Abstract
Rufinamide, a triazole derivative that is structurally distinct from currently marketed antiepileptic drugs (AEDs), is in development for the adjunctive treatment of Lennox-Gastaut syndrome (LGS) in children and adults. Rufinamide is well absorbed after oral administration, demonstrates low protein binding, and is metabolized by enzymatic hydrolysis without involvement of cytochrome P450 enzymes, conferring a low drug interaction potential. In a randomized, double-blind trial involving 138 adult and pediatric patients with LGS, compared with placebo, rufinamide 45 mg/kg/day resulted in significantly superior reductions in drop attacks (median change −42.5% vs +1.4% with placebo) and total seizures (−32.1% vs −11.7% with placebo), accompanied by significantly higher responder rates. These results are comparable with findings reported for other AEDs in randomized, controlled clinical trials in patients with LGS. Rufinamide produced statistically significant seizure reduction which was maintained during long-term therapy and accompanied by good tolerability. The most frequently reported adverse events from a pooled safety database evaluating short- and long-term therapy were headache (22.9% and 29.5%), dizziness (15.5% and 22.5%) and fatigue (13.6% and 17.7%). Rufinamide therefore presents a favorable efficacy and tolerability profile and is a promising candidate for the adjunctive therapy of LGS.
Keywords: antiepileptic drug, Lennox-Gastaut syndrome, pediatrics, rufinamide
Introduction
Rufinamide (1-[(2,6-difluorophenyl)methyl]-1hydro-1,2,3-triazole-4 carboxamide) is a novel antiepileptic drug (AED) that is structurally distinct from other AEDs available on the market (Levy et al 2002a). An application was filed by Eisai Ltd in 2005 for European approval of this agent as an adjunctive treatment for seizures associated with Lennox-Gastaut Syndrome (LGS) in children and adults (Eisai Co Ltd 2005).
Lennox-Gastaut syndrome is a catastrophic epileptic encephalopathy of childhood onset (typically at age 3–5 years), for which effective treatment options are limited. This syndrome is associated with multiple types of generalized seizures, especially drop attacks and tonic seizures, delayed psychomotor development, behavioral and personality disorders, and a characteristic electroencephalogram pattern containing both generalized slow spike wave activity and paroxysms of generalized fast rhythmic activity during sleep which reflect excessive neocortical excitability (Markand 2003; Nabbout and Dulac 2003; Guerrini 2006). The long-term prognosis is very poor, with persistence of seizures in more than 75% of patients and severe mental retardation in more than 50% of patients (Markand 2003; Dulac and Engel 2003; Schmidt and Bourgeois 2000). Patient quality of life is particularly affected by the drop attacks. Consequently, LGS is viewed as one of the most difficult epilepsies to treat.
Currently available treatment options for the epileptic encephalopathies in Europe center around the use of valproate, with adjunctive benzodiazepines, topiramate, lamotrigine, or felbamate (Guerrini 2006). However, a Cochrane review has concluded that no single AED could be considered highly efficacious for LGS (Hancock and Cross 2003). Indeed, the efficacy of valproate as a first-line treatment has been described as “unimpressive”, and its use in young children should be accompanied by great caution due to the risk of life-threatening hepatic toxicity (Schmidt and Bourgeois 2000). Other first-generation AEDs (carbamazepine, phenytoin, and phenobarbital) are not recommended for the treatment of LGS, due to the potential for aggravation of absence and myoclonic seizures by carbamazepine and phenytoin, and exacerbation of the behavioral problems seen in patients with LGS by barbiturates. Among the benzodiazepines, clobazam may be viewed as the best tolerated, although there is no head-to-head comparison to confirm this. However, for this class of drugs, development of tolerance is well documented in the literature (Levy et al 2002b). The only AEDs which have been evaluated in a randomized, controlled trial in LGS are lamotrigine, topiramate, and felbamate, the outcomes of which are discussed later in this paper. Although felbamate is licenced for the treatment of LGS in the USA and some other countries (eg, Germany), its use has been limited following reports of serious toxicity (Borowicz et al 2004). This has led to the recommendation that, in LGS, it should be used only in patients aged over 4 years who cannot be treated satisfactorily with other AEDs (Schmidt and Bourgeois 2000).
Thus, there is a clear need for new options in the management of this intractable condition. Rufinamide was granted orphan drug status by the European Medicines Agency (EMEA) and the US Food and Drug Administration (FDA) in 2005. This review will evaluate the relevant pharmacologic and clinical data for rufinamide, and explore its potential role in the future management of this severe form of epilepsy in the context of other products currently available for LGS.
Pharmacology and pharmacokinetics of rufinamide
An extensive preclinical evaluation has suggested that the principal mechanism involved in the antiepileptic activity of rufinamide is its ability to modulate the activity of sodium channels, limiting high-frequency firing of neuronal action potentials over a broad range of concentrations, as demonstrated in vitro (McLean et al 2005). In radioligand binding studies, rufinamide does not interact with other neurotransmitter systems, including monoamine, adenosine, acetylcholine, histamine, glycine, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainite, N-methyl-D-aspartate (NMDA), and γ-amino butyric acid (GABA) systems (Bialer et al 1996).
