Abstract
In this study, the authors examined age-related changes in participants’ ability to perceive global spatial structure defined by temporal fine structure among elements undergoing rapid, irregular change. Participants were also tested on a task involving form recognition from luminance contrast and on a task dependent on perception of 3-dimensional shape from motion. Compared with young adults, older individuals were less sensitive to spatial form defined by temporal structure. In contrast, older observers performed as well as young adults on the other two tasks that were not dependent on temporal sensitivity, ruling out nonsensory factors as the cause of the deficits on the temporal structure task. This selective deficit may reveal reduced sensitivity within the temporal impulse response of the aging visual system, a deficit that could be related to reduced effectiveness of neural inhibition.
Keywords: visual temporal structure, visual perception, vision and aging, perceptual organization, figure/ground segmentation
Getting older takes its toll on sensory–perceptual function. Even in the absence of disease, taste and smell are dulled, tactile sensitivity is blunted, and hearing is compromised. Aspects of vision, arguably our most important sense, likewise can change gradually as we age (see chapters in Sekuler, Kline, & Dismukes, 1982, and review by Jackson & Owsley, 2003). Some of those age-related changes in vision can be traced to optical (e.g., increased lens density) and/or structural (e.g., reduced density of rod photoreceptors) causes within the eye. Other changes, however, are thought to arise from neural reorganization (e.g., enlarged areal summation within visual receptive fields) in the retina and the brain.
One category of sensory–perceptual tasks susceptible to aging are those dependent on resolution of sensory stimuli occurring in close temporal proximity. For example, older individuals show reduced ability to perceive temporal modulation at high rates of visual flicker (e.g., Kline & Scheiber, 1982), and they have generally higher contrast thresholds (decreased sensitivity) for detection of two stimuli presented very closely in time (Shinomori & Werner, 2003). Older individuals also exhibit reduced temporal resolution on auditory tasks (e.g., Strouse, Ashmead, Ohde, & Grantham, 1998) and on tactile tasks involving dynamic stimulation (Verrillo, 1982). These ubiquitous changes in temporal processing with age may point to modifications in the strength or in the temporal precision of neural responses underlying the registration of time-varying stimulation. To examine one possible consequence of such a change in the case of vision, we have compared the abilities of young and older individuals to perform a figure/ground segmentation task in which figure and background are defined solely in terms of the temporal fine structure among the feature elements composing the figure and the background.
Visual grouping refers to the perceptual ability to combine, or “bind,” local features into coherent, global forms. Grouping is intimately related to figure/ground organization, whereby objects are segmented from their backgrounds. These two interrelated aspects of perception formed the core of Gestalt psychology, whose advocates documented the existence of a number of spatial factors supporting figure/ground segmentation and grouping. Among those spatial factors were proximity, continuity, and symmetry (reviewed by Palmer, 2002). The Gestalt psychologists also pointed out that common fate, a form of temporal structure, could also effectively unite spatially distributed elements into a common global form. In the Gestalt notion of common fate, individual objects that move in the same general direction at the same time tend to group into a coherent form, the textbook example being a flock of birds in flight. In addition to motion, other forms of temporal structure (TS) have been identified in recent years, the common thread among them being the tendency for spatially distributed elements that undergo change at the same time to group together over space (see review by Blake & Lee, 2005).
For our study of aging's impact on visual grouping from TS, we used animation displays in which form (spatial structure) is created by rapid, repetitive changes (TS) in stimulus elements defining the form and the background against which it appears (Lee & Blake, 1999a). Individual static frames in these animations contain no information specifying spatial structure. Within an array of non-overlapping apertures, contours move in one of two directions, with direction reversing randomly over time. When contours in one region reverse directions in synchrony, they stand out from surrounding regions of asynchrony. While there is disagreement concerning the specific neural events promoting grouping from TS (e.g., Adelson & Farid, 1999; Lee & Blake, 1999a, 1999b; Morgan & Castet, 2002), there is no doubt that this form of spatial grouping depends on the temporal resolving power of the visual system.
Using these unique displays, we compared results from older and young individuals tested on a form recognition task in which the form is defined by TS. Participants also completed a task to assess their ability to perceive form from luminance contrast (LUM). This allowed us to determine whether any general difference in form perception for static images existed between the groups. To assess possible group differences in sustained attention or general motivation, we also compared the performance of older participants with the standard performance of young participants on a difficult task involving perception of structure from motion (SFM); for this part of our study, we used data previously collected from the older participants during an unrelated research project conducted at the University of Iowa.
