Abstract
Increased vulnerability to psychosocial stressors likely predisposes individuals to decreased immune function and inability to control pathogens. While many factors influence the susceptibility to psychosocial stress, genetic polymorphisms may modify individual reactivity to environmental stressors. The present study evaluated how immune function was altered by the interaction of in polymorphisms in the gene that encodes the serotonin reuptake transporter (5HTT) and the psychosocial stress imposed by social subordination in adult female rhesus monkeys. Subjects were dominant and subordinate females that carried both alleles of the long promoter variant (l/l) of the 5HTT gene, and dominant and subordinate that had at least one allele for the short promoter length variant (l/s or s/s, s-variant). Plasma cortisol was higher in subordinate females in response to a social separation paradigm, confirming their increased reactivity to psychosocial stressors. Subordinate females exhibited increased T-cell activation and proliferation regardless of genotype. Despite these higher levels of T-cell proliferation and activation, subordinate females showed significantly lower frequency of T-cells. This latter finding may be due to an increased susceptibility to cell death, as indicated by higher levels of annexin-V+ CD4+ and CD8+ T-cells in s-variant subordinate compared to dominant females. These findings indicate that subordinate rhesus monkeys with the s-variant 5HTT genotype exhibit decreased T-cell numbers perhaps compromising their ability to mount an immune response to pathogens. These data underscore the importance for considering gene polymorphisms that influence emotional reactivity to better understand susceptibility to disease.
Keywords: Immune Function, 5HTTLPR, Social Status, Rhesus Monkeys
Introduction
Chronic exposure to stressors increases the risk for a wide range of adverse health outcomes, including accelerated progression of coronary artery disease (Kaplan et al., 1983; Rozanski et al., 1999); affective disorders (Bale, 2006; de Kloet et al., 2006) and addictive behaviors (Koob and Kreek, 2007); and reproductive dysfunction (Berga and Loucks, 2005). In addition, chronic activation of the stress axis also adversely affects immune function (Sapolsky, 1994), increasing vulnerability to opportunistic disease (Miller et al., 2002; Zozulya et al., 2008).
While a number of stress paradigms have been used to study alterations in immune function in animals (Koolhaas, 2008), a range of immune parameters are influenced by social status (Avitsur et al., 2002; Avitsur et al., 2001; Bohus et al., 1991b; de Groot et al., 2002; Sapolsky, 1994, 1995; Stefanski, 2000). For example, following establishment of a dominance hierarchy, subordinate rats exhibit decreased corticosteroid-binding globulin and decreased CD4 T-cells as compared to dominant animals (Stefanski, 2000). Another model shows subordinate mice who lost territory ownership have lower IgG proliferation and IL-2 levels (Bartolomucci et al., 2003). Additionally, social stressors also suppress lymphocyte proliferation, reduce control of latent herpes viruses, blunt humeral responses to immunization, and slow wound healing (Cohen, 1999; Herbert and Cohen, 1993; Kiecolt- Glaser et al., 1995; Kiecolt-Glaser et al., 1996; Marucha and Favagehi, 1998). These findings illustrate the relationship between exposure to social stressors and resulting immune compromise.
The integration of stress reactivity and immune function is, however, complicated by the large degree of individual variability that exists in response to stressors (Koolhaas, 2008). This variability likely depends on a number of factors, for example previous exposure to stressor and the exacerbated response to new stressors (Bhatnagar and Dallman, 1998; Ma and Morilak, 2005). Furthermore, polymorphisms in genes whose protein products influence stress reactivity may be important. Indeed, variations in the length of the promoter region of the gene (SLC6A4) that encodes the serotonin reuptake transporter (5HTT) affects susceptibility to psychosocial stress and may, thus, influence individual’s immune function. 5HTT regulates 5HT neurotransmission by recycling serotonin back into the presynaptic neurons (Soeby et al., 2005) and is a target of the selective serotonin reuptake inhibitor (SSRI) group of anti-depressant/anti-anxiety medications (Sen et al., 2004). The 5HTT repeat polymorphism (5HTTLPR) produces different transcriptional efficacies, with the long allele (l) being more transcriptionally active than the shorter allele (s) (Lesch et al., 1996; Sen et al., 2004). Thus, the presence of two l alleles has increased levels of mRNA, 5-HT binding, and 5-HT reuptake (Mazzanti et al., 1998) compared to the l/s or s/s (s-variant) genotype. The presence of the s allele is associated with a higher incidence of anxiety and depression in response to life stressors (Caspi et al., 2003; Lesch et al., 1996; Melke et al., 2001; Serretti et al., 2006; Veenstra-VanderWeele et al., 2000). Rhesus monkeys have homologous promoter length variations in the 5HTT gene (Lesch et al., 1997), and, like humans, the presence of the short allele has reduced transcriptional activity (Bennett et al., 2002). Studies of this species clearly show a gene by environment interaction, as animals with the short allele exposed to adverse rearing conditions show more anxiety-like behaviors in standardized tests of emotionality (Bethea et al., 2004; Champoux et al., 2002), increases in stress hormone response to social separation (Barr et al., 2004b), and heightened preference for alcohol consumption (Barr et al., 2004a). Furthermore, female rhesus monkeys with an s-variant 5HTTLPR genotype show a greater behavioral reactivity and more pronounce metabolic compromise to social subordination (Jarrell et al., 2008). Because the 5HT system is thought to contribute to T cell activation and function (Leon-Ponte et al., 2007), polymorphisms in the gene encoding 5HTT may alter immune function directly or by increasing reactivity to psychosocial stressors.
