Abstract
Ziconotide is a powerful analgesic drug that has a unique mechanism of action involving potent and selective block of N-type calcium channels, which control neurotransmission at many synapses. The analgesic efficacy of ziconotide likely results from its ability to interrupt pain signaling at the level of the spinal cord. Ziconotide is a peptidic drug and has been approved for the treatment of severe chronic pain in patients only when administered by the intrathecal route. Importantly, prolonged administration of ziconotide does not lead to the development of addiction or tolerance. The current review discusses the various studies that have addressed the in vitro biochemical and electrophysiological actions of ziconotide as well as the numerous pre-clinical studies that were conducted to elucidate its antinociceptive mechanism of action in animals. In addition, this review considers the pivotal Phase 3 (and other) clinical trials that were conducted in support of ziconotide’s approval for the treatment of severe chronic pain and tries to offer some insights regarding the future discovery and development of newer analgesic drugs that would act by a similar mechanism to ziconotide but which might offer improved safety, tolerability and ease of use.
Keywords: ziconotide, Prialt, analgesic drug, N-type calcium channel blocker, severe chronic pain
Introduction
Ziconotide, which is also known as SNX-111, is a novel non-opioid analgesic drug. It is a synthetic version of ω-conotoxin MVIIA (ω-MVIIA), which is a peptide that is found in the venom of the fish-eating marine snail, Conus magus. Ziconotide has only limited ability to cross the blood–brain barrier and so in order to achieve optimal analgesic efficacy with reduced potential for serious side-effects, it must be administered intrathecally to patients. This spinal route of administration permits ziconotide to reach its maximum local concentration in a short time, which encourages a rapid onset of analgesia. Following the successful completion of three pivotal double-blind, placebo-controlled trials, intrathecal infusion of ziconotide was recently approved by regulatory bodies worldwide as a therapeutic approach for the symptomatic management of severe chronic pain, particularly in patients who are refractory to treatment with morphine and for whom intrathecal therapy is a viable option. The “Ziconotide Intrathecal Infusion” product is marketed by Elan Pharmaceuticals as Prialt® and is intended for continuous delivery via a programmable surgically implanted variable rate infusion device such as the Medtronic SynchroMed® EL, the SynchroMed® II Infusion System, or the CADD-Micro® Ambulatory Infusion Pump. Alternatively, an external microinfusion device can be used temporarily. The use of an infusion pump allows the dose of ziconotide to be titrated incrementally according to patients’ personal needs and comfort in order to achieve an optimal balance of analgesic efficacy and side-effects.
Ziconotide’s pharmacological effects have been investigated extensively in pre-clinical in vivo and in vitro models. Briefly, intrathecal ziconotide is a powerful antinociceptive drug in several animal models of chronic pain and it appears to have a completely novel mechanism of action that involves potent and selective block of pre-synaptic neuronal N-type calcium channels in the spinal cord. In fact, it is the only selective N-type channel blocker that is currently approved for clinical use. Evidence suggests that ziconotide delivers its antinociceptive efficacy by reducing the release of pronociceptive neurotransmitters in the dorsal horn of the spinal cord, thereby inhibiting pain signal transmission. Intrathecal ziconotide’s clinical efficacy is consistent with the hypothesis that spinal N-type calcium channels are key regulators of nociceptive signaling in humans, although it is fair to say that its precise analgesic mechanism in humans remains unconfirmed at this time. There are several recent publications that are relevant to the topics in this review and they will be cited where appropriate.
Acute and chronic pain
Pain has been defined as “an unpleasant sensory and emotional experience that is associated with actual or potential tissue damage” (International Association for the Study of Pain®) and can be classified according to a variety of characteristics including its duration (acute or chronic) and intensity (mild, moderate, or severe). Acute pain is a normal experience that is usually short-lasting and serves to alert the body about ongoing tissue damage so that protective or evasive measures can be taken. Acute pain usually lessens over time as a consequence of the healing process. In contrast, chronic pain represents an abnormal experience that is long-lasting and persists in the absence of any apparent tissue damage. Chronic pain is not equivalent to long-lasting acute pain; it appears to serve no useful purpose and is often associated with diseases involving tissue inflammation (leading to chronic inflammatory pain) or damage to peripheral or central neurons (leading to chronic neuropathic pain). More complex chronic pain syndromes may exhibit signs of both inflammatory and neuropathic pain.
Pain is experienced through a complex neural network that has two anatomically defined and functionally interacting systems that control pain perception and pain modulation (Almeida et al 2004; Apkarian et al 2005). During normal pain sensation, components of the pain perception system are activated first and subsequently the pain modulation system may contribute inhibitory and/or facilitatory input to alter the strength and duration of the pain. During pain perception, the peripheral nerve endings of high-threshold mechanosensitive and polymodal nociceptive neurons, whose cell bodies are located in the dorsal root ganglia (DRG), are excited by noxious stimuli, leading to the generation and propagation of sodium channel-dependent action potentials along small diameter finely myelinated (Aδ fiber) or unmyelinated (C fiber) axons. The Aδ and C fibers project mainly to the superficial laminae of the dorsal horn in the spinal cord, where they make synaptic connections with secondary sensory neurons (Light and Perl 1979a, 1979b; Light et al 1979). In contrast, large diameter low-threshold mechanosensitive Aβ fibers, which encode ordinary tactile information, project mainly to the deeper laminae of the dorsal horn. When the action potentials reach the central terminals of the primary afferent neurons, calcium influx through pre-synaptic voltage-gated calcium channels triggers the release of pronociceptive neurotransmitters and neuromodulators such as substance P, calcitonin gene related peptide (CGRP), and glutamate (Levine et al 1993; Dickenson et al 1997; Bennett 2000). Under conditions of chronic pain, plastic changes in the nervous system may occur, possibly leading to overactivity in the pain perception system and/or an imbalance in the inhibitory and facilitatory components of the pain modulation system. Both peripheral and central maladaptive mechanisms may contribute to the generation of sensory deficits (Katz and Rothenberg 2005). Peripheral mechanisms include sensitization of Aδ and C fibers, phenotypic switching of Aβ fibers, and awakening of silent nociceptors. Central mechanisms include sensitization of secondary and tertiary sensory neurons, as well as spinal and cortical circuit reorganization.
Many medications are available to treat acute and chronic inflammatory pain, but options for treating chronic neuropathic pain are more limited. Mild to moderate acute pain often can be managed effectively by over-the-counter medications, such as acetaminophen, whereas severe acute pain requires stronger analgesics such as opioid drugs. The exact mechanism of action of acetaminophen is unknown and although it is a very safe drug with few side-effects, a recent study suggests that it may increase the serum levels of liver enzymes when taken at high doses (Watkins et al 2006). The opioid drugs are very effective pain relievers and exert their analgesic effects by agonizing μ, δ, and κ opioid receptors located at spinal and supraspinal sites in the central nervous system. Unfortunately, the opioids can produce serious side-effects, are prone to addiction and promote the development of tolerance with prolonged or repeated use. Drugs that have been used to treat pain associated with inflammation include non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen. These drugs are non-selective inhibitors of the two major isoforms of cyclo-oxygenase (COX), ie, constitutive COX-1 and inducible COX-2. The COX inhibitors work by decreasing the production of prostaglandins, which are endogenous agents that are known to sensitize peripheral and central sensory neurons (McMahon et al 2005). However, these non-selective drugs are associated with the development of gastric ulcers, probably as a result of COX-1 inhibition. In contrast, COX-2 selective inhibitors produce fewer gastrointestinal problems and were prescribed widely for several years, but following controversial revelations regarding potential cardiovascular risks, some COX-2 inhibitors have been withdrawn from the market and others now carry warnings about the potential dangers. Drugs that have been approved for the treatment of neuropathic pain include carbamazepine, gabapentin, pregabalin and duloxetine. In addition, several tricyclic antidepressant, antiepileptic, and antiarrhythmic drugs are commonly used off-label for the symptomatic relief of neuropathic pain. The majority of these drugs appear to act by inhibiting non-selectively the activity of neuronal voltage-gated sodium and calcium channels. However, these drugs usually require high doses, have a high incidence of non-responders and deliver suboptimal efficacy. Consequently, there are significant opportunities for the discovery and development of novel drugs for the treatment of severe and chronic pain conditions although it must be remembered that regulatory agencies will insist that drugs are very safe before granting market approval.