Method
Apparatus
The animations specifying form from LUM and from TS were presented on the video screen of an iMac computer (Apple, Cupertino, CA), which also controlled the timing of all trial-related events. The 14-in. (36-cm) viewable screen had a resolution of 600 × 800 pixels and a refresh rate of 95 Hz; it was viewed in an otherwise dark room. The SFM task was presented on a Macintosh IICi computer in an otherwise dark room. The 11-in. (28-cm) viewable screen had a resolution of 480 × 640 pixels and a refresh rate of 66 Hz. All responses were made on a standard computer keyboard.
Participants
Twenty-four older participants (mean age, 68.0 years; range, 60−76 years) were recruited from an ongoing longitudinal study of cognition in advanced age at the University of Iowa. Twenty-two young participants (mean age, 28.5 years; range, 17−40 years) were recruited as a comparison group from the surrounding community or the university student population. All participants gave informed consent to take part in this study, which was approved by the institutional review board committees of the University of Iowa and Vanderbilt University. All participants had normal or corrected-to-normal visual acuity, and none had previous experience with TS animations although many had participated in unrelated psychophysical experiments. None of the participants had active medical, neurological, or psychiatric problems, and none were taking psychoactive medications. No participant had glaucoma, macular degeneration, optic nerve disease, symptomatic cataracts, intraocular implants, or other disorders of the ocular media.
TS and LUM Stimuli
The TS and LUM stimuli, created using MATLAB (The Math-Works, Natick, MA) and the Psychophysics Toolbox (Brainard, 1997), consisted of a square array of small circular elements appearing against a homogenous gray background (see Figure 1). At the viewing distance of 72 cm, the 576 (24 × 24)–element array subtended 6.36° × 6.36° of visual angle. Each element consisted of a one-dimensional sinusoidal grating viewed through a circular window subtending a visual angle of 16 arc min. The orientation of each element was randomized for every trial, as was the luminance with amplitude variations of ±3.94 candelas per square meter (cd/m2) around the background gray of 70 cd/m2.The contrast of each element was randomly modulated during every trial on a frame-by-frame basis, with a mean contrast of 0.5 and amplitude of ±0.2. Each element's sinusoidal grating was phase shifted by 2π/6 radians per frame, a spatial displacement sufficient to produce smooth apparent motion of the grating within the circular aperture, with the direction of motion being in either of the two directions orthogonal to the grating's orientation. The direction of the phase shift in each frame was constrained so that no three consecutive frames contained either alternations between positive and negative phase shifts or continuous phase shifts in a single direction. This constraint, as well as the randomization of contrast and luminance among elements, was implemented to minimize potential LUM artifacts attributable to a temporal low-pass filter of the sort discussed by Adelson and Farid (1999). The target in each task was a 13 × 8–element region whose long axis was oriented either horizontally or vertically; this “rectangular target” could appear anywhere within the central 20 × 20–element region of the 24 × 24–element array. The location and orientation of the target were random in each trial. Each trial was presented for 526 ms (50 video frames always synchronized to the video monitor refresh cycle). The target region was defined either by LUM or by TS—these two stimulus conditions are described in the next two subsections.
Figure 1.

Temporal structure (TS) and luminance contrast (LUM) stimuli. The display comprised a square array of small circular elements. Each element was a phase-shifting sinusoidal grating, presented behind a circular window; the orientation of each grating was randomly determined. In the TS display (Panel a), a rectangular target region was defined by TS (target boundary shown here only for purposes of visualization). The four elements shown at the right represent elements from the background and target regions. Within each region, all elements had an identical TS determined by stochastic point processes that defined the direction of motion from frame to frame of the animation. Shown in the right-hand portion of the figure are eight series of filled and unfilled circles denoting points in the two alternative directions of motion of a given grating; points in a given sequence where the dots change from unfilled to filled (or vice versa) represent points in time when the direction of motion of the grating changed from one direction to the other. The target and background point processes differed to varying degrees, creating differential TS that could mediate perception of a rectangular target if the difference in TS between figure and ground was sufficiently strong (“difference” was indexed by the correlation between the two point processes). The average luminance of all gratings was identical, and the contrast of each element varied randomly (within limits) from frame to frame of the animation. In the LUM display (Panel b), the target region was defined by a difference in average luminance between gratings defining the target versus those within the background. In TS condition, all elements in the target and background regions obeyed the same point process (as illustrated on the right), creating coherent TS that provided no cue for the location or orientation of the target.
