Abstract
Objective
To review the pharmacokinetics, efficacy, safety, and cost-effectiveness of long-acting risperidone.
Methods
Studies published between January 2000 and October 2006 evaluating the pharmacokinetics, efficacy, safety, and cost-effectiveness of long-acting risperidone were reviewed, as identified from literature searches using Medline and EMBASE. Abstracts and posters on long-acting risperidone presented at key psychiatry congresses and available in the public domain during this time period were also reviewed.
Results
The unique pharmacokinetic profile of long-acting risperidone is derived from the encapsulation of risperidone in a glycolide/lactide matrix in the form of microspheres such that after a single intramuscular injection, significant plasma levels of the drug are achieved after week 3. Steady state, after repeated administration at 2-week intervals, is achieved after 3 injection cycles. Short- and long-term studies have demonstrated that long-acting risperidone (25, 37.5, or 50 mg) is both efficacious and well tolerated in a wide variety of patients with schizophrenia and related psychoses. Most patients can be switched from other oral and long-acting antipsychotic agents without compromising efficacy and safety. Long-acting risperidone may also reduce overall healthcare costs by decreasing rates of relapse and hospitalization.
Conclusion
The assured delivery of an atypical antipsychotic medication with long-acting risperidone has important implications for patient compliance, maintenance of stability, consistency of treatment, and improving patient outcomes including the achievement of remission.
Keywords: long-acting injectable risperidone, efficacy, safety, pharmacokinetics, pharmacoeconomics, schizophrenia
Introduction
The long-acting injectable antipsychotics were developed in the 1960s as an additional method of drug delivery aimed specifically at improving treatment compliance in patients with schizophrenia, as well as simplifying the medication process. Long-acting antipsychotics are convenient for the patients and their families, in that patients no longer have the burden of remembering to take daily medication, and families no longer have to regularly monitor and remind their family member about their medication. Moreover, from the clinician’s point of view, a critical advantage of long-acting antipsychotics is that if a patient does become non-compliant, the clinical team will know immediately (because an injection has been missed) and will be able to initiate efforts to deal effectively with the problem before symptoms reappear (Valenstein et al 2001). Long-acting injectable antipsychotics are not associated with “first-pass metabolism” and can be adjusted more reliably to the lowest effective dose, thereby further reducing the risk of adverse effects (Ereshefsky et al 1984; Ereshefsky and Mascarenas 2003). A less well recognized benefit of long-acting therapy is the regular contact between patients and treatment teams, which provides the opportunity for greater therapeutic alliance and psychosocial interventions. Consistent with this, data from a number of individual trials and meta-analyses have shown that, compared with oral antipsychotics, long-acting antipsychotics reduce relapse frequency and rehospitalization rates (Davis et al 1994; Gerlach 1994) and lower annual treatment costs (Glazer and Ereshefsky 1996). However, despite the attractiveness of this treatment option, the use of long-acting antipsychotics has fallen in recent years particularly in North America, likely as the result of the introduction and widespread use of the oral atypical antipsychotics.
The development of the atypical antipsychotic agents over the last decade was a major step forward in the pharmacological management of schizophrenia, with numerous studies demonstrating that atypical agents improve negative symptoms (Lindenmayer et al 1994; Marder and Meibach 1994; Boyer et al 1995; Beasley et al 1996) and cognitive function (Meyer-Lindenberg et al 1997; Keefe et al 1999; Bilder et al 2002; Barkic et al 2003) and exert a beneficial effect on affective symptoms (Tollefson et al 1998; Peuskens et al 2000, 2002; Buckley 2004). In addition, the atypical agents have also been shown to enhance quality of life, functional status, and patient satisfaction compared with conventional antipsychotics (Meltzer 1990; Franz et al 1997; Revicki et al 1999; Colonna et al 2000; Hamilton et al 2000). This is of particular relevance since patient satisfaction has been recognized as an important determinant of treatment success (Kane 2001; Lambert and Naber 2004). Furthermore, a number of meta-analyses have demonstrated that, compared with conventional agents, the use of atypical antipsychotics is associated with a lower incidence of extrapyramidal side effects (EPS) (Leucht et al 1999; Geddes et al 2000; Bagnall et al 2003). However, despite their identified benefits, the issue of non- and partial compliance with therapy has not been fully resolved with the newer atypical agents. More than 35% of patients begin to demonstrate compliance problems during their first 4–6 weeks of treatment and by 2 years, 75% are partially compliant (Weiden and Zygmunt 1997). Moreover, results from a study by Dolder et al, which compared compliance rates with oral atypical vs oral conventional antipsychotics, reported similar, low levels of compliance in both groups after 12 months (54.9±26.0% vs 50.1±30.6%; p=0.11) (Dolder et al 2002).
It is only recently, with the introduction of long-acting risperidone launched in North America in 2004, that clinicians have had access to a long-acting atypical antipsychotic. Since an earlier study reported that psychiatrists would be persuaded to use these agents if any atypical long-acting antipsychotics were available (Patel et al 2003), the introduction of long-acting risperidone raises new questions regarding the treatment of patients with schizophrenia. The aim of this review is to provide an evaluation of the pharmacokinetics, efficacy, safety and cost-effectiveness of long-acting risperidone for the treatment of schizophrenia and schizoaffective disorder.
Methods
A literature search was performed in two parts. Firstly, an electronic search of English language articles published between January 2000 and October 2006 that evaluated the pharmacokinetics, efficacy, safety, and cost-effectiveness of long-acting risperidone for the treatment of schizophrenia and schizoaffective disorder was performed using Medline and EMBASE. The primary search parameters were “long-acting injectable risperidone”, “schizophrenia”, “schizoaffective disorder”, “efficacy”, “tolerability”, “safety”, “quality of life”, “patient satisfaction”, “pharmacokinetics”, cost-effectiveness”, and “pharmacoeconomic”. Original research articles, reviews, and other articles of interest were reviewed. Secondly, abstracts and posters on long-acting risperidone presented at key psychiatry and schizophrenia congresses during this period were also reviewed, where available in the public domain.
Results
Overview of pharmacokinetic properties
Technology
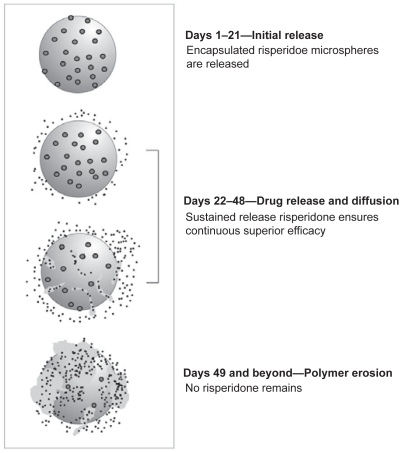
Long-acting risperidone is synthesized by a microsphere encapsulation process using static flow methods to incorporate risperidone inside a glycolide/lactide matrix, a commonly used medical polymer. In vitro studies have demonstrated that a small amount of risperidone at the surface of the microspheres is released by diffusion within 24 hours. This is followed by a latent period of approximately 3 weeks, while the majority of the release occurs by erosion of the glycolic acid-lacate polymer during weeks 4–6 (Ramstack et al 2003). Following administration, the copolymer gradually breaks down in the body, steadily releasing risperidone at a constant rate (Ramstack et al 2003; Eerdekens et al 2004). The final end products of long-acting risperidone are risperidone and naturally occurring glycolic and lactic acids, which are further metabolized to carbon dioxide and water (Nordstrom et al 1993). It is theorized that the sustained release of risperidone from the microspheres results in consistent and continuous antipsychotic coverage by reduction of the peaks and troughs observed with oral medication (Eerdekens et al 2004). The erosion of the microsphere with subsequent dispersion of risperidone is illustrated in Figure 1.
Figure 1.
The dispersion of risperidone from microspheres. Reproduced with permission of Wolters Kluwer Health from Ereshefsky L, Mannaert E. 2005. Pharmacokinetic profile and clinical efficacy of long-acting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs R D, 6:129–37. Figure 1 from page 131.
Pharmacokinetic profile
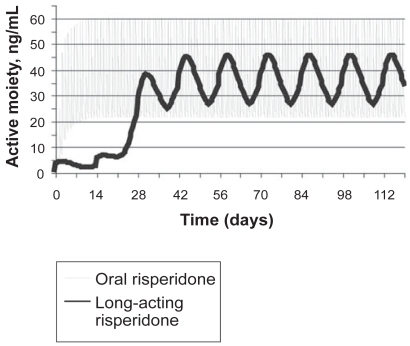
The release profile of risperidone microspheres in vitro has been confirmed in a number of clinical studies. Risperidone is metabolized principally by the cytochrome P450 2D6 (CYP) enzyme to the active metabolite 9-OH-risperidone with the sum of the two being frequently modeled for pharmacokinetic purposes and labelled the “active moiety”. The results of pharmacokinetic dose trials support the administration of long-acting risperidone every 2 weeks to maintain plasma levels of active moiety comparable to levels obtained with repeated oral dosing.
Single-dose studies have demonstrated that, starting from about week 3 after the injection, plasma levels of the active moiety gradually increase, peaking at 4–5 weeks and lasting approximately 7 weeks (Ereshefsky and Lacombe 1993). With repeated injections, steady-state levels are usually reached by 6–8 weeks from the start of therapy, with significantly reduced peak-trough fluctuations as compared with oral dosing (Figure 2). Oral risperidone is not required past the initial stabilization phase. The pharmacokinetic profile of long-acting risperidone has also been evaluated in multidose studies. Results from an open-label, non-randomized, Phase I study in 13 stable outpatients with schizophrenia who received long-acting risperidone 25, 50, or 75 mg once every 2 weeks for 5 injections demonstrated that stable plasma concentrations were reached after the third injection, maintained for 4–5 weeks and then declined rapidly with a half-life of 4–6 days (Gefvert et al 2005). An additional 15-week, open-label study was conducted to evaluate the pharmacokinetics of multiple doses of long-acting risperidone in 86 stable patients. Patients stabilized on 2, 4, or 6 mg/day of oral risperidone once-daily for at least 4 weeks were assigned to receive 25, 50, or 75 mg of long-acting risperidone respectively, every 2 weeks for 10 weeks (Eerdekens et al 2004). The 90% confidence intervals for the long-acting/oral ratios of the mean steady-state plasma area under the curve to day 14 demonstrated that they were within the range of 80%–125%, indicating bioequivalence between the two formulations. However, mean steady-state peak concentrations of the active moiety were 25%–32% lower with long-acting risperidone than oral dosing (p<0.05), and fluctuations in plasma active moiety levels were substantially lower with long-acting risperidone (range 56%–71%) compared with oral treatment (118%–129%). In addition, both a clinical improvement in symptom severity and a decrease in extrayramidal symptoms (EPS), as assessed by the Extrapyramidal Symptom Rating Scale (ESRS) were observed (Eerdekens et al 2004).
