Abstract
BACKGROUND AND OBJECTIVE
To correlate peripapillary retinal nerve fiber layer (NFL) loss and visual field defects in nonarteritic ischemic optic neuropathy (NAION).
PATIENTS AND METHODS
Patients with NAION and control subjects were enrolled in a case-control study. Participants were scanned with a Fourier domain optical coherence tomography (OCT) system. Peripapillary NFL thickness was averaged in hemispheric, quadrant, and octant divisions. Standard achromatic static perimetry was used to assess visual fields.
RESULTS
The reproducibility of peripapillary NFL parameters was excellent in both the healthy and NAION groups. Eyes in the NAION group showed a significant decrease of peripapillary NFL thickness in terms of the overall average, all quadrant averages, and all octants. There were statistically significant correlations between the peripapillary NFL and visual fields in terms of both overall averages and superior-inferior differences.
CONCLUSION
In NAION, the visual field and peripapillary NFL losses are correlated in both severity and location. Fourier domain OCT provides reproducible measurement of the peripapillary NFL and may be useful in the assessment of NAION.
INTRODUCTION
Nonarteritic ischemic optic neuropathy (NAION) is characterized by sudden, painless, nonprogressive visual loss of variable degree. It is initially unilateral, but there will often be later involvement of the fellow eye. NAION typically occurs in patients older than age 50. The visual fields in eyes affected by NAION are characterized by altitudinal visual field defects. However, there has been some controversy as to how “typical” are such visual fields in NAION.
NAION is a static optic neuropathy. Following the acute ischemic event, optic atrophy develops and loss of the retinal ganglion cells and their axons remains unchanged. NAION kills retinal ganglion cells rather than just making them less functional.1 However, a patient with glaucoma who has a retinal nerve fiber layer thickness greater than predicted may have ganglion cell axons that are malfunctioning but are alive.
Hood et al.1 described a simple linear model of the relationship between retinal nerve fiber layer thickness and standard automated perimetry field losses resulting from NAION and glaucoma and predicted that a complete loss of retinal ganglion cells in humans leaves a residual retinal nerve fiber layer thickness composed, at least in part, of glial cells and blood vessels.2,3 The thickness of this residual layer varies among individuals. This finding conflicts with the monkey model,4 which assumes that a complete loss of retinal ganglion cells reduces the thickness to zero.
Optical coherence tomography (OCT) is a high-resolution cross-sectional imaging modality5,6 that has been used to measure the peripapillary nerve fiber layer for the diagnosis of glaucoma7 and optic neuropathies.8 Recently, a new technology called Fourier domain OCT has greatly enhanced the speed and sensitivity of OCT imaging.9
The purpose of this article is to evaluate the use of Fourier domain OCT in the diagnosis of NAION. One aim is to determine the correlation between peripapillary nerve fiber layer thickness and visual field loss and to assess the reproducibility of the Fourier domain OCT peripapillary nerve fiber layer measurements in both healthy subjects and subjects with NAION. Additionally, this study is designed to assess the residual peripapillary nerve fiber layer thickness in patients in retinal regions corresponding to those areas of severe visual field loss.
PATIENTS AND METHODS
Patients with NAION and healthy subjects were enrolled in a case-control study. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of the University of Southern California.
The control subjects were selected from the healthy group of the Advanced Imaging for Glaucoma Study (www.AIGStudy.net). The inclusion criteria were intraocular pressure of less than 21 mm Hg for both eyes, a normal Humphrey SITA 24-2 visual field, a central corneal thickness of 500 μm or greater, a normal-appearing optic nerve head, a normal nerve fiber layer, an open anterior chamber angle, refraction between +3.00 and -7.00 diopters (D), and no history of chronic ocular or systemic corticosteroid use.
The patients with NAION had at least one eye with NAION that was tested at least 5 months after the ischemic attack. The 5-month cutoff was chosen to allow sufficient time to minimize the effects of optic disc swelling and to allow the retinal ganglion cell axons to degenerate. Ten of the patients had asymmetrical NAION (ie, only one eye had a documented attack of NAION).