TS display
In the TS display, the target and background regions were defined solely by differences in the points in time at which grating elements in the target versus the background reversed directions of motion (Figure 1a). For any given grating, these reversal times are defined as a point process: a time series specifying the irregular, frame-to-frame sequence of motion directions of that grating.1 All gratings within the 13 × 8–element rectangular target region obeyed the same point process, meaning that all moving gratings reversed direction of motion at the same time. Likewise, all gratings within the background region shared the same point process, thus reversing their directions of motion simultaneously over time. We stress that the orientations of all target and background elements, and thus the associated directions of motion, were random throughout the array—no spatial structure specified the location or the shape of the target relative to the background. Instead, target and background were distinguished solely by the degree of correlation between the two point processes associated with these regions. When the point process associated with the target elements is uncorrelated with the point process for the background elements, the target is maximally distinctive in location and shape. At the other extreme, when the two point processes are perfectly correlated (i.e., identical), the two regions share the same temporal structure, and therefore, the target is perforce invisible. Manipulating the correlation between the target point process and the background point process produces graded variations in the clarity of the target and, hence, in the observer's ability to judge the target's orientation on the two-alternative forced-choice (2AFC) task (“horizontal” vs “vertical”). Operationally, different degrees of correlation were produced by generating a point process assigned to the target elements and then shuffling that point process to produce a specified correlation value (±0.02 correlation units). This procedure does not introduce any kind of structured phase shift (i.e., uniform delay) between the two sets of elements but rather manipulates the percentage of direction reversals that are synchronized between target and background.
LUM display
In the LUM display (Figure 1b), the average luminance of the target elements was different from the average luminance of the background elements. On half of the trials, the target region had an average luminance higher than the background region, and on the remaining trials, the target had a lower average luminance than the background. The magnitude of the luminance contrast—the difference in average luminance between the rectangular target and background—determined the visibility of the target. LUM contrast (Michelson [1927] contrast) ranged from 0.26 to 0.01. All elements in the display shifted in phase from frame to frame of the animation and reversed direction of phase shift irregularly during the animation, in the same fashion as the TS display; however, all elements throughout the entire LUM display obeyed the same point process, producing uniform coherent temporal structure across the entire display. Thus, temporal structure in the LUM condition contained no information relevant to the location of the target.
TS and LUM Procedure
While seated in a darkened room, participants told an experimenter the orientation of the rectangular target within the square array of elements, guessing if necessary. The experimenter, who did not know the correct answer, then entered this information into the computer using the keyboard. This procedure was followed to minimize response errors, which introduce variability into the staircase procedure used to measure thresholds. Each experimental condition was explained to participants prior to the beginning of each session. Participants were free to look anywhere within the display during each stimulus presentation. Each trial consisted of a timed presentation of the stimulus followed by a blank screen. Trial-by-trial feedback was not given during training or testing, but a graphical representation of performance was presented at the end of each block.
All participants completed the LUM test followed by the TS test. Before each test, the participants completed 30 practice trials to gain familiarity with the tasks. The LUM and TS tests consisted of 60−80 trials administered in a standard 3:1 staircase procedure that converged onto the stimulus level yielding a performance of 81% correct. Each staircase began with an easily recognized version of the target, with progressively difficult presentations being introduced contingent on the observer's performance. Specifically, following three consecutive correct responses, the staircase progressed to a more difficult stimulus, and following each incorrect response, the staircase moved to an easier stimulus. Initial staircase steps were relatively large, but after the first incorrect response, step sizes were reduced to smaller increments and decrements, with the staircase being terminated after 12 reversals in staircase direction. The average stimulus value associated with the last 8 reversals was taken as the threshold estimate for that staircase run.