The present study used socially housed adult female rhesus monkeys to determine whether dominance status interacts with polymorphisms in the gene that encodes 5HTT to alter immune function. Social status is a major organizing feature in macaques living in social groups as subordinate animals are frequently harassed by dominant animals and typically lack control over their physical and social environments (Bernstein, 1976; Bernstein and Gordon, 1974). A consequence of social subordination is a dysregulation of the limbic - hypothalamic – pituitary –adrenal (LHPA) axis, resulting in hypercortisolemia (Abbott et al., 2003; Shively et al., 1997). Because the s-variant polymorphism in the SLC6A4 gene exacerbates the adverse consequences of social subordination on a number of behavioral and physiological systems (Jarrell et al., 2008), the present study tested the hypothesis that the consequence of psychosocial stress imposed by subordination would be exacerbated in females with the s-variant 5HTT polymorphism.
Methods
Subjects
Thirty adult (8 to 12 years of age) female rhesus monkeys (Macaca mulatta) housed at the Yerkes National Primate Research Center Field Station were used in this study. Females had been ovariectomized 12 months previously and were not receiving any exogenous hormone replacement. Animals were fed commercial monkey chow (Ralston Purina Company, St. Louis MO) ad libitum twice daily and seasonal fresh fruit and vegetables once a day. All procedures were approved by the Emory University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Humans Services “Guide for Care and Use of Laboratory Animals”.
All subjects had been previously screened for polymorphisms in the gene that encodes the 5HTT (Hoffman et al., 2007). Of the thirty subjects, 16 were homozygous for the long promoter length variant in the 5HTT gene (l/l) and 14 had at least one allele for the short promoter length variant (s-variant: l/s or s/s). Rather than being housed individually, the thirty females were members of one of eight small social groups consisting of five monkeys each that had been established for 8 months. Groups, regardless of size, are organized by a linear dominance hierarchy (Bernstein, 1976) that functions to maintain group stability. This is not accomplished through contact aggression but rather through continual harassment and the threat of aggression. Thus, each of the 30 subjects had a specific rank within their respective groups. These ranks were determined empirically based on the outcome of dyadic interactions in which a female clearly emitted a submissive response to another animal (Bernstein, 1976). Using previously established conventions (Kaplan et al., 1984), females ranked 1or 2 in their group were classified as dominant and those ranked 3, 4, or 5 were considered subordinate. Of the thirty females used in this analysis, 15 were dominant (eight females ranked 1 and seven females ranked 2) and 15 were subordinate (eight females ranked 4 and seven females ranked five). Because four of the groups were comprised entirely of females with an l/l 5HTT genotype and four with the s-variant genotype, the distribution of subjects was: eight dominant, l/l; seven dominant, s-variant; eight subordinate, l/l; and 7 subordinate, s-variant.
Glucocorticoid status
In order to characterize LHPA status in females, we performed a social separation test. Rhesus monkeys are xenophobic, emitting anxiety-like behaviors and increases in LHPA activity when separated from their groups and placed in novel environments with unfamiliar monkeys (Gordon et al., 1992; Gust et al., 1993; Gust et al., 1992). Consequently, each subject was removed from her group and placed in a cage in a remote room that contained unfamiliar monkeys also temporarily housed in a single cage. The separation test lasted 30 minutes and a plasma sample was obtained prior to the relocation immediately following the test for cortisol determination. Samples were assayed in the Yerkes Biomarker Core Lab using a commercially available kit (Beckman - Diagnostic Systems Laboratory, Webster TX) having a sensitivity of 0.5 μg/dl and an intra- and inter-coefficient of variation of 4.90% and 8.45%, respectively.
Sample Collection
Whole blood (30 ml) was collected from each subject following induction of anesthesia with ketamine (5 mg/kg, IM). The sample was collected in the morning following an overnight fast and represents a random, baseline sample from each subject. The sample was kept on ice (4°C) and was shipped overnight to the University of Pennsylvania. Prior to the shipment, an aliquot was taken for the determinant of complete blood counts CBCs using a Sysmex KX 21N instrument. Body weights were also obtained at the time of the blood sampling.
Lymphocyte studies and flow cytometry
Nine-color flow cytometric analysis was performed in the whole blood samples described above. The antibodies used were as follows: anti-CD62L FITC (clone SK11), anti-CD4-PerCP-Cy5.5 (clone SK3), anti-CD8- Pacific Blue (clone RTA-T8), anti-CD25 APC-Cy7 (clone M-A251), anti-HLA-DR-APC-Cy7 (clone L243) (all from BD Bioscience, San Jose CA); Ki67-FITC (clone B56), anti-CD3 Alexa700 (clone SP34-2), anti-CD95-PE-Cy5 (clone DX2) (all from BD Bioscience); anti-CD28 PE-Cy7 (clone 28.2) (eBiosciences, San Diego CA). Flow cytometric acquisition was performed on at least 100,000 events, gated on lymphocytes, on a LSR-II flow cytometer driven by the FACSDiVa software. Analysis data was performed using FlowJo software (Tree Star, Ashland OR).