Voltage-gated calcium channels, neurotransmission, and pain signaling
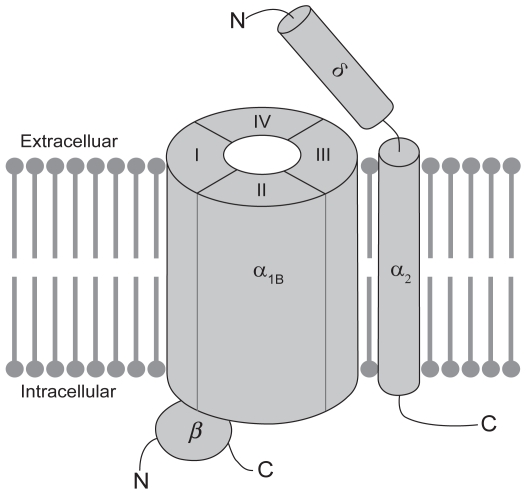
Various subtypes of voltage-activated calcium-permeable ion channels, including L-type, N-type, P/Q-type, and T-type channels have been identified throughout the mammalian nervous system. Most neuronal voltage-activated calcium channels are believed to exist as a complex of proteins (see Figure 1), comprising a large α1 subunit, which forms the pore of the channel and is responsible for defining the majority of its biophysical and pharmacological properties, as well as smaller auxiliary disulphide-linked α2δ and cytosolic β subunits, which regulate membrane insertion of the channel complex and modulate its functional properties (Arikkath and Campbell, 2003). So far 10 architecturally similar α1 subunits have been identified and structural elements have been identified that correlate with certain functions of the channel (Ertel et al 2000; Catterall et al 2003). The α1 subunit is organized into four homologous domains (DI-DIV), each of which contains six membrane-spanning segments (S1–S6). Membrane depolarization is sensed by positively charged amino acids in the so-called voltage-sensors that are located in the S4 transmembrane segment of each domain. The selectivity of the channel for calcium and the process of ion permeation are governed by four critical glutamate residues, one in each of the pore loops (P-loops) that are located between the S5 and S6 segments in each domain of the α1 subunit (Sather and McCleskey 2003). Of relevance to the current review, the molecular target of ziconotide appears to be the N-type calcium channel, which is a high-voltage-activated channel that contains the α1B subunit (also known as CaV2.2). The α1B subunit is subject to extensive splice variation (Lin et al 1997, 1999; Meadows and Benham 1999; Pan and Lipscombe 2000; Bell et al 2004; Castiglioni et al 2006), which enhances not only the molecular diversity of the N-type calcium channel but also its functional diversity, since there is the potential for altered biophysical and pharmacological properties. Perhaps with the exception of the α2δ subunit, which binds gabapentin and pregabalin, the α1B subunit contains most of the pharmacologically relevant binding sites on the N-type calcium channel. Calcium permeation can be modulated by agents that directly block the pore of the channel, such as divalent cations and peptides derived from venomous species, as well as by small molecule drugs that block the channel in a use-dependent manner, as a result of preferential interactions with activated and/or inactivated states of the channel (Winquist et al 2005).
Figure 1.
Schematic representation of the putative structure of the voltage-gated N-type calcium channel. N-type calcium channels are made up of a large pore-forming a1B subunit in association with one or more auxiliary subunits. The a1B subunit contains most determinants of channel function, including its biophysical and pharmacological properties. The proposed membrane topology of the a1B subunit is believed to involve four homologous domains (DI-DIV), each of which contains six transmembrane segments (S1–S6; not shown). The auxiliary subunits include the disulphide-linked a2d subunit, which is anchored in the membrane by a single membrane-spanning segment and the cytosolic b subunit, which interacts with the intracellular loop connecting DI to DII in the a1B subunit.
Voltage-activated calcium channels exhibit subtype-specific cellular and subcellular distributions in the nervous system and play distinct roles in controlling neuronal physiology. For instance, pre-synaptic calcium channels play a critical role in the biochemical cascade of events that leads to the exocytotic release of neurotransmitters via fusion of synaptic vesicles with the plasma membrane (Schneggenburger and Neher 2005). Immunocytochemical approaches have revealed that N-type and P/Q-type calcium channels are localized predominantly on pre-synaptic nerve terminals throughout the nervous system (Westenbroek et al 1998), where they associate with and are regulated by other components of the cellular machinery involved in synaptic transmission (Zhong et al 1999; Zamponi 2003). Although both subtypes are found pre-synaptically on the terminals of primary sensory neurons in the dorsal horn, only occasionally are they co-localized on the same nerve terminal (Westenbroek et al 1998). The N-type channels are evenly distributed throughout all the laminae of the dorsal horn and are in fact the predominant subtype in the superficial laminae (1 and 2), which is consistent with an involvement in Aδ and C fiber-mediated pain signaling (Gohil et al 1994). Furthermore, N-type channels are exclusively co-localized with substance P in presumptive C fiber terminals (Westenbroek et al 1998). In contrast, the P/Q-type channels are not found in lamina 1 of the dorsal horn, although their presence in lamina 2 suggests that they may also play a role in pain signal processing.
In accordance with these distribution data, the use of subtype-selective calcium channel blockers has confirmed that synaptic transmission in the peripheral and central nervous systems is triggered mainly by calcium influx through N-type and P/Q-type channels (Gaur et al 1994; Wheeler et al 1994; Mintz et al 1995), although additional subtypes may also contribute but to a lesser degree (Gasparini et al 2001). In the spinal cord, N-type and P/Q-type calcium channels contribute to both excitatory and inhibitory synaptic transmission. Interestingly, the N-type calcium channel is subject to direct regulation by G-protein βγ subunits (De Waard et al 2005) and a component of the spinal analgesic action of opioid drugs likely involves reduced release of pronociceptive neurotransmitters in the dorsal horn as a consequence of μ-opioid receptor activation and G-protein-dependent inhibition of N-type channels (North 1986). The importance of both N-type and P/Q-type calcium channels in the transmission and modulation of nociceptive signaling at the level of the spinal cord is further supported by in vivo pharmacological experiments with subtype-selective blockers, which will be discussed in more depth later (Chaplan et al 1994; Malmberg and Yaksh 1994; Diaz and Dickenson 1997; Matthews and Dickenson 2001). In addition, the reader is directed to several recent reviews that have discussed the relative contributions of N-type and other calcium channel subtypes to pain signaling (McGivern and McDonough 2004; McGivern 2006; Yaksh 2006).
Ziconotide: structural considerations and in vitro biochemical and electrophysiological studies
The ω-conotoxins, such as ω-GVIA, ω-MVIIA, ω-MVIIC, and ω-CVID, constitute a structurally related group of polypeptidic molecules that are found naturally in the venom of certain species of marine snail. In general, the ω-conotoxins bind with high affinity to voltage-gated calcium channels and potently block calcium flux. Despite structural conservation not only among the various ω-conotoxins but also among their binding sites on voltage-activated calcium channels, individual peptides actually exhibit distinguishing specificities for different channels.
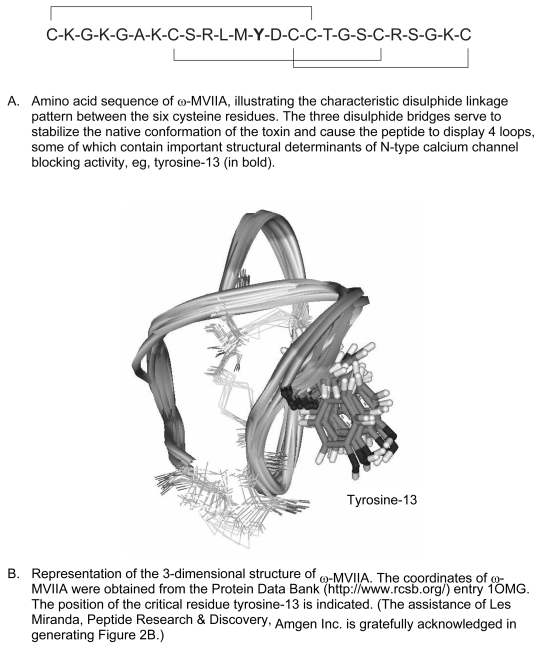
ω-MVIIA contains 25 amino acids, 6 of which are cysteine residues that are linked in pairs by 3 disulphide bonds (see Figure 2A). The disulphide bond linkage pattern is a characteristic feature of ω-conotoxins and serves to ensure correct folding of the peptide and stabilization of its structure in a compact, well-defined, native conformation (Chung et al 1995). Disruption of any one of the disulphide bridges greatly destabilizes the structure of ω-MVIIA and renders the remaining disulphide bonds more prone to reduction. Interestingly, the naturally occurring ω-MVIIA is synthesized by Conus magus as a precursor peptide that includes a C-terminally located glycine residue that becomes post-translationally converted to an amide group. This glycine appears to enhance the folding efficiency of the peptide in vivo by promoting molecular interactions that stabilize the native conformation with respect to other disulphide-bonded forms (Price-Carter et al 1996; Price-Carter et al 1998). The high resolution three dimensional structure of w-MVIIA has been determined by nuclear magnetic resonance (NMR) spectroscopy. The molecule displays a short triple-stranded anti-parallel β-sheet structure containing four loops, as illustrated in Figure 2B (Basus et al 1995; Kohno et al 1995).
Figure 2.
Putative structure of ziconotide.