Visual field was assessed with the Humphrey Visual Field Analyzer (Carl Zeiss Meditec, Inc., Dublin, CA) using the SITA 30-2 program.
Peripapillary nerve fiber layer thickness was measured using the Nerve Head Map 4 mm (NHM4) pattern (Fig. 1A) on the RTVue FD-OCT system (Optovue, Inc., Fremont, CA). The RTVue system has a depth resolution of 5 μm full-width-half-maximum and acquires 26,000 axial scans per second. Fourier domain OCT has a much higher scan speed than time domain OCT (TD-OCT) because it uses a spectrometer instead of a single detector at the detector arm of the interferometer. Thus, the slow physical scanning of the reference mirror is replaced by Fourier transform of the spectrum.5 The RTVue system works at a central wavelength of 830 nm.
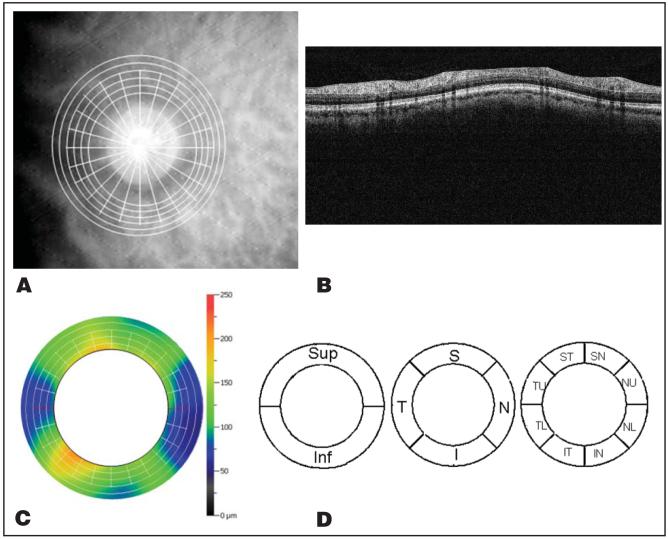
Figure 1.
Nerve Head Map 4 mm (NHM4) scan pattern and the automatic algorithm to create the peripapillary nerve fiber layer (ppNFL) thickness map. A, NHM4 scan pattern overlaid on the fundus image. B, The detected boundaries were overlaid with an optical coherence tomography image of one ring in Figure 1A. From top to bottom, three boundaries are detected: internal limiting membrane, outer ppNFL boundary, and inner segment and outer segment junction. The ppNFL thickness is the distance from the internal limiting membrane to the outer NFL. C, The ppNFL thickness map was interpolated from the ppNFL thickness profiles of six circular scans. D, The ppNFL thickness map was averaged over the whole ring, hemisphere, quadrant, and octant divisions. T = temporal; S = superior; N = nasal; I = inferior; U = upper; L = lower.
For the NHM4 scan, the scan depth is 2.3 mm and the transverse spot size is 15 μm. The NHM4 contains 12 radial scans with a diameter of 4.0 mm and 6 circular scans with diameters from 2.5 to 4.0 mm. The peripapillary nerve fiber layer thickness maps were obtained from the NHM4 scans using an automated image processing algorithm that we developed. The peripapillary nerve fiber layer thickness within a diameter of 2.5 to 4.0 mm was averaged in subsequent analysis.
To detect peripapillary nerve fiber layer thickness, only the six circular scans were used. First, the internal limiting membrane and inner segment and outer segment (IS/OS) junction were detected as the first and second gradient peak. The OCT images were then aligned according to IS/OS junction. Blood shadowing A-scans were removed. The usual intensity distribution pattern between the internal limiting membrane and IS/OS junction shows two or three white bands, including nerve fiber layer, inner plexiform layer, and outer plexiform layer band. The outer nerve fiber layer boundary is detected as the last gradient peak in front of the second white band. Peripapillary nerve fiber layer thickness is equal to the distance from the internal limiting membrane to the outer nerve fiber layer boundary (Fig. 1B). The peripapillary nerve fiber layer thickness map is then interpolated based on the peripapillary nerve fiber layer thickness profile of the six circular scans (Fig. 1C).