Figure 2.
Schematic representation of plasma concentrations following administration of oral risperidone and long-acting risperidone
Dopamine D2-receptor occupancy
Dopamine D2-receptor occupancy is an important consideration in determining efficacy and tolerability. Previous positron emission tomography (PET) studies have indicated that optimal clinical response occurs when at least 65% of striatal D2 receptors are occupied, while the risk of EPS increases notably at D2-receptor occupancy levels above 80% (Kapur et al 1999, 2000). To date, available PET data have suggested that dosing long-acting risperidone at 25–50 mg every 2 weeks is sufficient in attaining clinical response with minimal risk of EPS (Farde et al 2002; Gefvert et al 2005; Remington et al 2006).
One such PET study evaluated D2-receptor occupancy for long-acting risperidone at doses of 25, 50, or 75 mg administered every 2 weeks in 9 patients with schizophrenia or schizoaffective disorder (Remington et al 2006). Patients were scanned twice during the 2-week injection interval: within 3 days after injection (post-injection) and within 5 days before the next injection (pre-injection). Mean post-and pre-injection D2-receptor occupancy levels for the 25, 50, and 75 mg increased in a dose-dependent fashion and were 71.0% and 54.0%, 74.4%, and 65.4%, and 81.5% and 75.0%, respectively. Although all three doses of long-acting risperidone demonstrated peak D2-receptor occupancy levels above the 65% threshold associated with optimal clinical response, only the 75-mg dose approximated the 80% threshold linked to increased risk of EPS (Remington et al 2006). A PET study was undertaken by Gefvert et al to measure D2-receptor occupancy at steady state in 8 patients treated with doses of 25 (n=3), 50 (n=3), or 75 mg (n=2) of long-acting risperidone every 2 weeks (Gefvert et al 2005). Dose-proportional individual D2-receptor occupancy was 25%–48% in the 25-mg group, 59%–83% in the 50-mg group, and 62%–72% in the 75-mg group. The ranges of active moiety concentration were 4.4–8.8 ng/mL, 15.0–31.2 ng/mL, and 22.5–26.3 ng/mL for the three dosages, respectively. Although the D2-receptor occupancy at the 25-mg dose seems somewhat lower than what is considered necessary for an antipsychotic effect, it should be taken into account that the dopamine D2-receptor occupancies in this small trial were trough levels and do not exclude higher occupancies at peak plasma concentrations (Gefvert et al 2005). However, Farde et al estimated individual D2-receptor occupancy values of long-acting risperidone at both trough and peak concentrations in a large Phase III clinical trial by using plasma concentration and receptor binding data from the study by Gefvert et al (Farde et al 2002). Results suggested that most patients who responded well to the 25 mg dose had D2-receptor occupancy values of 50–70% at trough, shifting to values above 70% at peak. Patients in the 50 mg group had simulated D2-receptor occupancies covering the target range of 70–80%, while patients receiving the 75 mg dose had D2-receptor occupancies that were higher than desirable (57% of patients >80% D2-receptor occupancy) with no added clinical benefit (Farde et al 2002).
Clinical efficacy of long-acting risperidone
Long-acting risperidone is available in dosage strengths of 25, 37.5, or 50 mg. Although not commercially available, several pivotal studies have also investigated the use of a 75-mg dose. As such, results pertaining to the 75-mg dose are also discussed here. The short-term (vs placebo) and long-term clinical benefits of long-acting risperidone were explored in two large clinical trials, in which long-acting risperidone was administered by intramuscular injection at doses of 25, 50, and 75 mg every 2 weeks (Fleischhacker et al 2003; Kane et al 2003). A more recent study has also investigated the use of a 37.5-mg dose (Möller et al 2005).
Pivotal and switch studies with long-acting risperidone
In a 12-week, randomized, placebo-controlled trial by Kane et al, long-acting risperidone was associated with significantly greater improvements in Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression (CGI)-severity scores at endpoint compared with placebo (p<0.001) (Kane et al 2003). Significant improvements in total PANSS scores (p<0.01), positive symptoms (p<0.01), and negative symptoms (p<0.001) were also observed during an international, open-label, 1-year study conducted by Fleischhacker et al in patients with schizophrenia who were switched to long-acting risperidone from oral or long-acting conventional or oral atypical antipsychotics (Fleischhacker et al 2003). Results from a number of clinical studies and sub-analyses of the 1-year trial have also reported significant and sustained clinical improvement in patients switched, directly or indirectly, to long-acting risperidone from oral and long-acting antipsychotic agents. Overall, with the exception of the 4-year open-label extension study by Kushner et al, results from the pivotal studies reported continuation rates with long-acting risperidone (51%–92%) (Chue et al 2005a; Fleischhacker et al 2003; Kane et al 2003; Kushner et al 2004; Lindenmayer et al 2004; Turner et al 2004; Emsley et al 2005; Kissling et al 2005; Möller et al 2005). Furthermore, across the pivotal studies, withdrawals due to insufficient response (range 1.2%–12.2%) and lack of compliance (range 1.3%–5%) with long-acting risperidone were low. An overview of pivotal studies and sub-analyses that have evaluated the efficacy of long-acting risperidone in patients with schizophrenia and schizoaffective disorder is summarized in Table 1. It is recommended that the original studies be reviewed given the different designs, durations, and objectives of these studies. For example in the studies by Chue et al (2005a) and Fleischhacker et al (2003), the dose of long-acting risperidone was based upon clinical judgement thus, more severe patients tended to receive the higher dose potentially accounting for treatment failures at this dose. Furthermore, since the benefit of a long-acting antipsychotic is accrued in the longer-term (Gastpar et al 2005; Turner et al 2004; Lee et al 2006; Lindemayer et al 2006,), this may not be apparent initially in short-term studies especially when compared to patients switched from active treatment to placebo who can show a relative improvement to begin with that may be associated, for example, with the lack of medication side effects (Kane et al 2003). Switching antipsychotics is not without its complexities (Remington et al 2004) and the unique pharmacokinetics of long-acting risperidone may influence the outcome in short-term studies involving switching in stable patients (Lindenmayer et al 2004), or in patients that were previously on high dose or combination antipsychotic therapies (Kane et al 2003).
Table 1.
Overview of pivotal studies and sub-analyses evaluating the efficacy and safety of long-acting risperidone in patients with schizophrenia or schizoaffective disorder
| Study details | Treatment groups | Efficacy | Adverse events |
|---|---|---|---|
| Short-term studies | |||
Kane et al 2003
|
Placebo (n=98)
LAR doses included: 25 mg (n=99) 50 mg (n=103) 75 mg (n=100) |
|
|
Lauriello et al 2005
|
LAR 25 mg (n=52)
LAR 50 mg (n=57) LAR 75 mg (n=52) Placebo (n=53) |
|
|
Lindenmayer et al 2005
|
LAR 25 mg (n=52)
LAR 50 mg (n=57) LAR 75 mg (n=52) Placebo (n=53) |
Data previously presented by Kane et al 2003 |
|
Lindenmayer et al 2004
|
LAR 25 mg, 37.5 mg, and 50 mg (n=141) |
|
|
Turner et al 2004
|
LAR 25 mg, 37.5 mg, and 50 mg (n=166) |
|
|
Chue et al 2005
|
Oral risperidone 2, 4, and 6 mg/day (n=321)
LAR 25, 50, and 75 mg (n=319) |
|
|
| Long-term studies | |||
Rodriguez et al 2005
|
LAR 25 mg and 50 mg (n=323) |
|
|
Möller et al 2005
|
LAR 25 mg, 37.5 mg, and 50 mg (n=1876) |
|
|
Vauth et al 2004
|
LAR 25, 37.5, and 50 mg (n=119) |
|
|
Gastpar et al 2005
|
LAR 25, 37.5, and 60 mg (n=192) |
|
|
Kissling et al 2005
|
LAR 25, 37.5, and 50 mg (n=715) |
|
|
Fleischhacker et al 2003
|
LAR 25 mg (n=120)
LAR 50 mg (n=228) LAR 75 mg (n=267) |
|
|
van Os et al 2004
|
LAR 25 mg (n=18)
LAR 50 mg (n=16) LAR 75 mg (n=12) |
|
|
Lasser et al 2004a
|
LAR 25 mg (n=35)
LAR 50 mg (n=80) LAR 75 mg (n=73) |
|
|
Lasser et al 2005a
|
LAR 25 mg (n=79)
LAR 50 mg (n=125) LAR 75 mg (n=132) |
|
|
Gharabawi et al 2005
|
LAR 25, 50, and 75 mg (n=662) | Data previously presented by Fleischhacker et al 2003 |
|
Lindenmayer et al 2005
|
LAR 25 mg (n=120)
LAR 50 mg (n=228) LAR 75 mg (n=267) |
Data previously presented by Fleischhacker et al 2003 |
|
Lasser et al 2005b
|
LAR 25, 50, and 75 mg (n=578) |
|
|
Kushner et al 2004
|
LAR 25, 50, and 75 mg (n=271) |
|
|
Abbreviations: AE, adverse event; ANCOVA, Analysis of Covariance; BMI, body mass index; CGI-S, Clinical Global Impression-Severity; DAI, Drug Attitude Inventory; EPS, extrapyramidal symptoms; ESRS, Extrapyramidal Symptoms Rating Scale; GAF, Global Assessment of Function; HRQoL, health-related quality of life; LAR, long-acting risperidone; PANSS, Positive and Negative Syndrome Scale for Schizophrenia; SD, standard deviation; SE, standard error; SF-36, Short Form-36; VAS, visual analogue scale.