As already mentioned, the molecular target of ziconotide (ω-MVIIA) appears to be the N-type calcium channel. In support of this hypothesis, radioligand binding experiments have demonstrated that ziconotide binds rapidly, reversibly, and with high affinity (see Table 1A) to N-type calcium channels in membrane and synaptosome preparations of rat brain (Stoehr and Dooley 1993; Kristipati et al, 1994). Ziconotide displays a high degree of binding and functional selectivity (>1000-fold) for the N-type calcium channel (Olivera et al 1987; Nielsen et al 1999b; Lewis et al 2000), whereas in contrast ω-MVIIC is more selective for the P/Q-type calcium channel (Hillyard et al 1992). It is believed that the differential potencies of the toxins are determined largely by the relative positions of amino acid side chains on the exposed surface of the toxin peptides (Nielsen et al 1996). In the case of ω-MVIIA, it is the non-cysteine amino acids in the loops that determine its binding affinity and calcium-channel-blocking activity. In particular, the second loop located between cysteine-8 and cysteine-15 appears to be most important in directing the selectivity of ω-MVIIA towards N-type channels and away from P/Q-type channels, although the fourth loop also contributes to a lesser degree (Nielsen et al 1999b). Alanine substitution experiments have revealed that tyrosine-13 in ω-MVIIA is a critical determinant of binding to N-type calcium channels (Kim et al 1995). As one would expect, correct folding of the ω-MVIIA peptide is necessary to ensure appropriate positioning of tyrosine-13 and permit toxin binding to the N-type calcium channel (Kohno et al 1995). Furthermore, altering the chirality of tyrosine-13 appears to affect the positions of key residues in the second loop of ω-MVIIA, leading to a reduction in its ability to recognize the N-type calcium channel in a radioligand binding assay (Nielsen et al 1999a). In addition, individual amino acid substitutions and chimeric-toxin approaches have revealed the importance of other amino acids such as lysine-2 and arginine-21 as well as those residues in positions 9 through 12 in determining the binding of ω-MVIIA to the N-type calcium channel (Nadasdi et al 1995; Sato et al 1997, 2000).
Table 1.
Summary of in vitro studies with ziconotide
| A. Radioligand binding studies | |
| N-type calcium channels in rat brain membranes or synaptosomes | Saturation binding of 125I-w-MVIIA |
| - Kd 1.1 pM (Stoehr and Dooley 1993) | |
| - Kd 9 pM (Kristipati et al 1994) | |
| Kinetic analysis of binding of 125I-w-MVIIA | |
| - Kd 7 pM (Stoehr and Dooley 1993) | |
| - Kd 18 pM (Kristipati et al 1994) | |
| Displacement of 125I-w-MVIIA | |
| - Ki 1 pM (Kristipati et al 1994) | |
| - IC50 2 pM (Newcomb et al 1995) | |
| - IC50 7.2 pM (Wang et al 1998) | |
| - IC50 29 pM (Lewis et al 2000) | |
| Displacement of 125I-w-GVIA | |
| - IC50 45 pM (Nielsen et al 1999b) | |
| - IC50 55 pM (Lewis et al 2000) | |
| B. Electrophysiological studies | |
| Inhibition of native high-voltage-activated calcium currents | Human neuroblastoma, IMR32 cells |
| - 42% inhibition of total calcium current at 10 nM (Fox 1995) | |
| Rat superior cervical ganglion neurons | |
| - IC50 32 nM, with 90% inhibition (Sanger et al 2000) | |
| Rat hippocampal neurons | |
| - 30% inhibition of total calcium current at 3 μM (Wen et al 2005) | |
| Inhibition of recombinant a1B-mediated calcium currents | Human a1B |
| - HEK cells, 92% inhibition at 100 nM (Bleakman et al 1995) | |
| Rat a1B | |
| - HEK cells, IC50 72 nM (Sanger et al 2000) | |
| - tsa-201 cells, complete block by 100 nM (Feng et al 2001) | |
| - Xenopus oocytes, IC50 0.4–11 nM, depending on the a1B splice variant and the absence or presence of the b3 subunit (Lewis et al 2000) | |
| C. Neurotransmitter release studies | |
| Depolarization-evoked norepinephrine release | Rat hippocampus |
| - IC50 ~0.5 nM (Newcomb et al 1995) | |
| - IC50 5.5 nM (Wang et al 1998) | |
| Rat peripheral sympathetic efferent neurons | |
| - IC50 1.2 nM (Wang et al 1998) | |
| Depolarization-evoked substance P release | Rat dorsal root ganglion neurons |
| - IC50 63 nM (Smith et al 2002) | |
Ziconotide appears to bind to the pore region of the α1B subunit and may interfere with calcium permeation by physically occluding the channel. Electrophysiological experiments have demonstrated conclusively that ziconotide inhibits N-type calcium currents in native cells as well as in heterologous expression systems (see Table 1B). Most native cells express a variety of different calcium channels and as a result, ziconotide only partially reduces high-voltage-activated calcium currents in differentiated human neuroblastoma IMR32 cells (Fox 1995), rat superior cervical ganglion neurons (Sanger et al 2000), and rat hippocampal neurons (Wen et al 2005). Ziconotide also reduces calcium currents that result from expression of the α1B subunit in HEK cells (Bleakman et al 1995; Sanger et al 2000), tsa-201 cells (Feng et al 2003), and Xenopus laevis oocytes (Newcomb et al 1995; Lewis et al 2000; Luchian 2001). Mechanistically, ziconotide exhibits little or no use-dependence in its inhibitory effect, which probably reflects its equal binding affinity for resting, open, and inactivated states of the channel (Feng et al 2003). Interestingly, the inhibitory potency of ziconotide on α1B-mediated calcium currents may vary depending on whether or not α2d and/or β auxiliary subunits are co-expressed (Lewis et al 2000; Luchian 2001; Mould et al 2004).
Chimeric-calcium channel α1 subunit approaches along with amino acid substitution experiments have shed light on which regions of the large α1B subunit may define the binding site and determine the characteristics of block by ziconotide. The binding site for ziconotide appears to be overlapping with that of ω-GVIA, which is positioned close to the P-loop of DIII (Ellinor et al 1994). Notably, the α1B subunit contains an EF hand-like motif that is located close to the P-loop in DIII. This EF hand-like motif may serve to bind calcium and facilitate its permeation through the pore of the channel. In addition, this motif may also impact ω-conotoxin binding. Indeed, electrophysiological experiments have revealed that mutations at glycine-1326 and glutamate-1332 affect not only calcium permeation but also the channel blocking characteristics of ω-MVIIA. In particular, glycine-1326 appears to restrict access of the toxin to its binding site, thereby decreasing the rate of onset of block and enhancing the reversibility of block (Feng et al 2001). Interestingly, calcium channel block by ziconotide and ω-GVIA are affected differently by amino acid mutations downstream of the EF hand-like motif, supporting the idea that their respective binding sites are overlapping but not identical (Feng et al 2003).
Since it blocks N-type calcium channels so potently, ziconotide has proven to be an effective inhibitor of neurotransmitter release at multiple synapses in the nervous system (see Table 1C). In fact due to its subtype-specificity, ziconotide is often used as a tool to define the contribution of N-type calcium channels to synaptic transmission at central and peripheral synapses. Thus ziconotide inhibits norepinephrine release from hippocampal (Newcomb et al 1995; Wang et al 1998) and peripheral sympathetic efferent neurons (Wang et al 1998). Consistent with the co-localization of N-type calcium channels and substance P in the central nerve terminals of primary afferent neurons (Westenbroek et al 1998), ziconotide potently inhibits the depolarization-evoked release of substance P from spinal cord slices (Smith et al 2002). This result implicates N-type calcium channels in the central processing of pain signals and suggests that this mechanism may contribute to the antinociceptive efficacy of ziconotide. Due to the predominant role of P-type calcium channels in controlling neurotransmitter release at the neuromuscular junction (Llinas et al 1992), ziconotide does not inhibit most nerve-evoked muscle contractions (Bowersox et al 1995).
Ziconotide: pre-clinical in vivo studies
Ziconotide has been described as a potent and long-lasting antinociceptive drug when administered by the intrathecal route. Experimental evidence of ziconotide’s antinociceptive properties was first obtained in the early 1990s and since then extensive studies have been conducted to characterize its effects in multiple animal models of pain (see Tables 2 and 3 for details on efficacy and dosing). These studies have revealed that ziconotide is more potent than morphine and is particularly efficacious in models of persistent pain (duration measured in minutes to hours) and chronic pain (duration measured in hours to days). In comparison, it tends to be less effective in tests of acute pain (duration measured in minutes). It is also important to note that ziconotide can be efficacious under a variety of intrathecal dosing regimens, including single bolus injection and acute or chronic continuous infusion. Despite its potent efficacy, the therapeutic index of spinal ziconotide tends to be low and often its antinociceptive effects in animals are accompanied by motor deficits at higher doses. Although ziconotide does not easily cross the blood brain barrier in normal animals, it may cause hypotension if it enters the systemic circulation (Bowersox et al 1992; Wright et al 2000; Takahara et al 2002). This effect on blood pressure appears to be mediated at least partially by inhibition of sympathetic neurotransmission, probably as a result of N-type calcium channel blockade in sympathetic nerve terminals (Wang et al 1998).
Table 2.