To center the scans around the optic disc to ensure that the same location was evaluated every time, there was an internal fixation target in the RTVue system, which is a flash blue dot in the patient’s view. The operator saw a scan pattern overlaid on a video fundus image and adjusted the position of the scan pattern until it was centered on the disc.
The NHM4 scan pattern required only 0.39 second; therefore, the potential for motion error is less than other OCT systems where nerve fiber layer or disc scans typically require 1 second or more. The operator inspected the cross-sectional images on the computer display immediately after acquisition. If any of the images showed motion artifact, the operator would reject the scan and acquire a new one instead. The same operator performed all of the scans, and the eyes were dilated for OCT imaging.
The RTVue system provides a parameter named SSI for signal strength. For patients with NAION, the average SSI was 40. For healthy eyes, the average SSI was also 40. A scan was excluded if the SSI was less than 38 or the algorithm failed to detect the correct boundary. If all three repeat scans for the same patient were excluded, the patient was excluded from the final analysis. The image processing software did not adjust centration or magnification.
The peripapillary nerve fiber layer thickness was averaged over the entire map and also in hemispheric, quadrant, and octant divisions (Fig. 1D).
Study participants were scanned with the NHM4 three times during one visit. Intravisit repeatability was assessed by intraclass correlation, pooled standard deviation, and coefficient of variation.
The absolute thicknesses were compared between control patients and patients with NAION by using the Student’s t test. Standardized deviation is the ratio of the average peripapillary nerve fiber layer thickness of the NAION group minus the mean of the control group to the standardized deviation of the control group. It provided another criterion indicating how different the NAION and control groups were. The minimum peripapillary nerve fiber layer thickness was also calculated to be compared with other studies in the literature. The mean differences between control and NAION eyes were also calculated for all tested parameters. To statistically compare the mean differences, we calculated the deviation from the mean of the control group for each NAION eye parameter. Thus, the paired t test was performed to assess statistical significance for any pair of overall average, hemisphere, quadrant, and octant parameters.
To measure the correlation between visual field and peripapillary nerve fiber layer thickness, the superior and inferior hemisphere averages of total deviation and pattern deviation were correlated with the superior and inferior hemisphere averages of peripapillary nerve fiber layer thickness.
To adjust intereye correlation, linear mixed models or generalized estimating equation10 was used to account for the correlation between the left and right eyes of the same subject. Specifically, the robust variance estimation (Huber-White sandwich estimator),11,12 an option under the generalized estimating equation approach, was used to estimate the standard error of parameters.
RESULTS
Twenty-two eyes from 16 patients with NAION and 39 eyes from 20 healthy subjects were analyzed. Their demographic information is shown in Table 1. Both the healthy and NAION groups consisted of a majority of white subjects and a minority of African American subjects. A minority in both groups were Hispanic. The NAION group also included a few subjects of Middle Eastern descent. The age and gender distributions were not significantly different between the healthy and NAION groups.
TABLE 1. Demography and Visual Field Parameter of the Healthy and Nonarteritic Ischemic Optic Neuropathy Groups.
| Parameter/Groupa | Healthy | NAION | P |
|---|---|---|---|
| No. of subjects | 20 | 16 | - |
| No. of eyes | 39 | 22 | - |
| Age (y) | 63.5 (5.2) | 64.0 (9.0) | .9 |
| Female (% total) | 70% | 37.5% | .051 |
| Hyperopic (%) | 38.5% | 86.4% | .002 |
| MD (dB) | -0.1 | -13.4 | < .0001 |
| PSD (dB) | 1.5 (0.3) | 11.1 (3.6) | < .0001 |
| Spherical equivalent (D) | -0.4 (2.0) | 2.9 (1.5) | < .0001 |
NAION = non-arteritic ischemic optic neuropathy; MD = mean deviation on visual field; PSD = pattern standard deviation.