Intracellular cytokines staining
Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation according to standard procedures and resuspended to 1 × 106 cells/ml in complete RPMI 1640 medium. Cells were then incubated for 4.5 h at 37°C in medium containing PMA, A23187, and Golgi Stop. Following incubation, the cells were washed and surface stained with anti-CD3, anti-CD4, anti-CD8, anti-CD28, and anti-CD95 for 30 min in the dark at 4°C followed by fixation and permeabilization. After permeabilization, the cells were washed and stained intracellularly with anti-human IL-2-APC (clone MQ1-17H12), anti-human TNF-FITC (clone MAb11), and anti-human IFN-PE (clone B27) Abs (all from BD Biosciences) for 1 hour in the dark at 4°C. Following staining, the cells were washed and fixed in PBS containing 1% paraformaldehyde. In all experiments, at least 50,000 lymphocytes were acquired.
Cell death analysis
Levels of baseline (ex vivo), spontaneous (48 hours incubation without stimulus), and activation induced (48 hours incubation with concanavalin A) cell death was determined in PBMC isolated. Cell death was measured by multicolor flow cytometry in both CD3+CD4+ and CD3+CD8+ T-cells following staining with Annexin V-PE (BD Biosciences).
Statistical Analysis
Data are expressed as mean ± sem for each social status – 5HTT genotype group. Data were analyzed using analysis of variance models. Multiple comparisons used the Bonferroni post hoc test. Statistical values having a p < 0.05 were considered significant.
Results
Characteristics of the groups
There was a significant status by genotype interaction for body weight (p = 0.01). Dominant l/l females were significantly heavier (8.49 ± 0.49 kg) than dominant s-variant animals (7.08 ± 0.29 kg). While subordinate l/l females (6.52 ± 0.29 kg) and s-variant females 6.93 ± 0.59 kg) weighed the least. Results from CBCs for each of the groups are illustrated in Table 1. There was a significant status by genotype interaction for platelet counts (p=0.031), due to the subordinate s-variant females having significantly higher counts compared to the dominant s-variant females. Additionally, there was a significant main effect of status in the following parameters: (i) percentage of segmental neutrophils (segs) whereby subordinate females had a significantly higher percentage of segs than dominant females (p = 0.013); (ii) number of lymphocytes (lymphs) with subordinate females having a significantly lower number of lymphocytes compared to dominant subjects (p = 0.001); (iii) percentage of total white blood cells that are lymphocytes (lymphs %) with subordinate females having a significantly lower % of lymphs compared to dominant females (p = 0.02).
Table 1.
Results of complete blood counts (CBCs) (mean ± sem) for each social status – 5HTT genotype group. For a given measure, different lettered superscripts indicate groups differ significantly (P < 0.05).
| Dominant, l/l | Subordinate, l/l | Dominant, s- variant | Subordinate, s- variant | |
|---|---|---|---|---|
| RBC | 6.09 ± 0.16 | 5.82 ± 0.24 | 5.83 ± 0.19 | 5.66 ± 0.16 |
| WBC | 6625 ± 844 | 6871 ± 822 | 7663 ± 584 | 7700 ± 1573 |
| Hgb | 13.5 ± 0.45 | 12.8 ± 0.63 | 13.1 ± 0.41 | 12.5 ± 0.49 |
| Hct | 43.7 ± 1.24 | 41.7 ± 1.55 | 42.3 ± 1.40 | 41.0 ± 1.51 |
| MCV | 71.8 ± 0.78 | 72.0 ± 2.70 | 72.5 ± 0.97 | 72.4 ± 1.41 |
| MCH | 22.1 ± 0.38 | 22.2 ± 1.15 | 22.5 ± 0.43 | 22.1 ± 0.52 |
| MCHC | 30.8 ± 0.34 | 30.7 ± 0.60 | 31.1 ± 0.30 | 30.6 ± 0.20 |
| Pltx1000 | 413 ± 46a | 387 ± 35a | 313 ± 27b | 468 ± 48c |