Summary of experiments conducted with ziconotide in rat behavioral models of acute pain (all dosing was intrathecal)
| A. Formalin model studies | ||
| 5%-formalin injected into the paw | Phase 1 Response | Phase 2 Response |
| Bolus injection | Bolus injection | |
| (Malmberg and Yaksh 1994) | - ID50 3 pmol | - ID50 3 pmol |
| (Bowersox et al 1996) | - No significant effect | - 100 ng caused ~50% decrease |
| (Wang et al 2000a) | - No significant effect | - ID50 110 ng |
| (Malmberg and Yaksh 1995) | 2-day infusion | 2-day infusion |
| - 3 pmol/h →42.5% decrease | - 3 pmol/h →42.7% decrease | |
| - 30 pmol/h →61.2% decrease | - 30 pmol/h →86.0% decrease | |
| (Bowersox et al 1996) | 3-day infusion | 3-day infusion |
| - ID50 14 ng/h | - ID50 0.82 ng/h | |
| (Malmberg and Yaksh 1995) | 7-day infusion | 7-day infusion |
| - 3 pmol/h →20.4% decrease | - 3 pmol/h →59% decrease | |
| - 30 pmol/h →43.1% decrease | - 30 pmol/h →86.1% decrease | |
| B. Other acute pain studies | ||
| Paw incision | Bolus injection | |
| (Wang et al 2000b) | - 1 h pre-incision:1 μg prevented mechanical allodynia and heat hyperalgesia | |
| - 1 d post-incision: mechanical allodynia ID50 <0.3 μg and heat hyperalgesia ID50 0.1 μg | ||
| 52.5°C hot plate | Bolus injection | |
| (Malmberg and Yaksh 1994) | - 20% increase in paw withdrawal latency at 8 pmol | |
| (Wang et al 2000a) | - 22% increase in paw withdrawal latency at 30 ng/h | |
| Continuous infusion | ||
| (Malmberg and Yaksh 1995) | - 3 pmol/h →approximately doubled the paw withdrawal latency following 2-day and 7-day infusion | |
| - 30 pmol/h →approximately tripled the paw withdrawal latency following 2-day and 7-day infusion | ||
| Paw pressure (analgesymeter) | Bolus injection | |
| (Wang et al 2000a) | - ID50 0.6 μg | |
| Tail flick (infra-red heat) | Bolus injection | |
| (Scott et al 2002) | - No significant effect at 0.3–1.0 μg/kg | |
| Tail immersion (50°C water) | Continuous infusion | |
| (Wang et al 2000a) | - 0.03 μg/h →no significant effect following 7-day infusion | |
Table 3.
Summary of experiments conducted with ziconotide in rat behavioral models of chronic pain (all dosing was intrathecal)
| A. Inflammatory pain studies | |
| Kaolin (3%) and carrageenan (3%) injected into the knee-joint | Continuous infusion |
| (Sluka 1998) | - 1 h infusion pre-induction: 100 μM at 5 μL/min. prevented the development of secondary heat hyperalgesia |
| - Continuous infusion beginning 4 h post-induction: 100 μM at 5 μL/min. reversed established secondary heat hyperalgesia within 1 h | |
| Complete Freund’s adjuvant (CFA) injected into the paw | Bolus injection |
| (Smith et al 2002) | - 5 days post-CFA injection: ID50 16 pmol |
| B. Neuropathic Pain Studies | |
| Spinal nerve (L5/L6) ligation | Bolus injection |
| (Chaplan et al 1994) | - Mechanical allodynia: ID50 1000 ng |
| (Bowersox et al 1996) | - Mechanical allodynia: ID50 30–100 ng |
| (Scott et al 2002) | - Mechanical allodynia: ID50 320 ng/kg |
| Continuous infusion | |
| (Bowersox et al 1996) | - Mechanical allodynia: ID50 10 ng/h following 3-day infusion |
| Chronic constriction injury (sciatic) | Bolus injection |
| (Yamamoto and Sakashita 1998) | - Heat hyperalgesia: 100 pmol reversed heat hyperalgesia |
| Partial nerve injury (sciatic) | Bolus injection |
| (Yamamoto and Sakashita 1998) | - Heat hyperalgesia: no significant effect on heat hyperalgesia at 100 pmol |
Formalin is a chemical irritant that when injected subcutaneously into the rat paw evokes a complex behavioral response consisting of an early (acute) phase and a late (persistent) phase of paw flinching and licking. This biphasic behavioral response appears to be correlated temporally with formalin-evoked electrical activity in both primary afferents and secondary sensory neurons in the dorsal horn of the spinal cord (Dickenson and Sullivan 1987b). Electrophysiological experiments have revealed an initial period of high intensity C fiber firing, which is followed by a prolonged period of lower intensity C fiber discharging accompanied by facilitation (“wind up”) of dorsal horn neuronal responses. The early phase of neuronal firing appears to be due to the direct excitation of peripheral C fiber nerve endings by formalin (Dickenson and Sullivan 1987a), whereas the late phase, although dependent on the early phase for its induction, appears to require additionally the release of pronociceptive neurotransmitters and activation of post-synaptic N-methyl-D-aspartate (NMDA) subtype of glutamate receptors in the spinal cord (Haley et al 1990).
Consistent with their predominant role in controlling release of pronociceptive neurotransmitters in the dorsal horn, N-type calcium channels appear to be involved in defining both the electrophysiological and behavioral responses to peripheral injection of formalin (Malmberg and Yaksh 1994; Diaz and Dickenson 1997). In common with other ω-conotoxins such as ω-GVIA, ziconotide suppresses the formalin-induced hyperexcitability of dorsal horn neurons (Diaz and Dickenson 1997). These electrophysiological effects are consistent with the ability of the ω-conotoxins to exert antinociceptive effects in the formalin model (see Table 2A), as assessed during both phases of the behavioral response (Malmberg and Yaksh 1994, 1995; Bowersox et al 1996; Wang et al 2000a; Chen et al 2005). The antinociceptive efficacy of ziconotide can be observed under a variety of dosing regimens including administration prior to the injection of 5% formalin, either as single bolus injection (eg, 10 minutes before) or following acute (up to 2 days before) or chronic (up to 7 days before) continuous infusion. In general ziconotide is more efficacious when administered prophylactically, although it does have activity in the late phase of the behavioral response when administered 9 minutes after the injection of formalin. Overall, ziconotide appears to be more effective at inhibiting the late phase rather than the early phase of the behavioral response to formalin. Perhaps consistent with its lesser effects in the early phase of the formalin test, ziconotide is not very effective at increasing pain thresholds in more straightforward models of acute pain (see Table 2B), such as the hot plate, radiant heat, and tail immersion tests in rats (Malmberg and Yaksh 1994, 1995; Wang et al 2000a, 2000b; Scott et al 2002).
The antinociceptive efficacy of ziconotide was also studied in animal models of inflammatory pain and nerve injury-evoked pain. Typically, animal models of inflammatory pain employ biochemical agents to sensitize or activate primary afferent neurons, leading to spontaneous pain as well as hyperresponsiveness to noxious (hyperalgesia) and innocuous (allodynia) stimuli. On the other hand, animal models of nerve injury-evoked pain usually involve constriction, ligation or partial transection of a peripheral or spinal nerve, leading to the development of behavioral symptoms that mimic some of the sensory abnormalities that are reported by neuropathic pain patients. These sensory phenomena include hyperalgesia and allodynia, both of which can be assessed behaviorally and interpreted as symptoms of neuropathic-like pain. Ziconotide exerts potent antihyperalgesic and antiallodynic effects in models of acute and chronic inflammatory pain (see Table 3-A) and in models of neuropathic pain (see Table 3-B). In a model of acute inflammatory pain, intrathecal ziconotide is able to prevent (infusion initiated 1 hour before biochemical challenge) or reverse (continuous infusion initiated 4 hours after biochemical challenge) kaolin and carrageenan-induced secondary heat hyperalgesia in the knee joint (Sluka 1998). In a rat incisional model of post-operative pain, a single bolus injection of ziconotide is able to reverse established heat hyperalgesia and mechanical allodynia (Wang et al 2000b) and in a model of chronic inflammatory pain, an intrathecal bolus of ziconotide can reverse Freund’s complete adjuvant-induced mechanical hyperalgesia in the hind paw (Smith et al 2002). In models of neuropathic pain, including several that involve surgically-induced damage to the sciatic nerve or spinal nerve roots, ziconotide is effective at reversing established hyperalgesia and allodynia, either when administered by bolus injection or by continuous spinal infusion (Chaplan et al 1994, Bowersox et al 1996; Yamamoto and Sakashita 1998; Scott et al 2002; Urban et al 2005). Interestingly, ziconotide can also be efficacious when administered outside the spinal cord. For instance, significant antihyperalgesic and antiallodynic effects have been observed following local application of ziconotide (ID50 <1.0 μg) to the site of sciatic nerve injury in rats (Xiao and Bennett 1995). Additionally, antiallodynic effects can be produced following microinjection of ziconotide (ID50 2.8 pmol) into the rostral ventromedial medulla in rats with spinal nerve ligation (Urban et al 2005), presumably by inhibiting neurotransmitter-dependent activation of pain facilitatory neurons that, by way of their descending projections to the dorsal horn of the spinal cord, contribute to the maintenance of hypersensitivity in neuropathic pain states (Ossipov et al 2000; Porreca et al 2001).