Long-acting conventional antipsychotic and long-acting risperidone studies
Few studies have examined the efficacy of long-acting risperidone compared with a long-acting conventional agent. This likely due to the fact that randomization to conventional depot is no longer considered a standard of treatment for patients with schizophrenia, and such a study would not be acceptable to the majority of research ethics boards (certainly in North America and Canada) (Quraishi and David 2000). Thus, the two studies that have examined long-acting injectable treatments have been open-label comparisons in special populations. In the first 6-month study, 115 patients fulfilling the criteria for schizophrenia and substance abuse disorder were allocated to receive either long-acting risperidone (n=57) or long-acting zuclopenthixol (n=58). Overall, long-acting risperidone (mean dose 47.2 mg q15 days and 3.4 mg oral risperidone) was shown to be more effective than long-acting zuclopenthixol (mean dose 200 mg q21 days and 15 mg oral zuclopenthixol) in improving the symptoms of schizophrenia (mean [±SD] PANSS total scores improved from 93.79±22.9 at baseline to 64.39±19.9 at endpoint for the long-acting risperidone group vs 93.69±22.5 at baseline to 74.03±20.9 for the long-acting zuclopenthixol group). Long-acting risperidone was also more effective in treating substance abuse (mean [±SD] number of total positive tests for substance abuse: long-acting risperidone 8.67±3.0 vs 10.36±3.1 for long-acting zuclopenthixol, p=0.005). More patients receiving long-acting risperidone attended more than 75% of addiction counselling sessions compared with patients receiving long-acting zuclopenthixol (92.9% vs 67.8%, p=0.001) (Rubio et al 2006). In the second study of chronic hospitalized patients with schizophrenia followed up for one year, of 40 patients treated with long-acting risperidone, 83% were discharged and none readmitted. In contrast, of 54 patients treated with conventional depot, 58% were discharged and 26% re-admitted (Snaterse et al 2005).
Oral atypical antipsychotics and long-acting risperidone studies
The large and often contradictory literature examining the efficacy of oral atypical antipsychotics in head-to-head comparisons illustrates the difficulty in demonstrating substantial differences between efficacious agents particularly when dosing and titration are controlled. A non-inferiority analysis of oral risperidone and long-acting risperidone found that long-acting risperidone was as efficacious and as well tolerated as oral risperidone in a 20-week randomized, double-blind, double-dummy study (Chue et al 2005a). It is known from earlier studies with conventional antipsychotics comparing a long-acting injection to its oral equivalent, that benefits secondary to improved compliance are demonstrated with greater duration of study and maintenance of adequate serum levels (Jayaram et al 1986). Since non-compliant patients, although representing a significant proportion of real-world patients with schizophrenia and other psychoses, are rarely recruited to clinical trials, this poses problems in demonstrating the benefit of a treatment that is likely to be advantageous in that very population. The only other study presented to date comparing long-acting risperidone injection to an oral atypical antipsychotic is being conducted in first-episode patients. Malla et al have shown preliminary data on a 24-month, prospective, open-label, multi-center study in young adults (aged 18–30 years) with recent onset schizophreniform disorder, schizophrenia, and schizoaffective disorder (≤3 years) (Malla et al 2006). The effectiveness data set is very small (n=15), but mean changes in CGI-S (±SD) and total PANSS scores for long-acting risperidone were −0.7±1.3 and −16.1±16.2 respectively, and for oral atypical antipsychotics were −0.2± 0.8 and −5.0±12.9 respectively.
Achieving and maintaining remission in schizophrenia
Recently, operational criteria for remission in schizophrenia have been proposed by the Remission in Schizophrenia Working Group (Andreasen et al 2005). The consensus definition of remission was defined as achieving a score ≤3 (mild or less) simultaneously in eight core PANSS items: delusions [P1], conceptual disorganization [P2], hallucinations [P3], unusual thought content [G9], mannerisms and posturing [G5], blunted affect [N1], passive/apathetic social withdrawal [N4], and lack of spontaneity and flow of conversation [N6], sustained for a minimum duration of 6 months (Andreasen et al 2005).
To date, the consensus remission criteria have been retrospectively applied to two clinical studies, which primarily evaluated the efficacy of long-acting risperidone (Table 1) (Kissling et al 2005; Lasser et al 2005b). The first was a sub-analysis of the 1-year trial by Fleischhacker et al (2003) in patients with schizophrenia who were switched to long-acting risperidone from oral or long-acting conventional or oral atypical antipsychotics. Although all patients in this study were considered clinically “stable” at baseline, 68.2% (394/578) did not meet the symptom-severity component of remission at baseline (Lasser et al 2005b). Following treatment with long-acting risperidone, 20.8% (n=82) of non-remitted patients achieved symptom remission for at least 6 months, with significant decreases in mean PANSS total and subscale scores (p<0.0001). Finally, out of the 184 patients who met the symptom-severity component of the remission criteria at baseline, 84.8% (n=156) maintained their remitted status after 1 year of treatment (Lasser et al 2005b).
The second study assessed data from an extension phase of a 6-month trial in 715 stable psychotic patients who were transitioned directly from their previous antipsychotic regimen to long-acting risperidone (25, 37.5, or 50 mg), without an oral risperidone run-in (Kissling et al 2005). The proportion of patients who met the PANSS severity criteria for remission increased from 209 (29%) at baseline to 429 (60%) at endpoint. Among those patients who met the severity criteria at study entry, 84% met them at endpoint. Furthermore, 79% of those patients met both the severity and the duration criteria for remission at endpoint. Of the 506 patients who did not meet the severity criteria at baseline, 50% did so at endpoint. At 12 months 31% of those patients met both the severity and duration criteria and were therefore considered to be in remission (Kissling et al 2005).
Health-related quality of life, patient satisfaction and functioning
The concept of health-related quality of life (HRQoL) has not been a treatment outcome focused upon in studies until the last few years. Nonetheless, when patients are asked directly what is important to them in terms of treatment, it is in fact such measures as improvement in overall happiness, mental health and functionality (Hufnagel et al 2004). These patient benefits are not easily shown through standard studies conducted in the context of drug registration and approval, which are designed with very specific efficacy and tolerability perspectives.
Results from the 6-month study by Möller et al (2005) and a number of its sub-analyses have demonstrated that patients experience significant improvements in HRQoL and patient satisfaction following treatment with long-acting risperidone (Table 1). In addition, a number of studies have been specifically undertaken to examine the effect of long-acting risperidone on QoL and social functioning. A post-hoc analysis by Nasrallah et al measured HRQoL using the SF-36 scale in patients who participated in the 12-week study by Kane et al (2003) (Nasrallah et al 2004). At week 12, patients receiving long-acting risperidone 25 mg and 50 mg had improved significantly in five of the eight domains of the SF-36 (bodily pain, general health, social functioning, role-emotional, and mental health) compared with patients receiving placebo. No significant differences in seven of the eight measures were observed between the long-acting risperidone 25-mg subject group and US normal population scores (individuals aged 35–44 years). In contrast, subjects in the placebo group had a significantly poorer QoL compared with the US normal population in all measures (p<0.01). Similarly, a sub-analysis of the data from the 1-year study by Fleischhacker et al also found that long-acting risperidone resulted in a significant improvement on the SF-36 Mental Component Summary score and on the vitality and social functioning scales (Fleischhacker et al 2005). Recently a 1-year, randomized, double-blind, multicenter study was undertaken to examine the effects of long-acting risperidone on social functioning using the Personal and Social Performance (PSP) Scale (Rodriguez et al 2005). The PSP scale provides a clinician rating of personal and social functioning on a 100-point scale based on the assessment of patient’s functioning in four important domains: a) socially useful activities, including work and study; (b) personal and social relationships; (c) self-care; and (d) disturbing and aggressive behaviors (Morosini 2000). Results demonstrated significant improvements in the Personal and Social Performance Scale (PSP) scores from baseline to endpoint (p=0.003). In addition, assessments on the Strauss-Carpenter Level of Functioning Scale (LOF) indicated that patients had a greater frequency of social contacts, increased quality of relationships, and a improved overall level of function during maintenance treatment with long-acting risperidone.
Safety and tolerability of long-acting risperidone
On the basis of the results from the studies by Kane et al and Fleischhacker et al, long-acting risperidone generally appears to be well tolerated, with an overall incidence of adverse events similar to that with placebo in comparative trials. In the study by Kane et al, similar proportions of patients in the placebo and long-acting groups (80%–83%) reported adverse events, while serious adverse events were more frequent in the placebo group (23.5%) than in the 25, 50, and 75 mg long-acting risperidone groups (13%, 14%, and 15%, respectively) (Kane et al 2003). Likewise long-acting risperidone was also well tolerated in the long-term study by Fleischhacker et al with the percentage of patients reporting adverse events declining from 68% during months 1–3 of the study to 43% during months 10–12 (Fleischhacker et al 2003). Results from a number of studies have also reported that patients can be safely transitioned from oral conventional or atypical therapy and conventional long-acting agents to long-acting risperidone (Lasser et al 2004a; Lindenmayer et al 2004; Turner et al 2004; van Os et al 2004). Furthermore, one such study by Möller et al demonstrated that switching from existing antipsychotic therapy to long-acting risperidone, without an oral risperidone run-in, was well tolerated in a large cohort of patients with schizophrenia or other psychotic disorders (Möller et al 2005). Several studies have also reported that patients considered symptomatically stable on oral risperidone can be safely switched to long-acting risperidone (Chue et al 2005a; Lasser et al 2005a), thereby contradicting the perception of some clinicians and patients that adverse events are more common with long-acting than with oral formulations.