The distributions of continuous parameters are listed in the format: mean (standard deviation).
The RTVue system was able to measure peripapillary nerve fiber layer thickness with excellent reproducibility in both normal eyes and and eyes with NAION (Tables 2 and 3). The healthy and NAION groups had similar reproducibilities. The peripapillary nerve fiber layer average was the most reproducible parameter for both groups. In general, the reproducibility was best for the overall average and declined with finer divisions. The coefficient of variation was 2.9% for the overall average and ranged from 3.7% to 3.9% for hemisphere averages, from 4.9% to 7.6% for quadrant averages, and from 6.0% to 9.0% for octant averages.
TABLE 2. Repeatability of the Total, Hemisphere, and Quadrant Averages.
| Healthy |
NAION |
|||||
|---|---|---|---|---|---|---|
| Parameter | SD (μm) | CV (%) | ICC | SD (μm) | CV (%) | ICC |
| Overall average | 2.03 | 2.16 | 0.96 | 1.50 | 2.87 | 0.99 |
| Hemisphere | ||||||
| Superior | 2.68 | 2.80 | 0.92 | 1.93 | 3.88 | 0.99 |
| Inferior | 2.22 | 2.41 | 0.97 | 2.00 | 3.67 | 0.99 |
| Quadrant | ||||||
| Superior | 4.63 | 4.24 | 0.86 | 2.99 | 5.80 | 0.98 |
| Inferior | 3.33 | 2.87 | 0.96 | 3.13 | 4.94 | 0.99 |
| Temporal | 4.35 | 6.03 | 0.91 | 3.26 | 7.17 | 0.97 |
| Nasal | 6.30 | 8.00 | 0.88 | 3.63 | 7.58 | 0.95 |
NAION = nonarteritic ischemic optic neuropathy; SD = pooled standard deviation; CV = coefficient of variation; ICC = intraclass correlation.
TABLE 3. Repeatability of the Octant Averages.
| Healthy |
NAION |
|||||
|---|---|---|---|---|---|---|
| Octant | SD (μm) | CV (%) | ICC | SD (μm) | CV (%) | ICC |
| Superior temporal | 4.82 | 4.14 | 0.88 | 3.18 | 6.02 | 0.99 |
| Superonasal | 6.56 | 6.43 | 0.82 | 3.79 | 7.52 | 0.96 |
| Inferotemporal | 5.67 | 4.68 | 0.92 | 4.57 | 7.09 | 0.98 |
| Inferonasal | 6.11 | 5.51 | 0.93 | 4.78 | 7.65 | 0.98 |
| Temporal upper | 4.88 | 6.07 | 0.92 | 3.59 | 7.71 | 0.97 |
| Temporal lower | 4.75 | 7.41 | 0.89 | 4.02 | 9.04 | 0.95 |
| Nasal upper | 6.47 | 7.65 | 0.89 | 4.07 | 8.38 | 0.94 |
| Nasal lower | 6.69 | 9.16 | 0.88 | 4.00 | 8.47 | 0.95 |
NAION = nonarteritic ischemic optic neuropathy; SD = pooled standard deviation; CV = coefficient of variation; ICC = intraclass correlation.
The NAION group had a significantly thinner peripapillary nerve fiber layer in every quadrant and octant division compared with the healthy group (Tables 4 and 5). The relative loss was greatest in the superior quadrant and superotemporal octant. Based on paired t tests with the generalized estimating equation approach, the relative loss in the superior quadrant (Table 4) was significantly greater than in the other quadrants (P < .0001) except for the inferior quadrant (P = .14). Similarly, the relative loss in the superotemporal octant (Table 5) was significantly greater than in the other 6 octants (P < .002), except for the inferotemporal octant (P = .23). The typical pattern of loss is illustrated by examples of a typical NAION eye compared with a healthy eye in Figure 2. The most common pattern in this series is thinning of the peripapillary nerve fiber layer that is most severe in the superotemporal octant, correlating with visual field loss that is most severe in the inferonasal quadrant.