The anti-nociceptive efficacy of ziconotide in animal models of pain is obviously complex. Nevertheless, the results described above are particularly enlightening with respect to both the mechanism of action of ziconotide and the role of N-type calcium channels in controlling pain signal transmission. The differential efficacy of ziconotide in models of acute versus persistent pain may reflect changes in the expression level of calcium channel subunits under conditions of neuronal hyperexcitability. Both the α1B and α2δ subunits appear to be up-regulated in response to tissue inflammation or nerve injury (Luo et al 2001; Newton et al 2001; Abe et al 2002; Cizkova et al 2002; Yokoyama et al 2003). Consequently, N-type calcium channels might become functionally more important in hypersensitive states and their role in pain transmission could be greater under conditions of ongoing chronic pain rather than under conditions of acute pain. Alternatively, it is known that the pharmacology of the N-type calcium channel can change depending on its subunit composition, at least in heterologous expression systems (Lewis et al 2000; Luchian 2001; Mould et al 2004). If this mechanism were to be replicated in native neuronal N-type calcium channels, then tissue inflammation- or nerve injury-induced alterations in the subunit composition of the channels could lead to increased sensitivity to ziconotide’s blocking actions.
The antinociceptive profile of ziconotide in models of acute, persistent and chronic pain is worthy of comparison with that of P/Q-type calcium channel blockers, such as ω-agatoxin-IVA. The potency and efficacy of ziconotide suggests that although N-type calcium channels in the pain pathway contribute to the perception of acute pain (eg, first phase of the formalin test as well as the hot plate test), they play a more significant role in the development and maintenance of multiple hypersensitive painful states (eg, second phase of the formalin test as well as inflammatory and neuropathic pain models). In contrast, the antinociceptive profile of ω-agatoxin-IVA suggests that P/Q-type calcium channels contribute only to inflammation-associated painful conditions (Malmberg and Yaksh 1994; Diaz and Dickenson 1997; Nebe et al 1997, 1999). They appear to be involved neither in the perception of acute pain nor in the development and maintenance of nerve injury-associated hypersensitive painful states in animal models (Chaplan et al 1994; Yamamoto and Sakashita 1998). The antinociceptive profiles of subtype-selective calcium channel blockers may reflect the involvement of distinct populations of primary or secondary sensory neurons in the transmission and processing of different types of pain signals. This is a plausible contributory factor because although both N-type and P/Q-type calcium channels are found in the dorsal horn of the spinal cord, they actually display complementary neuronal distributions, with the same nerve terminal rarely containing both subtypes.
In summary, potent antinociceptive effects of ziconotide have been observed in several animal models of pain under a variety of dosing regimens, including acute and chronic administration. The demonstration that ziconotide retains its potent antinociceptive efficacy during chronic administration provides convincing evidence that, unlike the opioids, this drug is not associated with the development of tolerance. This observation has important implications for the long-term therapeutic use of ziconotide in the treatment of pain in patients. The experimental evidence also suggests that N-type calcium channels expressed at multiple sites along the pain pathway are functionally important in the transmission of pain signals. These locations may include the peripheral site of nerve injury, where the N-type calcium channels appear to be involved in the generation of persistent spontaneous neuronal activity under conditions of nerve injury. In addition, N-type calcium channels are important for the transmission of incoming nociceptive signals to secondary sensory neurons in the spinal cord whereas those in the rostral ventromedial medulla may be involved in the activation of descending pain facilitatory systems that have been shown to contribute to the maintenance of neuropathic pain states.
Ziconotide: clinical studies
The development path to regulatory approval of intrathecal ziconotide involved three large randomized, double-blind, placebo-controlled Phase 3 clinical trials that established the safety and analgesic efficacy of this drug in more than 600 patients (see Table 4A). All of the patients in these trials were suffering from severe chronic pain of malignant and/or non-malignant origins (Staats et al 2004; Rauck et al 2006; Wallace et al 2006) and in order for them to be accepted into the trials, it was necessary that their pain was inadequately controlled by other analgesic drugs, including opioids. These clinical trials evaluated the analgesic efficacy of ziconotide under chronic dosing paradigms (up to 3 weeks) in order to determine the potential for this drug to develop tolerance. In addition to these pivotal trials, a smaller placebo-controlled clinical trial demonstrated analgesic efficacy of ziconotide in a post-surgical setting (see Table 4A) and a number of open-label studies showed that ziconotide can be an effective therapy in the treatment of neuropathic pain (see Table 4B). There are several previously published reviews available that discuss the clinical experiences with ziconotide (Jain 2000; Heading 2001; Doggrell 2004; Miljanich 2004; Lyseng-Williamson and Perry 2006; Staats 2006).
Table 4.
Summary of clinical trials conducted with ziconotide
| A. Double-blind, blacebo-controlled studies | ||
| Study report | Ziconotide dosing | Clinical Observations |
| Intractable severe pain due to cancer or AIDS (Staats et al 2004) |
|
|
| Intractable non-malignant severe chronic pain (Wallace et al 2006) |
|
|
| Intractable severe chronic pain (Rauck et al 2006) |
|
|
| Post-operative pain (Atanassoff et al 2000) |
|
|
| B. Open-label studies | ||
| Study report | Ziconotide dosing, efficacy and adverse events | |
| Intractable de-afferentation pain (Brose et al 1997) |
|
|
| Neuropathic pain (Wermeling et al 2003) |
|
|
| Neuropathic pain (Wermeling and Berger, 2006) |
|
|
The first pivotal trial with ziconotide involved patients with chronic pain due to cancer or AIDS (Staats et al 2004). In this trial, 68 patients received ziconotide by continuous intrathecal infusion for an initial 5- to 6-day period, followed by a maintenance period for those who responded to treatment. The starting dose of ziconotide was low (≤0.1 or 0.4 μg/h), although it could be increased frequently (at 12- or 24-hour intervals) either until satisfactory pain relief was achieved, the maximum dose of 2.4 μg/h was reached or adverse events were reported. Moderate to complete pain relief was achieved for most patients during the initial phase, with an average 53% reduction in pain scores, as estimated on a visual analog scale of pain intensity (VASPI). Importantly there was no loss of analgesic efficacy during the maintenance phase, suggesting that humans do not develop tolerance to ziconotide. Adverse events were observed more frequently in the ziconotide group than in the placebo group and in general, their occurrence was reduced either by initiating the drug infusion at lower doses or by using smaller or less frequent dose increments.
The second pivotal trial evaluated the safety and efficacy of ziconotide in patients with chronic non-malignant (mostly neuropathic) pain (Wallace et al 2006). In this trial, 169 patients received ziconotide beginning at a low dose (≤0.1 or 0.4 μg/h) and during the subsequent several days, the dose could be doubled at 24-hour intervals either until satisfactory pain relief was achieved, the maximum dose was reached (2.4 or 7.0 μg/h, depending on starting dose) or adverse events were experienced. As in the first trial, patients receiving ziconotide experienced moderate to complete pain relief, although the average reduction in VASPI scores was lower in this second trial. Responders continued to receive drug during the maintenance period, during which the efficacy of ziconotide was maintained. Again, adverse events could be resolved by reducing the dose or frequency of titration or by discontinuation of the drug.
The third pivotal trial evaluated the safety and efficacy of ziconotide in 220 patients with intractable severe chronic pain, the majority of which was neuropathic (Rauck et al 2006). This trial was conducted in response to regulatory concerns that were raised about the high incidence and severity of the adverse events as well as the high rate of patient drop-out during the first two trials. Therefore the design of this third trial differed from the earlier trials in several important aspects: (1) a slower titration schedule was employed (increments of 0.1 μg/h no more frequently than every 24 hours); (2) a lower maximum dose was allowed (0.9 μg/h); and (3) the trial length was longer (3 weeks). Significant pain relief was achieved in the majority of patients receiving ziconotide and the average improvement in VASPI scores was estimated to be 15%. The magnitude of this reduction was smaller than what was reported in the previous trials and this seems to be consistent with the lower doses used. In addition, the patients in the ziconotide group consumed 24% less opioid drug compared to the placebo group. Adverse events were experienced at low therapeutic doses of ziconotide in this trial, but most of these were rated as mild or moderate and were slow to develop after the drug infusion was initiated.
A relatively small clinical trial evaluated the ability of ziconotide to reduce post-operative pain arising from major surgery (Atanassoff et al 2000). Low dose (0.7 μg/h) or high dose (7.0 μg/h) ziconotide was given to patients undergoing total abdominal hysterectomy, radical prostatectomy, or total hip replacement. In this trial, ziconotide infusion was initiated before surgical incision and was continued for up to 72 hours post-operatively. For those patients who received ziconotide, significant pain relief was experienced and morphine consumption was reduced. Side-effects, such as dizziness, blurred vision, nystagmus, and sedation, appeared to be more severe in the high-dose drug group and they resolved after discontinuation of the drug.