Overall, relatively few patients have withdrawn in studies with long-acting risperidone due to adverse events (range 1.2%–16%) (Fleischhacker et al 2003; Kane et al 2003; Kushner et al 2004; Lindenmayer et al 2004; Turner et al 2004; Chue et al 2005a; Lasser et al 2005a; Möller et al 2005; Emsley 2006). The most common adverse events reported with long-acting risperidone were headache (range 7%–28%), insomnia (range 7%–28%), anxiety (range 7%–24%), and psychosis (range 5%–31%). In the majority of studies the severity of movement disorders, as assessed by the ESRS, was unchanged or further reduced during treatment with long-acting risperidone. In addition, a sub-analysis of the 1-year study demonstrated that long-acting risperidone is associated with a low incidence of treatment-emergent dyskinesia (1.2% annually) (Gharabawi et al 2005). Three studies, which examined the effect of long-acting risperidone on serum prolactin levels, reported rates of hyperprolactinemia of 1.3%–7% (Lindenmayer et al 2004; Turner et al 2004; Chue et al 2005a).
Metabolic side-effects in patients treated with atypical antipsychotic agents are increasingly receiving more attention in the literature, with recent evidence suggesting that some atypical antipsychotics may increase risk factors for diabetes and cardiovascular disease, including increased adiposity and adverse effects on glucose and lipid metabolism (ADA 2004; Citrome 2004). On the basis of the limited evidence presented here from the pivotal trials, weight gain with long-acting risperidone was low, being in the range of 0.5–2 kg in the short-term (12 weeks) and around 3 kg after 1 year of treatment, with no further weight gain apparent in patients receiving long-acting risperidone for up to 4 years (Kushner et al 2004). In addition, results from a short-term study demonstrated that serum glucose and triglyceride levels were reducing during treatment with long-acting risperidone (Lindenmayer et al 2004), while a further study reported a low occurrence (0.3%) of glucose-related adverse events over 6 months (Möller et al 2005).
Finally, in the patient populations studied, including antipsychotic injection-naive patients, the perception of pain at the injection site was rated as mild and decreased over time (Fleischhacker et al 2003; Kane et al 2003; Lindenmayer et al 2004; Turner et al 2004; Chue et al 2005a; Lindenmayer et al 2005). It is postulated that the low incidence of injection site pain could be related to the fact Finally, in the patient populations studied, including antipsychotic injection-naive patients, the perception of pain at the injection site was rated as mild and decreased over time (Fleischhacker et al 2003; Kane et al 2003; Lindenmayer et al 2004; Turner et al 2004; Chue et al 2005a; Lindenmayer et al 2005). It is postulated that the low incidence of injection site pain could be related to the fact that long-acting risperidone is an isotonic water-based suspension has been shown to be easier and less painful to administer than the oil-based solutions of the conventional long-acting antipsychotic agents (Bloch et al 2001). A full overview of the safety data for long-acting risperidone is also shown in Table 1.
Efficacy and safety of long-acting risperidone in special populations
Certain populations such as the elderly, young adults or those with a first episode of schizophrenia, patients with schizoaffective disorder and patients of different ethnicity require special consideration when selecting pharmacotherapy. A number of studies have demonstrated that long-acting risperidone is both effective and well tolerated in these populations (Table 2). However, it is important to note that these results are based solely on sub-analyses and interim analyses and, as such, further controlled studies of long-acting risperidone in these vulnerable patient groups are warranted.
Table 2.
Overview of clinical studies examining the efficacy and safety of long-acting injectable risperidone in special patient populations with schizophrenia
| Study details | Treatment groups | Efficacy | Adverse events |
|---|---|---|---|
| Elderly patients | |||
Lasser et al 2004c
|
LAR 25 mg, 50 mg, and 75 mg (n=57) |
|
|
| Young and first-episode patients | |||
Emsley et al 2005
|
LAR 25 mg and 50 mg (n=20) |
|
|
Emsley et al 2006
|
LAR 25 mg and 50 mg (n=51) |
|
|
Lasser et al 2004d
|
LAR 25, 50, and 75 mg (n=100) |
|
|
Saleem et al 2004
|
LAR 25, 37.5, and 50 mg (n=119) |
|
|
Parellada et al 2005
|
LAR 25, 37.5, and 50 mg (n=382) |
|
|
| Schizoaffective disorder | |||
Mohl et al 2005
|
LAR 25, 37.5, and 50 mg (n=249) |
|
|
Lasser et al 2004b
|
LAR 25 mg (n=27)
LAR 50 mg (n=42) LAR 75 mg (n=41) |
|
|
| Obese patients | |||
Teijeiro et al 2004
|
LAR 25, 37.5, and 50 mg (n=119) |
|
|
| Patients of different ethnicity | |||
Ciliberto et al 2005
|
LAR 25, 50, and 75 mg (n=439) |
|
|
Abbreviations: AE, adverse event; ANCOVA, Analysis of Covariance; BMI, body mass index; CGI-S, Clinical Global Impression-Severity; DAI, Drug Attitude Inventory; EPS, extrapyramidal symptoms; ESRS, Extrapyramidal Symptoms Rating Scale; GAF, Global Assessment of Function; HRQoL, health-related quality of life; LAR, long-acting risperidone; PANSS, Positive and Negative Syndrome Scale for Schizophrenia; SD, standard deviation; SE, standard error; SF-36, Short Form-36; VAS, visual analogue scale.
Elderly patients
Elderly patients with schizophrenia have generally been neglected in the research literature, but often suffer from persistent symptoms and cognitive deficits (Davidson et al 2000). Elderly patients also have a greater sensitivity to treatment-related adverse effects, a higher rate of co-morbidities and an increased risk of medication interactions (Masand 2000). A sub-analysis of the 12-month study by Fleischhacker et al examined the efficacy and safety of long-acting risperidone in elderly patients (≥65 years) with schizophrenia and schizoaffective disorder (Lasser et al 2004c). Long-acting risperidone was associated with significant improvements as determined by PANSS (p<0.001) and CGI-S assessment scales. Moreover, despite the age of this population and high rate of polypharmacy, the incidence of adverse events of particular concern such as cardiac effects and movement disorders, was low. Importantly, no new cases of tardive dyskinesia were reported in this high-risk group and symptoms of movement disorders were reduced compared to baseline (Lasser et al 2004c).
Young patients or patients with a first episode of schizophrenia
The early recognition and management of first-episode schizophrenia is a challenging task. Although these patients are the most responsive to treatment, they are also very sensitive to adverse events and often lack “disease insight”, which contribute to poor compliance and high treatment discontinuation rates (Kasper 1999). A recent subanalysis of the 6-month study by Möller et al examined the efficacy and safety of long-acting risperidone in patients in the early phases of schizophrenia and schizoaffective disorders (Parellada et al 2005). Following initiation of long-acting risperidone statistically significant improvements were seen in the control of symptoms and the severity of schizophrenia (both PANSS and CGI-S), as well as Global Assessment of Functioning (GAF), HRQoL, patient satisfaction, and EPS (Parellada et al 2005). A number of other sub-analyses have also reported that long-acting risperidone was efficacious and well accepted in young adults with schizophrenia or schizoaffective disorder (Lasser et al 2004d; Saleem et al 2004) (Table 2). In addition, Emsley et al, has reported on the 6-month interim data on 51 patients with first episode psychosis with 74% of patients achieving ≥50% reduction in the PANSS (Emsley et al 2005).
Patients with schizoaffective disorder
Schizoaffective disorder is a complex disorder to both diagnose and successfully treat, often requiring a combination of different classes of medications including an antipsychotic and an antidepressant or mood stabiliser (Levinson et al 1999). Two analyses were identified which examined the efficacy of long-acting risperidone in stable patients with schizoaffective disorder (Lasser et al 2004b; Mohl et al 2005) (Table 2). Data from these two studies suggest that significant clinical benefits follow a switch to long-acting risperidone in symptomatically stable patients with schizoaffective disorder, despite the fact that PANSS scores were low at baseline and further clinical improvement would not necessarily be anticipated. Of particular note for patients with schizoaffective disorder were the significant improvements in the two symptom domains relating to mood – the anxiety/depression factor and the uncontrolled hostility/excitement factor (Lasser et al 2004b; Mohl et al 2005). The reduction in psychopathology symptoms was also accompanied by significant improvements in CGI-S scores, GAF scores and improvements in HRQoL, as assessed by the SF-36 (Lasser et al 2004b; Mohl et al 2005).
Patients of different ethnicity
Several studies have suggested that African-American patients may respond differently to treatment than do other racial or ethnic groups (Emsley et al 2002). The nature of these differences is poorly understood but may reflect genetic, pharmacokinetic, cultural, or environmental factors (Frackiewicz et al 1997; Poolsup et al 2000). To date, the impact of race on the efficacy and safety of long-acting risperidone in Caucasian, African-American and other patients with schizophrenia or schizoaffective disorder has been examined in a sub-analysis of the 12-week, placebo-controlled study by Kane et al (Ciliberto et al 2005) (Table 2). Results demonstrated that there was a significant effect of treatment (p<0.001), independent of race, on the improvement in mean PANSS total scores.
Obese patients
Weight gain and obesity have been reported in patients receiving both conventional and atypical antipsychotics, and are associated with increased risks for hypertension, stroke, osteoarthritis, and particularly for coronary heart disease and type 2 diabetes (Ganguli 1999). To date, one study has been undertaken to investigate the efficacy of long-acting risperidone in obese patients (body mass index of ≥30 kg/m2) with schizophrenia or schizoaffective disorder (Table 2). Results demonstrated that there were significant reductions from baseline in mean total PANSS scores at 1 month and these continued throughout the trial. Overall, the efficacy of long-acting risperidone in obese patients was comparable to that reported for schizophrenia patients in general (Marder et al 1997). Importantly, bodyweight remained stable throughout the 6-month study period, with a mean increase of 0.5 kg. Furthermore, despite the high BMI of the patients, approximately half of the patients remained on the starting dose of long-acting risperidone throughout the study.
