has been shown to be modulated by their acetylation state (5,43,44). It is possible that this occurs as a consequence of a direct acetylation of the protein. However, in preliminary experiments, we were unable to show changes in HXK1 acetylation in cells treated with HDACI. HXK activity is also regulated by mitochondrial association, a process increased by oncogenic pathways associated with increased resistance to apoptosis (45,46). However, despite a dramatic induction of HXK1 expression, we have not observed an alteration in the association of this enzyme with mitochondria in response to HDACI. Previously, it has been demonstrated that CoA metabolites of VPA directly modulate enzyme activity, and CoA conjugation of VPA is known to take place in both mitochondria and cytosol, suggesting that VPA or one of its derivatives is directly inhibiting HXK1 activity (47). Finally, we have observed that HDACI effectively down-regulate the expression of several growth factor receptors (not shown). It is possible therefore that the activity of one of these receptors is required for HXK and/or GLUT1 activity, a possibility that is currently under investigation.
It is worth mentioning that the HDACI we have used in this study are relatively nonselective and can inhibit the activity of most of the classical HDAC. Although HDAC appear to be relatively interchangeable in vitro, recent work has established unique and essential roles for select HDAC within the cell (48,49). To this end, we believe that it will be of use to determine whether the metabolic responses to HDACI are mediated by a single HDAC or whether there is redundancy in the system. Furthermore, the cellular targets that link HDACI to metabolism need to be identified and incorporated into assays for the selection and optimization of the next generation of HDACI. Regardless, our studies suggest that cancers that rely on GLUT1 and HXK1 for glycolysis and energy production might be particularly responsive to HDACI therapy.
Materials and Methods
Cell lines
OPM2 cells were provided by E. Brad Thompson (Baylor College of Medicine, Houston, TX). NCI-H929, RPMI 8226, and U266 cells came from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in modified RPMI 1640 (ATCC catalog item 30-2001) supplemented with 12% fetal bovine serum.
Reagents
VPA, Dox, Dex, ATO, maltose, and 2-DOG came from Sigma-Aldrich (St. Louis, MO). SAHA acid was a gift from William Zuercher (GlaxoSmithKline, Research Triangle Park, NC). Valpromide came from Lancaster Synthesis (Pelham, NH). Amino acid supplement came from Invitrogen (Carlsbad, CA). 2-Deoxy-d-[2,6-3H]glucose and 3-O-methyl-d-[1-3H]glucose came from GE Healthcare (Piscataway, NJ).
[3H]Acetate distribution
Cells (1 × 106) were incubated 1 h in medium containing 50 μm (25 μCi/ml) [3H]acetate and then treated as indicated with continuous presence of [3H]acetate before washing in PBS plus 1% BSA. Cells were resuspended in water and lysed by repeated freeze/thaw and DNA shearing. Protein was precipitated by addition of methanol and cleared by centrifugation. Supernatant containing soluble counts was retained, and pellets were solubilized in sterile water. Counts present in both fractions were detected by scintillation counting.
Amino acid and acylcarnitine analysis
Cells (3 × 106) were treated as indicated. Lysates were prepared and analyzed by targeted MS-MS as previously described (50,51), using cocktails of stable isotope-labeled acylcarnitines or amino acids as internal standards. Amino acid and acylcarnitine levels were normalized to total protein.
HDAC inhibition
Nuclear extracts prepared from untreated OPM2 cells were incubated with indicated concentrations of HDACI, and HDAC activity was analyzed using kit AK-500 (Bio-Mol International, Plymouth Meeting, PA) per manufacturer’s instructions.
Glucose uptake
Glucose uptake was measured essentially as previously described (32) with some modifications. Treated cells were washed in PBS plus 1% BSA and resuspended at 2 × 106 intact cells/ml (per trypan blue staining) in warmed KRH buffer [20 mm HEPES (pH 7.4), 1.25 mm MgSO4, 1.25m CaCl2, 140 mm NaCl, 5 mm KCl, 2% BSA], and 100 μl was combined with 150 μl KRH containing 2 μCi 2-deoxy-d-[2,6-3H]glucose (GE Healthcare) or 3-O-methyl-d-[1-3H]glucose (GE Healthcare). After 0–30 min incubation at 37 C, glucose transport was stopped by addition of 200 μl ice-cold KRH buffer containing 200 μm phloretin (Calbiochem, San Diego, CA). Stopped samples were incubated on ice before washing the cells in PBS plus 1% BSA. Samples were lysed by addition of 200 μl 1 n NaOH. Retained radioactivity was detected by scintillation counting.