A number of open-label clinical trials have also been conducted with ziconotide. In one of the earliest reported trials, ziconotide was administered to a patient who had been suffering for more than 20 years from intractable deafferentation pain as a result of brachial plexus avulsion and limb amputation (Brose et al 1997). This patient was given ziconotide by continuous, constant rate, intrathecal infusion and complete pain relief was achieved, even after the dose was lowered to 2 ng/kg hourly to alleviate the patient’s side-effects of dizziness, blurred vision, and nystagmus. Another trial evaluated the analgesic efficacy, safety, and pharmacokinetic properties of intrathecal ziconotide in patients with chronic neuropathic pain (Wermeling et al 2003). Ziconotide was infused over a 1-hour period at doses of 1, 5, 7.5, or 10 μg. The analgesic efficacy of ziconotide was dose-related and was correlated with drug exposure in the cerebrospinal fluid. Moreover, efficacy developed rapidly (within 1 hour of initiation of drug infusion) and lasted for up to 48 hours. Most of the adverse events in this study were mild to moderate and serious events were only reported in the highest dose group. Another open-label trial evaluated the ability of ziconotide to relieve the symptoms of long-standing neuropathic pain of various origins in 3 patients (Wermeling and Berger 2006). Single dose administration (in 2 patients) or continuous infusion (in 1 patient) of ziconotide alleviated the pain considerably. The patients who received the single dose reported only mild side-effects, whereas the patient who received continuous infusion experienced more severe neurological side-effects. Interestingly, this patient could feel the imminent side-effects and was able to avoid them by reducing the rate of drug infusion. Regarding the pharmacokinetic profile of ziconotide in the cerebrospinal fluid of humans, the measured t½ is around 4½ hours, which is similar to the slow component of drug elimination that has been observed in rats and monkeys (Bowersox et al 1997). Ziconotide was not detectable in the plasma of the majority of patients, supporting the idea that this drug cannot easily cross the blood–brain barrier.
In summary, intrathecal ziconotide is a novel, potent and long-lasting analgesic therapy that can be used for the symptomatic relief of severe chronic pain of malignant and non-malignant origins. It is also effective for the prevention of surgically-induced pain. Since ziconotide is administered intrathecally to patients, it is tempting to speculate that its therapeutic mechanism of action primarily involves block of pre-synaptic N-type calcium channels in the spinal cord, leading to a reduction in the release of pronociceptive neurotransmitters from primary afferent nerve terminals and reduced synaptic excitation of secondary sensory neurons in the dorsal horn. Importantly, ziconotide is non-addictive and does not appear to induce the development of tolerance. Therefore it represents an analgesic therapy that is suitable for long-term use and in at least 1 case, a patient continued taking the drug for more than 7 years. Despite the use of an infusion pump to deliver drug directly into the intrathecal space, it is very difficult to predict or control the local concentration of ziconotide that can access the N-type calcium channels located on the central terminals of the primary sensory neurons in the dorsal horn. Therefore the optimal dose of drug needs to be determined empirically. Nevertheless, the therapeutic index of ziconotide tends to be low and adverse events (primarily psychiatric and neurological) may be experienced, particularly if the drug is infused rapidly, a high dose is given or the dose is escalated too frequently. However, the good news is that when adverse events are experienced, they usually resolve when the dose is lowered or the frequency of dose escalation is reduced. In order to minimize the incidence and severity of adverse events in new patients, the manufacturer recommends a “start low, go slow” approach to the use of ziconotide. The current recommendation is to initiate the infusion at a low dose of ≤0.1 μg/h and to titrate the dose upwards no more frequently than 2–3 times/week. This approach has been shown to produce fewer serious adverse events and to reduce the incidence of drug discontinuation by patients.
Future prospects and concluding remarks
Ziconotide represents a great achievement for current pain therapy but despite its potent analgesic efficacy there remains significant opportunity for improvement. The opportunity derives primarily from the peptidic nature of the drug and its requirement for intrathecal administration in order to yield analgesic efficacy with reduced potential for systemic and central nervous system side-effects. Consequently, drug discovery researchers are considering various approaches to identify and develop novel orally active, N-type calcium channel-selective blockers that have the potential to be superior to ziconotide.
High analgesic efficacy and improved safety and tolerability, relative to both ziconotide and the opioids, are essential properties of a next generation N-type calcium channel blocking drug. This goal could be achieved by exploring approaches to identify compounds that display greater selectivity for sensory neuron-specific splice variants of the N-type calcium channel and/or that possess a use-dependent mechanism of calcium channel block. Regarding the former approach, multiple kinetically distinct splice variants of the calcium channel α1B subunit are known to exist, some of which appear to be exclusive to peripheral neurons (Lin et al 1997, 1999). In particular, a dorsal root ganglion-specific variant was recently identified in rat (Bell et al 2004; Castiglioni et al 2006) and this discovery offers hope that human N-type calcium channels might also exhibit sensory neuron-specific splice variants that could be targeted selectively in the search for safer and more efficacious pain therapeutics. Regarding use-dependent N-type calcium channel blockers, one idea is to identify compounds that bind preferentially to open and/or inactivated states of the channel (Winquist et al 2005). If successful, this approach is likely to lead to the identification of molecules that might inhibit calcium influx more effectively during high-frequency neuronal firing, which occurs in hypersensitive pain states, and less effectively during low-frequency neuronal firing. The hope is that novel molecules displaying a use-dependent mechanism of action will offer both high analgesic efficacy and an improved therapeutic index relative to ziconotide. Neuromed Pharmaceuticals is pioneering efforts in this arena and recently they have partnered with Merck & Co. to develop NMED-160, an orally-available, use-dependent blocker of N-type calcium channels that is in Phase 2 clinical trials for a variety of pain conditions. In pre-clinical testing, this molecule displayed a broad efficacy profile in animal models of neuropathic and inflammatory pain and also had a good safety profile (Snutch et al 2003; Snutch 2004). However, it still remains to be shown that this drug is analgesic in patients with severe chronic pain.
Improved ease of administration is also a desirable property of a novel drug that could negate the requirement for intrathecal therapy that currently hinders widespread testing and use of ziconotide. Indeed, if a novel N-type calcium channel blocker could be delivered systemically then this would not only enable easier delivery to existing patients but could also increase the size of the patient population that stands to benefit from analgesia by this mechanism. An additional benefit of systemic delivery would be to reduce the risk of infection that is associated with a surgically implanted drug delivery device. Although it is theoretically possible to identify peptidic molecules that can cross the blood brain barrier, eg, the ziconotide analog SNX-194 (Newcomb et al 2000) the development hurdles would be difficult to overcome due to the access such a molecule would have to N-type calcium channels throughout the entire nervous system, including the sympathetic neurons that are involved in the control of blood pressure. In addition, due to their widespread distribution in the endocrine system (Sher et al 2003; Olsen et al 2005; Takahashi et al 2005), it must be appreciated that drugs targeting N-type calcium channels could have myriad effects on multiple organs that rely on these channels to carry out their normal physiological functions. Therefore based on current knowledge, a structurally novel, orally active small molecule with a use-dependent mechanism of action is considered to be the most desirable target profile for a next generation N-type calcium channel blocking drug for use in the treatment of severe pain.
Acknowledgments
The author is grateful to Barton Manning, Amgen Inc., and George Miljanich, Airmid LLC for useful discussion and constructive feedback on this manuscript.