SFM Display and Procedure
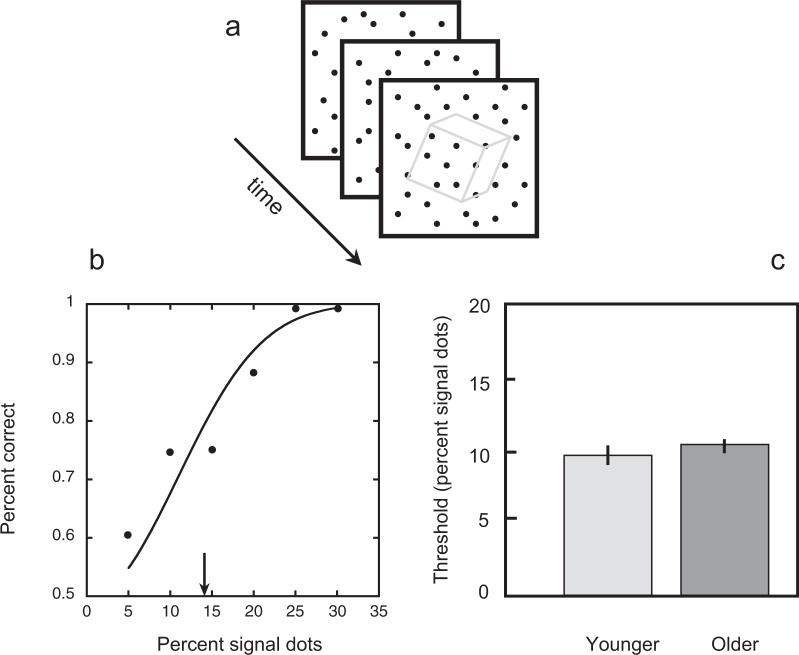
We assessed perception of SFM using a 2AFC shape-identification task in which the observer had to report the shape of a three-dimensional (3D) object defined exclusively in terms of motion parallax of dots moving on the surface of the object (Rizzo & Nawrot, 1998). These SFM objects were created by the orthogonal projection of small (2′ × 2′) white dot s onto a mathematically defined, rotating 3D sphere or 3D cube (Figure 2a). The motion-defined object, whether a sphere or a cube, subtended approximately 2.8° of visual arc in diameter, and the object appeared canted 45° about the x and z axes to stand on a vertex. The figure rotated slowly (66 angular degrees/sec) about either the horizontal or the vertical axis, with the axis of rotation varied randomly over presentations. The motion-defined object appeared within a square region 8° × 8°) that was filled with 1,000 small (2′ × 2′) white “noise” dots that each moved at 3°/sec everywhere within the square region; noise dots could change their directions of motion haphazardly. The exact position of the SFM object within the noise field was jittered spatially over trials, and each trial consisted of a single 5.4-sec presentation of one of the two shapes. In the absence of noise dots, the two shapes were easily distinguished, but when embedded in noise dots, the shapes were difficult or, in the extreme cases, impossible to perceive. In the actual experiment, the percentage of dots defining the figure was varied randomly over trials from 5% to 35% using a method of constant stimuli. Each participant completed a total of 48 trials. From the percentage-correct performance at each signal level, we determined the threshold (defined as 75% correct for a 2AFC task) using probit analysis.
Figure 2.
Structure from motion task. a. Three successive frames from an animation in which a subset of dynamic dots define a rotating object, in this case a cube; the arrow denotes the passage of time. The dots defining the figure were contained within a larger array of “noise” dots that moved randomly from frame to frame. The percentage of dots defining the figure could be varied to find the minimum number necessary to identify the object at some criterion level of performance. b. Representative psychometric function (older observer) for the structure from motion task wherein the observer judged on each trial whether a dot-defined figure embedded in noise was a cube or a sphere. Chance performance was 50% on this two-alternative forced-choice task, and threshold is defined as the percentage of signal dots associated with 75% correct performance (estimated from the best-fit Weibull function). c. Average thresholds for young and older observers.
Results
SFM
Figure 2b shows a representative psychometric function for one older observer tested on the identification task involving discrimination of SFM (sphere vs cube). Thresholds were defined as the signal level yielding 75% correct performance (chance performance being 50%). Among the 19 older participants in the SFM experiment, these thresholds ranged from 6.4% to 14%, with the average across older observers being 10.25% (SE = 0.45). This value is indistinguishable from the thresholds obtained from the young observers tested on this task, whose average was 9.82% (SE = 0.88). Figure 2c shows the average thresholds for the observer groups on this task. It should be noted that the task was difficult except at the highest signal levels, with performance falling to near-chance levels when signal strength was at its lowest value. Hence, the failure to find differences between young and older observers on this task is not attributable to a ceiling effect whereby the task is too simple to leave room for differentiation among groups.
Form From LUM
Figure 3a shows a representative staircase record from one young observer and one from one older observer tested on the LUM task, and Figure 4a shows the average thresholds for the two groups of observers (young and older). Both groups produced comparable thresholds for discriminating shape from luminance contrast (older: 0.12 [SE = 0.04] vs. young: 0.11 [SE = 0.02]).
Figure 3.
Representative staircase records from the luminance contrast (LUM) and the temporal structure (TS) tasks. Each record shows trial-by-trial variations in the stimulus dimension distinguishing figure from background. a. Staircase records from one young adult and one older adult on the LUM task. The ordinate plots LUM of the figure relative to the background—the task is more difficult when LUM values are smaller, which is why the staircase starts at high contrast values and descends to contrast values over trials. b. Staircase records from one young adult and two older adults on the TS tasks. The two staircases for the older adults show the two extremes in performance among the population of older observers. The ordinate plots the correlation in TS between figure and background elements—the task is more difficult when correlation is high (i.e., figure and background TS are more similar), which is why the staircase starts at low correlation values and ascends to higher values over trials.