Rufinamide has demonstrated broad spectrum anticonvulsant activity in animal models of epilepsy, elevating both electrically and chemically induced seizure thresholds and preventing seizure spread following oral and intraperitoneal administration in mice and rats (White et al 2005). No tolerance to this activity was observed following chronic administration over 5 days. Furthermore, the protective index for rufinamide (ratio of the concentration required for 50% anticonvulsant efficacy to that for neurotoxicity) was superior to that of each of the comparator AEDs examined (phenytoin, phenobarbital, ethosuximide, and valproate).
The pharmacokinetic properties of rufinamide have been established both in healthy volunteers and in patients with epilepsy. In a study of 3 healthy volunteers, an oral dose of 600 mg rufinamide demonstrated high absorption, and monoexponential elimination with a mean half-life (t½) of 9 hours (Waldmeier et al 1996). Excretion was largely renal (85%) and complete (98%) within 7 days. Following administration of 400 mg rufinamide as two 200-mg tablets or 400-mg oral suspension after a standard meal, mean maximum plasma concentrations (Cmax) of 3.03 and 3.32 μg/mL were reached after 6.6 and 7.2 hours, respectively, leading to elimination with a terminal t½ of 8.8 and 9.1 hours, respectively (Cheung et al 1995). In a further study, the absorption of a single dose of 400 mg rufinamide was accelerated in fed subjects compared with fasting conditions, resulting in a 43% increase in exposure (96-hour exposure), without affecting the t½ (Cardot et al 1998). However, no effect of food was observed with repeat dosing (Eisai, data on file), and no difference in rufinamide pharmacokinetics has been found between older (aged 66–77 years) and younger (18–40 years) healthy subjects in either single- or multiple-dosing conditions (Chang et al 1998). Rufinamide pharmacokinetics in a study of 16 children aged between 2 and 17 years treated with 10 or 30 mg/kg daily rufinamide for 2 weeks were also comparable to those reported elsewhere for adult patients (Sachdeo et al 1998). Overall, evaluation of these data suggests that no age-related dose adjustments are likely to be required for either pediatric or elderly populations (Bialer et al 2001).
The pharmacokinetic parameters of single ascending doses of rufinamide (0, 400, 800, 1200 mg) have also been compared between 3 healthy subjects and 16 patients with epilepsy receiving enzyme-inducing AEDs (Brunner et al 1994). Absorption rates were comparable, and both groups showed dose-related increases in Cmax and exposure. Rufinamide elimination was accelerated in AED-treated patients compared with healthy subjects, but t½ was independent of the dose administered in both groups. The pharmacokinetics of rufinamide have also been evaluated in 129 pediatric and adult patients with LGS (Critchley et al 2005). Rufinamide clearance was not affected by concomitant lamotrigine or topiramate, and was also unaffected by liver or kidney function. However, rufinamide concentrations were increased by concomitant valproate: 40% in children and 11% in adults. Therefore, the rufinamide dosage may require adjustment during the addition or withdrawal of concomitant valproate therapy.
Rufinimade is extensively metabolized (2% excreted unchanged in urine and 2% in feces), predominantly via hydrolysis of the carboxylamide group to yield an inactive metabolite (CGP-27292), and is largely excreted in urine (84.7% of dose) (Bialer et al 1999). Thus, rufinamide has a low propensity for drug–drug interactions, based on its low protein binding (approximately 34%) and absence of metabolism via hepatic cytochrome P450 or inhibition of the major enzyme subclasses (Bialer et al 1996; Kapeghian et al 1996). This is supported by the absence of clinically relevant alterations of concomitant AED levels observed in a population pharmacokinetic drug–drug interaction analysis (Fuseau et al 2005). This analysis was conducted using data from 5 double-blind studies involving pediatric and adult patients with inadequately controlled seizures despite previous treatment with up to 3 AEDs. In total, 903 patients were receiving carbamazepine, 588 valproate, 200 lamotrigine, 299 phenytoin, 149 phenobarbital, and 69 topiramate. Rufinamide co-administration did not affect the clearance of topiramate or valproate, but increased the clearance of carbamazepine and lamotrigine and decreased clearance of phenobarbital and phenytoin. However, all produced effects were small, were considered unlikely to be of clinical significance, and were independent of age. At a rufinamide plasma concentration of 15 μg/mL, representing steady-state concentrations following doses of 45 mg/kg daily in children or 3200 mg/day in adults, changes in clearance were under 18% of the values without rufinamide (Fuseau et al 2005). Conversely, in a similar study involving 471 patients, valproate co-administration decreased rufinamide clearance by approximately 22%, while any combination of phenytoin, phenobarbital, or primidone increased rufinamide clearance by approximately 25%, compared with patients not receiving these AEDs (Bialer et al 2001). Rufinamide also resulted in a small increase in clearance of the oral contraceptive Ortho-Novum® (Ortho-Mcneil Pharmaceutical, Inc. Raritan, NJ, USA) 1/35 in healthy female volunteers, though the clinical relevance of this effect is not known (Svendsen et al 1998).