The cost-effectiveness of long-acting risperidone
Healthcare costs in schizophrenia are disproportionately high. Although the illness affects approximately 1% of the world’s population, it accounts for up to 3% of health expenditure (Knapp 2000). Relapses increase refractoriness to future treatment leading to more frequent and prolonged hospitalization, and contribute significantly to the economic burden of schizophrenia. Results from a recent study demonstrated a four-fold increase in costs among patients experiencing relapse, compared to those who did not (Almond et al 2004). Overall, 79% of the direct costs of schizophrenia result from hospitalization or other residential care, while medications represent only a small fraction (1%–6%) of the total cost of schizophrenia (Foster and Goa 1998; Davies and Drummond 1994; Goeree et al 2005). It is expected that the introduction of long-acting risperidone with its potential to improve compliance and decrease relapse should lead to lower levels of healthcare resource use.
Hospitalization rates, healthcare resource utilization, and relapse rates with long-acting risperidone
Hospitalization rates were assessed as part of a 1-year international, open-label trial of long-acting risperidone in inpatients and outpatients with stable schizophrenia or schizoaffective disorder (Chue et al 2005b). Of the 397 patients who received a modal dose of long-acting risperidone 25 mg or 50 mg, 24% were inpatients and 76% were outpatients at baseline. Results demonstrated that the number of patients requiring hospitalization decreased continuously and significantly from 38% in the 3 months before treatment to 12% during the last 3 months of treatment (p<0.001). Of baseline inpatients, 71% were discharged during treatment. Overall, the 1-year re-hospitalization rate was 17.6%, with a rate of 15.9% for baseline outpatients (Chue et al 2005b). A further analysis of this study examined healthcare resource utilization during 1-year treatment with long-acting risperidone (Leal et al 2004). Results demonstrated that mean hospitalization length during the study was 30.5 days (outpatients, 4.9 days; inpatients 110 days). The need for partial hospitalization (day or night clinics) decreased significantly over the 12-month period, from 7% during the 12 weeks before treatment to 3% during the last 12 weeks (p=0.002). The need for outpatient consultations also decreased significantly from 70% in the 12 weeks before treatment to 30% (p<0.0001) during the first 12 weeks of treatment. The need for outpatient consultation remained stable throughout the remainder of the treatment period. Overall, only 9% of patients required an emergency room visit, mostly for non-psychiatric conditions (Leal et al 2004).
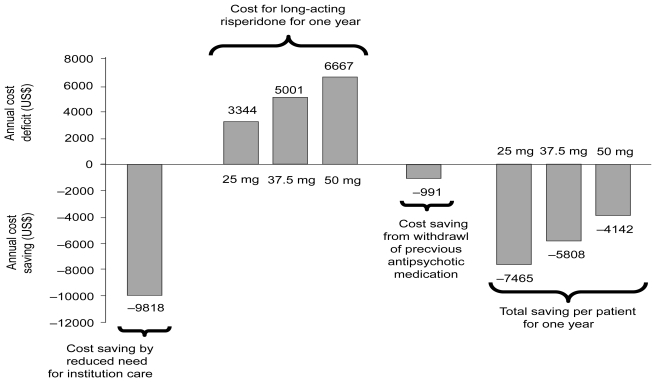
Results from a multicenter, Canadian retrospective study reported that following a switch to long-acting risperidone 92% fewer patients (4.3%) were hospitalized post-initiation compared with prior (50.7%), (p<0.0001). Furthermore, total duration of hospitalization days decreased by 99% (p<0.0001) and anticholinergic and anxiolytic use fell by 22% (p=0.0719) and 38% (p=0.0252), respectively (Chue et al 2005d). Of note, preliminary data from a Swedish multicenter study in 92 patients have demonstrated that for patients treated with long-acting risperidone, the total number of hospitalizations was reduced by 38% (p=0.0004) compared with the same observational period when treated with their previous antipsychotic therapy (Eriksson et al 2003). Using an empirical economic model, based on the Swedish costs, the mean annual cost savings can be calculated per patient following a switch to long-acting risperidone within the recommended dose range (Figure 3). Finally, a 1-year mirror image observational study was undertaken to investigate predictors of relapse (defined as hospital admission) for patients (n=142) on long-acting risperidone (Patel et al 2006). Results demonstrated that patients who discontinued long-acting risperidone (0–12 months) were 3 times more likely to relapse than continuers at 1 year (odds ratio [OR] 3.08, 95% confidence interval [CI]: 1.39–6.81, p=0.003). Clinically unstable patients (those admitted in the year preceding long-acting risperidone treatment) were much more likely to relapse than those who were clinically stable (OR 6.58, 95% CI: 2.77–15.66, p<0.001). No statistically significant differences were found for relapse in terms of sociodemographic factors, diagnosis and illness duration, medication history and clinical indication for long-acting risperidone (Patel et al 2006). As such, patients who receive consistent and continuous treatment with long-acting risperidone may be expected to have a lower incidence of relapse rates and, therefore, an improved long-term prognosis.
Figure 3.
Health economic benefits model from the reduced need for institutional care in patients with schizophrenia and schizoaffective disorder that are switched to treatment with long-acting risperidone.
Pharmacoeconomic evaluations of long-acting risperidone
The use of modeling as a means of assessing the economics of health interventions has increased considerably in recent years. A number of studies have employed either discrete event simulation or decision analytic models, both of which theoretically address the heterogeneous and real-world characteristics of patients with schizophrenia, to examine the costs of long-acting risperidone compared with oral and conventional long-acting agents in the US, Canada, Belgium, and Germany (Chue et al 2005c; De Graeve et al 2005; Edwards et al 2005; Laux et al 2005).
In the US, a decision analytical model captured rates of patient compliance, rates, frequency and duration of relapse, incidence of adverse events, and healthcare resource utilization and associated costs with 7 treatment alternatives (Edwards et al 2005). Primary outcomes were expressed in terms of percentage of patients relapsing per year, number of relapse days per year, and total direct 2003 medical cost per patient per year. On the basis of model projections, patients receiving long-acting risperidone will have the best clinical outcomes in terms of the frequency and duration of relapses. Over a period of 1 year, this would translate into direct medical cost-savings with long-acting risperidone compared with oral risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, and long-acting haloperidol of US$161, 1425, 508, 259, 1068, and 8224, respectively (Edwards et al 2005).
In Canada, a discrete-event model was designed to evaluate the cost-effectiveness of long-acting risperidone for patients with schizophrenia at a high risk of non-compliance, compared with 2 standard treatment alternatives (oral risperidone and long-acting haloperidol) over a 5-year period (Chue et al 2005c). On the basis of 3000 simulated patient characteristics, the model generated individual patient histories. Outcomes included the number and duration of psychotic episodes, the cumulative PANSS score and direct medical costs. The time horizon of the model was 5 years and a 5% discount rate was used for costs and effects. In this model, initiating treatment with long-acting risperidone was the dominant strategy. After 5 years, treatment with long-acting risperidone saved Can$6908 and Can$13130 (discounted) and avoided 0.28 and 0.54 relapses per patient, compared with long-acting haloperidol and oral risperidone, respectively (Chue et al 2005c).
In the Belgian model, a decision tree model was created to compare the cost effectiveness of three first-line treatment strategies in a sample of young schizophrenia patients who had been treated for 1 year and whose disease had not been diagnosed for longer than 5 years (De Graeve et al 2005). This model used a time horizon of 2 years, with health state transition probabilities, resource use and cost estimates derived from clinical trials, expert opinion, and published prices. The principal efficacy measure was the proportion of patients successfully treated, defined as those who responded to initial treatment and who had none to two episodes of clinical deterioration without needing a change of treatment over the 2-year period. A greater proportion of patients were successfully treated with long-acting risperidone (82.7%) for 2 years, compared with those treated with oral olanzapine (74.8%) or long-acting haloperidol (57.3%). Total mean costs per patient over 2 years were • 16 406 with long-acting risperidone, • 17 074 with olanzapine, and • 21 779 with long-acting haloperidol (year of costing 2003) (De Graeve et al 2005). Similarly, the mean cost-effectiveness ratios were • 19 839, • 22 826, and • 38 008 per successfully treated patient for long-acting risperidone, olanzapine, and haloperidol, respectively. Cost savings were also observed when long-acting risperidone was compared with oral olanzapine and long-acting haloperidol in a German study (Laux et al 2005). In this study a discrete-event simulation model was developed to compare the benefits and costs of three pharmacological treatment strategies from the perspective of major third party payers (sickness funds and social security) over a 5-year period in Germany. The model accounted for fixed patient characteristics, and on the basis of these, simulated patient histories according to several time-dependent variables. In accordance with German guidelines, costs and effects were discounted by between 3% and 10%. Outcomes were expressed in terms of the number and duration of psychotic episodes, cumulative symptom scores, costs, and quality-adjusted-life-years (QALY). The long-acting risperidone strategy was calculated to avoid 0.23 and 0.33 relapses per patient, decrease the cumulative symptom score by 25 and 33 points, and decrease costs by • 2017 and • 6096 per patient (• 1608 and • 5422 discounted), compared with the long-acting haloperidol and olanzapine strategies respectively, over 5 years (Laux et al 2005).
Discussion
It is recognized that long-acting antipsychotics facilitate compliance with medication, and may help to prevent relapse and improve functional outcomes in patients with schizophrenia (Gerlach 1994). Accordingly, the guidelines for long-acting antipsychotic treatment that were developed by a European Neuropsychopharmacology Consensus Conference recommend that: “any patient for whom long-term antipsychotic treatment is indicated should be considered for depot drugs” (Kane et al 1998). The development of long-acting risperidone, which combines the benefits of an atypical agent with the advantages of a long-acting formulation, represents an important new option for the long-term management of patients with schizophrenia.