| Segs | 3451 ± 991a | 4315 ± 799a | 3160 ± 244a | 5246 ± 1610a |
| Segs % | 46.8 ± 7.0a | 60.9 ± 5.2b | 43.1 ± 4.6a | 60.9 ± 6.7b |
| Lymphs | 2754.4 ± 206a | 2146.7 ± 227b | 4065.9 ± 538a | 2164.6 ± 104b |
| Lymphs % | 46.3 ± 5.8a | 33.4 ± 4.8b | 51.4 ± 4.0a | 34.4 ± 6.0b |
| Monos | 159.4 ± 36 | 195.9 ± 50 | 159.5 ± 58 | 132.3 ± 26 |
| Monos % | 2.5 ± 0.5 | 2.9 ± 0.7 | 2.0 ± 0.7 | 2.0 ± 0.5 |
| Eos | 253 ± 64.5 | 214 ± 70.0 | 257 ± 80.2 | 158 ± 45.6 |
| Eos % | 4.4 ± 1.24 | 2.86 ± 0.70 | 3.25 ± 0.94 | 2.71 ± 0.86 |
| Baso | 7.13 ± 7.13 | 0 ± 0 | 10.4 ± 10.4 | 0 ± 0 |
| Baso % | 0.13 ± 0.13 | 0 ± 0 | 0.13 ± 0.13 | 0 ± 0 |
| Bands | 0 ± 0 | 0 ± 0 | 9.75 ± 9.75 | 0 ± 0 |
| Bands % | 0 ± 0 | 0 ± 0 | 0.13 ± 0.13 | 0 ± 0 |
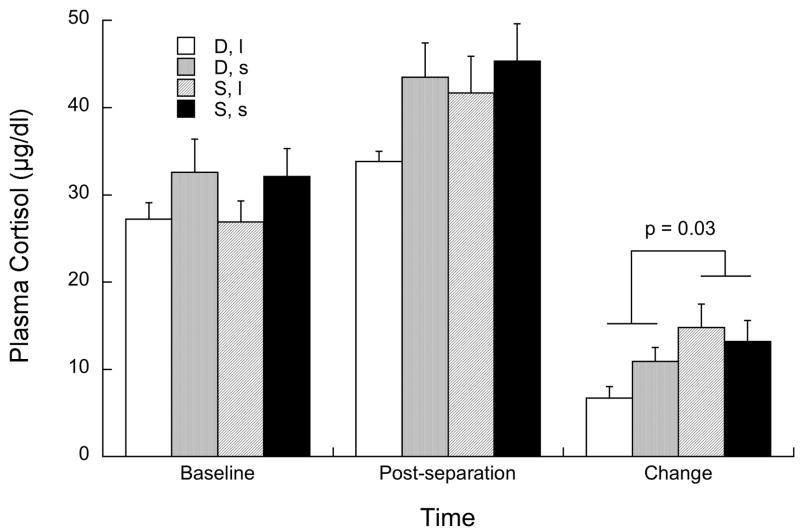
Figure 1 illustrates the response in plasma cortisol to the social separation test. As can be seen, subordinate females showed a significantly greater increase in cortisol compared with dominant females (p = 0.03). There was no significant effect of genotype (p = 0.44) or status by genotype interaction p = 0.14).
Figure 1.
Mean ±sem plasma cortisol at baseline, immediately following the social separation test, and the change from baseline to the post separation sample in dominant (“D”) and subordinate (“S”) female of both 5HTT genotypes (l/l or “l” and s-variant or “s”).
Levels of T cells and their main subsets
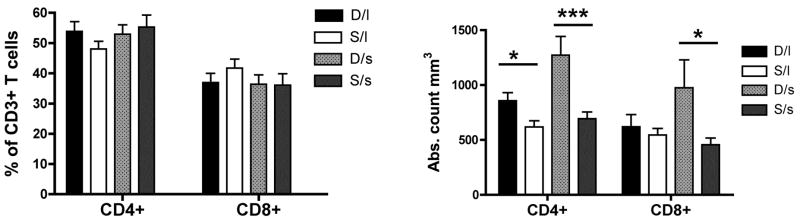
Multiparametric flow cytometry analysis of CD3+ CD4+ and CD3+ CD8+ T-cells in the peripheral blood was performed to assess how status and genotype influenced the peripheral compartment of T lymphocytes. As shown in Figure 2, the percentage of T-cells expressing CD4+ and CD8+ did not differ between groups (left panel). However, subordinate animals showed a significant reduction in the absolute number of CD4+ (p<0.05: dominant, l/l vs. subordinate l/l; p<0.001: dominant, s-variant vs. subordinate, s-variant) and CD8+ (p<0.05: dominant, s-variant vs. subordinate, s-variant) T-cells compared to the dominant females, independent of 5HTT polymorphism (right panel).
Figure 2.
Social status (dominant or “D” vs. subordinate or “S”), independently by 5HTT polymorphism l/l or “l” vs s-variant or “s”), affects the level of the T cell compartment. (A) Levels (mean ± S.D.) of CD4+ and CD8+ T lymphocytes are showed in the four studied groups both as percentage of the total CD3+ T cell population (left panel), and absolute number for mm3 (right panel). Statistically significant differences were indicated with * for p<0.05 and *** for p<0.001.
The distribution of different T-cell subtypes in both CD4+ and CD8+ T-lymphocytes based on the expression of CD28 and CD95 was assessed as previously described (Pitcher et al., 2002). CD4+ and CD8+ are defined as naïve (TN,), central memory (TCM) and effector memory (TEM) T-cells based on their functionality and the expression of specific surface markers. Specifically, naïve cells were identified as CD28+ CD95-, and memory cells as CD95+; memory T-cells were further divided in TCM and TEM based on their ability to express (TCM) or not express (TEM) CD62L. No group differences were observed, as all the subsets, i.e. TN, TCM, and TEM, were present at the same levels in the four groups of animals in both CD4+ and CD8+ lymphocytes. Taken together, these data suggest that the size of the T-cell compartment, but not the balance in the different subpopulations, is affected by social status, independent of 5HTT polymorphism.