Rufinamide in Lennox-Gastaut syndrome
The efficacy of rufinamide as an adjunctive therapy for treatment-resistant LGS has been evaluated in a multicenter, randomized, 12-week, double-blind, placebo-controlled, parallel-group study in 138 patients aged 4–37 years (mean age 14.1 years), followed by an open-label extension phase (Glauser et al 2005a, 2005b). Patients diagnosed with LGS, experiencing ≥90 seizures in the month prior to study entry, and receiving stable treatment with 1–3 concomitant AEDs, were evaluated. After a 28-day baseline, patients randomized to twice-daily oral treatment with rufinamide (n=74) or placebo (n=64) entered a 14-day titration period (rufinamide target dose 45 mg/kg daily), followed by 70 days of double-blind maintenance treatment. The reduction from baseline in the frequency of tonic-atonic seizures (drop attacks) per 28 days was significantly greater with rufinamide than placebo (median change −42.5% vs +1.4%, respectively, p<0.0001). Similarly, the reduction from baseline in total seizure frequency per 28 days was significantly greater with rufinamide than placebo treatment (median change −32.7% vs −11.7%, respectively, p=0.0015). These improvements were associated with significantly greater proportions of responders (patients achieving ≥50% reduction in seizures per 28 days) with rufinamide than placebo treatment, both for tonic-atonic seizures (42.5% vs 16.7%, p=0.0020) (Glauser et al 2005a) and total seizure frequency (31.1% vs 10.9%, p=0.0045) (Kluger et al 2006).
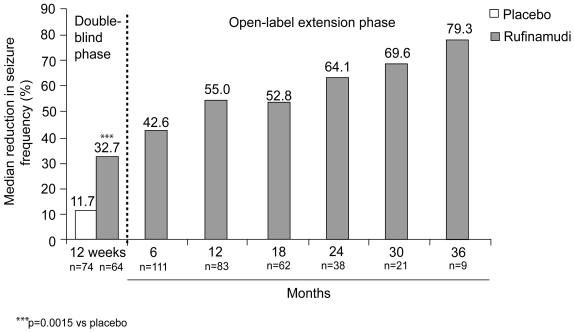
Following completion of the double-blind study, 123 patients continued to receive open-label treatment in the extension phase, at a median dose of 1800 mg/day (range 103–4865 mg/day) for a median duration of 432 days (range 10–1149 days) (Glauser et al 2005b). The reduction in median total seizure frequency observed at 12 weeks was maintained, with some further improvement noted with continued treatment for up to 3 years (see Figure 1). Similarly, the responder rate for total seizures was maintained, with 36.9% of patients achieving a reduction in total seizures of at least 50% overall during the extension study. Furthermore, 21.3% of patients achieved a ≥75% reduction in total seizures overall during the extension study. There was no evidence for tolerance to rufinamide treatment.
Figure 1.
Median percentage reduction from baseline in total seizure frequency during 12-week double-blind and subsequent open-label rufinamide treatment (Glauser 2005a, 2005b; Eisai, data on file). Data shown separately for patients receiving rufinamide (n=74) or placebo (n=64) during double-blind treatment (Glauser 2005a), and combined for patients continuing in the extension study and receiving open-label rufinamide treatment (n=124) (Glauser 2005b).
Tolerability and safety of rufinamide
During the double-blind study of rufinamide in patients with LGS, the most common adverse events (reported by ≥10% patients in either treatment group) were: somnolence (24.3% rufinamide, 12.5% placebo), vomiting (21.6% rufinamide, 6.3% placebo), pyrexia (13.5% rufinamide, 17.2% placebo), and diarrhea (5.4% rufinamide, 10.9% placebo) (Glauser et al 2005a). Notably, rufinamide treatment was associated with a lower incidence of cognitive/psychiatric adverse events (such as psychomotor hyperactivity and lethargy) than placebo treatment (17.6% vs 23.4%, respectively). There was no clinically significant change in clinical laboratory tests, physical examinations or vital signs. During the subsequent open-label extension study, the most commonly reported all-causality adverse events were vomiting (30.6%), pyrexia (25.8%), upper respiratory tract infection (21.8%), and somnolence (21.0%) (Glauser et al 2005b). There were two deaths, which were not considered to be related to rufinamide treatment. Thus, long-term treatment of patients with LGS does not appear to be associated with any increase in central nervous system (CNS) adverse events, with somnolence noted with somewhat lower frequency than during the double-blind phase.