Results from the studies presented here have demonstrated that long-acting risperidone 35, 37.5, or 50 mg, administered once every 2 weeks, is both effective and well tolerated in patients with schizophrenia or schizoaffective disorder (Fleischhacker et al 2003; Kane et al 2003; Lindenmayer et al 2004; Turner et al 2004; Chue et al 2005a; Möller et al 2005). In addition, several patient groups, including the young, the elderly, patients with schizoaffective disorder, and patients of different ethnicity have also been shown to derive significant benefit from long-acting risperidone. None of these trials, as with studies of conventional long-acting antipsychotics (Adams et al 2001), specifically recruited non-compliant patients due to the difficulties in maintaining such patients in a study thus, the positive outcomes reported are potentially subject to some bias. Nonetheless, the favorable efficacy and tolerability profile of long-acting risperidone is also associated with improvements in quality of life and patient satisfaction, thereby helping to reduce the burden on family members and caregivers and promote social integration (Nasrallah et al 2004; Fleischhacker et al 2005). Recently, The Remission in Schizophrenia Working Group defined remission as “a state in which patients have experienced an improvement in core signs and symptoms to the extent that any remaining symptomatology is of such low intensity that it no longer interferes significantly with behavior, and is below the threshold typically utilized in justifying an initial diagnosis of schizophrenia” (Andreasen et al 2005). Although these newly proposed remission criteria require further refinement, results from two retrospective analyses have demonstrated long-acting risperidone may help patients achieve and maintain remission (Kissling et al 2005; Lasser et al 2005b). Prospective studies utilizing the consensus remission criteria as a primary outcome are now eagerly awaited.
Non- and partial compliance with antipsychotic therapy remains widespread (Weiden and Zygmunt 1997). The combination of symptomatic improvement and better tolerability with long-acting risperidone is expected to improve compliance with therapy. This is of particular relevance to young patients or patients with a first episode of schizophrenia, who are particularly sensitive to adverse events and are lacking in disease insight, leading to poor compliance and treatment discontinuation. Indeed, a study of first-episode patients with schizophrenia demonstrated that partial compliance increased by nearly five-fold the risk of both first and second relapse (Robinson et al 1999). Evidence that long-acting risperidone improves compliance comes from the finding that across the pivotal studies only 1.3%–5% of patients discontinued treatment with long-acting risperidone because of poor compliance. Overall, the high retention rates in these studies (51%–92%) probably reflects a number of factors: the low incidence of adverse events (only 1.2%–16% of patients discontinued as a results of adverse events), the improvement in symptoms, HRQoL, and patient functioning experienced by patients with long-acting risperidone treatment, and the fact that long-acting risperidone was well accepted by patients, supported by the low rating of subjective pain and absence of objective changes at the injection site (Lindenmayer et al 2005). This is in contrast to the overall 74% all-cause discontinuation rate recently reported from the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) (Lieberman et al 2005). Although some clinicians believe that long-acting agents are old fashioned, stigmatizing, and less acceptable to patients (Patel et al 2003), results from a systematic review of patient preference reported that in 5 out of the 6 studies analyzed, the majority of patients preferred to receive their medication via a long-acting formulation than in tablet form (Walburn et al 2001).
Interestingly, in a number of studies with long-acting risperidone it has been noted that there has been a reduction in concomitant medications including side effect medications (anticholinergics, anxiolytics, hypnotics, sedatives), antipsychotics used in combination, and other classes of psychotropics such as antidepressants and mood stabilizers (Fleischhacker et al 2003; Taylor et al 2004; Chue et al 2005d; E-STAR 2005; Snaterse 2005). This may be regarded as a proxy measure of both efficacy and tolerability in real-world patients and warrants further study.
Medications that can reduce relapse rates and improve compliance leading to lower levels of healthcare resource use, particularly hospitalization, are an important part of the management of schizophrenia. A number of pharmacoeconomic models and early clinical data have consistently demonstrated that long-acting risperidone reduces the number of relapses, compared with oral atypical or conventional long-acting agents (Eriksson et al 2003; Chue et al 2005c; De Graeve et al 2005; Edwards et al 2005; Laux et al 2005). Applying country-specific economic data, this improvement in the number of relapses has consistently translated into various levels of cost savings despite very different healthcare systems (Chue et al 2005c; De Graeve et al 2005; Edwards et al 2005; Laux et al 2005). These findings suggest that long-acting risperidone is potentially a cost-effective first-line strategy for managing schizophrenia and reducing the burden related to the disease (Annemans 2005). However, it is recognized that for long-acting risperidone the results drawn from pharmacoeconomic model data are limited and must be supported by real-world observational findings before definitive conclusions can be made, particularly when the cost-effectiveness of conventional long-acting antipsychotics for schizophrenia has not been consistently demonstrated (Knapp et al 2002; Kilian and Angemeyer, 2004). Furthermore, an analysis of 22 pharmacoeconomic evaluations of different drug therapies showed that non-compliance always resulted in a reduction in efficacy, but the impact on costs varied substantially (Hughes et al 2001).
It is acknowledged that this review has several limitations. Firstly, this review includes initial data published at international congresses that are not yet subject to peer review, but nonetheless provide clinicians with early and important information. In addition, since this review analyzed a large number of clinical trials, encompassing a wide range of study designs from randomized, control to open-label, switch studies, variable patients numbers, and duration of follow-up, it is difficult to make direct comparisons across studies. The results of the efficacy of long-acting risperidone in special populations (young patients, elderly patients, patients with schizoaffective disorder, and patients of different ethnicity) were based solely on sub-analyses. As such, further controlled studies of long-acting risperidone in these difficult-to-treat populations are warranted. Likewise, the hypothesis that switching patients from oral or long-acting conventional or oral atypical antipsychotics may result in significant improvements also requires further investigation because the majority of these results were also based on sub-analyses. Finally, no head-to-head studies with other atypical antipsychotic agents have been published to date. However, it remains important to consider all levels of clinical evidence when evaluating the overall effectiveness of medication since randomized clinical trials in select study populations for registration purposes may not always reflect current and relevant clinical practice objectives.
The unique pharmacokinetics of long-acting risperidone does have implications when initiating and titrating therapy, particularly when compared to conventional long-acting antipsychotics. However, clinical recommendations concerning dosing are beyond the scope of this review, but are discussed in a number of clinical articles and in the Product Monograph (Marder et al 2003; Keith et al 2004; Viner et al 2006).
Conclusion
This review indicates that long-acting risperidone is suitable for a wide range of patients, including those who are deemed clinically stable, to further improve symptom control and enhance tolerability. The combination of improved efficacy and good tolerability may have important implications for patient compliance to therapy and subsequent positive long-term outcomes including the achievement of remission.
Acknowledgments
The author would like to thank Frances Gambling, Medicus International, for her editorial assistance. Editorial support was funded by an unrestricted educational grant from Janssen.
References
- Adams CE, Fenton MK, Quraishi S, David AS. Systematic meta-review of depot antipsychotic drugs for people with schizophrenia. Br J Psychiatry. 2001;179:290–9. doi: 10.1192/bjp.179.4.290. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–72. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- Almond S, Knapp M, Francois C. Relapse in schizophrenia: Costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–51. doi: 10.1192/bjp.184.4.346. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Carpenter W, Kane J, et al. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–9. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Annemans L. Cost effectiveness of long-acting risperidone: what can pharmacoeconomic models teach us? Pharmacoeconomics. 2005;23(Suppl 1):1–2. doi: 10.2165/00019053-200523001-00001. [DOI] [PubMed] [Google Scholar]
- Bagnall AM, Jones L, Ginnelly L, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess. 2003;7:1–193. doi: 10.3310/hta7130. [DOI] [PubMed] [Google Scholar]
- Barkic J, Filakovic P, Radanovic-Grguric L, et al. The influence of risperidone on cognitive functions in schizophrenia. Coll Antropol. 2003;27(Suppl 1):111–8. [PubMed] [Google Scholar]
- Beasley C, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol. Acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14:111–23. doi: 10.1016/0893-133X(95)00069-P. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–28. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bloch Y, Mendlovic S, Strupinsky S, et al. Injections of depot antipsychotic medications in patients suffering from schizophrenia: do they hurt? J Clin Psychiatry. 2001;62:855–9. doi: 10.4088/jcp.v62n1104. [DOI] [PubMed] [Google Scholar]
- Boyer P, Lecrubier Y, Puech A, et al. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry. 1995;166:68–72. doi: 10.1192/bjp.166.1.68. [DOI] [PubMed] [Google Scholar]
- Buckley P. Efficacy of quetiapine for the treatment of schizophrenia: a combined analysis of three placebo-controlled trials. Curr Med Res Opin. 2004;20:1357–63. doi: 10.1185/030079904125004510. [DOI] [PubMed] [Google Scholar]
- Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005a;15:111–7. doi: 10.1016/j.euroneuro.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Chue PS, Heeg B, Buskens E, et al. Modelling the impact of compliance on the costs and effects of long-acting risperidone in Canada. Pharmacoeconomics. 2005c;23(Suppl 1):62–74. doi: 10.2165/00019053-200523001-00006. [DOI] [PubMed] [Google Scholar]
- Chue P, Lam A, Chandra K, et al. Hospitalization and medication use in schizophrenia patients receiving risperidone long-acting injectable or oral atypical antipstychotic medication. PA202. Presented at ISPOR 8th Annual Congress; Florence, Italy. 2005d. [Google Scholar]
- Chue P, Llorca P, Duchesne I, et al. Hospitalization rates in patients during long-term treatment with long-acting risperidone injection. J Appl Res. 2005b;5:266–74. [Google Scholar]
- Ciliberto N, Bossie CA, Urioste R, et al. Lack of impact of race on the efficacy and safety of long-acting risperidone versus placebo in patients with schizophrenia or schizoaffective disorder. Int Clin Psychopharmacol. 2005;20:207–12. doi: 10.1097/00004850-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Citrome LL. The increase in risk of diabetes mellitus from exposure to second-generation antipsychotic agents. Drugs Today (Barc) 2004;40:445–64. doi: 10.1358/dot.2004.40.5.850492. [DOI] [PubMed] [Google Scholar]
- Colonna L, Saleem P, Dondey-Nouvel L, et al. Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Amisulpride Study Group. Int Clin Psychopharmacol. 2000;15:13–22. doi: 10.1097/00004850-200015010-00002. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Vervarcke J, et al. A long-term, multicenter, open-label study of risperidone in elderly patients with psychosis. On behalf of the Risperidone Working Group. Int J Geriatr Psychiatry. 2000;15:506–14. doi: 10.1002/1099-1166(200006)15:6<506::aid-gps146>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Davies L, Drummond M. Economics and schizophrenia: the real cost. Br J Psychiatry. 1994;165:18–21. [PubMed] [Google Scholar]
- Davis J, Metalon L, Watanabe M, et al. Depot antipsychotic drugs. Place in therapy. Drugs. 1994;47:741–73. doi: 10.2165/00003495-199447050-00004. [DOI] [PubMed] [Google Scholar]
- De Graeve D, Smet A, Mehnert A, et al. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a Belgian cost-effectiveness analysis. Pharmacoeconomics. 2005;23(Suppl 1):35–47. doi: 10.2165/00019053-200523001-00004. [DOI] [PubMed] [Google Scholar]
- Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159:103–8. doi: 10.1176/appi.ajp.159.1.103. [DOI] [PubMed] [Google Scholar]
- Edwards NC, Locklear JC, Rupnow MF, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics. 2005;23(Suppl 1):75–89. doi: 10.2165/00019053-200523001-00007. [DOI] [PubMed] [Google Scholar]
- Eerdekens M, Karcher K, Remmerie B, et al. Pharmacokinetics and tolerability of long-acting injectable risperidone in schizophrenia. Schizophr Res. 2004;70:91–100. doi: 10.1016/j.schres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Electronic Schizophrenia Treatment Adherence Registry (E-STAR). Janssen-Ortho Inc. Data on file. 2005.