References
- Abe M, Kurihara T, Han W, et al. Changes in expression of voltage-dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine. 2002;27:1517–24. doi: 10.1097/00007632-200207150-00007. discussion 25. [DOI] [PubMed] [Google Scholar]
- Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways:a neuroanatomical review. Brain Res. 2004;1000:40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP. Auxiliary subunits:essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Atanassoff PG, Hartmannsgruber MW, Thrasher J, et al. Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Reg Anesth Pain Med. 2000;25:274–8. doi: 10.1016/s1098-7339(00)90010-5. [DOI] [PubMed] [Google Scholar]
- Basus VJ, Nadasdi L, Ramachandran J, et al. Solution structure of omega-conotoxin MVIIA using 2D NMR spectroscopy. FEBS Lett. 1995;370:163–9. doi: 10.1016/0014-5793(95)00819-u. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Thaler C, Castiglioni AJ, et al. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–38. doi: 10.1016/s0896-6273(03)00801-8. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. Update on the neurophysiology of pain transmission and modulation:focus on the NMDA-receptor. J Pain Symptom Manage. 2000;19:S2–6. doi: 10.1016/s0885-3924(99)00120-7. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Bowman D, Bath CP, et al. Characteristics of a human N-type calcium channel expressed in HEK293 cells. Neuropharmacology. 1995;34:753–65. doi: 10.1016/0028-3908(95)00078-k. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Gadbois T, Singh T, et al. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J Pharmacol Exp Ther. 1996;279:1243–9. [PubMed] [Google Scholar]
- Bowersox SS, Mandema J, Tarczy-Hornoch K, et al. Pharmacokinetics of SNX-111, a selective N-type calcium channel blocker, in rats and cynomolgus monkeys. Drug Metab Dispos. 1997;25:379–83. [PubMed] [Google Scholar]
- Bowersox SS, Miljanich GP, Sugiura Y, et al. Differential blockade of voltage-sensitive calcium channels at the mouse neuromuscular junction by novel omega-conopeptides and omega-agatoxin-IVA. J Pharmacol Exp Ther. 1995;273:248–56. [PubMed] [Google Scholar]
- Bowersox SS, Singh T, Nadasdi L, et al. Cardiovascular effects of omega-conopeptides in conscious rats:mechanisms of action. J Cardiovasc Pharmacol. 1992;20:756–64. [PubMed] [Google Scholar]
- Brose WG, Gutlove DP, Luther RR, et al. Use of intrathecal SNX-111, a novel, N-type, voltage-sensitive, calcium channel blocker, in the management of intractable brachial plexus avulsion pain. Clin J Pain. 1997;13:256–9. doi: 10.1097/00002508-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Castiglioni AJ, Raingo J, Lipscombe D. Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J Physiol. 2006;576:119–34. doi: 10.1113/jphysiol.2006.115030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Striessnig J, Snutch TP, et al. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels:calcium channels. Pharmacol Rev. 2003;55:579–81. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–23. [PubMed] [Google Scholar]
- Chen JQ, Zhang YQ, Dai J, et al. Antinociceptive effects of intrathecally administered huwentoxin-I, a selective N-type calcium channel blocker, in the formalin test in conscious rats. Toxicon. 2005;45:15–20. doi: 10.1016/j.toxicon.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Chung D, Gaur S, Bell JR, et al. Determination of disulfide bridge pattern in omega-conopeptides. Int J Pept Protein Res. 1995;46:320–5. doi: 10.1111/j.1399-3011.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Cizkova D, Marsala J, Lukacova N, et al. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp Brain Res. 2002;147:456–63. doi: 10.1007/s00221-002-1217-3. [DOI] [PubMed] [Google Scholar]
- De Waard M, Hering J, Weiss N, et al. How do G proteins directly control neuronal Ca2+ channel function? Trends Pharmacol Sci. 2005;26:427–36. doi: 10.1016/j.tips.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Diaz A, Dickenson AH. Blockade of spinal N- and P-type, but not L-type, calcium channels inhibits the excitability of rat dorsal horn neurones produced by subcutaneous formalin inflammation. Pain. 1997;69:93–100. doi: 10.1016/s0304-3959(96)03271-x. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28:633–8. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat:differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987a;30:349–60. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett. 1987b;83:207–11. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Intrathecal ziconotide for refractory pain. Expert Opin Investig Drugs. 2004;13:875–7. doi: 10.1517/13543784.13.7.875. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Zhang JF, Horne WA, et al. Structural determinants of the blockade of N-type calcium channels by a peptide neurotoxin. Nature. 1994;372:272–5. doi: 10.1038/372272a0. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–5. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Doering CJ, Winkfein RJ, et al. Determinants of inhibition of transiently expressed voltage-gated calcium channels by omega-conotoxins GVIA and MVIIA. J Biol Chem. 2003;278:20171–8. doi: 10.1074/jbc.M300581200. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Hamid J, Doering C, et al. Residue Gly1326 of the N-type calcium channel alpha 1B subunit controls reversibility of omega-conotoxin GVIA and MVIIA block. J Biol Chem. 2001;276:15728–35. doi: 10.1074/jbc.M100406200. [DOI] [PubMed] [Google Scholar]
- Fox JA. Irreversible and reversible blockade of IMR32 calcium channel currents by synthetic MVIIA and iodinated MVIIC omega-conopeptides. Pflugers Arch. 1995;429:873–5. doi: 10.1007/BF00374813. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, et al. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–21. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S, Newcomb R, Rivnay B, et al. Calcium channel antagonist peptides define several components of transmitter release in the hippocampus. Neuropharmacology. 1994;33:1211–9. doi: 10.1016/s0028-3908(05)80012-7. [DOI] [PubMed] [Google Scholar]
- Gohil K, Bell JR, Ramachandran J, et al. Neuroanatomical distribution of receptors for a novel voltage-sensitive calcium-channel antagonist, SNX-230 (omega-conopeptide MVIIC) Brain Res. 1994;653:258–66. doi: 10.1016/0006-8993(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–26. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Heading CE. Ziconotide (Elan Pharmaceuticals) IDrugs. 2001;4:339–50. [PubMed] [Google Scholar]
- Hillyard DR, Monje VD, Mintz IM, et al. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Jain KK. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expert Opin Investig Drugs. 2000;9:2403–10. doi: 10.1517/13543784.9.10.2403. [DOI] [PubMed] [Google Scholar]
- Katz WA, Rothenberg R. Section 3:The nature of pain:pathophysiology. J Clin Rheumatol. 2005;11:S11–5. doi: 10.1097/01.rhu.0000158686.43637.af. [DOI] [PubMed] [Google Scholar]
- Kim JI, Takahashi M, Ohtake A, et al. Tyr13 is essential for the activity of omega-conotoxin MVIIA and GVIA, specific N-type calcium channel blockers. Biochem Biophys Res Commun. 1995;206:449–54. doi: 10.1006/bbrc.1995.1063. [DOI] [PubMed] [Google Scholar]
- Kohno T, Kim JI, Kobayashi K, et al. Three-dimensional structure in solution of the calcium channel blocker omega-conotoxin MVIIA. Biochemistry. 1995;34:10256–65. doi: 10.1021/bi00032a020. [DOI] [PubMed] [Google Scholar]
- Kristipati R, Nadasdi L, Tarczy-Hornoch K, et al. Characterization of the binding of omega-conopeptides to different classes of non-L-type neuronal calcium channels. Mol Cell Neurosci. 1994;5:219–28. doi: 10.1006/mcne.1994.1026. [DOI] [PubMed] [Google Scholar]
- Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–86. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ, Nielsen KJ, Craik DJ, et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J Biol Chem. 2000;275:35335–44. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979a;186:133–50. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979b;186:117–31. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–71. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lin Z, Haus S, Edgerton J, et al. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 1997;18:153–66. doi: 10.1016/s0896-6273(01)80054-4. [DOI] [PubMed] [Google Scholar]
- Lin Z, Lin Y, Schorge S, et al. Alternative splicing of a short cassette exon in alpha1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci. 1999;19:5322–31. doi: 10.1523/JNEUROSCI.19-13-05322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Hillman DE, et al. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–5. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Luchian T. The influence exerted by the beta(3) subunit on MVIIA omega-conotoxin binding to neuronal N-type calcium channels. Biochim Biophys Acta. 2001;1512:329–34. doi: 10.1016/s0005-2736(01)00336-4. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–75. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseng-Williamson KA, Perry C. Ziconotide. CNS Drugs. 2006;20:331–8. doi: 10.2165/00023210-200620040-00007. [DOI] [PubMed] [Google Scholar]
- McGivern JG. Targeting N-type and T-type calcium channels for the treatment of pain. Drug Discov Today. 2006;11:245–53. doi: 10.1016/S1359-6446(05)03662-7. [DOI] [PubMed] [Google Scholar]
- McGivern JG, McDonough SI. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr Drug Targets CNS Neurol Disord. 2004;3:457–78. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–62. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing:blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994;14:4882–90. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of omega-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92:235–46. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Meadows HJ, Benham CD. Sensitivity to conotoxin block of splice variants of rat alpha 1B (rbBII) subunit of the N-type calcium channel coexpressed with different beta subunits in Xenopus oocytes. Ann N Y Acad Sci. 1999;868:224–7. doi: 10.1111/j.1749-6632.1999.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Miljanich GP. Ziconotide:neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029–40. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–88. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Mould J, Yasuda T, Schroeder CI, et al. The alpha2delta auxiliary subunit reduces affinity of omega-conotoxins for recombinant N-type (Cav2.2) calcium channels. J Biol Chem. 2004;279:34705–14. doi: 10.1074/jbc.M310848200. [DOI] [PubMed] [Google Scholar]