These tolerability and safety data in patients with LGS are further supported by a pooled analysis of rufinamide tolerability and safety in a large population of epilepsy patients, in which the data were evaluated separately for patients receiving short-term or long-term treatment (Krauss et al 2005). The short-term safety population comprised 1240 patients treated with rufinamide (mean age 31.7 years; mean rufinamide dose 1373 mg/day; mean duration of treatment 2.8 months) and 635 placebo recipients (mean age 28.6 years, mean duration of treatment 3.0 months). Somnolence was reported somewhat less frequently in this population than in the LGS study, with an incidence of 11.8% for rufinamide and 9.1% among placebo recipients. The timing of events in this pooled analysis indicated a pattern of early onset and rapid resolution, and a tolerability profile not substantially different from placebo; the incidence of the most frequently reported adverse events with rufinamide vs placebo were headache (22.9% vs 18.9%), dizziness (15.5% vs 9.4%), fatigue (13.6% vs 9.0%), somnolence (11.8% vs 9.1%), and nausea (11.4% vs 7.6%).
This favorable tolerability and safety profile was maintained with long-term administration. The pooled tolerability and safety analysis included 1978 patients assessed for long-term tolerability and safety (mean age 31.3 years, mean rufinamide dose 1700 mg/day, maximum dose 7200 mg/day), 47% of whom were treated with rufinamide for at least 12 months; the most frequently reported adverse events in this population were headache (29.5%), dizziness (22.5%), and fatigue (17.7%) (Krauss et al 2005).
Rufinamide in context
Other randomized controlled trials in LGS
Few randomized, controlled clinical trials have been conducted in patients with LGS, none of which is a head-to-head comparison. Consequently, the available treatments for LGS cannot be compared directly, and only limited conclusions may be drawn from indirect comparisons.
The most robust approaches for indirect comparisons include systematic reviews and meta-analyses, such as those conducted by the Cochrane Group. A systematic (Cochrane) review of randomized, controlled trials in LGS (Hancock and Cross 2003) retrieved only 5 evaluable studies: one each conducted with topiramate, lamotrigine, felbamate, cinromide, and a thyrotropin releasing hormone (TRH) derivative, respectively (Anonymous 1989, 1993; Inanaga et al 1989; Motte et al 1997; Sachdeo et al 1999). This review concluded that no single agent was found to be highly efficacious in LGS, and that of those evaluated only lamotrigine, topiramate and felbamate were considered potentially efficacious as add-on therapy (Hancock and Cross 2003).
Data derived from this Cochrane analysis for each of the clinical trials are summarized in Table 1, together with data from the more recently reported rufinamide study (Glauser et al 2005a). Although this provides an indirect comparison, it is of interest to note that rufinamide produced a particularly marked effect in the reduction in drop attacks (tonic-atonic seizures), since this is also the parameter viewed as the primary outcome variable for LGS according to guidelines from the American Academy of Neurology (French et al 2004). The median reduction in tonic-atonic seizures was 42.5% with rufinamide, which compares well with rates observed in the separate studies with topiramate (14.8% reduction) or lamotrigine (34% reduction); the effect of felbamate on drop attacks was not reported. When corrected for the magnitude of placebo response, the median effect size for rufinamide on drop attacks was 41.1%, compared with 19.9% for topiramate and 14.8% for lamotrigine. Similarly, the proportion of patients who could be considered responders to therapy (achieving at least a 50% decrease in drop attacks) was 42.5% with rufinamide, 45.6% with topiramate, and 37.3% with lamotrigine, or 25.8%, 25.6%, and 14.8%, respectively, when corrected for placebo response.
Table 1.