- Emsley RA. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Risperidone Working Group. Schizophr Bull. 1999;25:721–9. doi: 10.1093/oxfordjournals.schbul.a033413. [DOI] [PubMed] [Google Scholar]
- Emsley R, Oosthuizen P, Koem L, et al. A trial of risperidone long-acting injectable in first episode psychosis. Presented at the Annual Meeting of the American Psychiatric Association; May 21–26, 2005; Atlanta, GA, USA. 2005. [Google Scholar]
- Emsley R, Oosthuizen P, Koem L, et al. Safety and efficacy of long-acting risperidone as a first-line treatment for first-episode psychosis: 6-month interim analysis [abstract]. Abstracts of the XIIIth Biennial Winter Workshop of Schizophrenia Research; February 4–10, 2006; Davos, Switzerland. 2006. [Google Scholar]
- Emsley RA, Roberts MC, Rataemane S, et al. Ethnicity and treatment response in schizophrenia: a comparison of 3 ethnic groups. J Clin Psychiatry. 2002;63:9–14. doi: 10.4088/jcp.v63n0103. [DOI] [PubMed] [Google Scholar]
- Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatry. 1993;38(Suppl 3):S80–8. [PubMed] [Google Scholar]
- Ereshefsky L, Mannaert E. Pharmacokinetic profile and clinical efficacy of long-acting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs R D. 2005;6:129–37. doi: 10.2165/00126839-200506030-00001. [DOI] [PubMed] [Google Scholar]
- Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(Suppl 16):18–23. [PubMed] [Google Scholar]
- Ereshefsky L, Saklad SR, Jann MW, et al. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry. 1984;45:50–9. [PubMed] [Google Scholar]
- Eriksson L, Almqvist A, Mehnert A, et al. Long-acting risperidone significantly reduces the need for institutional psychiatric care. Presented at American College of Neuropsychopharmacologists, 42nd Annual meeting; December 7 2003; San Juan, Puerto Rico. 2003. [Google Scholar]
- Farde L, Eerdekens M, Eriksson B, et al. Relationship between plasma concentrations, D2-receptor occupancy and efficacy of Risperdal Consta. Presented at American College of Neuropsychopharmacologists, 41st Annual Meeting; December 8–12, 2002; San Juan, Puerto Rico. 2002. [Google Scholar]
- Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64:1250–7. doi: 10.4088/jcp.v64n1017. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Lasser R, Mehnert A. Perceived functioning and well-being and association with psychiatric symptomatology in clinically stable schizophrenia patients treated with long-acting risperidone for 1 year. Br J Psychiatry. 2005;187:131–6. doi: 10.1192/bjp.187.2.131. [DOI] [PubMed] [Google Scholar]
- Foster RH, Goa KL. Risperidone. A pharmacoeconomic review of its use in schizophrenia. Pharmacoeconomics. 1998;14:97–133. doi: 10.2165/00019053-199814010-00009. [DOI] [PubMed] [Google Scholar]
- Frackiewicz EJ, Sramek JJ, Herrera JM, et al. Ethnicity and antipsychotic response. Ann Pharmacother. 1997;31:1360–9. doi: 10.1177/106002809703101114. [DOI] [PubMed] [Google Scholar]
- Franz M, Lis S, Pluddemann K, et al. Conventional versus atypical neuroleptics: subjective quality of life in schizophrenic patients. Br J Psychiatry. 1997;170:422–5. doi: 10.1192/bjp.170.5.422. [DOI] [PubMed] [Google Scholar]
- Ganguli R. Weight gain associated with antipsychotic drugs. J Clin Psychiatry. 1999;60(Suppl 21):20–4. [PubMed] [Google Scholar]
- Gastpar M, Masiak M, Latif MA, Frazzingaro S, Medori R, Lombertie ER. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol. 2005;19:32–8. doi: 10.1177/0269881105056598. [DOI] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000;321:1371–6. doi: 10.1136/bmj.321.7273.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefvert O, Eriksson B, Persson P, et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta™) in patients with schizophrenia. Int J Neuropsychopharmacol. 2005;8:27–36. doi: 10.1017/S1461145704004924. [DOI] [PubMed] [Google Scholar]
- Gerlach J. Oral versus depot administration of neuroleptics in relapse prevention. Acta Psychiatr Scand Suppl. 1994;382:28–32. doi: 10.1111/j.1600-0447.1994.tb05862.x. [DOI] [PubMed] [Google Scholar]
- Gharabawi GM, Bossie CA, Zhu Y, et al. An assessment of emergent tardive dyskinesia and existing dyskinesia in patients receiving long-acting, injectable risperidone: Results from a long-term study. Schizophr Res. 2005;77:129–39. doi: 10.1016/j.schres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Glazer WM, Ereshefsky L. A pharmacoeconomic model of outpatient antipsychotic therapy in “revolving door” schizophrenic patients. J Clin Psychiatry. 1996;57:337–45. [PubMed] [Google Scholar]
- Goeree R, Farahati F, Burke N, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opinion. 2005;21:2017–28. doi: 10.1185/030079905X75087. [DOI] [PubMed] [Google Scholar]
- Hamilton SH, Edgell ET, Revicki DA, et al. Functional outcomes in schizophrenia: a comparison of olanzapine and haloperidol in a European sample. Int Clin Psychopharmacol. 2000;15:245–55. doi: 10.1097/00004850-200015050-00001. [DOI] [PubMed] [Google Scholar]
- Hufnagel E, Locklear J, Caruso R, et al. Treatment goal expectations of physicains and patients with schizophrenia. Presented at the 56th Institute on Psychiatric Services Scientific Meeting; October 6–10, 2004; Atlanta, GA USA. 2004. [Google Scholar]
- Jayaram G, Coyle J, Tune L. Relapse in chronic schizophrenics treated with fluphenazine decanoate is associated with low serum neuroleptic levels. J Clin Psych. 1986;47:247–8. [PubMed] [Google Scholar]
- Kane J. Progress defined–short-term efficacy, long-term effectiveness. Int Clin Psychopharmacol. 2001;16(Suppl 1):S1–8. doi: 10.1097/00004850-200101001-00002. [DOI] [PubMed] [Google Scholar]
- Kane JM, Aguglia E, Altamura AC, et al. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Eur Neuropsychopharmacol. 1998;8:55–66. doi: 10.1016/s0924-977x(97)00045-x. [DOI] [PubMed] [Google Scholar]
- Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotics. Am J Psychiatry. 2003;160:1125–32. doi: 10.1176/appi.ajp.160.6.1125. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–93. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kasper S. First-episode schizophrenia: the importance of early intervention and subjective tolerability. J Clin Psychiatry. 1999;60(Suppl 23):5–9. [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, et al. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–22. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv. 2004;55:997–1005. doi: 10.1176/appi.ps.55.9.997. [DOI] [PubMed] [Google Scholar]
- Kilian R, Angemeyer MC. The impact of antipsychotic medication on the incidence and the costs of inpatient treatment in people with schizophrenia: results from a prospective observational study. Psychiatr Prax. 2004;31:138–46. doi: 10.1055/s-2003-812599. [DOI] [PubMed] [Google Scholar]
- Kissling W, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone—analysis of long-term efficacy. J Psychopharmacol. 2005;19:15–21. doi: 10.1177/0269881105056514. [DOI] [PubMed] [Google Scholar]
- Knapp M. Schizophrenia costs and treatment cost-effectiveness. Acta Psychiatr Scand Suppl. 2000:15–8. doi: 10.1046/j.1467-0658.2001.00137.x-i1. [DOI] [PubMed] [Google Scholar]
- Knapp M, Ilson S, David A. Depot antipsychotic preparations in schizophrenia: the state of the economic evidence. Int Clin Psychopharmacol. 2002;17:135–40. doi: 10.1097/00004850-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Kushner S, Khan A, Eerdekens M, et al. Treatment with long-acting risperidone in patients with schizophrenia for up to four years. Presented at Institute on Psychiatric Services; October 6–10, 2004; Atlanta, GA, USA. 2004. [Google Scholar]
- Lambert M, Naber D. Current issues in schizophrenia: overview of patient acceptability, functioning capacity and quality of life. CNS Drugs. 2004;18(Suppl 2):5–17. 41–3. doi: 10.2165/00023210-200418002-00002. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie C, Gharabawi G, et al. Patients with schizophrenia previously stabilized on conventional depot antipsychotics experience significant clinical improvements following treatment with long-acting risperidone. Eur Psychiatry. 2004a;19:219–25. doi: 10.1016/j.eurpsy.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord. 2004b;83:263–75. doi: 10.1016/j.jad.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie C, Gharabawi G, et al. Clinical improvement in 336 stable chronically psychotic patients changed from oral to long-acting risperidone: A 12-month open trial. Int J Neuropsychopharmacol. 2005a;8:1–12. doi: 10.1017/S1461145705005225. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie CA, Gharabawi GM, et al. Remission in schizophrenia: Results from a 1-year study of long-acting risperidone injection. Schizophr Res. 2005b;77:215–27. doi: 10.1016/j.schres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie CA, Zhu Y, et al. Efficacy and safety of long-acting risperidone in elderly patients with schizophrenia and schizoaffective disorder. Int J Geriatr Psychiatry. 2004c;19:898–905. doi: 10.1002/gps.1184. [DOI] [PubMed] [Google Scholar]
- Lasser R, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with schizophrenia or schizoaffective disorder. Presented at American Psychiatric Association; 1– 6 May 2004; New York, USA. 2004d. [Google Scholar]
- Laux G, Heeg B, van Hout B, et al. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics. 2005;23:49–61. doi: 10.2165/00019053-200523001-00005. [DOI] [PubMed] [Google Scholar]