Figure 4.
Histograms showing summary of performance on the luminance contrast (LUM) and temporal structure (TS) tasks. a. Average threshold for identifying form from LUM for young and older observers. Higher values of LUM = poorer performance. b. Average correlation threshold for identifying form from TS for young and older observers. Higher values of correlation = better performance (therefore, vertical axis is labeled sensitivity).
From earlier measurements, we had estimates of contrast sensitivity for each of the older observers, allowing us to compute the correlation between contrast sensitivity and LUM thresholds. That correlation turned out to be −0.64 ( p < .01): Individuals with higher contrast sensitivity had lower LUM thresholds, which makes sense because the target figure in the LUM display was defined by luminance contrast.
Form From TS
Reproduced in Figure 3b are staircase records for one young adult and two older adults on the TS task, including results from one older adult whose performance fell near the average of the older group and another older adult who exhibited the best performance among all older participants tested on the TS task. These staircase records plot the changes in correlation value over trials dependent on the observer's performance, as dictated by the 3:1 staircase rule. Keep in mind that the correlation index is inversely related to task difficulty: Very low correlation values mean that the TS defining the figural region bore little relation to the TS defining the background, whereas high correlation values mean that TS in both regions was highly similar (making segmentation of figure from ground difficult). Not surprisingly, the particulars of the staircase varied among observers, but in all instances, performance tended to rise to a given range of correlation values and then fluctuate haphazardly within that range until the requisite number of staircase reversals terminated the series.
Figure 4b shows the group-averaged thresholds and associated standard errors of the mean. In this figure, one can see that older participants showed poorer performance (lower correlation thresholds) on the TS task than the young participants (0.18 [SE = 0.13] vs. 0.51 [SE = 0.20]; p < .0001). TS thresholds among the older observers were negatively correlated with age, such that older observers tended to have higher thresholds (r = −.36, p < 01); among the young group, this correlation was negligible (r = .09, ns). We also calculated the correlation between TS thresholds and LUM thresholds for our 20 older observers, and that correlation was nil (r = .01). Obviously, these two tasks—TS and LUM—are tapping different aspects of visual perception.
Effect of Reduced Retinal Illumination
It is well known that the size of the pupil in response to a given light level is smaller in older people (the condition called pupillary miosis). Could the reduced level of light reaching the eyes of our older observers cause their impaired sensitivity to TS? It is also known that sensitivity to temporal frequency is somewhat lower in older adults, as evidenced by modest increases in the threshold luminance modulation necessary to perceive repetitive sinusoidal flicker. Some of this loss in flicker sensitivity is attributable to reduced retinal illuminance caused by pupillary miosis (Kim & Mayer, 1994).
We doubt, however, whether reduced illuminance accounts for the deficits seen in our study. For one thing, the time-varying signals in our study were well above threshold and, therefore, easily seen, whereas the losses in flicker sensitivity with aging pertain to time-varying signals whose amplitudes are at the limits of visibility. Moreover, older observers can perceptually group a subset of elements on the basis of their common TS when TS is conspicuously different in figure and ground. Still, we were compelled to evaluate whether reductions in retinal illuminance could indeed reduce sensitivity to form from TS.
To accomplish this, we retested 5 of the young observers (mean age, 28.8 years) on the TS task while they wore glasses containing 0.50 neutral density filters; the resulting level of light attenuation approximates the reduced retinal illumination caused by pupillary miosis in a 65-year-old adult. Among these 5 young individuals, the average TS thresholds without the filters was 0.59, and with the filters it was 0.61; among the 5 observers, the correlation between TS thresholds with and without filters was highly positive even though the range of thresholds was relatively small among observers (r = .81).
The measurements with filters in place were all performed after these observers had already been tested on the TS task, so their performance with the filters could have included some improvement owing to practice (Aslin, Blake, & Chun, 2002). To minimize possible practice effects, we retested 3 of these individuals on six successive staircase tests, alternating between normal viewing and filtered viewing. For none of the 3 individuals did practice change the pattern of results: Performance with the filters was equivalent to performance without the filters (Participant 1: 0.62 vs. 0.58, respectively; Participant 2: 0.69 vs. 0.72; Participant 3: 0.81 vs. 0.84).