T cell activation and proliferation
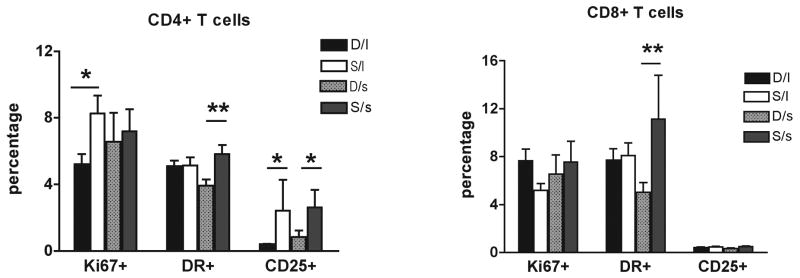
The level of immune activation in CD4+ and CD8+ T-lymphocytes, using common surface (HLA-DR, CD25, CD69) and intracellular (Ki67) markers of activation and proliferation was assessed by multicolor flow cytometric analysis. Comparing the fraction of CD4+ and CD8+ T-cells that express those markers in all the studied groups, showed that subordinate animals, independent of 5HTT polymorphism, had increased levels of T-cell activation and proliferation compared to dominant females (Figure 3, left panel for CD4, right panel for CD8). In particular, subordinate l/l females had a significantly greater expression of Ki67 and CD25 in CD4+ T-lymphocytes (p<0.05) compared to dominant l/l females while subordinate, s-variant showed a significant increased expression of CD25 on CD4+ (p<0.05) and HLA-DR in both CD4+ and CD8+ T-cells (p<0.01) compared to dominant s-variant females. Collectively, these findings indicate that the social subordination results in increased levels of activation and proliferation of their main T-cell subsets. Interestingly, this increased activating/proliferating status does not result in an overall expansion but rather in a reduction of T-cell number (Figure 2).
Figure 3.
Social status (dominant or “D” vs. subordinate or “S”), independently by 5HTT polymorphism l/l or “l” vs s-variant or “s”), affects the level of activation and proliferation of T cells. The percentage (mean ± S.D.) of CD4+ (left panel) and CD8+ (right panel) T cells expressing the activation markers DR and CD25 and the proliferating markers Ki67 are reported in the four studied groups. Statistically significant differences were indicated with * for p<0.05 and ** for p<0.01.
T lymphocytes susceptibility to activation induced cell death
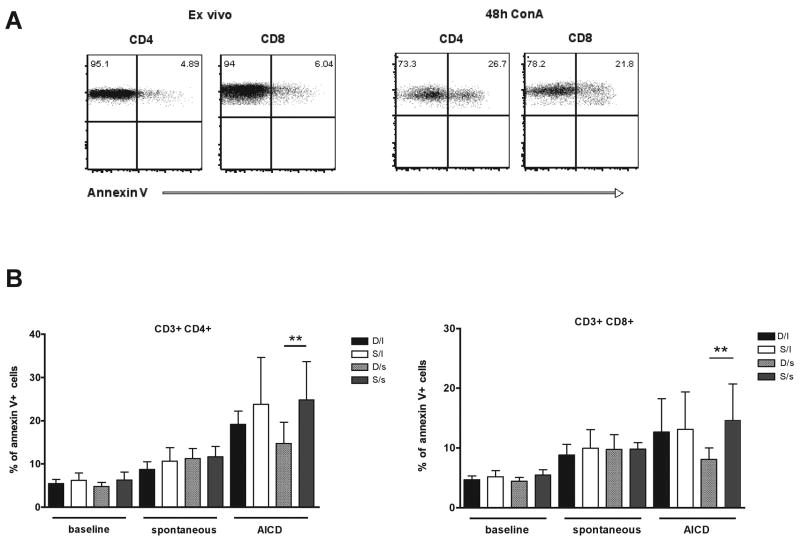
When T-cells are activated and proliferate, they become more susceptible to activation-induced cell death (AICD) (Krammer et al., 2007). To determine if the increased activation found in subordinate animals results in increased susceptibility to AICD, T-cells were assessed for their responsiveness to annexin-V, a molecule with high affinity for phosphotydilserine extensively used to identify apoptotic cells. This determination was performed ex vivo (baseline cell death) and after 48 hours in culture without (spontaneous cell death) or with a mitogenic (Concanavalin A, Con A) stimulus (activation induced cell death, AICD). Annexin-V staining in CD4+ and CD8+ T-cells ex vivo and after 48 hours stimulation with Con A is shown in a representative subject (Figure 4, panel A). Consistent with the increased levels of immune activation, subordinate females showed high levels of annexin V+ expression after 48 hours in culture with Con A compared to dominant animals. Specifically, subordinate s-variant females had significantly (p<0.01) higher levels of both CD4+ and CD8+ T-lymphocytes expressing annexin-V compared with dominant s-variant females (panel B). These data, combined with those reported in Figures 2 and 3, indicate that the psychosocial stress experienced by subordinate animals may profoundly impact the homeostasis of their immune system, resulting in abnormal levels of activation, proliferation, and cell death of the T-cell compartment that are ultimately associated with reduced size of the overall T-cell pool.
Figure 4.
Social status (dominant or “D” vs. subordinate or “S”) affects T lymphocytes susceptibility to activation induced cell death. (A) Annexin V staining is showed in CD4+ and CD8+ T cells from a representative rhesus monkey ex vivo and after 48 hours stimulation with Concanavalin A (Con A). (B) The percentage (mean ± S.D.) of CD4+ (left panel) and CD8+ (right panel) T cells expressing annexin V is reported in the four studied groups at three different conditions: ex vivo (baseline), after 48 hours in culture without (spontaneous) or with Con A stimulus (activation induced cell death, AICD). Statistically significant differences were indicated with ** for p<0.01.