- Lauriello J, McEvoy JP, Rodriquez S, et al. Long-acting risperidone vs. placebo in the treatment of hospital inpatients with schizophrenia. Schizophr Res. 2005;72:249–58. doi: 10.1016/j.schres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Leal A, Rosillon D, Mehnert A, et al. Healthcare resource utilization during 1-year treatment with long-acting, injectable risperidone. Pharmacoepidemiol Drug Saf. 2004;13:811–6. doi: 10.1002/pds.978. [DOI] [PubMed] [Google Scholar]
- Lee MS, Ko YH, Lee SH, et al. Long-term treatment with long-acting risperidone in Korean patients with schizophrenia. Hum Psychopharmacol. 2006;21:399–407. doi: 10.1002/hup.782. [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, et al. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138–48. doi: 10.1176/ajp.156.8.1138. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Eerdekens E, Berry SA, et al. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry. 2004;65:1084–9. [PubMed] [Google Scholar]
- Lindenmayer JP, Grochowski S, Mabugat L. Clozapine effects on positive and negative symptoms: a six-month trial in treatment-refractory schizophrenics. J Clin Psychopharmacol. 1994;14:201–4. [PubMed] [Google Scholar]
- Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol. 2005;20:213–21. doi: 10.1097/00004850-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Khan A, Eerdekens M, Van Hove I, Kushner S. Long-term safety and tolerability of long-acting injectable risperidone in patients with schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol. 2006;16 doi: 10.1016/j.euroneuro.2006.08.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Presented at 61st Annual Convention Society of Biological Psychiatry; May 18–20, 2006; Toronto Canada. 2006. [Google Scholar]
- Marder SR, Conley R, Ereshefsky L, Kane JM, Turner MS. Clinical guidelines: Dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(Suppl 16):41–6. [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–46. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Marder S, Meibach R. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–35. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- Masand PS. Side effects of antipsychotics in the elderly. J Clin Psychiatry. 2000;61(Suppl 8):43–9. 50–1. [PubMed] [Google Scholar]
- Meltzer H, Beurnett S, Bastani B. Effects of six months clozapine treatment of quality of life of chronic schizophrenic patients. Hosp Community Psychiatry. 1990;41:892–7. doi: 10.1176/ps.41.8.892. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Gruppe H, Bauer U, et al. Improvement of cognitive function in schizophrenic patients receiving clozapine or zotepine: results from a double-blind study. Pharmacopsychiatry. 1997;30:35–42. doi: 10.1055/s-2007-979481. [DOI] [PubMed] [Google Scholar]
- Mohl A, Westlye K, Opjordsmoen S, et al. Long-acting risperidone in stable patients with schizoaffective disorder. J Psychopharmacol. 2005;19:22–31. doi: 10.1177/0269881105056515. [DOI] [PubMed] [Google Scholar]
- Möller H, Llorca PM, Sachetti E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol. 2005;20:121–30. doi: 10.1097/00004850-200505000-00001. [DOI] [PubMed] [Google Scholar]
- Morosini PL. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–9. [PubMed] [Google Scholar]
- Nasrallah H, Duchesne I, Mehnert A, et al. Health-related quality of life with schizophrenia during treatment with long-acting risperidone injection. J Clin Psychiatry. 2004;65:531–6. doi: 10.4088/jcp.v65n0412. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Wiesel FA, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227–35. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19:5–14. doi: 10.1177/0269881105056513. [DOI] [PubMed] [Google Scholar]
- Patel MX, Nikolaou V, David AS. Psychiatrists’ attitudes to maintenance medication for patients with schizophrenia. Psychol Med. 2003;33:83–9. doi: 10.1017/s0033291702006797. [DOI] [PubMed] [Google Scholar]
- Patel MX, Young C, David A, et al. Risperidone long-acting injection: predictors of relapse at one year. Abstracts of the XIIIth Biennial Winter Workshop on Schizophrenia Research; February 4–10, 2006; Davos, Switzerland. 2006. [Google Scholar]
- Peuskens J, Moller HJ, Puech A. Amisulpride improves depressive symptoms in acute exacerbations of schizophrenia: comparison with haloperidol and risperidone. Eur Neuropsychopharmacol. 2002;12:305–10. doi: 10.1016/s0924-977x(02)00031-7. [DOI] [PubMed] [Google Scholar]
- Peuskens J, Van Baelen B, De Smedt C, et al. Effects of risperidone on affective symptoms in patients with schizophrenia. Int Clin Psychopharmacol. 2000;15:343–9. doi: 10.1097/00004850-200015060-00005. [DOI] [PubMed] [Google Scholar]
- Poolsup N, Li Wan Po A, Knight TL. Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther. 2000;25:197–220. doi: 10.1046/j.1365-2710.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Quraishi S, David A. Depot haloperidol for schizophrenia. Cochrane Database Syst Rev. 2000;2:CD001361. doi: 10.1002/14651858.CD001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstack J, Grandolfi G, D’Hoore P, et al. Long-acting risperidone: Prolonged-release injectable delivery of risperidone using Medisorb® microsphere technology. Schizophr Research. 2003;60:314. [Google Scholar]
- Remington G, Chue P, Kopala L, Stip E. Switching atypical antipsychotics: a review. Acta Neuropsychiatrica. 2004;16:301–13. doi: 10.1111/j.0924-2708.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163:396–401. doi: 10.1176/appi.ajp.163.3.396. [DOI] [PubMed] [Google Scholar]
- Revicki DA, Genduso LA, Hamilton SH, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and other psychotic disorders: quality of life and clinical outcomes of a randomized clinical trial. Qual Life Res. 1999;8:417–26. doi: 10.1023/a:1008958925848. [DOI] [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–7. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Locklear J, Turkoz I, et al. Maintenance therapy with long-acting risperidone: functioning and quality of life in patients with schizophrenia and schizoaffective disorder. Presented at American Psychiatric Association; 22–26 May, 2005; Atlanta, GA, USA. 2005. [Google Scholar]
- Rubio G, Martinez I, Ponce G, et al. Long-acting injectable risperidone versus zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can J Psychiatry. 2006;51:35–43. doi: 10.1177/070674370605100808. [DOI] [PubMed] [Google Scholar]
- Saleem P, Firmino H, Parellada E, et al. Young patients 18–30 years) with schizophrenia and schizoaffective disorder: results of direct switching to long-acting injectable risperidone (StoRMi trial). Presented at the 12th Biennial Winter Workshop on Schizophrenia; 7– 13 February 2004; Davos, Switzerland. 2004. [Google Scholar]
- Snaterse M, Welch R, Chue P. Effectiveness and tolerability outcomes of a risperidone long-acting injection compared to conventional depot antipsychotics in a Canadian teaching hospital. Presented at 44th American College of Neuropsychopharmacology Annual Meeting; 11–15 December 2005; Hawaii, USA. [Google Scholar]
- Taylor DM, Young CL, Mace S, Patel MX. Early clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patients. J Clin Psychiatry. 2004;65:1076–83. doi: 10.4088/jcp.v65n0808. [DOI] [PubMed] [Google Scholar]
- Teijeiro R, Turner M, Bouhours P, et al. Obese patients with schizophrenia and schizoaffective disorder: efficacy of risperidone long-acting injectable. Presented at the 12th Biennial Winter Workshop on Schizophrenia; 7–13 February 2004; Davos, Switzerland. 2004. [Google Scholar]
- Tollefson GD, Sanger TM, Lu Y, et al. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. Arch Gen Psychiatry. 1998;55:250–8. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]
- Turner M, Eerdekens E, Jacko M, et al. Long-acting injectable risperidone: safety and efficacy in stable patients switched from conventional depot antipsychotics. Int Clin Psychopharmacol. 2004;19:241–9. doi: 10.1097/01.yic.0000133500.92025.20. [DOI] [PubMed] [Google Scholar]
- Valenstein M, Copeland LA, Owen R, et al. Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. J Clin Psychiatry. 2001;62:545–51. doi: 10.4088/jcp.v62n07a08. [DOI] [PubMed] [Google Scholar]
- van Os J, Bossie C, Lasser R. Improvements in stable patients with psychotic disorders switched from oral conventional antipsychotics therapy to long-acting risperidone. Int Clin Psychopharmacol. 2004;19:229–32. doi: 10.1097/01.yic.0000122861.35081.16. [DOI] [PubMed] [Google Scholar]
- Vauth R, Justers J, Braendle D, et al. Direct switch from atypical antipsychotic to long-acting injectable risperidone in schizophrenia and schizoaffective disorders [abstract]. Abstracts of the Biennial Winter Workshop of Schizophrenia Research; 7–13 February; Davos, Switzerland. 2004. [Google Scholar]
- Viner MW, Matuszak JM, Knight LJ. Initial dosing strategies for long-acting injectable risperidone. J Clin Psychiatry. 2006;67:1310–1. doi: 10.4088/jcp.v67n0821e. [DOI] [PubMed] [Google Scholar]
- Walburn J, Gray R, Gournay K, et al. Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry. 2001;179:300–7. doi: 10.1192/bjp.179.4.300. [DOI] [PubMed] [Google Scholar]
- Weiden P, Zygmunt A. Medication noncompliance in schizophrenia. Part I. Assessment. J Practical Psychiatry and Behavioural Health. 1997;3:106–10. [Google Scholar]