Discussion
Our results reveal that older individuals are less sensitive to spatial form defined by TS, when sensitivity is indexed by the degree of similarity between TS in the “figure” region compared with TS in the “background” region. This deficit is not attributable to an inability to understand the nature of the task, for the older participants performed accurately at very low correlation values when figure and background elements differed substantially in TS. Rather, their difficulties emerged as this correlation value increased (i.e., as the differential TS between figure and background grew increasingly weaker). Also, older participants performed just as well as young participants on our tasks involving perception of form from LUM and SFM. These tasks, like the TS task, involved visual discriminations whose difficulty varied from easy to very difficult over trials; all three of these tasks were equally demanding in terms of sustained attention, motivation, and working memory. Thus the deficit in perception of TS in the older participants cannot be attributed to general cognitive deficits that adversely affect performance on difficult visual tasks (Hoyer & Plude, 1982).
When comparing visual performance of young versus older individuals, one always worries about possible ocular and/or retinal differences between these two categories of observers. Our participants all possessed normal or corrected-to-normal visual acuity measured at the light level used during the psychophysical tests. To the extent that acuity provides an index of ocular and retinal health, we have no reason to believe our older participant population had difficulty seeing the visual displays. Moreover, our control experiments showed no variations in TS sensitivity with reduced retinal illumination, thus ruling out senile miosis as the cause of the reduced sensitivity to TS among our older observers.
Older people are known to experience difficulties on tasks involving motion perception. Thus, for example, older individuals exhibit deficits in detection of low-contrast moving contours (Sekuler, Hutman, & Owsley, 1980), in perception of optic flow (Atchley & Andersen, 1998), in perception of self-motion from optic flow (Warren, Blackwell, & Morris, 1989), in detection of coherent motion in the presence of noise (Bennett, Sekuler, & Sekuler, 2007; Gilmore, Wenk, Baylor, & Stuve, 1992; Snowden & Kavahagh, 2006), and in the discrimination of differences in motion speed (Norman, Ross, Hawkes & Long, 2003; Snowden & Kavanagh, 2006). Could impaired motion perception be at the root of our older participants’ performance on the TS task? For several reasons, we think not. It is true that the sets of elements comprising figure and background in the LUM and TS displays were contours moving orthogonal to their orientations. However, those contours were high contrast; they were not presented within noise and their changes in direction of translational motion were very conspicuous. Moreover, the individual motion stimuli were identical across all levels of correlation between figure and background, from correlations when the task was trivially easy to correlations when the task was impossible. The critical cue for task performance was not the motion of the elements that distinguished figure from background but, rather, the points in time at which those elements changed their directions of motion. Indeed, form from TS can also be readily perceived in displays in which changes other than motion are used to define TS (Guttman, Gilroy, & Blake, 2005).
What, then, does underlie the impaired ability of older people to perform a figure/ground segmentation task based on TS? Recent modeling work on perception of form from TS suggests to us that the differences in performance between our two participant groups on the TS task may have to do with the strength or the precision of the time-varying neural signals generated by TS in these displays. The following paragraphs summarize the reasoning leading us to propose this hypothesis, starting with an overview of a general model of the visual system's response to time-varying stimulation.
Neural responses governing temporal sensitivity can be modeled using what is termed a biphasic filter (also sometimes called an impulse response function); Watson (1986) provided a detailed description of this class of models and their success in predicting human performance on a wide variety of visual tasks. In overview, brief, transient visual stimulation produces biphasic neural responses, the term biphasic referring to an initial excitatory response followed closely in time by an inhibitory component. The contribution of the inhibitory component is to sharpen the peak of the excitatory response in time; without this second, inhibitory component, the filter's response would be largely sustained (Watson, 1986). Parameters of the model can be estimated from psychophysical data or from physiological data (see Cao, Zele, & Pokorny, 2007, and/or Shinomori & Werner, 2003, for excellent examples of work based on this model).
Applied to perception of form from TS (e.g., Adelson & Farid, 1999; Guttman, Gilroy, & Blake, 2007; Lee & Blake, 1999b), biphasic filters can predict with reasonable accuracy human performance on TS tasks like the one used in our study. This success is not surprising, for biphasic temporal filtering is specifically designed to highlight abrupt, transient changes in a stimulus. The transient events within a TS animation produce patterns of responses within biphasic filters that are strongly correlated with the temporal structure contained in the TS display. Given this general conceptualization of biphasic filters and their involvement in registration of TS, how might we interpret the reduced sensitivity to TS that accompanies aging?