Ability of T cells to produce cytokines
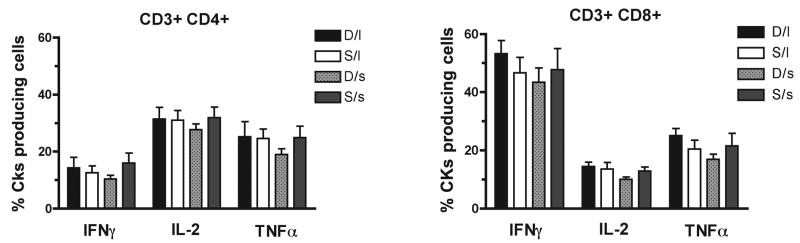
Finally, the ability of T-cells to produce cytokines was assessed as a function of status and genotype. Using intracellular cytokine staining assay, the fraction of CD4+ and CD8+ T-cells producing IFNγ, IL-2, and TNFα following in vitro activation with PMA and Ionomycin was determined. As shown in Figure 5, the fraction of T-cells producing cytokines were similar in all studied groups, indicating that neither social status nor 5HTT polymorphisms affect this function of T-cells.
Figure 5.
Effects of social status (dominant or “D” vs. subordinate or “S”) and 5HTT polymorphism l/l or “l” vs s-variant or “s”) on the levels of T cell cytokines production. Percentage (mean ± S.D.) of CD4+ (left panel) and CD8+ (right panel) T cells producing IFNγ, IL-2, and TNFα following in vitro activation was assessed by intracellular cytokine staining in the four studied groups.
Discussion
The purpose of this study was to determine whether social status interacts with a polymorphism in the gene encoding 5HTT to help explain the individual variability observed in female rhesus monkeys’ immune function. We hypothesized the s-variant 5HTT genotype may be associated with altered immune function because of heightened stress reactivity. However, our assessment of LHPA function showed that plasma cortisol in response to the social separation test was significantly elevated in subordinate females compared to dominant animals regardless of genotype. While subordinate females as a group were more compromised than dominant animals, we still observed significant status by genotype interactions on several parameters of immune function, suggesting indeed the s-variant genotype can exacerbate the consequences of social subordination. Characteristically, subordinate females showed significantly greater reactivity to a psychosocial stressor (Abbott et al., 2003; Sapolsky, 2005) as well as lower body weight, increased percentage of segmental neutrophils, decreased levels of lymphocytes (both as absolute numbers and percentage of white blood cells), and increased platelet count, specifically in s-variant females. Significant differences between dominant and subordinate animals were observed in levels of lymphocyte production, T-cell activation and proliferation, and T-cell death. The s-variant 5HTT genotype did not exacerbate the effects of social status on T-cell production, activation and proliferation, but it did influence the level of T-cell cell death in dominant versus subordinate animals. Specifically, subordinate s-variant animals had significantly higher levels of both CD4+ and CD8+ T-lymphocytes expressing annexin V+ compared to dominant s-variant females, thus indicating that T-cells of s-variant subordinate females were most susceptible to activation induced cell death.
Despite the fact that the percentage of both CD4+ and CD8+ T-cells were similar in all groups, subordinate animals showed a significant reduction in the absolute number of CD4+ and CD8+ T-cells compared to dominant animals, independent of 5HTT. The reduction in T-cell numbers observed in subordinate animals has been well documented in social confrontation studies conducted in Long-Evans rats. After two days of continuous social confrontation, total numbers of CD4+ and CD8+ T-cells in subordinate males declined significantly compared to control males that were kept undisturbed in pairs (Stefanski and Engler, 1999). Similarly, assembled colonies of unfamiliar rats resulted in establishment of a dominance system whereby a marked decrease of CD4+ and CD8+ T-cells was observed in subordinate rats (Stefanski et al., 2001). These studies, in conjunction with the findings of the present study, indicate that subordinate animals subjected to chronic social stress exhibit altered immunological status and reduced numbers of T-cells. Because T-helper cells (CD4+) are the key orchestrators of the immune response and are necessary for the activation of major effector cells, including cytotoxic T-cells (CD8+) and antibody producing B-cells, a decrease in CD4+ T-cell production may result in subordinate animals having a weakened immune response to invading pathogens and thus, an increased susceptibility to disease.
Although absolute CD4+ and CD8+ T-cell counts were significantly reduced in subordinate female rhesus monkeys, the balance between the different T-cell subsets was not affected by a subject’s 5HTT genotype or social status. The distribution of naïve, central memory, and effector memory T-cell subsets was consistent amongst all four groups of animals and suggests that neither social status nor 5HTT genotype influences T cell subtype balance. However, in contrast to T-cell subtype balance, social status influenced levels of CD4+ and CD8+ T-lymphocyte activation and proliferation as measured by expression of common surface (HLA-DR, CD25, CD69) and intracellular (Ki67) markers of activation and proliferation. Specifically, the subordinate females who showed increased secretion in cortisol in response to a psychosocial stressor had increased levels of activation and proliferation of their T-cell compartments. Previous studies conducted in male and female rats in colonies found an almost complete suppression of all lymphocyte subclasses and lower proliferation to the mitogens ConA and phytohemagglutinin A (PHA) in subordinate rats (Bohus et al., 1991a; Bohus et al., 1993). Similarly, a decrease in T-cell proliferation was observed in response to the mitogen ConA in subordinate male rats in colonies of unfamiliar rats who established a dominance system (Stefanski et al., 2001). It is important to note that the aforementioned rodent studies considered in vitro proliferative potential, i.e. the ability of cells to proliferate in response to a mitogenic stimulus. The present study examined ex vivo proliferation, i.e. the levels of cells that are proliferating in the blood of the animals, and thus differences between in vitro and ex vivo proliferation may exist.