- Nadasdi L, Yamashiro D, Chung D, et al. Structure-activity analysis of a Conus peptide blocker of N-type neuronal calcium channels. Biochemistry. 1995;34:8076–81. doi: 10.1021/bi00025a013. [DOI] [PubMed] [Google Scholar]
- Nebe J, Ebersberger A, Vanegas H, et al. Effects of omega-agatoxin IVA, a P-type calcium channel antagonist, on the development of spinal neuronal hyperexcitability caused by knee inflammation in rats. J Neurophysiol. 1999;81:2620–6. doi: 10.1152/jn.1999.81.6.2620. [DOI] [PubMed] [Google Scholar]
- Nebe J, Vanegas H, Neugebauer V, et al. Omega-agatoxin IVA, a P-type calcium channel antagonist, reduces nociceptive processing in spinal cord neurons with input from the inflamed but not from the normal knee joint—an electrophysiological study in the rat in vivo. Eur J Neurosci. 1997;9:2193–201. doi: 10.1111/j.1460-9568.1997.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Abbruscato TJ, Singh T, et al. Bioavailability of Ziconotide in brain:influx from blood, stability, and diffusion. Peptides. 2000;21:491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, et al. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95:1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Palma A, Fox J, et al. SNX-325, a novel calcium antagonist from the spider Segestria florentina. Biochemistry. 1995;34:8341–7. doi: 10.1021/bi00026a015. [DOI] [PubMed] [Google Scholar]
- Nielsen KJ, Adams DA, Alewood PF, et al. Effects of chirality at Tyr13 on the structure-activity relationships of omega-conotoxins from Conus magus. Biochemistry. 1999a;38:6741–51. doi: 10.1021/bi982980u. [DOI] [PubMed] [Google Scholar]
- Nielsen KJ, Adams D, Thomas L, et al. Structure-activity relationships of omega-conotoxins MVIIA, MVIIC and 14 loop splice hybrids at N and P/Q-type calcium channels. J Mol Biol. 1999b;289:1405–21. doi: 10.1006/jmbi.1999.2817. [DOI] [PubMed] [Google Scholar]
- Nielsen KJ, Thomas L, Lewis RJ, et al. A consensus structure for omega-conotoxins with different selectivities for voltage-sensitive calcium channel subtypes:comparison of MVIIA, SVIB and SNX-202. J Mol Biol. 1996;263:297–310. doi: 10.1006/jmbi.1996.0576. [DOI] [PubMed] [Google Scholar]
- North RA. Opioid receptor types and membrane ion channels. Trends Neurosci. 1986;9:114–7. [Google Scholar]
- Olivera BM, Cruz LJ, de Santos V, et al. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry. 1987;26:2086–90. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- Olsen HL, Theander S, Bokvist K, et al. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146:4861–70. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, et al. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Pan JQ, Lipscombe D. Alternative splicing in the cytoplasmic II-III loop of the N-type Ca channel alpha 1B subunit:functional differences are beta subunit-specific. J Neurosci. 2000;20:4769–75. doi: 10.1523/JNEUROSCI.20-13-04769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, et al. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–8. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Carter M, Gray WR, Goldenberg DP. Folding of omega-conotoxins. 2. Influence of precursor sequences and protein disulfide isomerase. Biochemistry. 1996;35:15547–57. doi: 10.1021/bi9615755. [DOI] [PubMed] [Google Scholar]
- Price-Carter M, Hull MS, Goldenberg DP. Roles of individual disulfide bonds in the stability and folding of an omega-conotoxin. Biochemistry. 1998;37:9851–61. doi: 10.1021/bi9803978. [DOI] [PubMed] [Google Scholar]
- Rauck RL, Wallace MS, Leong MS, et al. A Randomized, Double-Blind, Placebo-Controlled Study of Intrathecal Ziconotide in Adults with Severe Chronic Pain. J Pain Symptom Manage. 2006;31:393–406. doi: 10.1016/j.jpainsymman.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Ellis ES, Harries MH, et al. Rank-order inhibition by omega-conotoxins in human and animal autonomic nerve preparations. Eur J Pharmacol. 2000;388:89–95. doi: 10.1016/s0014-2999(99)00830-4. [DOI] [PubMed] [Google Scholar]
- Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–59. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- Sato K, Raymond C, Martin-Moutot N, et al. Binding of chimeric analogs of omega-conotoxin MVIIA and MVIIC to the N- and P/Q-type calcium channels. FEBS Lett. 1997;414:480–4. doi: 10.1016/s0014-5793(97)01056-9. [DOI] [PubMed] [Google Scholar]
- Sato K, Raymond C, Martin-Moutot N, et al. Binding of six chimeric analogs of omega-conotoxin MVIIA and MVIIC to N- and P/Q-type calcium channels. Biochem Biophys Res Commun. 2000;269:254–6. doi: 10.1006/bbrc.2000.2284. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–74. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur J Pharmacol. 2002;451:279–86. doi: 10.1016/s0014-2999(02)02247-1. [DOI] [PubMed] [Google Scholar]
- Sher E, Giovannini F, Codignola A, et al. Voltage-operated calcium channel heterogeneity in pancreatic beta cells:physiopathological implications. J Bioenerg Biomembr. 2003;35:687–96. doi: 10.1023/b:jobb.0000008032.49504.48. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J Pharmacol Exp Ther. 1998;287:232–7. [PubMed] [Google Scholar]
- Smith MT, Cabot PJ, Ross FB, et al. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–27. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- Snutch TP. N-type channels, small organic molecules and pain. Spring Pain Research Conference; Grand Cayman, BWI. 2004. [Google Scholar]
- Snutch TP, Feng ZP, Belardetti F, et al. Novel N-type calcium channel blockers efficacious in animal models of chronic pain. 226th American Chemical Society National Meeting; New York, NY. 2003. [Google Scholar]
- Staats P. Ziconotide:a viewpoint by peter staats. CNS Drugs. 2006;20:339–41. [Google Scholar]
- Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS:a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- Stoehr SJ, Dooley DJ. Characteristics of [125I]omega-conotoxin MVIIA binding to rat neocortical membranes. Neurosci Lett. 1993;161:113–6. doi: 10.1016/0304-3940(93)90153-c. [DOI] [PubMed] [Google Scholar]
- Takahara A, Koganei H, Takeda T, et al. Antisympathetic and hemodynamic property of a dual L/N-type Ca(2+) channel blocker cilnidipine in rats. Eur J Pharmacol. 2002;434:43–7. doi: 10.1016/s0014-2999(01)01521-7. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ito M, Miyamoto N, et al. Increased glucose tolerance in N-type Ca2+ channel alpha(1B)-subunit gene-deficient mice. Int J Mol Med. 2005;15:937–44. [PubMed] [Google Scholar]
- Urban MO, Ren K, Sablad M, et al. Medullary N-type and P/Q-type calcium channels contribute to neuropathy-induced allodynia. Neuroreport. 2005;16:563–6. doi: 10.1097/00001756-200504250-00009. [DOI] [PubMed] [Google Scholar]
- Wallace MS, Charapata SG, Fisher R, et al. Intrathecal Ziconotide in the Treatment of Chronic Nonmalignant Pain:A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Neuromodulation. 2006;9:75–86. doi: 10.1111/j.1525-1403.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Wang YX, Bezprozvannaya S, Bowersox SS, et al. Peripheral versus central potencies of N-type voltage-sensitive calcium channel blockers. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:159–68. doi: 10.1007/pl00005150. [DOI] [PubMed] [Google Scholar]
- Wang YX, Gao D, Pettus M, et al. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain. 2000a;84:271–81. doi: 10.1016/s0304-3959(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Wang YX, Pettus M, Gao D, et al. Effects of intrathecal administration of ziconotide, a selective neuronal N-type calcium channel blocker, on mechanical allodynia and heat hyperalgesia in a rat model of postoperative pain. Pain. 2000b;84:151–8. doi: 10.1016/s0304-3959(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily:a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- Wen L, Yang S, Qiao H, et al. SO-3, a new O-superfamily conopeptide derived from Conus striatus, selectively inhibits N-type calcium currents in cultured hippocampal neurons. Br J Pharmacol. 2005;145:728–39. doi: 10.1038/sj.bjp.0706223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermeling DP, Berger JR. Ziconotide infusion for severe chronic pain:case series of patients with neuropathic pain. Pharmacotherapy. 2006;26:395–402. doi: 10.1592/phco.26.3.395. [DOI] [PubMed] [Google Scholar]
- Wermeling D, Drass M, Ellis D, et al. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J Clin Pharmacol. 2003;43:624–36. [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–30. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–11. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Winquist RJ, Pan JQ, Gribkoff VK. Use-dependent blockade of Cav2.2 voltage-gated calcium channels for neuropathic pain. Biochem Pharmacol. 2005;70:489–99. doi: 10.1016/j.bcp.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Wright CE, Robertson AD, Whorlow SL, et al. Cardiovascular and autonomic effects of omega-conotoxins MVIIA and CVID in conscious rabbits and isolated tissue assays. Br J Pharmacol. 2000;131:1325–36. doi: 10.1038/sj.bjp.0703701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Synthetic omega-conopeptides applied to the site of nerve injury suppress neuropathic pains in rats. J Pharmacol Exp Ther. 1995;274:666–72. [PubMed] [Google Scholar]
- Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N- and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998;794:329–32. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Kurihara T, Makita K, et al. Plastic change of N-type Ca channel expression after preconditioning is responsible for prostaglandin E2-induced long-lasting allodynia. Anesthesiology. 2003;99:1364–70. doi: 10.1097/00000542-200312000-00019. [DOI] [PubMed] [Google Scholar]
- Zamponi GW. Regulation of presynaptic calcium channels by synaptic proteins. J Pharmacol Sci. 2003;92:79–83. doi: 10.1254/jphs.92.79. [DOI] [PubMed] [Google Scholar]
- Zhong H, Yokoyama CT, Scheuer T, et al. Reciprocal regulation of P/Q-type Ca2+ channels by SNAP-25, syntaxin and synaptotagmin. Nat Neurosci. 1999;2:939–41. doi: 10.1038/14721. [DOI] [PubMed] [Google Scholar]