As pointed out earlier, the filters’ outputs are governed by an interaction between excitatory and inhibitory components that constitute distinct neural processes. Reductions in the strengths of either of these components or their relative timings (phase) can reduce the amplitude or the temporal precision of the outputs of these filters. From the results of our experiment, we cannot unequivocally pinpoint which of these factors actually causes the reduced sensitivity to TS in older people. Based on other work, however, it is tempting to believe that the strength of the inhibitory component may be a prime source of this reduced sensitivity. Specifically, Shinomori and Werner (2003) derived estimates of the strengths of the excitatory and inhibitory components of the responses to pairs of visual patterns presented in close temporal proximity, and from those measures, they concluded that the inhibitory component is smaller in amplitude, but not retarded in time, in older individuals. Their result dovetails nicely with physiological evidence showing that levels of the inhibitory neurotransmitter γ-amino butyric acid (GABA) are reduced within the aging brain (Leventhal, Wang, Pu, Zhou, & Ma, 2003). Reduced inhibition would lower the strength of the inhibitory component of the responses of biphasic filters and, thereby, compromise the ability to resolve discrete events occurring closely in time. Such events, of course, are the essence of temporal structure. This hypothesis leads to a clear, testable prediction: Performance by older observers on our TS task should correlate significantly with their performance on the two-pulse, contrast-detection task utilized by Shinomori and Werner (2003).
This hypothesis, even if corroborated in future work, does not specify in which stages of visual processing we should find reduced inhibition with aging. There is, however, growing evidence suggesting that declines of visual function in old age may result from degraded information handling in visual cortical areas. In this vein, older monkeys show delayed intracortical and intercortical transfer of information throughout visual area V2 and parts of area V1 outside layer 4 (Wang, Zhou, Ma, & Leventhal, 2005). These timing impairments coincide with degraded intracortical inhibition associated with reduced levels of GABA, and this degradation may be reversible with pharmacologic interventions (Leventhal et al., 2003). Incidentally, biphasic neural responses of the sort thought to underlie performance on TS tasks have also been proposed as neural mechanisms of temporal resolution in other sensory domains including hearing and touch. It is tempting to wonder whether the losses in sensory function seen in hearing, touch, and vision may all stem from a common neurological consequence of aging (i.e., reduced neural inhibition).
Finally, it is natural to ask whether the reduced ability to perceive form from TS has serious practical implications for visuomotor behavior of older adults. This ability could be crucial in natural settings where the surface qualities of an object (e.g., a threatening animal) blend in seamlessly with the surface qualities of that object's background (e.g., foliage in which the animal is hiding). When the object moves relative to its background, its presence is disclosed by the synchronized movements of its body parts relative to those of the background. Fortunately, we rarely encounter threatening, camouflaged objects during our everyday lives, so reduced sensitivity to TS probably has minimal visual consequences when it comes to detection of such objects. However, TS in the optical input to vision may also be important for grouping of visual features of a given object when viewing multiple, partially overlapping objects (Suzuki & Grabowecky, 2002). Grouping of this kind is required routinely in a cluttered visual environment, and it is thought by some that such grouping is mediated by temporal synchrony in distributed neural activity evoked by spatially extended features (Samonds, Zhou, Bernard, & Bonds, 2006; Singer & Gray, 1995). To the extent that perception of TS taps into the same neural mechanisms, it is tempting to speculate that the difficulties older people can experience when searching for objects in cluttered scenes (e.g., Ho, Sciafla, Caird, & Graw, 2001; Plude & Doussard-Roosevelt, 1989) may arise, in part, from difficulties associated with feature integration that is reliant on TS. It remains for future work to evaluate this possibility.
Acknowledgments
This research was supported by National Institutes of Health Grants NIH EY07760 and NIA AG17717.
Footnotes
Readers may see examples of TS displays by navigating to the following Web site: http://psych-s1.psy.vanderbilt.edu/faculty/blaker/TS/TS.html
Contributor Information
Randolph Blake, Department of Psychology, Vanderbilt University.
Matthew Rizzo, Division of Neuroergonomics, Department of Neurology, Carver College of Medicine, University of Iowa.
Sean McEvoy, Department of Neurology, University of Iowa Medical Center..