Overview of randomized, double-blind, controlled trials of adjunctive drug treatment for Lennox-Gastaut syndrome: data from evaluable studies in Cochrane reviewa (Hancock and Cross 2003) and a recent double-blind trial of rufinamide (Glauser et al 2005a, Kluger et al 2006)
| Study reference | Patients and inclusion criteria | Treatments and duration | Key outcomesb |
|---|---|---|---|
| Glauser et al 2005a | n=138 (74 rufinamide, 64 placebo)
Age 4–37 years (mean 14.1) 390 seizures/month prior to baseline Taking 1–3 AEDs at fixed doses |
28-day baseline
14-day titration (placebo or rufinamide 45 mg/kg daily in 2 divided doses) 70 days of maintenance treatment (median dose 1800 mg/day) |
Oucomes for rufinamide vs placebo: Median reduction in tonic-atonic seizure frequency from baseline: 42.5% vs 1.4% (p<0.0001)
Responder ratec for tonic-atonic seizures: 42.5% vs 16.7% (p=0.0020) Median reduction in total seizure frequency: 32.7% vs 11.7% (p=0.0015) Patients with improvement in seizure frequency: 53.4% vs 30.6% (p=0.0041) Discontinuations due to adverse events: 6 (3 vomiting, 2 somnolence, 2 rash) vs 0 No deaths reported |
| Sachdeo et al 1999 | n=112 (48 topiramate, 50 placebo)
Age 1–30 years Slow spike and wave on EEG; 360 seizures/month prior to baseline (including drop attacks and atypical absence seizures) Taking 1 or 2 AEDs |
4-week baseline
11 weeks of placebo or topiramate (titrated over 3 weeks up to 6 mg/kg daily or maximal tolerated dose) |
Outcomes for topiramate vs placebo: Median change in drop attacks: 14.8% reduction vs 5.1% increase (p=0.041)
Patients achieving complete cessation of drop attacks: 2.2% vs 0 (RR 3.3, CI 0.1–7.8) Responder ratec for drop attacks: 45.6% vs 20.0% (RR=2.9, CI 0.8–10.2 for 375% reduction; RR=2.0, CI 0.9–4.6 for 50–74% reduction) Median reduction in total seizures 20.6% vs 8.8% (p=0.037) No reported discontinuations due to adverse effects or deaths |
| Motte et al 1997 | n=179 (79 lamotrigine, 90 placebo)
Age 3–25 years >1 type of predominantly generalized seizure for 31 year; <11 years old at onset; 31 seizure every 2 days; intellectual impairment and EEG with slow-wave complexes |
4-week baseline
16 weeks of placebo or lamotrigine (dose depending on concomitant valproate usage and body weight) |
Outcomes for lamotrigine vs placebo: Median reduction in drop attacks: 34% vs 9%
Responder ratec for drop attacks: 37.3% vs 22.5% (p=0.04, RR=1.7, CI=1.0–2.7) Responder ratec for tonic-clonic seizures: 43.3% vs 20.3% (RR=2.1, CI=1.2–3.8) Responder ratec for total seizures: 33.3% vs 15.7% (RR=2.1, CI=1.2–3.8) Median change in total seizure frequency: 32% reduction vs 9% increase Discontinuations: 3 (1 worsening seizures; 2 rash) vs 7 (6 worsening seizures; 1 rash) No deaths reported |
| Anonymous 1993 | n=73 (37 felbamate, 36 placebo) 390 atonic or atypical absence seizures/month during 8 weeks prior to baseline
Taking £2 AEDs |
28-day baseline
14-day titration (placebo or felbamate 15 mg/kg daily increasing to 30 mg/kg at 7 days and the lower of either 14 mg/kg daily or 3600 mg/day at 14 days) 56 days of maintenance treatment |
Outcomes for felbamate vs placebo: Patients achieving complete cessation of seizures: 10.8% vs 2.7% (p=0.2, RR=3.9, CI=0.5–33.2)
Patients achieving complete cessation of atonic seizures: 17.9% vs 0% (p=0.1, RR=5.7, CI=0.5–149.8) Patients achieving complete cessation of tonic-clonic seizures: 43.7% vs 7.7% (p=0.08, RR=5.7, CI=0.8–40.5) Mean change in total seizure frequency: 19% reduction vs 4% increase (p=0.002) Discontinuations: 1 (somnolence and ataxia) vs 1 (pancreatitis) No deaths reported |
| Anonymous 1989 | n=73 (26 cinromide, 30 placebo)
Age 2–18 years 340 seizures/2 weeks during baseline; predominantly generalized slow spike and wave discharges on EEG Taking 33 marketed AEDs |
6-week baseline 18
weeks of placebo or cinromide (20–40 mg/kg daily in 4 divided doses, titrated up to 83–109 mg/kg as required) |
Outcomes for cinromide vs placebo: Responder ratec for total seizures: 26.9% vs 23.3%
Patients achieving complete cessation of seizures: 0 vs 0 No significant benefit in any Cochrane analyses of RR No reported discontinuations due to adverse events, or deaths |
| Inanaga et al 1989 | n=98 with LGS (48 low dose TRH, 50 high dose TRH)
Age >2 years or weight >15kg Stable condition No excess AED-induced sedation |
8 weeks of treatment with low or high dose TRH DN-1417 (0.4 mg/kg daily or 1.6 mg/kg daily) | Outcomes for high dose vs low dose: Responder ratec for absence seizures: 19.2% vs 4.8%
Responder ratec for tonic seizures: 20.8% vs 12.1% Responder ratec for atonic seizures: 21.7% vs 20.0% No significant RR of treatment effect with high-dose TRH; significant RR with low-dose TRH only for achievement of 0–24% reduction in tonic seizures (RR=2.0, CI=0.1–46.1) No reported discontinuations due to adverse events, or deaths |
Evaluable studies as identified in a Cochrane review (Hancock and Cross 2003), plus recently reported randomized, double-blind trial of rufinamide (Glauser et al 2005a).
Key outcomes as reported by Glauser et al (2005a) for rufinamide study, and derived from data reported in Cochrane review (Hancock and Cross 2003) for all other studies.
Responder rate defined as % patients achieving
50% reduction from baseline in seizure frequency.