TABLE 4. Population Averages at the Overall, Hemisphere, and Quadrant Divisions and the Difference Between the Healthy and Nonarteritic Ischemic Optic Neuropathy Groups.
| Healthy |
NAION |
NAION-Normal |
||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Average | Pop SD | Average | Pop SD | Minimum | Difference (μm) | SD | P |
| Overall average | 93.5 | 10.3 | 52.8 | 18.8 | 32.4 | -40.7 | -4.0 | < .001 |
| Hemisphere | ||||||||
| Superior | 95.8 | 9.3 | 51.1 | 20.5 | 31.5 | -44.8 | -4.8 | < .001 |
| Inferior | 91.3 | 12.5 | 54.5 | 20.1 | 32.1 | -36.8 | -2.9 | < .001 |
| Quadrant | ||||||||
| Superior | 110.3 | 11.6 | 53.3 | 23.6 | 32.4 | -57.0 | -4.9 | < .001 |
| Inferior | 114.4 | 16.8 | 63.0 | 28.9 | 34.2 | -51.4 | -3.1 | < .001 |
| Temporal | 72.2 | 14.3 | 46.7 | 19.5 | 26.4 | -25.5 | -1.8 | < .001 |
| Nasal | 77.1 | 15.5 | 48.1 | 15.8 | 27.0 | -29.0 | -1.9 | < .001 |
NAION = nonarteritic ischemic optic neuropathy; Pop SD = population standard deviation; SD = standard deviation.
TABLE 5. Octant Averages and Differences Between Healthy and Nonarteritic Ischemic Optic Neuropathy Groups.
| Healthya |
NAIONa |
NAION-Healthy |
||||||
|---|---|---|---|---|---|---|---|---|
| Octant | Average | Pop SD | Average | Pop SD | Minimum | Difference | SD | P |
| Superotemporal | 116.7 | 13.7 | 54.5 | 27.5 | 29.7 | -62.2 | -4.6 | < .001 |
| Superonasal | 103.9 | 14.1 | 52.0 | 20.8 | 30.3 | -51.9 | -3.7 | < .001 |
| Inferotemporal | 121.3 | 19.7 | 63.9 | 29.9 | 34.4 | -57.4 | -2.9 | < .001 |
| Inferonasal | 107.4 | 20.4 | 62.0 | 30.5 | 32.0 | -45.4 | -2.2 | < .001 |
| Temporal upper | 79.7 | 17.2 | 48.3 | 23.7 | 21.9 | -31.4 | -1.8 | < .001 |
| Temporal lower | 64.8 | 13.4 | 45.0 | 19.0 | 25.1 | -19.8 | -1.5 | < .001 |
| Nasal upper | 82.9 | 17.5 | 49.3 | 16.4 | 27.6 | -33.6 | -1.9 | < .001 |
| Nasal lower | 71.5 | 15.2 | 47.0 | 16.0 | 26.4 | -24.5 | -1.6 | < .001 |
NAION = nonarteritic ischemic optic neuropathy; Pop SD = population standard deviation; SD = standard deviation.
Population averages and standard deviations were shown.