Cell viability and apoptosis
Cells (1 × 105/ml) were treated as indicated and then washed in PBS and stained with phycoerythrin (PE)-conjugated annexin V and 7-AAD per manufacturer’s instructions (Pharmingen, San Diego, CA) before FACS analysis.
RTqPCR
Cells (0.5–2 ×106) treated as indicated were washed in PBS before lysis. RNA isolation (Bio-Rad, Hercules, CA) and reverse transcription (iScript; Bio-Rad) were performed per kit manufacturer’s instructions. RTqPCR of cDNA was done using iQ SYBR Green Supermix (Bio-Rad) per kit instructions and performed using the iCycler optical system with associated software (Bio-Rad). mRNA abundance was calculated using the ΔΔCT method as previously described (52). Primer sequences included: GLUT1 forward 5′-CTTTTCTGTTGGGGGCATGATTG-3′ and reverse 5′-CCGCAGTACACACCGATGAT-3′, HXK1 forward 5′-GGACTGGACCGTCTGAATGT-3′ and reverse 3′-ACAGTTCCTTCACCGTCTGG-3′, and 36B4 forward 5′-GGACATGTTGCTGGCCAATAA-3′ and reverse 3′-GGGCCCGAGACCAGTGTT-3′.
Immunoblot analysis
SDS-PAGE and immunoblot analysis was performed per instructions from Bio-Rad using antibodies to GLUT1 (Abcam, Cambridge, MA) or HXK1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Retrovirus construction
FLAG-tagged rat GLUT1 cDNA (32) was cloned into pMINR retroviral expression vector, which allows cocystronic internal ribosome entry site-mediated expression of the extracellular domain of NGFR. Amphitrophic retrovirus was produced by cotransfection of pMINR-GLUT1 with pVSVg (CLONTECH, Mountain View, CA) into GP2 293 packaging cells (CLONTECH). After 48 h infection, OPM2 cells were stained with a PE-conjugated antibody to NGFR (Pharmingen) and sorted by FACS analysis. Sorted cells were maintained as a polyclonal population, and expression of FLAG-GLUT1 was monitored by immunostaining for FLAG (Sigma) and FACS analysis.
HXK activity
HXK activity was analyzed as previously described (53) and normalized to total protein.
ATP content
Treated cells were washed in PBS, and 1.25 × 104 intact cells (per trypan blue staining) per sample were analyzed using a luciferase-based kit per manufacturer’s instructions (Roche, Basel, Switzerland).
Supplementary Material
Acknowledgments
We thank Michelle Jansen, Ph.D., for optimizing the in vitro HDAC inhibition assay and the Duke University Medical Center flow cytometry facility for experimental analysis.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant T32 CA 059365 (S.E.W.), NIH Grant PO1 DK58398 (C.B.N. and O.R.I.), and National Institute of Allergy and Infectious Diseases Grant RO1 A1063345 (J.C.R. and H.L.W.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 23, 2008
Abbreviations: ATO, Arsenic trioxide; CoA, coenzyme A; Dex, dexamethasone; 2-DOG, 2-deoxyglucose; Dox, doxorubicin; FACS, fluorescence-activated cell sorting; GLUT1, glucose transporter 1; HDACI, histone deacetylase inhibitor; HIF-1, hypoxia-inducible factor; HXK, hexokinase; MS-MS, tandem mass spectrometry; NGFR, nerve growth factor receptor; 3-OMG, 3-O-methylglucose; PE, phycoerythrin; ROS, reactive oxygen species; RTqPCR, real-time quantitative PCR; SAHA, suberoylanilide hydroxamic acid; VPA, valproate.