Abbreviations: AED, antiepileptic drug; CI, 95% confidence interval; EEG, electroencephalogram; RR, relative risk; TRH, thyrotropin releasing hormone.
As with drop attacks, the effects of these agents on total seizure frequency produced some interesting findings. The rufinamide, topiramate, and lamotrigine studies demonstrated 32.7%, 20.6%, and 32.0% median reductions in total seizures, and treatment effect sizes (adjusted for placebo) of 20.0%, 12.6%, and 17.6%, respectively. In the felbamate study, outcomes were reported as mean rather than median values, resulting in a 19% mean reduction in total seizures, and a 23% mean effect size. The number of patients achieving complete cessation of seizures has only been reported with felbamate, and although these results are favorable, cannot be compared with the other AEDs in the studies available to date.
Of course, the overall benefit of treatment comprises a balance between reduction in (or cessation of) seizures and tolerability/safety. The reported tolerability of treatment was broadly comparable among the studies described in Table 1, with relatively small differences in rates of discontinuations due to adverse events.
Additional tolerability and safety considerations
Each of these currently available agents has been associated with warnings relating to severe adverse events. The use of felbamate has been restricted in many countries following reports of idiosyncratic aplastic anemia and hepatic failure, which led to its initial withdrawal in the USA, although limited use was granted subsequently (FDA 1994). Similarly, the efficacy of lamotrigine must be balanced against the risk of serious skin reactions in susceptible individuals, for which boxed warnings or safety alerts have been issued (Committee on Safety of Medicines 1996; FDA 1997), while the use of topiramate is associated with warnings relating to oligohydrosis and hyperthermia in children, and also metabolic acidosis and glaucoma (FDA 2003; Janssen-Cilag 2006). In addition, factors such as cognitive slowing and weight loss may be issues with the use of topiramate (Levy et al 2002c).
The timing of these warnings, some time after the initial marketing of these AEDs, illustrates the difficulties in identifying such events during clinical trials, and that further evaluation is required to confirm the long-term safety of rufinamide in comparison with the marketed drugs.
An additional consideration in the long-term use of AEDs is cardiac safety. Indeed, increased QT interval dispersion has been demonstrated in children with epilepsy, potentially predisposing them to serious cardiac events (Akalin et al 2003). Rufinamide has demonstrated excellent cardiovascular tolerability, with no increase in QT intervals in a cardiac safety study conducted in 15 healthy volunteers treated with placebo or ascending doses of rufinamide (from 800 to 7200 mg/day) over 18 days (Marchand et al 2005). On the contrary, rufinamide produced a small dose-dependent decrease in QT.
Conclusions
LGS is a catastrophic and debilitating age-specific epileptic disorder, for which a substantial unmet need exists for highly effective treatment options. The data reviewed here indicate that adjunctive therapy with rufinamide, a new AED that is structurally unrelated to other currently available AEDs, significantly reduces seizure frequency in patients with LGS, accompanied by good tolerability, both of which are maintained during long-term use. In particular, the reduction in drop attacks adds substantially to patient quality of life.
The efficacy of rufinamide is most likely derived from its ability to block sodium channels, thereby limiting high-frequency action potential firing, a mechanism shared by some other AEDs including phenytoin, carbamazepine, lamotrigine, and oxcarbazepine (McLean et al 2005). However, it is commonplace for patients with LGS to remain symptomatic despite optimal therapy, and it is notable that, in the studies reviewed, adjunctive rufinamide treatment was able to exert significant improvement in a treatment-resistant population receiving 1–3 other AEDs at baseline.
In an indirect comparison with randomized controlled trials examining other AEDs, the results with rufinamide appear impressive: a median reduction in drop attacks of 42.5% with rufinamide, compared with 14.8% with topiramate and 34% for lamotrigine, representing treatment effect sizes, after adjustment for placebo response, of 41.1%, 19.9%, and 14.8%, respectively. This was associated with a median reduction in total seizures of 32.7% with rufinamide, compared with 20.6% in the topiramate study and 32% in the lamotrigine study. Although the full safety data for the rufinamide study in LGS have yet to be reported, data from a pooled safety database suggest a good tolerability profile for both short- and long-term use of rufinamide, with a pattern of early onset and early resolution of events. Furthermore, this tolerability profile is accompanied by a “clean” cardiac safety profile, based on a dose-ranging study in healthy volunteers which included QT analysis. However, the full spectrum of efficacy as well as the safety profile of rufinamide will become clear with accumulation of long-term clinical experience and usage.
Overall, these findings suggest that rufinamide may be a valuable option for the adjunctive treatment of LGS.
Acknowledgments
Publication of this paper was supported by Eisai. Editorial and project management services were provided by ACUMED® (Tytherington, Cheshire, UK).