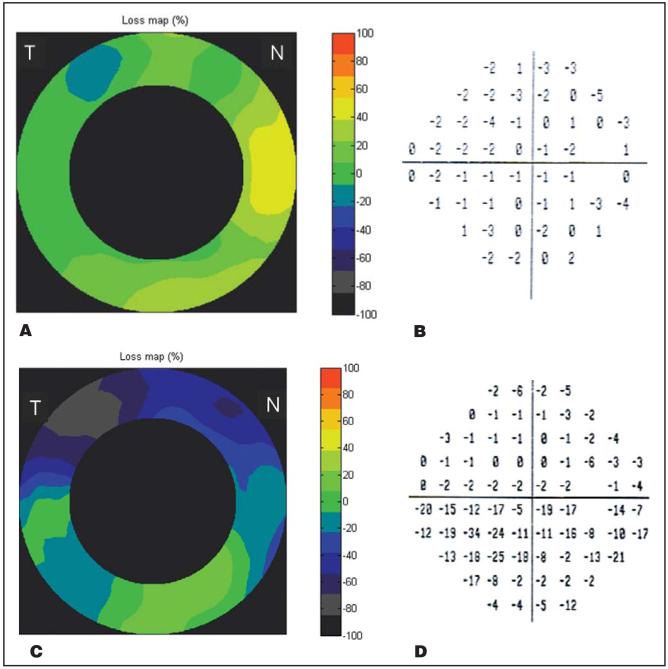
Figure 2.
Examples of a healthy eye and an eye affected by NAION. Both are right eyes. The NAION eye showed severe loss of superior peripapillary nerve fiber layer that correlated well with defects in the inferior visual field. A, Peripapillary nerve fiber layer thickness loss map of a healthy eye (unit: % of normal). The small area of superotemporal loss was within the 5 percentile limit. B, Visual field total deviation (TD) plot of the healthy eye. All visual field parameters were within normal: mean deviation = -1.08 dB, pattern standard deviation = 1.46 dB. C, The NAION eye’s peripapillary nerve fiber layer thickness loss map showed severe (up to 80%) loss in the superior hemisphere. D, Visual field TD plot of the NAION eye. The Glaucoma Hemifield Test was outside normal limits; mean deviation = -7.81 dB, P < 0.5%; pattern standard deviation = 9.18 dB, P < 0.5%. T = temporal; N = nasal.
The correlation between the visual field mean deviation (MD) and the peripapillary nerve fiber layer thickness was plotted in Figure 3. The severity of visual field loss was significantly correlated with peripapillary nerve fiber layer thinning in general. Each micron of peripapillary nerve fiber layer thinning was correlated with approximately 0.26 dB of visual field sensitivity loss. The correlations between the visual field total deviation, superior-inferior difference (SID), and the peripapillary nerve fiber layer SID were plotted in Figure 4. The superior-inferior hemispheric asymmetry of visual field loss and peripapillary nerve fiber layer thinning was also significantly correlated.
Figure 3.
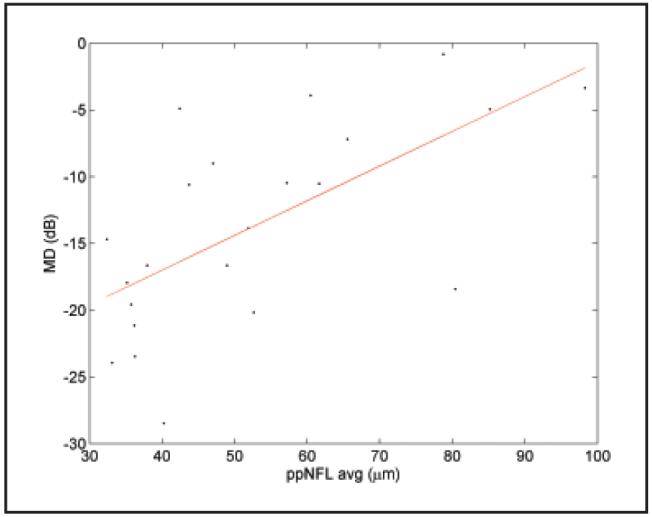
Plot of visual field mean deviation (MD) versus peripapillary nerve fiber layer overall average (ppNFL AVG). R2 = 0.37, slope = 0.26 dB/μm, P < .0001.
Figure 4.