References
- Van Lint C, Emiliani S, Verdin E 1996 The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5:245–253 [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Zavrski I, Sterz J, Jakob C, Fleissner C, Kloetzel P-M, Sezer O, Heider U 2006 The effects of the histone deacetylase inhibitor valproic acid on cell cycle, growth suppression and apoptosis in multiple myeloma. Haematologica 91:248–251 [PubMed] [Google Scholar]
- Duan H, Heckman C, Boxer L 2005 Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol 25:1608–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind R, Marks P 1998 A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. J Cell Biol 95:3003–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Packman K, Jeffrey R, Tenniswood M 2005 Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ 12:482–491 [DOI] [PubMed] [Google Scholar]
- Takai N, Kawamata N, Gui D, Said J, Miyakawa I, Koeffler H 2004 Human ovarian carcinoma cells: histone deacetylase inhibitors exhibit antiproliferative activity and potently induce apoptosis. Cancer 101:2760–2770 [DOI] [PubMed] [Google Scholar]
- Aoyagi S, Archer T 2005 Modulating molecular chaperone Hsp90 functions through reversible acetylation. Trends Cell Biol 15:565–567 [DOI] [PubMed] [Google Scholar]
- Bannister A, Miska E, Gorlich D, Kouzarides T 2000 Acetylation of importin-α nuclear import factors by CBP/p300. Curr Biol 10:467–470 [DOI] [PubMed] [Google Scholar]
- Blagosklonny M, Robey R, Sackett D, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates S 2002 Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther 1:937–941 [PubMed] [Google Scholar]
- Xu M, Nie L, Kim S, Sun X 2003 STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPβ. EMBO J 22:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy V, Van Lint C 2004 Regulation at multiple levels of NF-κB-mediated transactivation by protein acetylation. Biochem Pharmacol 68:1221–1229 [DOI] [PubMed] [Google Scholar]
- Yuan Z, Guan Y, Chatterjee D, Chin Y 2005 Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307:269–273 [DOI] [PubMed] [Google Scholar]
- Acharya M, Sparreboom A, Venitz J, Figg W 2005 Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol 68:917–932 [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Issa J 2005 Histone deacetylase inhibitors: a review of their clinical status as antineoplastic agents. Cancer Invest 23:635–642 [DOI] [PubMed] [Google Scholar]
- Bruserud O, Stapnes C, Tronstad K, Ryningen A, Anensen N, Gjertsen B 2006 Protein lysine acetylation in normal and leukaemic haematopoiesis: HDACs as possible therapeutic targets in adult AML. Expert Opin Ther Targets 10:51–68 [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Kawagoe H, Sano S 2002 Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and -independent apoptotic signaling pathways. Leuk Res 26:495–502 [DOI] [PubMed] [Google Scholar]
- Silva M, Ruiter J, Ijlst L, Jakobs C, Duran M, de Almeida I, Wanders J 2001 Differential effect of valproate and its Δ2- and Δ4-unsaturated metabolites, on the β-oxidation rate of long-chain and medium-chain fatty acids. Chem Biol Interact 137:203–212 [DOI] [PubMed] [Google Scholar]
- Ponchaut S, van Hoof F, Veitch K 1992 Cytochrome aa3 depletion is the cause of the deficient mitochondrial respiration induced by chronic valproate administration. Biochem Pharmacol 43:644–647 [DOI] [PubMed] [Google Scholar]
- Kibayashi M, Nagao N, Chiba S 1999 Influence of valproic acid on the expression of various acyl-CoA dehydrogenases in rats. Pediatr Int 41:52–60 [DOI] [PubMed] [Google Scholar]
- Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R 2004 Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ 11:S45–S55 [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades C, Richardson P, McMullan C, Poulaki V, Fanourakis G, Schlossman R, Chauhan D, Munshi N, Hideshima T, Richon V, Marks P, Anderson K 2003 Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood 101:4055–4062 [DOI] [PubMed] [Google Scholar]
- Mitsiades C, Mitsiades N, McMullan C, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann T, Richon V, Marks P, Anderson K 2004 Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA 101:540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle D, Chen Y, Hankewych M, DeSimone J 2001 Histone deacetylase inhibitors increase p21(WAF1) and induce apoptosis of human myeloma cell lines independent of decreased IL-6 receptor expression. Am J Hematol 68:170–178 [DOI] [PubMed] [Google Scholar]
- Silva M, Cornelius J, Duran M, de Almeida I, Wanders R 2001 Valproate induces in vitro accumulation of long-chain fatty acylcarnitines. Mol Genet Metab 73:358–361 [DOI] [PubMed] [Google Scholar]
- Ponchaut S, van Hoof F, Veitch K 1992 In vitro effects of valproate and valproate metabolites on mitochondrial oxidations. Relevance of CoA sequestration to the observed inhibitions. Biochem Pharmacol 43:2435–2442 [DOI] [PubMed] [Google Scholar]