References
- Adelson EH, Farid H. Filtering reveals form in temporally structured displays. Science. 1999 December 17;286:2231a. [Google Scholar]
- Aslin C, Blake R, Chun M. Perceptual learning of temporal structure. Vision Research. 2002;42:3019–3030. doi: 10.1016/s0042-6989(02)00386-3. [DOI] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13:297–308. doi: 10.1037//0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler A. The effects of aging on motion detection and direction identification. Vision Research. 2007;47:799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Blake R, Lee S-H. The role of temporal structure in human vision. Behavioral and Cognitive Neuroscience Reviews. 2005;4:21–42. doi: 10.1177/1534582305276839. [DOI] [PubMed] [Google Scholar]
- Brainard D. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Cao D, Zele AJ, Pokorny J. Linking impulse response functions to reaction time: Rod and cone reaction time data and a computational model. Vision Research. 2007;47:1060–1074. doi: 10.1016/j.visres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore GC, Wenk HE, Baylor LA, Stuve TA. Motion perception and aging. Psychology and Aging. 1992;7:654–660. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- Guttman S, Gilroy L, Blake R. Mixed messengers, unified message: Spatial grouping from temporal structure. Vision Research. 2005;45:1021–1030. doi: 10.1016/j.visres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Guttman S, Gilroy L, Blake R. Spatial grouping in human vision: Temporal structure trumps temporal synchrony. Vision Research. 2007;47:219–230. doi: 10.1016/j.visres.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho G, Sciafla CT, Caird JK, Graw T. Visual search for traffic signs: The effects of clutter, luminance, and aging. Human Factors. 2001;43:194–207. doi: 10.1518/001872001775900922. [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Plude DJ. Aging and the allocation of attentional resources in visual information-processing. In: Sekuler R, Kline D, Dismukes K, editors. Aging and human visual function. Liss; New York: 1982. pp. 245–263. [Google Scholar]
- Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurologic Clinics. 2003;21:709–728. doi: 10.1016/s0733-8619(02)00107-x. [DOI] [PubMed] [Google Scholar]
- Kim CBY, Meyer MJ. Foveal sensitivity in healthy aging eyes: II. Cross-sectional aging trends from 18 through 77 years of age. Journal of the Optical Society of America A: Optics, Image Science, and Vision. 1994;11:1958–1969. doi: 10.1364/josaa.11.001958. [DOI] [PubMed] [Google Scholar]
- Kline D, Scheiber H. Visual persistence and temporal resolution. In: Sekuler R, Kline D, Dismukes K, editors. Aging and human visual function. Liss; New York: 1982. pp. 231–244. [Google Scholar]
- Lee S-H, Blake R. Visual form created solely from temporal structure. Science. 1999a May 14;284:1165–1168. doi: 10.1126/science.284.5417.1165. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Blake R. Reply to “Filtering reveals form in temporal dynamic displays.”. Science. 1999b December 17;286:2231a. [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improve visual cortical function in senescent monkeys. Science. 2003 May 2;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Michelson AA. Studies in optics. University of Chicago Press; Chicago: 1927. [Google Scholar]
- Morgan M, Castet E. High temporal frequency synchrony is insufficient for perceptual grouping. Proceedings of the Royal Society B: Biological Sciences. 2002;269:513–516. doi: 10.1098/rspb.2001.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JF, Ross HE, Hawkes LM, Long JR. Aging and the perception of speed. Perception. 2003;32:85–96. doi: 10.1068/p3478. [DOI] [PubMed] [Google Scholar]
- Palmer SE. Vision science: Photons to phenomenology. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Plude D, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology and Aging. 1989;1:4–10. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Nawrot M. Perception of movement and shape in Alzheimer's disease. Brain. 1998;121:2259–2270. doi: 10.1093/brain/121.12.2259. [DOI] [PubMed] [Google Scholar]
- Samonds JM, Zhou Z, Bernard MR, Bonds AB. Synchronous activity in cat visual cortex encodes collinear and cocircular contours. Journal of Neurophysiology. 2006;95:2602–2616. doi: 10.1152/jn.01070.2005. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Hutman LP, Owsley CJ. Human aging and spatial vision. Science. 1980 September 12;209:1255–1256. doi: 10.1126/science.7403884. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Kline D, Dismukes K. Aging and human visual function. Liss; New York: 1982. [PubMed] [Google Scholar]
- Shinomori K, Werner JS. Senescence of the temporal impulse response to a luminous pulse. Vision Research. 2003;43:617–627. doi: 10.1016/S0042-6989(03)00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham W. Temporal processing in the aging auditory system. Journal of the Acoustical Society of America. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Grabowecky M. Overlapping features can be parsed on the basis of rapid temporal cues that produce stable emergent percepts. Vision Research. 2002;42:2669–2692. doi: 10.1016/s0042-6989(02)00326-7. [DOI] [PubMed] [Google Scholar]
- Verrillo RT. Effects of aging on the suprathreshold responses to vibration. Perception & Psychophysics. 1982;32:61–68. doi: 10.3758/bf03204869. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cerebral Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Warren WH, Blackwell AW, Morris MW. Age differences in perceiving the direction of self-motion from optical flow. Journal of Gerontology. 1989;44:147–153. doi: 10.1093/geronj/44.5.p147. [DOI] [PubMed] [Google Scholar]
- Watson AB. Temporal sensitivity. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of perception and human performance. Wiley; New York: 1986. pp. 6.1–6.43. [Google Scholar]