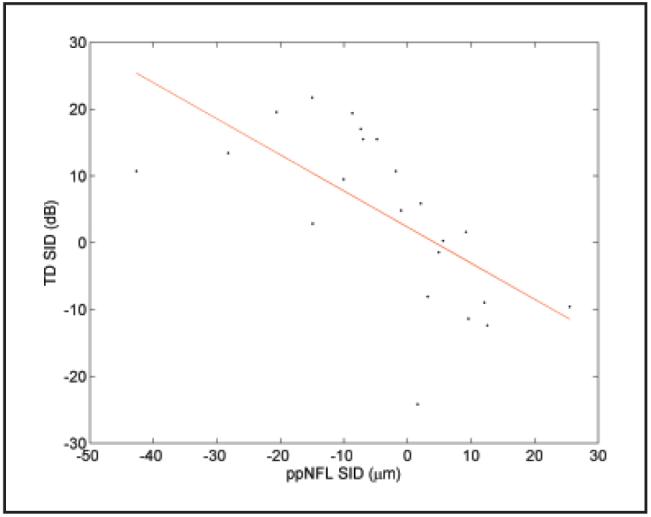
Plot of visual field total deviation (TD) superior-inferior difference (SID) versus peripapillary nerve fiber layer (ppNFL) SID. R2 = 0.38, slope = -0.54 dB/μm, P = .0002.
The correlations between the peripapillary nerve fiber layer hemispheric averages and both the visual field pattern deviation and total deviation hemispheric averages were calculated (Table 6). The superior peripapillary nerve fiber layer and the inferior visual field were significantly correlated on both pattern deviation and total deviation. The inferior peripapillary nerve fiber layer and the superior visual field were significantly correlated on total deviation.
TABLE 6. Correlation Between Hemispheric Averages of Peripapillary Nerve Fiber Layer and Visual Field.
| R Square |
||
|---|---|---|
| Parameter | ppNFL Superior | ppNFL Inferior |
| PD superior | 0.005 | 0.15 |
| PD inferior | 0.26* | 0.01 |
| TD superior | 0.04 | 0.27* |
| TD inferior | 0.36* | 0.09 |
ppNFL = peripapillary nerve fiber layer; PD = pattern deviation on visual field; TD = total deviation on visual field.
P < .05 for the slope of ppNFL parameter.
The most severe hemispheric visual field loss was associated with a residual peripapillary nerve fiber layer thickness of 32 μm (34%).
DISCUSSION
Our results show that a complete loss of retinal ganglion cells in humans leaves a residual retinal nerve fiber layer thickness that is at least in part the result of ganglion cells and blood vessels. The thickness of this residual layer varies among individuals. This is in concert with previous studies.2,3
NAION occurs acutely and, after a period of axonal swelling and then thinning, the optic neuropathy remains static after atrophy of retinal ganglion cells has taken place.13 The current study supports the value of OCT as an assessment tool after the ischemic attack of NAION had subsided and following a required period to allow sufficient time for the reversal of the effects of optic disc swelling and resolution of retinal ganglion cells axonal degeneration.
Visual field testing is useful in the evaluation of NAION in the acute stage. However, large scale studies demonstrate that the Humphrey Visual Field test shows significant intersubject variance and less of the characteristic altitudinal visual field defect that conventional wisdom dictates.14 This inter-subject variance is greater than that seen in OCT measurements.15
The RTVue system is one of a new generation of OCT instruments that use Fourier domain technology to greatly increase speed and sensitivity. Because Fourier domain OCT can map a fundus area in a fraction of a second, its results may be more precise than older time domain technology. Our results showed that the RTVue system was able to measure peripapillary nerve fiber layer thickness with excellent reproducibility in both healthy eyes and eyes with NAION. We also showed that the severity and location of visual field defects were significantly correlated with peripapillary nerve fiber layer thinning. We expect the RTVue system to become a valuable tool for the evaluation of optic neuropathies such as NAION in the chronic stage.
The proprietary image processing algorithm developed by the authors for peripapillary nerve fiber layer thickness measurement was licensed to Optovue, Inc. and released in RTVue software 3.0.