- Silva M, Ruiter J, Ijlst L, Allers P, ten Brink H, Jakobs C, Duran M, de Almeida I, Wanders R 2001 Synthesis and intramitochondrial levels of valproyl-coenzyme A metabolites. Anal Biochem 290:60–67 [DOI] [PubMed] [Google Scholar]
- Silva M, Ruiter J, Overmars H, Bootsma A, van Gennip A, Jakobs C, Duran M, de Almeida I, Wanders R 2002 Complete β-oxidation of valproate: cleavage of 3-oxovalproyl-CoA by a mitochondrial 3-oxoacyl-CoA thiolase. Biochem J 362:755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan T, Millington D, Roe C, Yergey A, Liberto D 1984 A novel metabolite of valproic acid. Ann Neurol 16:394–398 [Google Scholar]
- Schwartz C, Palissot V, Aouali N, Wack S, Brons N, Leners B, Bosseler M, Berchem G 2007 Valproic acid induces non-apoptotic cell death mechanisms in multiple myeloma cell lines. Int J Oncol 30:573–582 [PubMed] [Google Scholar]
- Coude F, Grimber G, Pelet A, Benoit Y 1983 Action of the antiepileptic drug, valproic acid, on fatty acid oxidation in isolated rat hepatocytes. Biochem Biophys Res Commun 115:730–736 [DOI] [PubMed] [Google Scholar]
- Wong H, Chu T, Lai J, Fung K, Fok T, Fujii T, Ho Y 2005 Sodium valproate inhibits glucose transport and exacerbates Glut1-deficiency in vitro. J Cell Biochem 96:775–785 [DOI] [PubMed] [Google Scholar]
- Wieman H, Wofford J, Rathmell J 2007 Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift A, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick D 2007 Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol 292:C125–C136 [DOI] [PubMed] [Google Scholar]
- Lu H, Forbes R, Verma A 2002 Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 277:23111–23115 [DOI] [PubMed] [Google Scholar]
- Abbott Laboratories 2006 Depakote tablets: divalproex sodium delayed-release tablets. http://www.rxabbott.com/pdf/depakote/pdf. North Chicago, IL: Abbott Laboratories [Google Scholar]
- Cancer Therapy Evaluation Program 2004 CTEP solicitation for Phase I and II trial: letters of intent—suberoylanilide hydroxamic acid (NSC 701852). http://dctd.cancer.gov/FeaturedAgents/pdfs/SAHASolicitation2rev_cpvrjc13.pdf. Bethesda, MD: National Cancer Institute [Google Scholar]
- Verrotti A, Trotta D, Morgese G, Chiarelli F 2002 Valproate-induced hyperammonemic encephalopathy. Metab Brain Dis 17:367–373 [DOI] [PubMed] [Google Scholar]
- Gomceli Y, Kutlu G, Cavdar L, Sanivar F, Inan L 2007 Different clinical manifestations of hyperammonemic encephalopathy. Epilepsy Behav 10:583–587 [DOI] [PubMed] [Google Scholar]
- Abdelmohsen K, Pullmann RJ, Lal A, Kim H, Galban S, Yang X, Blethrow J, Walker M, Shubert J, Gillespie D, Furneaux H, Gorospe M 2007 Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25:543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Arinze I 2006 Valproic acid-induced gene expression through production of reactive oxygen species. Cancer Res 66:6563–6569 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y 2004 Induction of glucose transporter 1 expression through hypoxia-inducible factor 1α under hypoxic conditions in trophoblast-derived cells. J Endocrinol 183:145–154 [DOI] [PubMed] [Google Scholar]
- Kim S, Jeong J, Park J, Lee J, Seo J, Jung B, Bae M, Kim K 2007 Regulation of the HIF-1α stability by histone deacetylases. Oncol Rep 17:647–651 [PubMed] [Google Scholar]
- Tang Y, Zhao W, Chen Y, Zhao Y, Gu W 2008 Acetylation is indispensable for p53 activation. Cell 133:612–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K, Herrera J, Saito S, Miki T, Bustin M, Vassilev A, Anderson C, Appella E 1998 DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev 12:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J, Fox C, Plas D, Hammerman P, Cinalli R, Thompson C 2003 Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23:7315–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Bhaskar P, Coy P, Skeen J, Gottlob K, Chandel N, Thompson C, Robey R, Hay N 2004 Hexokinase-mitochondrial interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16:819–830 [DOI] [PubMed] [Google Scholar]
- Silva M, Aires C, Luis P, Ruiter J, Ijlst L, Duran M, Wanders J, de Almeida I 2008 Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis 31:205–216 [DOI] [PubMed] [Google Scholar]
- Farooq M, Sulochana K, Pan X, To J, Sheng D, Gong Z, Ge R 2007 Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Dev Biol 317:336–353 [DOI] [PubMed] [Google Scholar]
- Bhaskara S, Chyla B, Amann J, Knutson S, Cortez D, Sun Z, Hiebert S 2008 Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell 30:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Muoio D, Shiota M, Fujimoto Y, Cline G, Shulman G, Koves T, Stevens R, Millington D, Newgard C 2004 Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10:268–274 [DOI] [PubMed] [Google Scholar]
- Rashed M, Ozand P, Bucknall M, Little D 1995 Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res 38:324–331 [DOI] [PubMed] [Google Scholar]
- Livak K, Schmittgen T 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Bauer D, Harris M, Plas D, Lum J, Hammerman P, Rathmell J, Riley J, Thompson C 2004 Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J 18:1303–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.