References
- Akalin F, Tirtir A, Yilmaz Y. Increased QT dispersion in epileptic children. Acta Paediatr. 2003;92:916–20. doi: 10.1080/08035250310003550. [DOI] [PubMed] [Google Scholar]
- Anonymous Double-blind, placebo-controlled evaluation of cinromide in patients with the Lennox-Gastaut syndrome. The Group for the Evaluation of Cinromide in the Lennox-Gastaut syndrome. Epilepsia. 1989;30:422–9. doi: 10.1111/j.1528-1157.1989.tb05321.x. [DOI] [PubMed] [Google Scholar]
- Anonymous Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). The Felbamate Study Group in Lennox Gastaut syndrome. N Engl J Med. 1993;328:29–33. doi: 10.1056/NEJM199301073280105. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the third Eilat conference. Epilepsy Res. 1996;25:299–319. doi: 10.1016/s0920-1211(96)00081-2. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the fourth Eilat conference. Epilepsy Res. 1999;34(1):1–41. doi: 10.1016/s0920-1211(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the fifth Eilat conference. Epilepsy Res. 2001;43:11–58. doi: 10.1016/s0920-1211(00)00171-6. [DOI] [PubMed] [Google Scholar]
- Borowicz KK, Piskorska B, Kimber-Trojnar Z, et al. Is there any future for felbamate treatment? Pol J Pharmacol. 2004;56:289–94. [PubMed] [Google Scholar]
- Brunner LA, Harrigan EP, John VA, Powell ML. Pharmacokinetics of a new anticonvulsant (CGP 33101) in epileptic male patients and healthy male subjects after single ascending oral doses of 400–1200 mg. Am J Ther. 1994;1:215–20. doi: 10.1097/00045391-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Cardot JM, Lecaillon JB, Czendlik C, et al. The influence of food on the disposition of the antiepileptic rufinamide in healthy volunteers. Biopharm Drug Dispos. 1998;19:259–62. doi: 10.1002/(sici)1099-081x(199805)19:4<259::aid-bdd98>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chang SW, Choi L, Karoplchyk MA. A pharmacokinetic evaluation of rufinamide in elderly and younger subjects [abstract] Epilepsia. 1998;39(Suppl 6):59. (2.110) [Google Scholar]
- Cheung WK, Kianifard F, Wong A, et al. Intra- and inter-subject variabilities of CGP 33101 after replicate single oral doses of two 200-mg tablets and 400-mg suspension. Pharm Res. 1995;12:1878–82. doi: 10.1023/a:1016275402723. [DOI] [PubMed] [Google Scholar]
- Committee on Safety of Medicines. Current problems in pharmacovigilance. [Accessed 21 June 2006.];Reminder: Lamotrigine (Lamictal) and serious skin reactions [online] 1996 URL: http://www.mhra.gov.uk/home/groups/pl-p/documents/publication/con2023237.pdf.
- Critchley D, Fuseau E, Perdomo C, Arroyo S. Pharmacokinetic and pharmacodynamic parameters of adjunctive rufinamide in patients with Lennox-Gastaut Syndrome [abstract] Epilepsia. 2005;46(Suppl 7):2.351. [Google Scholar]
- Dulac O, Engel J. Lennox-Gastaut Syndrome. [Accessed 21 June 2006];Report of the International League against Epilepsy (updated 23 August 2003) [online] 2003 URL: http://www.ilae-epilepsy.org/ctf/lennox_gastaut.html.
- Eisai Co Ltd. [Accessed 21 June 2006];Annual Report 2005 [online] 2005 URL: http://www.eisai.co.jp/pdf/eannual/epdf200504an.pdf.
- FDA document T94-46. [Accessed 21 June 2006];Felbatol update [online] 27 September 1994. 1994 URL: http://www.fda.gov/bbs/topics/ANSWERS/ANS00605.html.
- FDA ‘Dear Doctor’ letter. [Accessed 21 June 2006];[online] 1997 URL: http://www.fda.gov/medwatch/safety/1997/lamict.htm.
- FDA ‘Dear Doctor’ letter. [Accessed 21 June 2006];[online] 2003 URL: http://www.fda.gov/medwatch/SAFETY/2003/topamax.htm.