In this study, we showed that the division between affected and nonaffected hemispheres in NAION is more complicated than depicted by the altitudinal defects usually seen on the Humphrey Visual Field test. Furthermore, the correlation between OCT and the Humphrey Visual Field test in this study suggests that the Humphrey visual field picture is oversimplified. Expected strong correlations were found between superior visual field defects and inferior peripapillary nerve fiber layer OCT maps and between inferior visual field defects and superior peripapillary nerve fiber layer OCT maps.
There remain many points of controversy regarding the pathophysiology of NAION.16 What is clear is that NAION involves an apoplectic ischemic event. It is also accepted that this is watershed (nonthrombotic or embolic) ischemia. However, it remains unclear as to what are the exact mechanical and vascular factors and their potentially complex interplay.17,18
One feature of NAION, that the visual field loss is altitudinal, is frequently cited in the many purported NAION pathophysiologic explanations. The conclusions of this study, most particularly as related to the variance from strict altitudinal borders between affected and non-affected zones, should be taken into account in further models of NAION.
Mapping of the peripapillary nerve fiber layer with the RTVue system may provide valuable and complementary anatomic information to be used together with the visual field in the diagnosis and understanding of NAION.
Acknowledgments
Supported by NIH grants R01 EY013516 and P30 EY03040; a grant from Optovue, Inc.; and an unrestricted grant from Research to Prevent Blindness, Inc.
Drs. Tan and Huang receive grant support and patent royalty from Optovue, Inc. Dr. Huang also received stock options and travel support from Optovue.
REFERENCES
- 1.Hood DC, Anderson S, Rouleau J, et al. Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. Ophthalmology. 2008;115:904–910. doi: 10.1016/j.ophtha.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood DC. Relating retinal nerve fiber thickness to behavioral sensitivity in patients with glaucoma: application of a linear model. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1426–1430. doi: 10.1364/josaa.24.001426. [DOI] [PubMed] [Google Scholar]
- 3.Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: a test of a linear model. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.06-1401. In press. [DOI] [PubMed] [Google Scholar]
- 4.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., 3rd. The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Tan O, Fujimoto J, et al. Optical coherence tomography. In: Huang D, Kaiser PK, Lowder CY, Traboulsi EI, et al., editors. Retinal Imaging. Mosby; Philadelphia: 2006. pp. 47–65. [Google Scholar]
- 6.Drexel W, Morgner U, Ghanta RK, Kartner FX, Schuman JS, Fujimoto JG. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–507. doi: 10.1038/86589. Erratum in Nat Med. 2001;7:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Miller NR. Peripapillary nerve fiber layer thickness measured by optical coherence tomography in patients with no light perception from long-standing nonglaucomatous optic neuropathies. J Neuroophthalmol. 2007;27:176–179. doi: 10.1097/WNO.0b013e31814b1ac4. [DOI] [PubMed] [Google Scholar]
- 9.Cense B, Chen TC, Nassif N, et al. Ultra-high speed and ultra-high resolution spectral-domain optical coherence tomography and optical Doppler tomography in ophthalmology. Bull Soc Belge Ophtalmol. 2006;302:123–132. [PubMed] [Google Scholar]
- 10.Liang KY, Zeger SL. Longitudinal data analysis using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 11.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 12.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 13.Savini G, Bellusci C, Carbonelli M, et al. Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using Stratus OCT. Arch Ophthalmol. 2006;124:1111–1117. doi: 10.1001/archopht.124.8.1111. [DOI] [PubMed] [Google Scholar]
- 14.Feldon SE, Levin L, Scherer RW, et al. Development and validation of a computerized expert system for evaluation of automated visual fields from the Ischemic Optic Neuropathy Decompression Trial. BMC Ophthalmol. 2006;6:34. doi: 10.1186/1471-2415-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., 3rd. The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 16.Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23:157–163. doi: 10.1097/00041327-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Hayreh SS. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4:251–263. [PubMed] [Google Scholar]
- 18.Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305.e2. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]