While we observed significant differences in the proliferative capacity of CD4 and CD8 positive cells, no group differences in cytokine production were detected. Several factors may account for this discrepancy. First, levels of proliferation were assessed ex vivo, whereas cytokine production was measured after in vitro stimulation with PMA and Ionomycin. Another factor to take in account is that the changes induced by the stress of subordination on cytokine production may be below the limit of detection of the technique used. Furthermore, proliferating T cells are more susceptible to activation induced cell death, which may render these cells more susceptible to die after in vitro stimulation, thus reducing their ability to produce cytokines upon restimulation.
Although T cell proliferation was elevated in the subordinate female rhesus monkeys, ultimate expression of absolute numbers of CD4+ and CD8+ T-cells was reduced. This phenomenon may be explained by the significantly higher levels of annexin-V expression observed in subordinate monkeys, indicating that these subjects’ CD4+ and CD8+ T-cells were more susceptible to die of activation induced cell death (AICD). These findings suggest that subordinate rhesus monkeys may react differently than subordinate rodents who experienced a decreased T-cell proliferation, to chronic psychosocial stress by initially mounting an elevated immune response by increasing T cell activation and proliferation followed by AICD and suppression of the immune response as evidenced by a reduction in absolute numbers of T-cells. It is important to note that the higher levels of annexin-V+ expression observed in subordinate females compared to dominant females was especially prominent in those females with the s-variant 5HTT polymorphism. Thus, it seems that the s-variant polymorphism may exacerbate a subordinate rhesus monkey’s susceptibility to T-lymphocyte cell death through AICD. T-lymphocyte cell death through AICD may thus be specific to the s-variant polymorphism, as this mechanism did not account for the reduction in T-cell numbers observed in subordinate l/l females.
Recently, the presence of 5HTT mRNA and protein was observed in different lymphocyte subsets in rhesus monkeys (Yang et al., 2007). CD3+CD4+ T-lymphocytes, in particular, were are 5HTT immunoreactive, by multicolor flow cytometry (Yang et al., 2007). This finding, in conjunction with our observation of increased susceptibility to AICD in subordinate s-variant females’ T-lymphocytes, suggests that 5HT may influence the degree of T-lymphocyte cell death through 5HTT. Indeed, the number of lymphocytes expressing the 5HTT is reduced in patients with major depression (Fazzino et al., 2008), a psychiatric condition thought to be due to LHPA dysregulation (Ressler and Nemeroff, 2000). Similar to the observations made in the present study, depressed patients also show increased T cell proliferation that is not influenced by ConA (Fazzino et al., 2008). Consequently, the genetic polymorphism in 5HTT resulting in s-variant genotypes may be more likely to exhibit altered immune function as a result of increased T-cell cell death and hence may be more susceptible to diseases caused by invading pathogens. These data are consistent with a number of studies showing that 5HT contributes to T cell activation and function (Frick et al., 2008; Leon-Ponte et al., 2007). Importantly, 5HT attenuates cell death in human monocytes (Soga et al., 2007) and there is some evidence that increased 5HT activity resulting from treatment with SSRIs can increase natural killer cell immunity and protect against opportunistic infection (Evans et al., 2008). Because diminished 5HT neurotransmission is a typical characteristic of the s-variant 5HTT genotype (Lesch et al., 1997), one could have predicted a main effect of genotype on immune status in our subjects, independent of social status. However, the prominent effect of genotype we observed in this analysis was evident in its interaction with social status. This could be the result of heightened stress reactivity in subordinate, s-variant females. However, the cortisol response to the social separation test did not show any differences in subordinate females as a function of genotype.
Alternatively, because the 5HT system can be compromised by chronic exposure to psychosocial stressors (Dinan, 1996; Graeff et al., 1996), 5HT may be most compromised in subordinate s-variant females resulting in a greater immune system dysfunction. This would account for the increased susceptibility to cell death exhibited by subordinate s-variant females.
The present study provides a unique model to study how psychosocial stress may interact with genetic polymorphisms to influence individual variability in T-cell phenotype and function. Given the increased activation of the LHPA axis that results from the imposition of social subordination, this model provides an ethologically relevant paradigm of chronic psychosocial stress in individuals. Additionally, the ability to evaluate the genetic contribution to this vulnerability contributes to the utility of this model. We must emphasize that our sample size was not adequate to fully determine the genetic contributions of polymorphisms in the gene encoding the 5HTT and it is entirely likely that many genes influence the phenotypes examined in this study. The data from the present study can best serve as the foundation for broader linkage and association analyses to understand individual differences in immune function and susceptibility to disease. In future studies, this unique model may be utilized to answer key questions that could help elucidate mechanisms linking environmental stressors with altered immune function in addition to explaining co-morbidities such as altered neurotransmitter function seen in depression and decreased immune function.