- French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society [online] [Accessed 21 June 2006];Neurology. 2004 62(8):1261–73. doi: 10.1212/01.wnl.0000123695.22623.32. URL: http://www.neurology.org/cgi/content/full/62/8/1261. [DOI] [PubMed]
- Fuseau E, Critchley D, Perdomo C, Arroyo S. Population pharmacokinetic drug–drug interaction analyses of rufinamide studies in patients with epilepsy [abstract] Epilepsia. 2005;46(Suppl 8):2.356. [Google Scholar]
- Glauser TA, Kluger G, Sachdeo R, et al. Efficacy and safety of rufinamide adjunctive therapy in patients with Lennox-Gastaut Syndrome (LGS): A multicenter, randomized, double-blind, placebo-controlled parallel trial [abstract] Neurology. 2005a;64(10):1862. (LBS.001) [Google Scholar]
- Glauser T, Kluger G, Sachdeo R, et al. Open-label extension study of the efficacy and safety of rufinamide adjunctive therapy in patients with Lennox-Gastaut syndrome [abstract] Epilepsia. 2005b;46(Suppl 6):408. 1356. [Google Scholar]
- Guerrini R. Epilepsy in children. Lancet. 2006;367(9509):499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- Hancock E, Cross H. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev. 2003;3:CD003277. doi: 10.1002/14651858.CD003277. [DOI] [PubMed] [Google Scholar]
- Inanaga K, Kumashiro H, Fukyama Y, et al. Clinical study of oral administration of DN-1417, a TRH analog, in patients with intractable epilepsy. Epilepsia. 1989;30:438–45. doi: 10.1111/j.1528-1157.1989.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Janssen-Cilag Ltd. Topamax® [online] [Accessed 21 June 2006];High Wycombe, UK 20 January 2006. 2006 URL: http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=6768.
- Kapeghian JC, Madan A, Parkinson A, et al. Evaluation of rufinamide (CGP 33101), a novel anticonvulsant, for potential drug interactions in vitro [abstract] Epilepsia. 1996;37(Suppl 5):26. (1.96) [Google Scholar]
- Kluger G, Glauser T, Sachdeo R, et al. Short-term and long-term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox-Gastaut syndrome. Poster presented at the 7th European Congress on Epileptology; Helsinki, Finland. 2–6 July 2006.2006. [Google Scholar]
- Krauss GL, Perdomo C, Arroyo S. Short-term and long-term safety of rufinamide in patients with epilepsy [abstract] Epilepsia. 2005;46(Suppl 7):2.363. [Google Scholar]
- Levy RH, Manson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5th ed . Philadelphia: Lippincott Williams and Wilkins; 2002a. pp. 906–12. [Google Scholar]
- Levy RH, Manson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5th ed . Philadelphia: Lippincott Williams and Wilkins; 2002b. pp. 206–14. [Google Scholar]
- Levy RH, Manson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. 5th ed . Philadelphia: Lippincott Williams and Wilkins; 2002c. pp. 760–764. [Google Scholar]
- McLean MJ, Schmutz M, Pozza M, et al. The Influence of rufinamide on sodium currents and action potential firing in rodent neurons [abstract] Epilepsia. 2005;46(Suppl 7):3.062. [Google Scholar]
- Marchand M, Critchley D, Nagy C, Fuseau E. The effect of rufinamide concentration on the QT interval in healthy subjects treated during 18 days with multiple ascending doses: a population PKPD analysis. Abstract presented at the Annual Meeting of the Population Approach Group in Europe 2005 [online]; (A785); 2005. [Accessed 21 June 2006]. URL: http://www.page-meeting.org/default.asp?abstract=785. [Google Scholar]
- Markand ON. Lennox-Gastaut syndrome (childhood epileptic encephalopathy) J Clin Neurophysiol. 2003;20:426–41. doi: 10.1097/00004691-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Motte J, Trevathan E, Arvidsson JF, et al. Lamotrigine for generalised seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. N Engl J Med. 1997;337:1807–12. doi: 10.1056/NEJM199712183372504. [DOI] [PubMed] [Google Scholar]
- Nabbout R, Dulac O. Epileptic encephalopathies: a brief overview. J Clin Neurophysiol. 2003;20:393–7. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Sachdeo RC, Rosenfeld WE, Choi L, et al. Pharmacokinetics and safety of adjunctive rufinamide therapy in pediatric patients with epilepsy [abstract] Epilepsia. 1998;39(Suppl 6):166–7. (5.081) [Google Scholar]
- Sachdeo RC, Glauser TA, Ritter F, et al. A double-blind, randomized trial of topiramate in Lennox-Gastaut syndrome. Topiramate YL Study Group. Neurology. 1999;52:1882–7. doi: 10.1212/wnl.52.9.1882. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Bourgeois B. A risk-benefit assessment of therapies for Lennox-Gastaut syndrome. Drug Saf. 2000;22:467–77. doi: 10.2165/00002018-200022060-00005. [DOI] [PubMed] [Google Scholar]
- Svendsen KD, Choi L, Chen BL, et al. Single center, open-label, multiple-dose pharmacokinetic trial investigating the effect of rufinamide administration on Ortho-Novum 1/35 in healthy women [abstract] Epilepsia. 1998;39(Suppl 6):59. (2.111) [Google Scholar]
- Waldmeier F, Gschwind H-P, Rouan M-C, et al. Metabolism of the new anticonvulsive trial drug rufinamide (CGP 33101) in healthy male volunteers [abstract] Epilepsia. 1996;37(Suppl 5):167. (6.60) [Google Scholar]
- White S, Schmutz M, Pozza M, et al. The anticonvulsant profile and tolerability of rufinamide in mice and rats [abstract] Epilepsia. 2005;46(Suppl 7):3.088. [Google Scholar]