Acknowledgments
We thank Jeff Fisher, Jennifer Whitley, and Holly Jarrell for their expert technical assistance in collecting the samples. This work was supported by NIH grants HD46501 and RR00165. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004a;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Sacerdote P, Panerai AE, Peterzani T, Palanza P, Parmigiani S. Chronic psychosocial stress-induced down-regulation of immunity depends upon individual factors. Journal of Neuroimmunology. 2003;141:58–64. doi: 10.1016/s0165-5728(03)00220-0. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berga SL, Loucks TL. The diagnosis and treatment of stress-induced anovulation. Minerva Ginecol. 2005;57:45–54. [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious Behavior and Fenfluramine-Induced Prolactin Secretion in Young Rhesus Macaques with Different Alleles of the Serotonin Reuptake Transporter Polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bohus B, Koolhaas JM, De Ruiter AJ, Heijnen CJ. Stress and differential alterations in immune system functions: conclusions from social stress studies in animals. The Netherlands journal of medicine. 1991a;39:306–315. [PubMed] [Google Scholar]
- Bohus B, Koolhaas JM, de Ruiter AJH, Heinen CJ. Stress and differential alterations in immune functions: conclusions from social stress studies in animals. Ned J Med. 1991b;39:306–315. [PubMed] [Google Scholar]
- Bohus B, Koolhaas JM, Heijnen CJ, de Boer O. Immunological responses to social stress: dependence on social environment and coping abilities. Neuropsychobiology. 1993;28:95–99. doi: 10.1159/000119008. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social status and susceptibility to respiratory infections. Ann N Y Acad Sci. 1999;896:246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- de Groot J, Boersma W, Scholten JW, Koolhaas JM. Social stress in male mice impairs long-term anti-viral immunity selectively in wounded subjects. Physiol Behav. 2002;75:277–285. doi: 10.1016/s0031-9384(01)00677-1. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996;58:1683–1694. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Evans DL, Lynch KG, Benton T, Dube B, Gettes DR, Tustin NB, Lai JP, Metzger D, Douglas SD. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino F, Montes C, Urbina M, Carreira I, Lima L. Serotonin transporter is differentially localized in subpopulations of lymphocytes of major depression patients. Effect of fluoxetine on proliferation. J Neuroimmunol. 2008;196:173–180. doi: 10.1016/j.jneuroim.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Frick LR, Palumbo ML, Zappia MP, Brocco MA, Cremaschi GA, Genaro AM. Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem Pharmacol. 2008;75:1817–1826. doi: 10.1016/j.bcp.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Gordon TP, Gust DA, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiol Behav. 1992;51:467–472. doi: 10.1016/0031-9384(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK. Response to removal from and return to a social group in adult male rhesus monkeys. Physiol Behav. 1993;53:599–602. doi: 10.1016/0031-9384(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Brodie AR, Ahmed-Ansari A, McClure HM. Removal from natal social group to peer housing affects cortisol levels and absolute numbers of T cell subsets in juvenile rhesus monkeys. Brain Behav Immun. 1992;6:189–199. doi: 10.1016/0889-1591(92)90018-j. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: A meta-analystic review. Psychosomatic Medicine. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of serotonin transporter polymorphism (5HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DT, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- Kiecolt- Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein SMWB, Sheridan JF. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–667. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nature reviews. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Leon-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Marucha PTKGJK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- Melke J, Landen M, Baghei F, Rosmond R, Holm G, Bjorntorp P, Westberg L, Hellstrand M, Eriksson E. Serotonin transporter gene polymorphisms are associated with anxiety-related personality traits in women. Am J Med Genet. 2001;105:458–463. doi: 10.1002/ajmg.1434. [DOI] [PubMed] [Google Scholar]
- Miller GE, Ritchey AK, Cohen S. Chronic Psychological Stress and the Regulation of Pro-Inflammatory Cytokines: A Glucocorticoid-Resistance Model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan JR. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Individual differences and the stress response. Semin Neurosci. 1994;6:261–269. [Google Scholar]
- Sapolsky RM. Social subordinance as markers of hypercortisolism. Some unexpected subtleties. Ann NY Acad Sci. 1995;771:626–639. doi: 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: a comprehensive review. Curr Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Ann N Y Acad Sci. 1997;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- Soeby K, Larsen SA, Olsen L, Rasmussen HB, Werge T. Serotonin transporter: evolution and impact of polymorphic transcriptional regulation. Am J Med Genet B Neuropsychiatr Genet. 2005;136:53–57. doi: 10.1002/ajmg.b.30184. [DOI] [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000;25:389–406. doi: 10.1016/s0306-4530(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Engler H. Social stress, dominance and blood cellular immunity. J Neuroimmunol. 1999;94:144–152. doi: 10.1016/s0165-5728(98)00242-2. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Knopf G, Schulz S. Long-term colony housing in Long Evans rats: immunological, hormonal, and behavioral consequences. J Neuroimmunol. 2001;114:122–130. doi: 10.1016/s0165-5728(00)00464-1. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Anderson GM, Cook EH., Jr Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- Yang GB, Qiu CL, Aye P, Shao Y, Lackner AA. Expression of serotonin transporters by peripheral blood mononuclear cells of rhesus monkeys (Macaca mulatta) Cell Immunol. 2007;248:69–76. doi: 10.1016/j.cellimm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Zozulya AA, Gabaeva MV, Sokolov OY, Surkina ID, Kost NV. Personality, coping style, and constitutional neuroimmunology. J Immunotoxicol. 2008;5:221–225. doi: 10.1080/15476910802131444. [DOI] [PubMed] [Google Scholar]